The Antiarrhythmic and Hypotensive Effects of S-61 and S-73, the Pyrrolidin-2-one Derivatives with α1-Adrenolytic Properties
Abstract
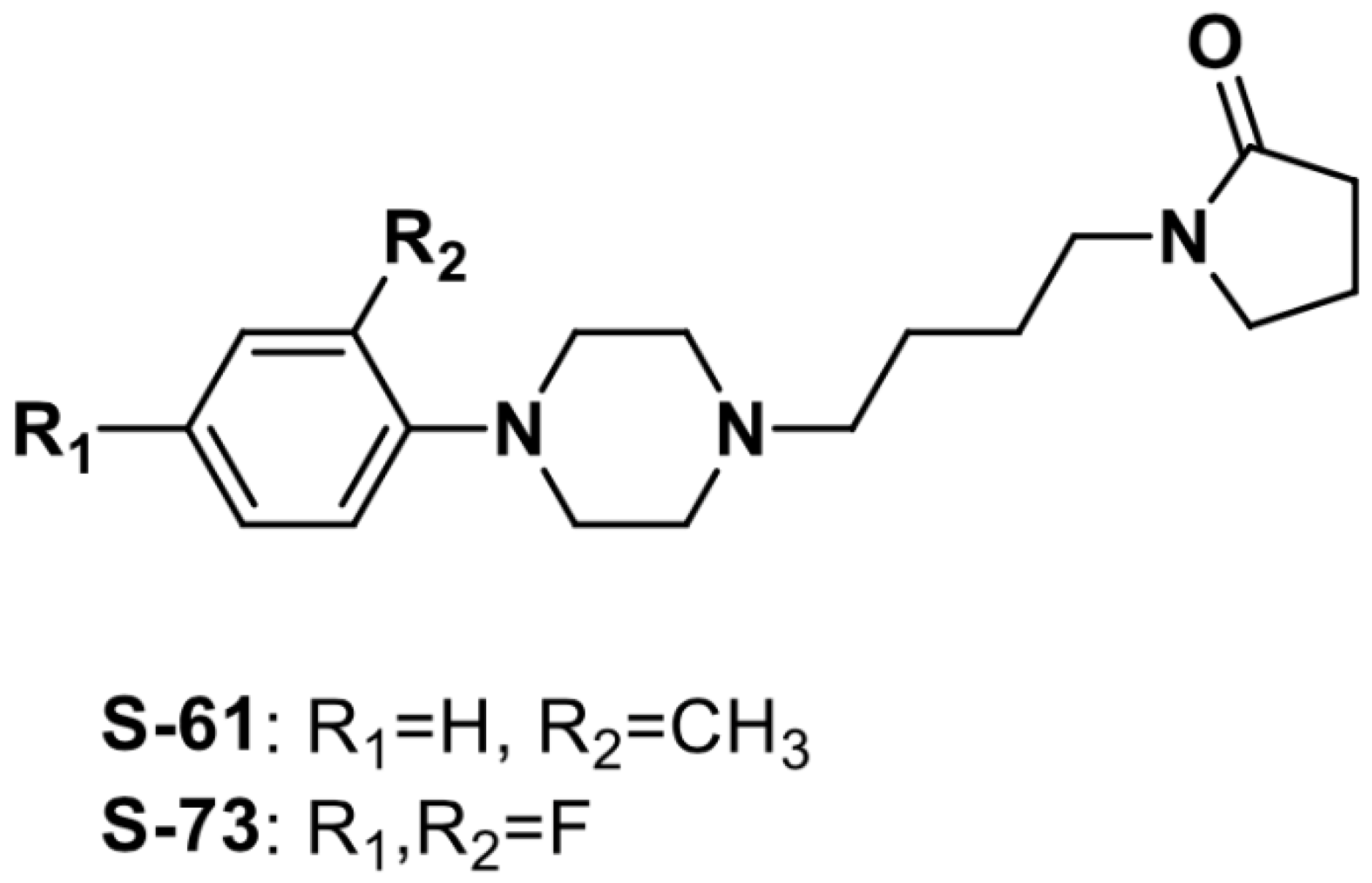
:1. Introduction
2. Results
2.1. S-61 and S-73 Did Not Show Affinity for β1-Adrenergic Receptors
2.2. S-61 and S-73 Showed Prophylactic Antiarrhythmic Activity in Arrhythmia Models Induced by Adrenaline, but Not by Calcium Chloride and Aconitine
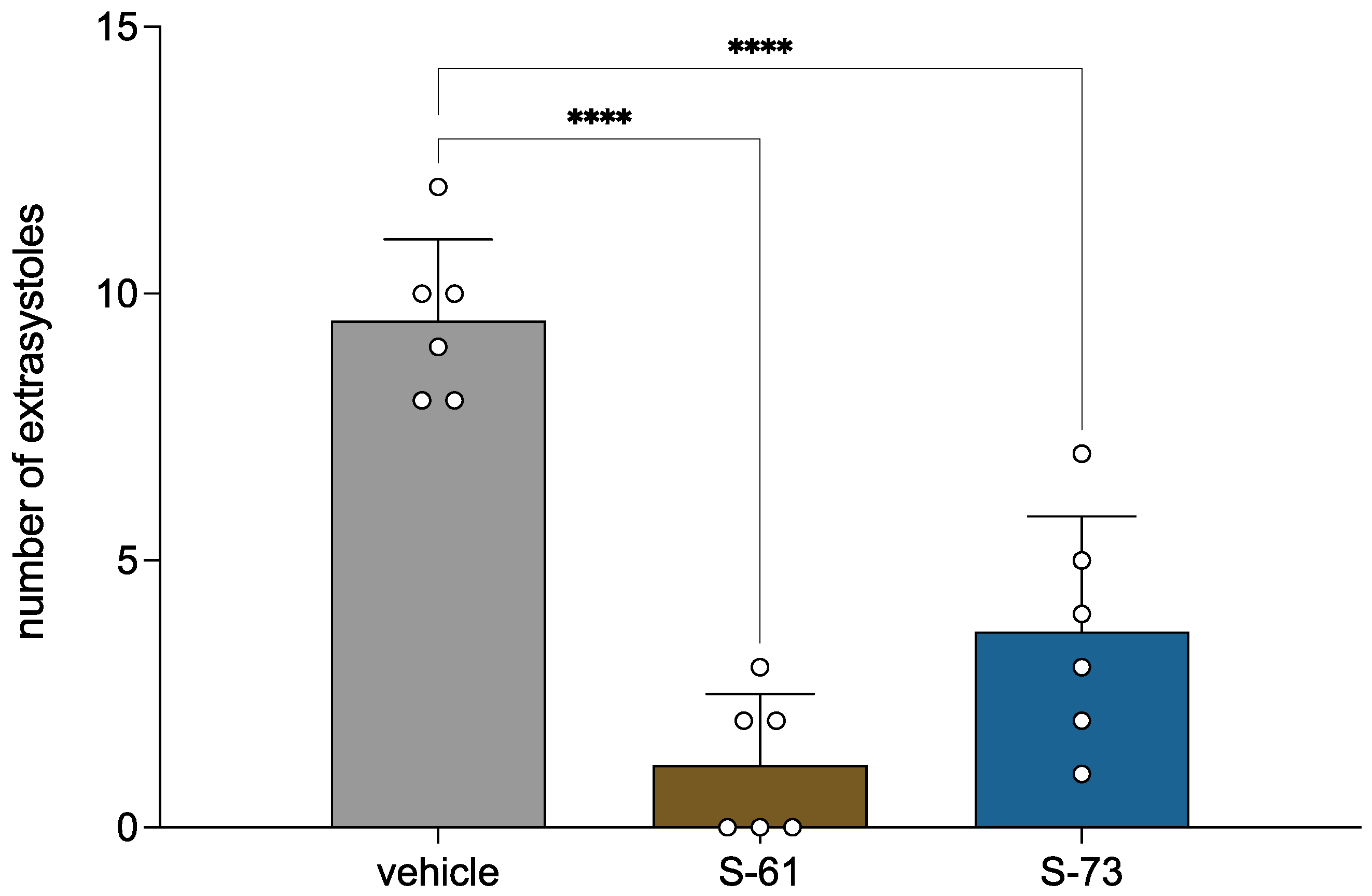
2.3. S-61 and S-73 Showed Therapeutic Antiarrhythmic Activity in Adrenaline-Induced Arrhythmia Model
2.4. S-61 and S-73 Decreased the Heart Rate in the Normal ECG in Rats
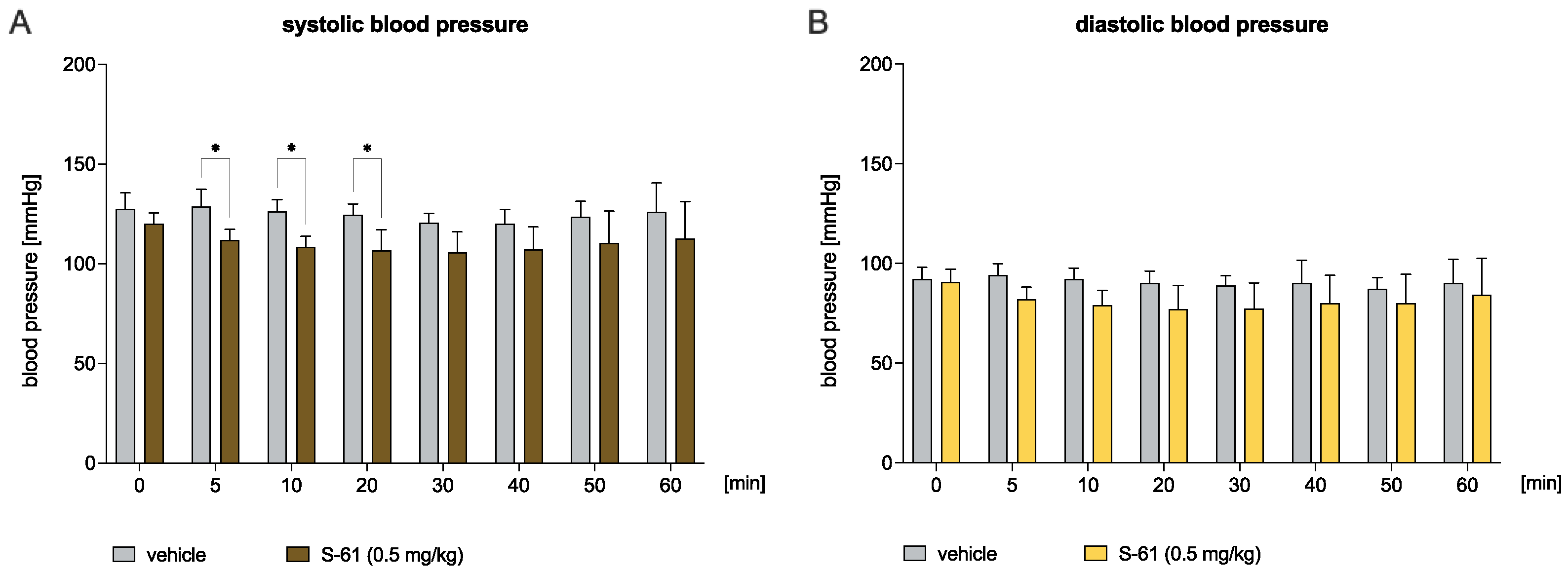
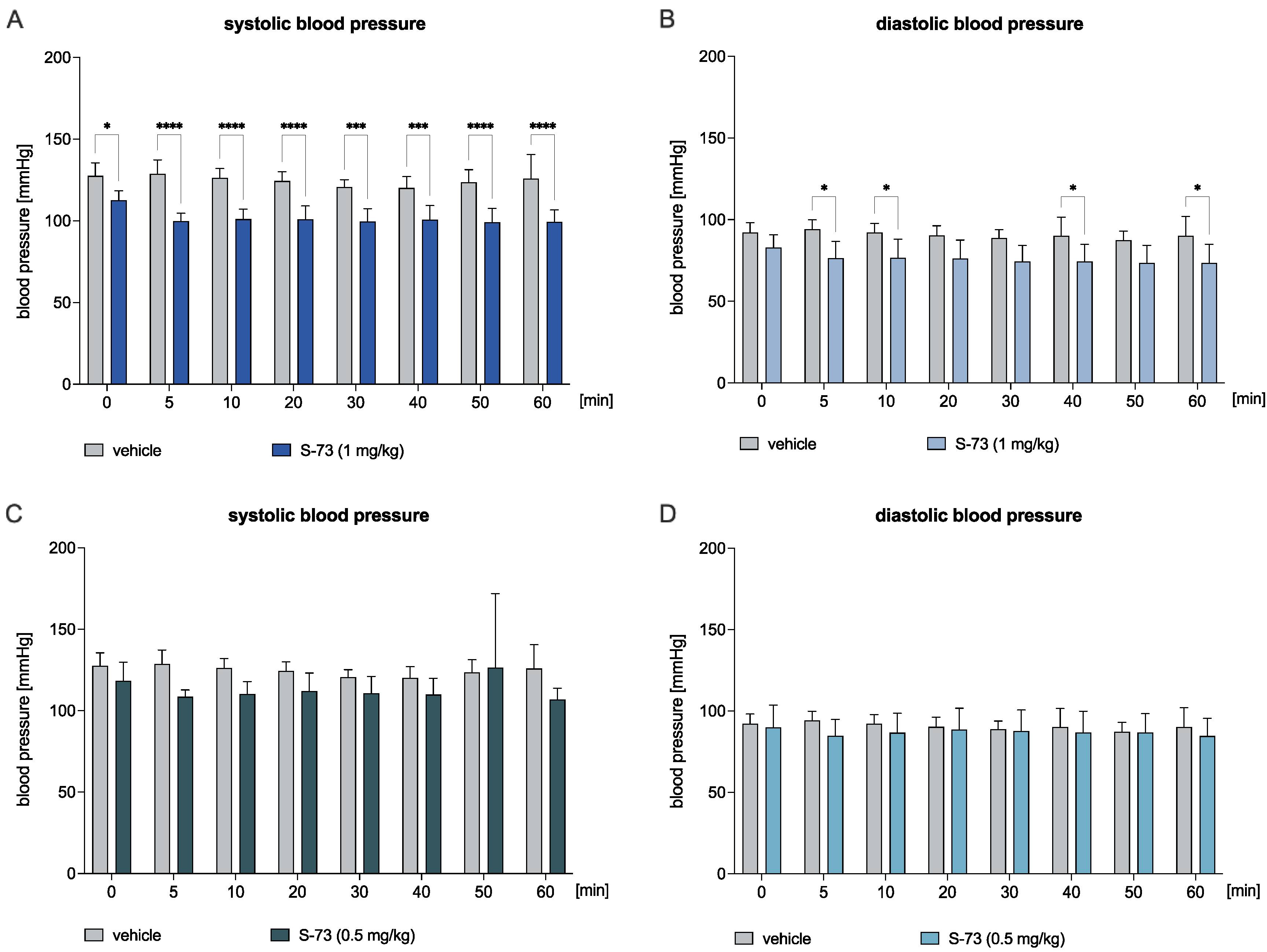
2.5. S-61 and S-73 Decreased the Blood Pressure in the Normotensive Rats
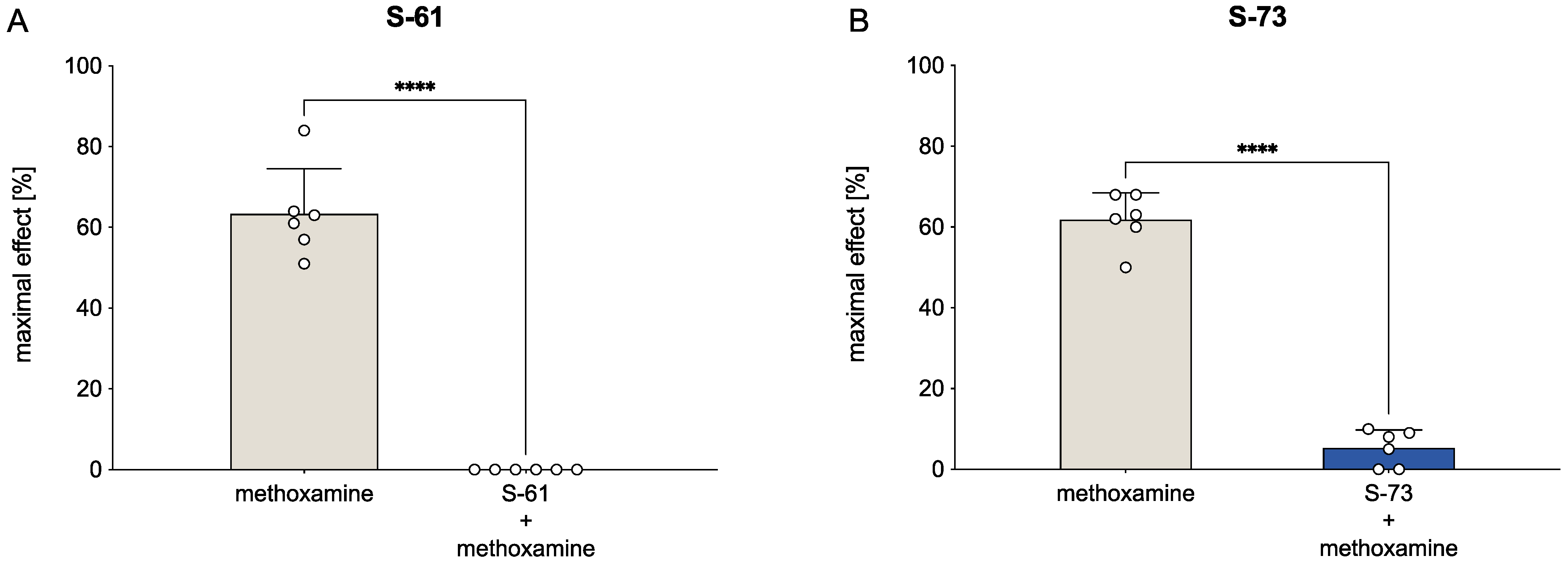
2.6. S-61 and S-73 Abolished the Pressic Effect of Methoxamine
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Animals
4.3. Radioligand Binding Assay
4.4. Prophylactic Antiarrhythmic Activity in Adrenaline-, Aconitine-, and Calcium Chloride-Induced Arrhythmia
4.5. Therapeutic Antiarrhythmic Activity in Adrenaline-Induced Arrhythmia
4.6. Effect on a Normal Electrocardiogram in Rats
4.7. ECG Waveform Analysis
4.8. Influence on Blood Pressure in Normotensive Rats
4.9. Influence on Blood Vasopressor Response in Rats
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuriachan, V.P.; Sumner, G.L.; Mitchell, L.B. Sudden Cardiac Death. Curr. Probl. Cardiol. 2015, 40, 133–200. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.T.; Schilling, R.J. Sudden cardiac death and arrhythmias. Arrhythmia Electrophysiol. Rev. 2018, 7, 111–117. [Google Scholar] [CrossRef]
- Isbister, J.; Semsarian, C. Sudden cardiac death: An update. Intern. Med. J. 2019, 49, 826–833. [Google Scholar] [CrossRef]
- Podrid, P.J. Proarrhythmia, a serious complication of antiarrhythmic drugs. Curr. Cardiol. Rep. 1999, 1, 289–296. [Google Scholar] [CrossRef]
- Mankad, P.; Kalahasty, G. Antiarrhythmic Drugs: Risks and Benefits. Med. Clin. N. Am. 2019, 103, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Wooten, J.M.; Earnest, J.; Reyes, J. Review of common adverse effects of selected antiarrhythmic drugs. Crit. Care Nurs. Q. 2000, 22, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Yamada, K.A.; DaTorre, S.D.; Corr, P.B. Alpha1adrenergic system and arrhythmias in ischaemic heart disease. Eur. Heart J. 1991, 12, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, U.; Tsuruya, Y.; Yaginuma, T. Alpha 1-adrenergic stimulation is coupled to cardiac myocyte hypertrophy. Am. J. Physiol. 1991, 260, H953–H956. [Google Scholar] [CrossRef]
- Terzic, A.; Pucéat, M.; Clément, O.; Scamps, F.; Vassort, G. Alpha 1-adrenergic effects on intracellular pH and calcium and on myofilaments in single rat cardiac cells. J. Physiol. 1992, 447, 275–292. [Google Scholar] [CrossRef]
- Hirano, S.; Kusakari, Y.; O-Uchi, J.; Morimoto, S.; Kawai, M.; Hongo, K.; Kurihara, S. Intracellular mechanism of the negative inotropic effect induced by alpha1-adrenoceptor stimulation in mouse myocardium. J. Physiol. Sci. 2006, 56, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Owens, W.A.; Chen, S.; Stevens, M.E.; Kesteven, S.; Arthur, J.F.; Woodcock, E.A.; Feneley, M.P.; Graham, R.M. Targeted alpha(1A)-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ. Res. 2001, 89, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Alpha-adrenergic mechanisms in myocardial ischemia. Circulation 1990, 81, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Corr, P.B.; Witkowski, F.X. Arrhythmias associated with reperfusion: Basic insights and clinical relevance. J. Cardiovasc. Pharmacol. 1984, 6, S903–S909. [Google Scholar] [PubMed]
- Lamontagne, D.; Yamaguchi, N.; Nadeau, R.; De Champlain, J.; Godin, D.; Campeau, N. Effects of sotalol, (-)-propranolol and prazosin on reperfusion-induced arrhythmias and increased cardiac norepinephrine release. Eur. J. Pharmacol. 1986, 123, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Colucci, W.S. Alpha-adrenergic receptor blockade with prazosin. Consideration of hypertension, heart failure, and potential new applications. Ann. Intern. Med. 1982, 97, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Tölg, R.; Kurz, T.; Ungerer, M.; Schreieck, J.; Görge, B.; Richardt, G. Influence of alpha- and beta-adrenoceptor antagonists on ventricular fibrillation in ischemic rat hearts. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1997, 356, 62–68. [Google Scholar] [CrossRef]
- Bralet, J.; Didier, J.; Moreau, D.; Opie, L.H.; Rochette, L. Effect of alpha-adrenoceptor antagonists (phentolamine, nicergoline and prazosin) on reperfusion arrhythmias and noradrenaline release in perfused rat heart. Br. J. Pharmacol. 1985, 84, 9–18. [Google Scholar]
- Bernauer, W.; Ernenputsch, I. Antagonistic effects of alpha-adrenoceptor blocking agents on arrhythmias, enzyme release, and myocardial necrosis in isolated rat hearts with coronary occlusion and reperfusion. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1988, 338, 88–95. [Google Scholar] [CrossRef]
- Lustyk, K.; Sałaciak, K.; Zaręba, P.; Siwek, A.; Sapa, J.; Pytka, K. The Antiarrhythmic Activity of Novel Pyrrolidin-2-one Derivative S-75 in Adrenaline-Induced Arrhythmia. Pharmaceuticals 2021, 14, 1065. [Google Scholar] [CrossRef]
- Sapa, J.; Nowaczyk, A.; Kulig, K. Antiarrhythmic and antioxidant activity of novel pyrrolidin-2-one derivatives with adrenolytic properties. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 13–25. [Google Scholar] [CrossRef]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [PubMed]
- De Clerck, F.; Van de Water, A.; D’Aubioul, J.; Lu, H.R.; van Rossem, K.; Hermans, A.; Van Ammel, K. In vivo measurement of QT prolongation, dispersion and arrhythmogenesis: Application to the preclinical cardiovascular safety pharmacology of a new chemical entity. Fundam. Clin. Pharmacol. 2002, 16, 125–140. [Google Scholar] [CrossRef]
- Gupta, M.; Ojha, M.; Yadav, D.; Pant, S.; Yadav, R. Novel Benzylated (Pyrrolidin-2-one)/(Imidazolidin-2-one) Derivatives as Potential Anti-Alzheimer’s Agents: Synthesis and Pharmacological Investigations. ACS Chem. Neurosci. 2020, 11, 2849–2860. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Dong, S.; Chen, X.; Qian, K.; Wang, H.; Quan, H.; Zhang, Z.; Zuo, Y.; Huang, L.; Li, D.; et al. Design, Synthesis, Biological Evaluation, Homology Modeling and Docking Studies of (E)-3-(benzo[d][1,3]dioxol-5-ylmethylene) Pyrrolidin-2-one Derivatives as Potent Anticonvulsant Agents. Bioorg. Med. Chem. Lett. 2018, 28, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Bristow, L.J.; Gulia, J.; Weed, M.R.; Srikumar, B.N.; Li, Y.W.; Graef, J.D.; Naidu, P.S.; Sanmathi, C.; Aher, J.; Bastia, T.; et al. Preclinical characterization of (R)-3-((3S,4S)-3-fluoro-4-(4-hydroxyphenyl)piperidin-1-yl)-1-(4-methylbenzyl)pyrrolidin-2-one (BMS-986169), a novel, intravenous, glutamate N-methyl-D-aspartate 2B receptor negative allosteric modulator with potential in major depressive disorder. J. Pharmacol. Exp. Ther. 2017, 363, 377–393. [Google Scholar] [PubMed]
- Malawska, K.; Rak, A.; Gryzło, B.; Sałat, K.; Michałowska, M.; Żmudzka, E.; Lodarski, K.; Malawska, B.; Kulig, K. Search for new potential anticonvulsants with anxiolytic and antidepressant properties among derivatives of 4,4-diphenylpyrrolidin-2-one. Pharmacol. Rep. 2017, 69, 105–111. [Google Scholar] [CrossRef]
- Sifferlen, T.; Boller, A.; Chardonneau, A.; Cottreel, E.; Gatfield, J.; Treiber, A.; Roch, C.; Jenck, F.; Aissaoui, H.; Williams, J.T.; et al. Substituted pyrrolidin-2-ones: Centrally acting orexin receptor antagonists promoting sleep. Part 2. Bioorg. Med. Chem. Lett. 2015, 25, 1884–1891. [Google Scholar] [CrossRef]
- Shirota, H.; Chiba, K.; Ono, H.; Yamamoto, H.; Kobayashi, S.; Terato, K.; Ikuta, H.; Yamatsu, I.; Katayama, K. Pharmacological properties of the novel non-steroidal antiinflammatory agent N-methoxy-3-(3,5-di-tert-butyl-4-hydroxybenzylidene)pyrrolidin-2 -one. Arzneimittelforschung 1987, 37, 930–936. [Google Scholar]
- Muralidharan, V.P.; Alagumuthu, M.; Iyer, S.K. Iodine catalyzed three component synthesis of 1-((2-hydroxy naphthalen-1-yl)(phenyl)(methyl))pyrrolidin-2-one derivatives: Rationale as potent PI3K inhibitors and anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 2510–2514. [Google Scholar] [CrossRef]
- Ramachandran, G.; Karthikeyan, N.S.; Giridharan, P.; Sathiyanarayanan, K.I. Efficient iodine catalyzed three components domino reaction for the synthesis of 1-((phenylthio)(phenyl)methyl)pyrrolidin-2-one derivatives possessing anticancer activities. Org. Biomol. Chem. 2012, 10, 5343–5346. [Google Scholar] [CrossRef]
- Dascalu, A.E.; Ghinet, A.; Lipka, E.; Furman, C.; Rigo, B.; Fayeulle, A.; Billamboz, M. Design, synthesis and evaluation of hydrazine and acyl hydrazone derivatives of 5-pyrrolidin-2-one as antifungal agents. Bioorganic Med. Chem. Lett. 2020, 30, 127220. [Google Scholar] [CrossRef]
- Okaniwa, M.; Shibata, A.; Ochida, A.; Akao, Y.; White, K.L.; Shackleford, D.M.; Duffy, S.; Lucantoni, L.; Dey, S.; Striepen, J.; et al. Repositioning and Characterization of 1-(Pyridin-4-yl)pyrrolidin-2-one Derivatives as Plasmodium Cytoplasmic Prolyl-tRNA Synthetase Inhibitors. ACS Infect. Dis. 2021, 7, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Kulig, K.; Spieces, C.; Sapa, J.; Caspers, C.; Filipek, B.; Malawska, B. Synthesis and pharmacological evaluation of pyrrolidin-2-one derivatives as antiarrhythmic, antihypertensive and alpha-adrenolytic agents. Pharmacol. Rep. 2010, 62, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Kulig, K.; Sapa, J.; Nowaczyk, A.; Filipek, B.; Malawska, B. Design, synthesis and pharmacological evaluation of new 1-[3-(4-arylpiperazin-1-yl)-2-hydroxy-propyl]-3,3-diphenylpyrrolidin-2-one derivatives with antiarrhythmic, antihypertensive, and alpha-adrenolytic activity. Eur. J. Med. Chem. 2009, 44, 3994–4003. [Google Scholar] [CrossRef]
- Zaręba, P.; Dudek, M.; Lustyk, K.; Siwek, A.; Starowicz, G.; Bednarski, M.; Nowiński, L.; Raźny, K.; Sapa, J.; Malawska, B.; et al. α-Adrenoceptor antagonistic and hypotensive properties of novel arylpiperazine derivatives of pyrrolidin-2-one. Bioorganic Med. Chem. 2015, 23, 2104–2111. [Google Scholar] [CrossRef]
- Hattori, Y.; Kanno, M. Role of alpha1-adrenoceptor subtypes in production of the positive inotropic effects in mammalian myocardium: Implications for the alpha1-adrenoceptor subtype distribution. Life Sci. 1998, 62, 1449–1453. [Google Scholar] [CrossRef]
- Zhang, S.; Takahashi, R.; Yamashita, N.; Teraoka, H.; Kitazawa, T. Alpha(1B)-adrenoceptor-mediated positive inotropic and positive chronotropic actions in the mouse atrium. Eur. J. Pharmacol. 2018, 839, 82–88. [Google Scholar] [CrossRef]
- Kurtzwald-Josefson, E.; Hochhauser, E.; Bogachenko, K.; Harun-Khun, S.; Katz, G.; Aravot, D.; Seidman, J.G.; Seidman, C.E.; Eldar, M.; Shainberg, A.; et al. Alpha blockade potentiates CPVT therapy in calsequestrin-mutant mice. Heart Rhythm 2014, 11, 1471–1479. [Google Scholar] [CrossRef]
- Muresan, L.; Cismaru, G.; Muresan, C.; Rosu, R.; Gusetu, G.; Puiu, M.; Mada, R.O.; Martins, R.P. Beta-blockers for the treatment of arrhythmias: Bisoprolol—A systematic review. Ann. Pharm. Fr. 2022, 80, 617–634. [Google Scholar] [CrossRef]
- Dorian, P. Antiarrhythmic action of beta-blockers: Potential mechanisms. J. Cardiovasc. Pharmacol. Ther. 2005, 10 (Suppl. S1), S15–S22. [Google Scholar] [CrossRef]
- Kubacka, M.; Szkaradek, N.; Mogilski, S.; Pańczyk, K.; Siwek, A.; Gryboś, A.; Filipek, B.; Żmudzki, P.; Marona, H.; Waszkielewicz, A.M. Design, synthesis and cardiovascular evaluation of some aminoisopropanoloxy derivatives of xanthone. Bioorganic Med. Chem. 2018, 26, 3773–3784. [Google Scholar] [CrossRef] [PubMed]
- Richer, L.-P.; Vinet, A.; Kus, T.; Cardinal, R.; Ardell, J.L.; Armour, J.A. Alpha-adrenoceptor blockade modifies neurally induced atrial arrhythmias. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1175–R1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pytka, K.; Lustyk, K.; Żmudzka, E.; Kotańska, M.; Siwek, A.; Zygmunt, M.; Dziedziczak, A.; Śniecikowska, J.; Olczyk, A.; Gałuszka, A.; et al. Chemically Homogenous Compounds with Antagonistic Properties at All α1-Adrenoceptor Subtypes but not β1-Adrenoceptor Attenuate Adrenaline-Induced Arrhythmia in Rats. Front. Pharmacol. 2016, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- De Vecchis, R.; Di Maio, M.; Noutsias, M.; Rigopoulos, A.G.; Ariano, C.; Di Biase, G. High Prevalence of Proarrhythmic Events in Patients With History of Atrial Fibrillation Undergoing a Rhythm Control Strategy: A Retrospective Study. J. Clin. Med. Res. 2019, 11, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Reiffel, J.A. Antiarrhythmic Drugs for Atrial Fibrillation: Selected Features of Ventricular Repolarization That Facilitate Proarrhythmic Torsades de Pointes and Favor Inpatient Initiation. J. Innov. Card. Rhythm Manag. 2021, 12, 4600–4605. [Google Scholar] [CrossRef]
- Szekeres, L.; Papp, J. Experimental Cardiac Arrhythmias” in Experimental Production of Diseases, Part 3, Heart and Circulation; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 1975. [Google Scholar]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Savalia, S.; Acosta, E.; Emamian, V.; Savalia, S.; Acosta, E.; Emamian, V. Classification of Cardiovascular Disease Using Feature Extraction and Artificial Neural Networks. J. Biosci. Med. 2017, 5, 64–79. [Google Scholar] [CrossRef]
- Ghorbani Afkhami, R.; Azarnia, G.; Tinati, M.A. Cardiac arrhythmia classification using statistical and mixture modeling features of ECG signals. Pattern Recognit. Lett. 2016, 70, 45–51. [Google Scholar] [CrossRef]
| Treatment | Adrenergic Receptors—pKi |
|---|---|
| β1 | |
| S-61 | nc |
| S-73 | nc |
| Propranolol | 7.82 |
| Treatment | Dose (mg/kg) | Fibrillations (%) | Extrasystoles (%) | Bradycardia (%) | Blocks (%) | Mortality (%) |
|---|---|---|---|---|---|---|
| Adrenaline-Induced Arrhythmia | ||||||
| Control | - | - | 100 | 100 | 100 | 100 |
| S-61 | 0.5 | - | 50 | 33 | 33 | 0 |
| 0.25 | - | 83 | 17 | 17 | 0 | |
| 0.125 | - | 100 | 100 | 100 | 33 | |
| S-73 | 0.5 | - | 67 | 33 | 67 | 33 |
| 0.25 | - | 67 | 67 | 83 | 33 | |
| 0.125 | - | 100 | 67 | 83 | 50 | |
| Calcium Chloride-Induced Arrhythmia | ||||||
| Control | - | 100 | 100 | 100 | 100 | 100 |
| S-61 | 5 | 100 | 67 | 100 | 100 | 100 |
| S-73 | 5 | 83 | 100 | 100 | 100 | 100 |
| Aconitine-Induced Arrhythmia | ||||||
| Control | - | 100 | 100 | 100 | 100 | 100 |
| S-61 | 5 | 100 | 100 | 100 | 100 | 100 |
| S-73 | 5 | 67 | 100 | 100 | 83 | 100 |
| Treatment | Dose (mg/kg) | Bradycardia (%) | Blocks (%) | Mortality (%) |
|---|---|---|---|---|
| Control | - | 100 | 83 | 67 |
| S-61 | 5 | 50 | 33 | 0 |
| S-73 | 5 | 50 | 67 | 0 |
| Treatment | Dose (mg/kg) | Parameters | Time of Observation [min] | |||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | |||
| S-61 | 5 | PQ | 42.89 ± 1.31 | 43.44 ± 2.25 | 41.33 ± 1.12 | 44.89 ± 1.50 |
| QRS | 35.44 ± 0.98 | 37.11 ± 3.17 | 37.89 ± 1.36 | 36.11 ± 1.15 | ||
| QTc | 178.2 ± 3.93 | 171.8 ± 6.53 | 178.6 ± 6.69 | 179.9 ± 5.05 | ||
| QT | 80.57 ± 0.69 | 81.53 ± 4.44 | 82.47 ± 5.35 | 83.90 ± 4.24 | ||
| Rate | 312.2 ± 4.39 | 272.0 ± 1.66 **** | 279.2 ± 7.56 **** | 307.0 ± 7.21 | ||
| S-73 | 5 | PQ | 47.11 ± 2.14 | 48.33 ± 2.22 | 47.56 ± 1.38 | 48.11 ± 2.08 |
| QRS | 29.44 ± 1.77 | 30.33 ± 2.30 | 30.67 ± 1.40 | 30.67 ± 1.52 | ||
| QTc | 185.9 ± 11.35 | 186.4 ± 15.84 | 180.0 ± 18.47 | 180.2 ± 20.72 | ||
| QT | 80.57 ± 0.69 | 81.53 ± 4.44 | 82.47 ± 5.40 | 83.90 ± 4.24 | ||
| Rate | 320.0 ± 13.81 | 278.1 ± 19.31 **** | 277.1 ± 7.77 **** | 269.0 ± 10.95 **** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lustyk, K.; Sałaciak, K.; Siwek, A.; Sapa, J.; Zaręba, P.; Gałuszka, A.; Pytka, K. The Antiarrhythmic and Hypotensive Effects of S-61 and S-73, the Pyrrolidin-2-one Derivatives with α1-Adrenolytic Properties. Int. J. Mol. Sci. 2022, 23, 10381. https://doi.org/10.3390/ijms231810381
Lustyk K, Sałaciak K, Siwek A, Sapa J, Zaręba P, Gałuszka A, Pytka K. The Antiarrhythmic and Hypotensive Effects of S-61 and S-73, the Pyrrolidin-2-one Derivatives with α1-Adrenolytic Properties. International Journal of Molecular Sciences. 2022; 23(18):10381. https://doi.org/10.3390/ijms231810381
Chicago/Turabian StyleLustyk, Klaudia, Kinga Sałaciak, Agata Siwek, Jacek Sapa, Paula Zaręba, Adam Gałuszka, and Karolina Pytka. 2022. "The Antiarrhythmic and Hypotensive Effects of S-61 and S-73, the Pyrrolidin-2-one Derivatives with α1-Adrenolytic Properties" International Journal of Molecular Sciences 23, no. 18: 10381. https://doi.org/10.3390/ijms231810381
APA StyleLustyk, K., Sałaciak, K., Siwek, A., Sapa, J., Zaręba, P., Gałuszka, A., & Pytka, K. (2022). The Antiarrhythmic and Hypotensive Effects of S-61 and S-73, the Pyrrolidin-2-one Derivatives with α1-Adrenolytic Properties. International Journal of Molecular Sciences, 23(18), 10381. https://doi.org/10.3390/ijms231810381






