Rapid Detection of Available Nitrogen in Soil by Surface-Enhanced Raman Spectroscopy
Abstract
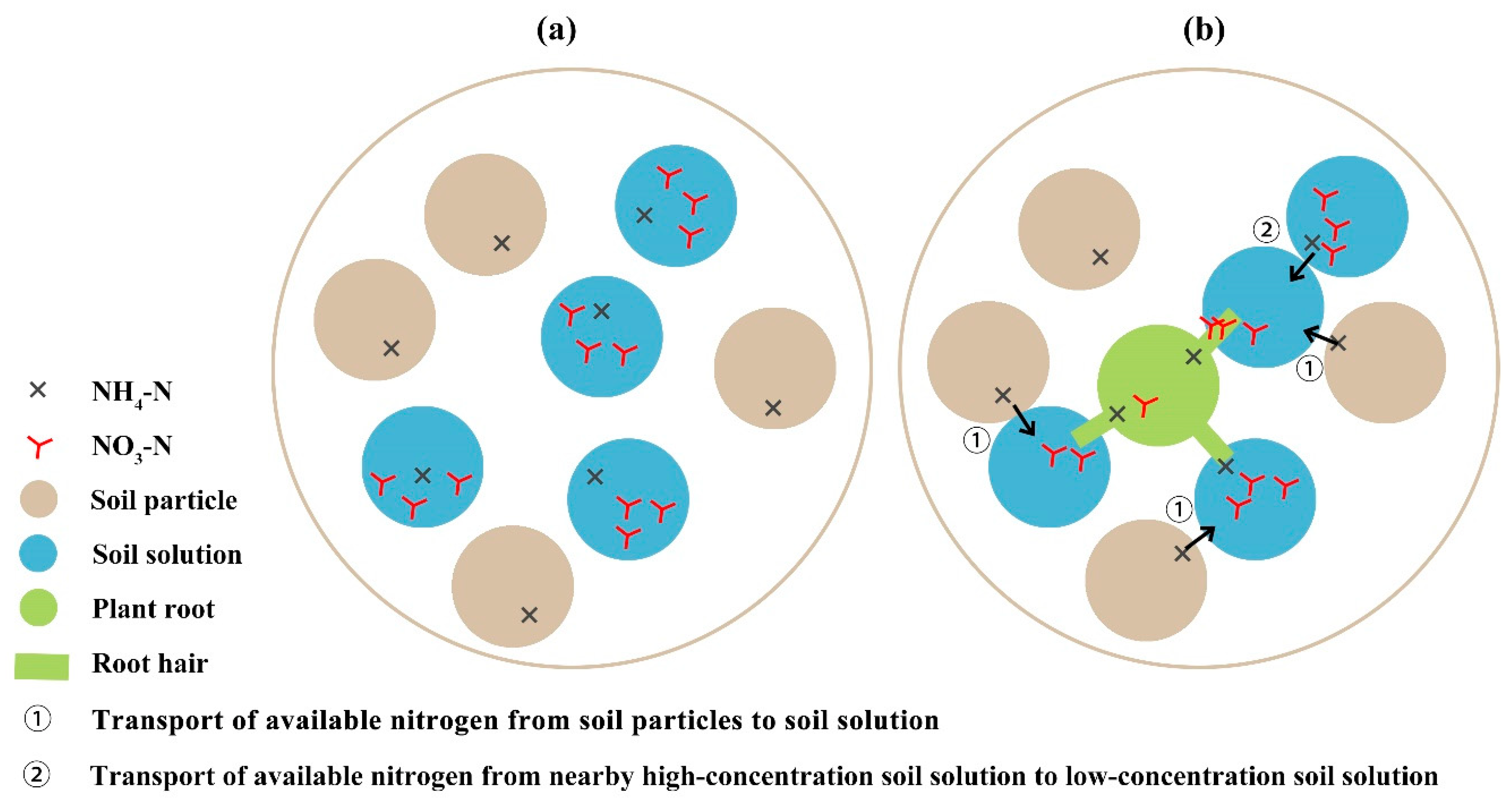
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties Analysis of Soil
2.2. Characterization of Nanosol Substrate
2.3. Spectral Feature of Soil-Available Nitrogen
2.4. Model Analysis of Characteristic Peak
2.5. Model Analysis of Full Band
2.6. Prospects for Application and Implementation
3. Materials and Methods
3.1. Materials and Apparatus
3.2. Ammonium Adsorption Experiment
3.3. Nanosol Substrate Synthesis
3.4. Assay Sample Preparation
3.5. Raman Spectra Acquisition
3.6. Spectral Data Modeling
3.7. Model Performance Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Havlin, J.L. Soil: Fertility and Nutrient Management. In Landscape and Land Capacity, 2nd ed.; Wang, Y., Ed.; Soil; CRC Press: Boca Raton, FL, USA, 2020; pp. 251–265. [Google Scholar]
- Takuji, O. Nitrogen as a major essential element of plants. In Nitrogen Assimilation in Plants; Ohyama, T., Sueyoshi, K., Eds.; Research Signpost: Kerala, India, 2010; Volume 37, pp. 1–18. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers; Pearson Education India: Uttar Pradesh, India, 2016. [Google Scholar]
- Keenan, S.W.; Schaeffer, S.M.; Jin, V.L.; DeBruyn, J.M. Mortality hotspots: Nitrogen cycling in forest soils during vertebrate decomposition. Soil Biol. Biochem. 2018, 121, 165–176. [Google Scholar] [CrossRef]
- Scarsbrook, C.E. Nitrogen Availability. In Soil Nitrogen; Bartholomew, W.V., Clark, F.E., Eds.; Wiley Online Library: Hoboken, NJ, USA, 1965; Volume 10. [Google Scholar] [CrossRef]
- Hirel, B.; Lea, P.J. Ammonia assimilation. In Plant Nitrogen; Lea, P.J., Morot-Gaudry, J.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 79–99. [Google Scholar] [CrossRef]
- Chapin, F.S.; Moilanen, L.; Kielland, K. Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature 1993, 361, 150–153. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.A.; Rentsch, D.; Robinson, N.; Christie, M.; Webb, R.I.; Gamage, H.K.; Carroll, B.J.; Schenk, P.M.; Schmidt, S. Plants can use protein as a nitrogen source without assistance from other organisms. Proc. Natl. Acad. Sci. USA 2008, 105, 4524–4529. [Google Scholar] [CrossRef]
- Kojima, S.; Bohner, A.; Wirén, N.v. Molecular Mechanisms of Urea Transport in Plants. J Membr. Biol 2006, 212, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Z.; Zhang, F.-Z. Activities of nitrate reductase and glutamine synthetase in rice seedlings during cyanide metabolism. J. Hazard. Mater. 2012, 225–226, 190–194. [Google Scholar] [CrossRef]
- Ebbs, S.D.; Kosma, D.K.; Nielson, E.H.; Machingura, M.; Baker, A.J.M.; Woodrow, I.E. Nitrogen supply and cyanide concentration influence the enrichment of nitrogen from cyanide in wheat (Triticum aestivum L.) and sorghum (Sorghum bicolor L.). Plant Cell Environ. 2010, 33, 1152–1160. [Google Scholar] [CrossRef]
- Schrader, L.E.; Domska, D., Jr.; Hung, P.E.J.; Peterson, L.A. Uptake and Assimilation of Ammonium-N and Nitrate-N and Their Influence on the Growth of Corn (Zea mays L.). Agron. J. 1972, 64, 690–695. [Google Scholar] [CrossRef]
- Chance, W.O.I.; Somda, Z.C.; Mills, H.A. Effect of nitrogen form during the flowering period on zucchini squash growth and nutrient element uptake. J. Plant Nutr. 1999, 22, 597–607. [Google Scholar] [CrossRef]
- Lin, L.; Gao, Z.; Liu, X. Estimation of soil total nitrogen using the synthetic color learning machine (SCLM) method and hyperspectral data. Geoderma 2020, 380, 114664. [Google Scholar] [CrossRef]
- Kjeldahl, J.G. Neue methode zur bestimmung des stickstoffs in organischen körpern. Z. Für Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis: Part 3 Chemical Methods, 5.3, 1st ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Bigham, J.M., Bartels, J.M., Eds.; SSSA Book Series; Soil Science Society of America, Inc.; American Society of Agronomy, Inc.: Madison, WI, USA, 1996. [Google Scholar] [CrossRef]
- Nordin, A.; Schmidt, I.K.; Shaver, G.R. Nitrogen Uptake by Arctic Soil Microbes and Plants in Relation to Soil Nitrogen Supply. Ecology 2004, 85, 955–962. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Schrader, L.E.; Youngs, V.L. Analysis by Digestion and Colorimetric Assay of Total Nitrogen in Plant Tissues High in Nitrate. Crop. Sci. 1974, 14, 854–856. [Google Scholar] [CrossRef]
- Garland, N.T.; McLamore, E.S.; Cavallaro, N.D.; Mendivelso-Perez, D.; Smith, E.A.; Jing, D.; Claussen, J.C. Flexible Laser-Induced Graphene for Nitrogen Sensing in Soil. ACS Appl. Mater. Interfaces 2018, 10, 39124–39133. [Google Scholar] [CrossRef]
- Nicolodelli, G.; Cabral, J.; Menegatti, C.R.; Marangonia, B.; Senesi, G.S. Recent advances and future trends in LIBS applications to agricultural materials and their food derivatives: An overview of developments in the last decade (2010–2019). Part I. Soils and fertilizers. TrAC Trends Anal. Chem. 2019, 115, 70–82. [Google Scholar] [CrossRef]
- Ehsani, M.R.; Upadhyaya, S.K.; Slaughter, D.; Shafii, S.; Pelletier, M. A NIR Technique for Rapid Determination of Soil Mineral Nitrogen. Precis. Agric. 1999, 1, 219–236. [Google Scholar] [CrossRef]
- Tahmasbian, I.; Xu, Z.; Boyd, S.; Zhou, J.; Esmaeilani, R.; Che, R.; Bai, S.H. Laboratory-based hyperspectral image analysis for predicting soil carbon, nitrogen and their isotopic compositions. Geoderma 2018, 330, 254–263. [Google Scholar] [CrossRef]
- Hossen, M.A.; Diwakar, P.K.; Ragi, S. Total nitrogen estimation in agricultural soils via aerial multispectral imaging and LIBS. Sci. Rep. Vol. 2021, 11, 12693. [Google Scholar] [CrossRef]
- Kozik, A.; Pavlova, M.; Petrov, I.; Bychkov, V.; Kim, L.; Dorozhko, E.; Cheng, C.; Rodriguez, R.D.; Sheremet, E. A review of surface-enhanced Raman spectroscopy in pathological processes. Anal. Chim. Acta 2021, 1187, 338978. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Ultrasensitive Chemical Analysis by Raman Spectroscopy. Chem. Rev. 1999, 99, 2957–2976. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Chatelain, L.; Scopelliti, R.; Živković, I.; Mazzanti, M. Nitrogen reduction and functionalization by a multimetallic uranium nitride complex. Nature 2017, 547, 332–335. [Google Scholar] [CrossRef]
- FAO; IFA. Fertilizers and Their Use, 4th ed.; FAO: Rome, Italy, 2000. [Google Scholar]
- Maaten, L.v.d.; Hinton, G. Visualizing Data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. Available online: https://www.jmlr.org/papers/volume9/vandermaaten08a/vandermaaten08a.pdf. (accessed on 5 August 2022).
- Verstraete, W.; Focht, D.D. Biochemical Ecology of Nitrification and Denitrification. In Advances in Microbial Ecology, 1st ed.; Alexander, M., Ed.; Springer: Boston, MA, USA, 1977; Volume 1, pp. 135–214. [Google Scholar] [CrossRef]
- Oades, J.M. The Retention of Organic Matter in Soils. Biogeochemistry 1988, 5, 35–70. [Google Scholar] [CrossRef]
- Lützow, M.v.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—Review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Nommik, H.; Vahtras, K. Retention and Fixation of Ammonium and Ammonia in Soils. In Nitrogen in Agricultural Soils; Stevenson, F.J., Ed.; Agronomy Monographs; Wiley Online Library: Hoboken, NJ, USA, 1982; Volume 22, pp. 123–171. [Google Scholar] [CrossRef]
- Bolster, C.H.; Hornberger, G.M. On the Use of Linearized Langmuir Equations. Soil Sci. Soc. Am. J. 2007, 71, 1796–1806. [Google Scholar] [CrossRef]
- Xu, X.; Li, A.; Wang, X.; Ding, C.; Qiu, S.; He, Y.; Lu, T.; He, F.; Zou, B.; Liu, R. The high-accuracy prediction of carbon content in semi-coke by laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2020, 35, 984–992. [Google Scholar] [CrossRef]
- Li, Y.; Wei, M.; Liu, L.; Xue, Q.; Yu, B. Adsorption of toluene on various natural soils: Influences of soil properties, mechanisms, and model. Sci. Total Environ. 2020, 740, 140104. [Google Scholar] [CrossRef]
- Elfanssi, S.; Ouazzani, N.; Mandi, L. Soil properties and agro-physiological responses of alfalfa (Medicago sativa L.) irrigated by treated domestic wastewater. Agric. Water Manag. 2018, 202, 231–240. [Google Scholar] [CrossRef]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Yılmaz, D.; Günaydın, B.N.; Yüce, M. Nanotechnology in food and water security: On-site detection of agricultural pollutants through surface-enhanced Raman spectroscopy. Emergent Mater. 2022, 5, 105–132. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Cheng, L.; Lee, S.-T.; Liu, Z. Noble Metal Coated Single-Walled Carbon Nanotubes for Applications in Surface Enhanced Raman Scattering Imaging and Photothermal Therapy. J. Am. Chem. Soc. 2012, 134, 7414–7422. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Duyne, R.P.V. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Stamplecoskie, K.G.; Scaiano, J.C.; Tiwari, V.S.; Anis, H. Optimal Size of Silver Nanoparticles for Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2011, 115, 1403–1409. [Google Scholar] [CrossRef]
- Tsuboi, M.; Takenishi, T. Infrared Spectrum of α-Aminoisobutyric Acid and the Assignment of the Vibrational Frequencies. Bull. Chem. Soc. Jpn. 1959, 32, 1044–1050. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wharton, D.; Hickey, L.; Frost, R.L. Infrared and Raman study of interlayer anions CO32–, NO3–, SO42– and ClO4– in Mg/Al-hydrotalcite. Am. Mineral. 2002, 87, 623–629. [Google Scholar] [CrossRef]
- Misra, A.K.; Sharma, S.K.; Acosta, T.E.; Porter, J.N.; Lucey, P.G.; Bates, D.E. Portable standoff Raman system for fast detection of homemade explosives through glass, plastic, and water. In Proceedings of the SPIE Defense, Security, and Sensing, Baltimore, MD, USA, 23 April 2012; pp. 261–270. [Google Scholar] [CrossRef]
- Galvão, R.K.H.; Araujo, M.C.U.; José, G.E.; Pontes, M.J.C.; Silva, E.C.; Saldanha, T.C.B. A method for calibration and validation subset partitioning. Talanta 2005, 67, 736–740. [Google Scholar] [CrossRef]
- Chung, P.-R.; Tzeng, C.-T.; Ke, M.-T.; Lee, C.-Y. Formaldehyde Gas Sensors: A Review. Sensors 2013, 13, 4468–4484. [Google Scholar] [CrossRef]
- Umpleby, R.J.; Baxter, S.C.; Chen, Y.; Shah, R.N.; Shimizu, K.D. Characterization of Molecularly Imprinted Polymers with the Langmuir-Freundlich Isotherm. Anal. Chem. 2001, 73, 4584–4591. [Google Scholar] [CrossRef]
- Chow, M.K.; Zukoski, C.F. Gold Sol Formation Mechanisms: Role of Colloidal Stability. J. Colloid Interface Sci. 1994, 165, 97–109. [Google Scholar] [CrossRef]
- Munro, C.H.; Smith, W.E.; Garner, M.; Clarkson, J.; White, P.C. Characterization of the Surface of a Citrate-Reduced Colloid Optimized for Use as a Substrate for Surface-Enhanced Resonance Raman Scattering. Langmuir 1995, 11, 3712–3720. [Google Scholar] [CrossRef]
- Yan, H.; Li, P.-H.; Zhou, G.-S.; Wang, Y.-J.; Bao, B.-H.; Wu, Q.-N.; Huang, S.-L. Rapid and practical qualitative and quantitative evaluation of non-fumigated ginger and sulfur-fumigated ginger via Fourier-transform infrared spectroscopy and chemometric methods. Food Chem. 2021, 341, 128241. [Google Scholar] [CrossRef]
- Brereton, R.G.; Jansen, J.; Lopes, J.; Marini, F.; Pomerantsev, A.; Rodionova, O.; Roger, J.M.; Walczak, B.; Tauler, R. Chemometrics in analytical chemistry—part II: Modeling, validation, and applications. Anal. Bioanal. Chem. 2018, 410, 6691–6704. [Google Scholar] [CrossRef]
- Peris-Díaz, M.D.; Krężel, A. A guide to good practice in chemometric methods for vibrational spectroscopy, electrochemistry, and hyphenated mass spectrometry. TrAC Trends Anal. Chem. 2021, 135, 116157. [Google Scholar] [CrossRef]
- Peter, S.C.; Dhanjal, J.K.; Malik, V.; Radhakrishnan, N.; Jayakanthan, M.; Sundar, D. Quantitative Structure-Activity Relationship (QSAR): Modeling Approaches to Biological Applications. In Encyclopedia of Bioinformatics and Computational Biology; Ranganathan, S., Gribskov, M., Nakai, K., Schönbach, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 2, pp. 661–676. [Google Scholar] [CrossRef]
- Wold, S.; Ruhe, A.; Wold, H.; Dunn, W.J. The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. SIAM J. Sci. Stat. Comput. 1984, 5, 735–743. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Suykens, J.A.K.; Vandewalle, J. Least Squares Support Vector Machine Classifiers. Neural Processing Lett. 1999, 9, 293–300. [Google Scholar] [CrossRef]
- Brabanter, K.D.; Karsmakers, P.; Ojeda, F.; Alzate, C.; Brabanter, J.D.; Pelckmans, K.; Moor, B.D.; Vandewalle, J.; Suykens, J.A.K. LS-SVMlab Toolbox User’s Guide Version 1.8; Department of Electrical Engineering: Leuven, Belgium, 2011; Available online: https://www.esat.kuleuven.be/sista/lssvmlab/downloads/tutorialv1_8.pdf (accessed on 5 August 2022).
- Kennedy, J.; Eberhart, R. Particle Swarm Optimization. In Proceedings of the ICNN’95-International Conference on Neural Networks, Perth, WA, Australia, 27 November–1 December 1995; pp. 1942–1948. [Google Scholar] [CrossRef]


















| Soil Sample | pH | Electrical Conductivity (µm/cm) | Organic Matter (%) | Available Nitrogen (mg/kg) | Available Potassium (mg/kg) | Available Phosphorus (mg/kg) |
|---|---|---|---|---|---|---|
| Soil1 | 6.41 | 25.80 | 16.63 | 348.25 | 5632.87 | 480.60 |
| Soil2 | 4.70 | 31.20 | 2.51 | 59.06 | 226.19 | 212.83 |
| Soil3 | 5.60 | 15.96 | 3.50 | 19.47 | 80.21 | 74.34 |
| Soil | Soil1 | Soil2 | Soil3 | |
|---|---|---|---|---|
| Index | ||||
| pH | 3 | 1 | 2 | |
| Electrical conductivity | 2 | 3 | 1 | |
| pH + electrical conductivity | 5 | 4 | 3 | |
| pH × electrical conductivity | 6 | 3 | 2 | |
| Element | At. No | Net | Atom (%) | Mass (%) | Mass Norm. (%) | Abs. Error (%) (1 Sigma) | Rel. Error (%) (1 Sigma) |
|---|---|---|---|---|---|---|---|
| O | 8 | 339 | 34.96 | 2.28 | 7.39 | 0.75 | 32.82 |
| Ag | 47 | 13,879 | 65.04 | 28.64 | 92.61 | 0.97 | 3.38 |
| Total | / | / | 100.00 | 30.92 | 100.00 | / | / |
| Dataset | Sample Set | Single Variable Linear Regression Equation | Rp2 | RMSEr | RPDr |
|---|---|---|---|---|---|
| DH1 | Soil1 NH4-N | y = 37.0533 + 1082.7043x | 0.984 | 0.046 | 5.022 |
| DH2 | Soil2 NH4-N | y = 55.0753 + 1272.4329x | 0.974 | 0.048 | 6.405 |
| DH3 | Soil3 NH4-N | y = 26.0325 + 1126.2949x | 0.975 | 0.062 | 4.445 |
| DO1 | Soil1 NO3-N | y = 267.8691 + 2242.9603x | 0.959 | 0.083 | 2.722 |
| DO2 | Soil2 NO3-N | y = 619.2549 + 1987.4874x | 0.963 | 0.128 | 1.693 |
| DO3 | Soil3 NO3-N | y = 303.2366 + 1404.7394x | 0.963 | 0.085 | 1.767 |
| Dataset | Sample Set | Multiple Linear Regression Equation | Rp2 | RMSEr | RPDr |
|---|---|---|---|---|---|
| DH | Soil1 NH4-N | z = 124.6083 + 1255.5384x − 0.66569y | 0.9763 | 0.03903 | 6.436 |
| Soil2 NH4-N | |||||
| Soil3 NH4-N | |||||
| DO | Soil1 NO3-N | z = −6894.454 + 2615.5929x + 43.0878y | 0.9377 | 0.06098 | 3.887 |
| Soil2 NO3-N | |||||
| Soil3 NO3-N |
| Dataset | Sample Set | Model | Rc2 | RMSEc | Rp2 | RMSEp | RPD |
|---|---|---|---|---|---|---|---|
| DH1 | Soil1 NH4-N | PLS | 0.9989 | 0.0859 | 0.9769 | 0.2158 | 6.6950 |
| BPNN | 0.9973 | 0.1475 | 0.9905 | 0.1429 | 10.0700 | ||
| LSSVM | 1.0000 | 0.0063 | 0.9962 | 0.0912 | 16.0400 | ||
| DH2 | Soil2 NH4-N | PLS | 0.9994 | 0.0712 | 0.9985 | 0.0849 | 24.7400 |
| BPNN | 0.9985 | 0.1177 | 0.9956 | 0.1464 | 14.4300 | ||
| LSSVM | 1.0000 | 0.0040 | 0.9982 | 0.0924 | 22.9500 | ||
| DH3 | Soil3 NH4-N | PLS | 0.9980 | 0.1370 | 0.9885 | 0.1356 | 7.6820 |
| BPNN | 0.9987 | 0.1125 | 0.9839 | 0.1433 | 7.9710 | ||
| LSSVM | 1.0000 | 0.0047 | 0.9933 | 0.1023 | 10.4200 | ||
| DO1 | Soil1 NO3-N | PLS | 0.9992 | 0.0831 | 0.9949 | 0.0731 | 13.6000 |
| BPNN | 0.9951 | 0.2290 | 0.9345 | 0.3055 | 3.7830 | ||
| LSSVM | 1.0000 | 0.0091 | 0.9980 | 0.0462 | 21.6600 | ||
| DO2 | Soil2 NO3-N | PLS | 0.9893 | 0.3197 | 0.9741 | 0.3213 | 5.4110 |
| BPNN | 0.9984 | 0.1225 | 0.9866 | 0.2141 | 8.7510 | ||
| LSSVM | 1.0000 | 0.0045 | 0.9997 | 0.0386 | 47.8000 | ||
| DO3 | Soil3 NO3-N | PLS | 0.9988 | 0.1049 | 0.9943 | 0.1147 | 12.2000 |
| BPNN | 0.9972 | 0.1625 | 0.9727 | 0.2394 | 5.4510 | ||
| LSSVM | 1.0000 | 0.0039 | 0.9989 | 0.0560 | 24.7500 |
| Sample Set | Nitrogen Concentration (wt%) | Sample Size |
|---|---|---|
| Soil1 NH4-N | 1.371, 1.874, 2.377, 2.880, 3.384, 3.887, 4.390, 4.894, 7.410, 9.926 | 100 |
| Soil1 NO3-N | 0.140, 0.685, 1.230, 1.776, 2.321, 2.867, 3.412, 3.958, 6.685, 9.412 | 100 |
| Soil2 NH4-N | 0.459, 0.960, 1.461, 1.962, 2.463, 2.964, 3.966, 4.968, 7.473, 9.978 | 100 |
| Soil2 NO3-N | 0.108, 0.620, 1.133, 1.645, 2.157, 2.669, 3.694, 4.718, 7.280, 9.841 | 100 |
| Soil3 NH4-N | 0.497, 0.997, 1.497, 1.997, 2.497, 2.998, 3.998, 4.998, 7.498, 9.998 | 100 |
| Soil3 NO3-N | 0.427, 0.929, 1.431, 1.933, 2.436, 2.938, 3.943, 4.947, 7.459, 9.970 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, R.; Zhang, Y.; Ren, S.; Nie, P. Rapid Detection of Available Nitrogen in Soil by Surface-Enhanced Raman Spectroscopy. Int. J. Mol. Sci. 2022, 23, 10404. https://doi.org/10.3390/ijms231810404
Qin R, Zhang Y, Ren S, Nie P. Rapid Detection of Available Nitrogen in Soil by Surface-Enhanced Raman Spectroscopy. International Journal of Molecular Sciences. 2022; 23(18):10404. https://doi.org/10.3390/ijms231810404
Chicago/Turabian StyleQin, Ruimiao, Yahui Zhang, Shijie Ren, and Pengcheng Nie. 2022. "Rapid Detection of Available Nitrogen in Soil by Surface-Enhanced Raman Spectroscopy" International Journal of Molecular Sciences 23, no. 18: 10404. https://doi.org/10.3390/ijms231810404





