Mast Cells Are Activated in the Giant Earlobe Keloids: A Case Series
Abstract
:1. Introduction
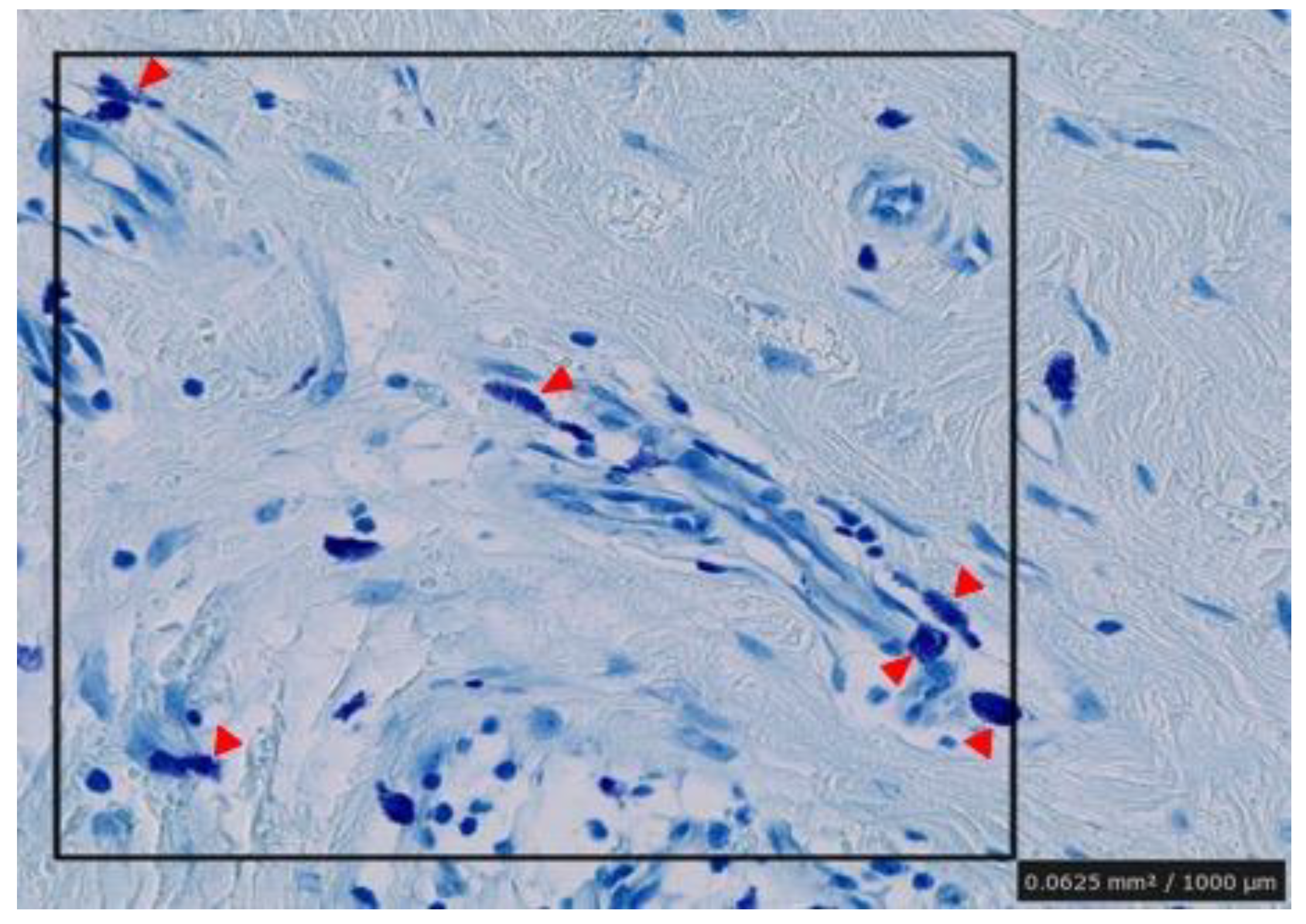
2. Results
3. Discussion
4. Materials and Methods
4.1. Histopathology
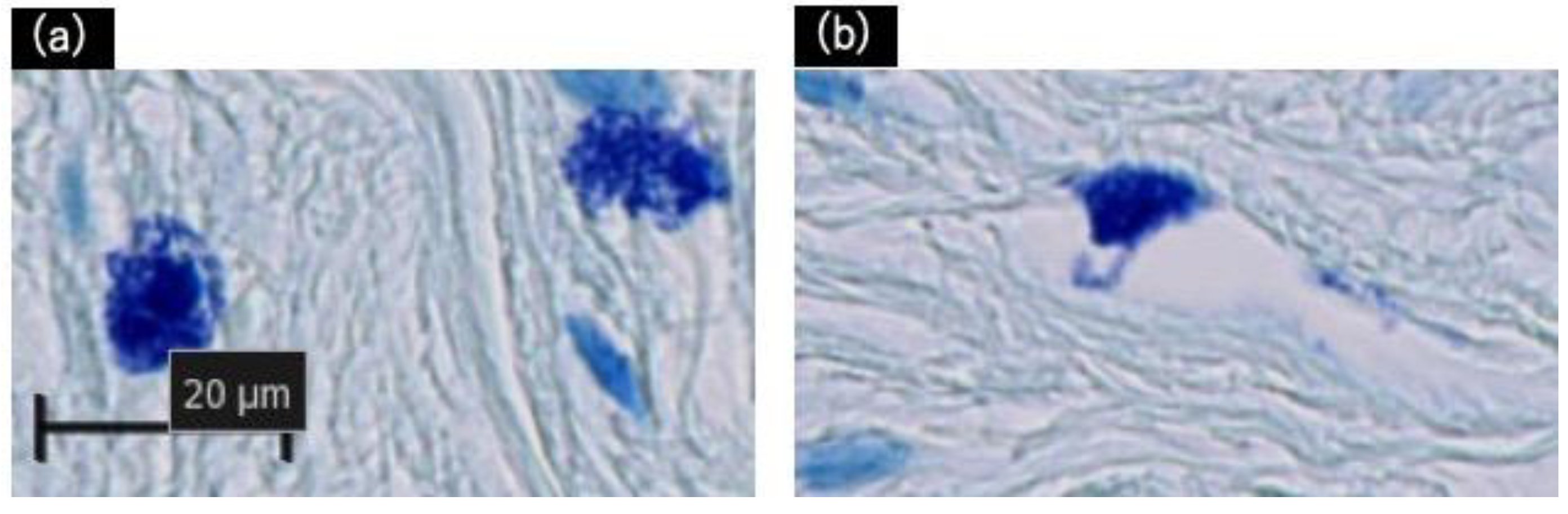
4.2. Immunohistochemistry
4.3. Visual Collagen Quantitations and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nangole, F.W.; Ouyang, K.; Anzala, O.; Ogeng’o, J.; Agak, G.W.; Zuriel, D. Does Keloid Histology Influence Recurrence? Am. J. Dermatopathol. 2021, 43, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef] [PubMed]
- Shaker, S.A.; Ayuob, N.N.; Hajrah, N.H. Cell Talk: A Phenomenon Observed in the Keloid Scar by Immunohistochemical Study. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Nangole, F.W.; Agak, G.W. Keloid Pathophysiology: Fibroblast or Inflammatory Disorders? JPRAS Open 2019, 22, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, C.; Ma, S.; Wen, H. Mast Cell Chymase in Keloid Induces Profibrotic Response via Transforming Growth Factor-β1/Smad Activation in Keloid Fibroblasts. Int. J. Clin. Exp. Pathol. 2014, 7, 3596–3607. [Google Scholar] [PubMed]
- Yamamoto, T.; Hartmann, K.; Eckes, B.; Krieg, T. Mast Cells Enhance Contraction of Three-Dimensional Collagen Lattices by Fibroblasts by Cell-Cell Interaction: Role of Stem Cell Factor/c-kit. Immunology 2000, 99, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Kunder, C.A.; St John, A.L.; Li, G.; Leong, K.W.; Berwin, B.; Staats, H.F.; Abraham, S.N. Mast Cell-Derived Particles Deliver Peripheral Signals to Remote Lymph Nodes. J. Exp. Med. 2009, 206, 2455–2467. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, Y.; Dong, Y.; Zhang, D.; Xia, L.; Sun, X.; Li, H.; Han, C.; Wang, H.; Xu, G. Mast Cell and Heparin Promote Adipogenesis in Superficial Fascia of Rats. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159024. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hartmann, K.; Eckes, B.; Krieg, T. Role of Stem Cell Factor and Monocyte Chemoattractant Protein-1 in the Interaction between Fibroblasts and Mast Cells in Fibrosis. J. Dermatol. Sci. 2001, 26, 106–111. [Google Scholar] [CrossRef]
- Nakamura, Y.; Oscherwitz, J.; Cease, K.B.; Chan, S.M.; Muñoz-Planillo, R.; Hasegawa, M.; Villaruz, A.E.; Cheung, G.Y.; McGavin, M.J.; Travers, J.B.; et al. Staphylococcus δ-Toxin Induces Allergic Skin Disease by Activating Mast Cells. Nature 2013, 503, 397–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Divoux, A.; Sun, J.; Zhang, J.; Clément, K.; Glickman, J.N.; Sukhova, G.K.; Wolters, P.J.; Du, J.; Gorgun, C.Z.; et al. Genetic Deficiency and Pharmacological Stabilization of Mast Cells Reduce Diet-Induced Obesity and Diabetes in Mice. Nat. Med. 2009, 15, 940–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
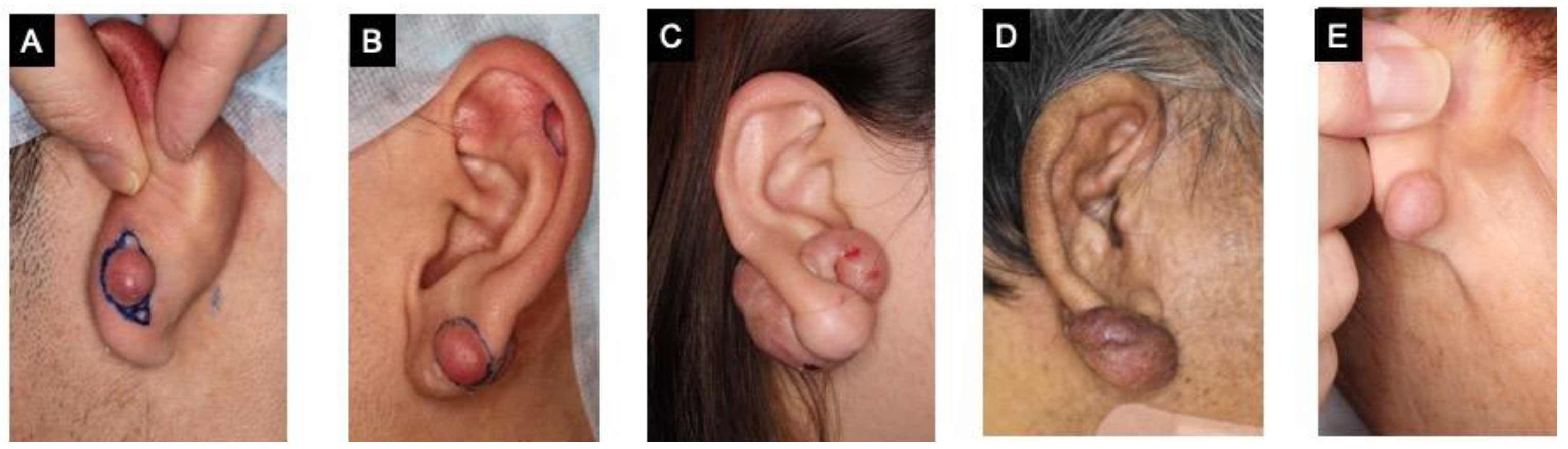
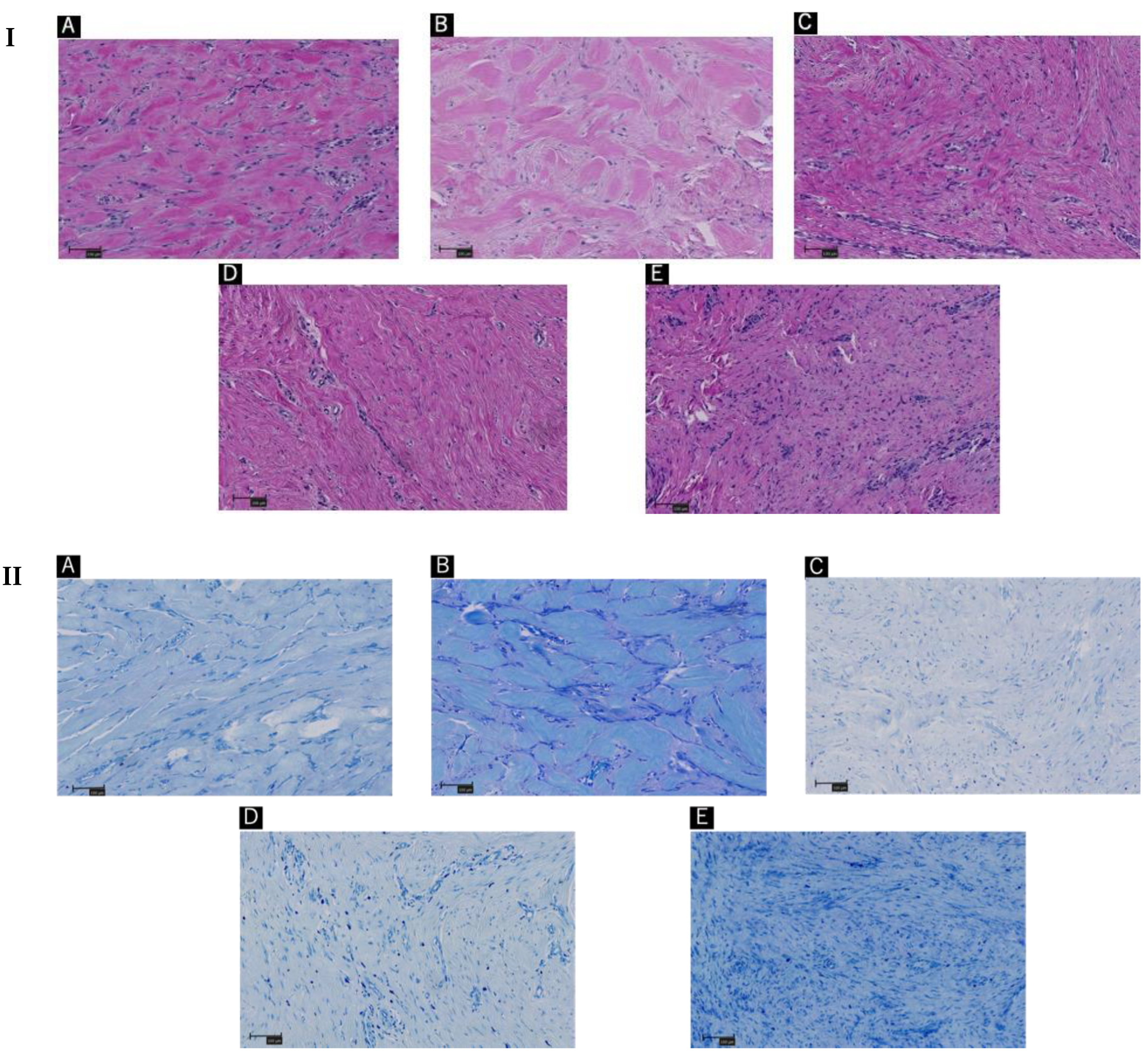
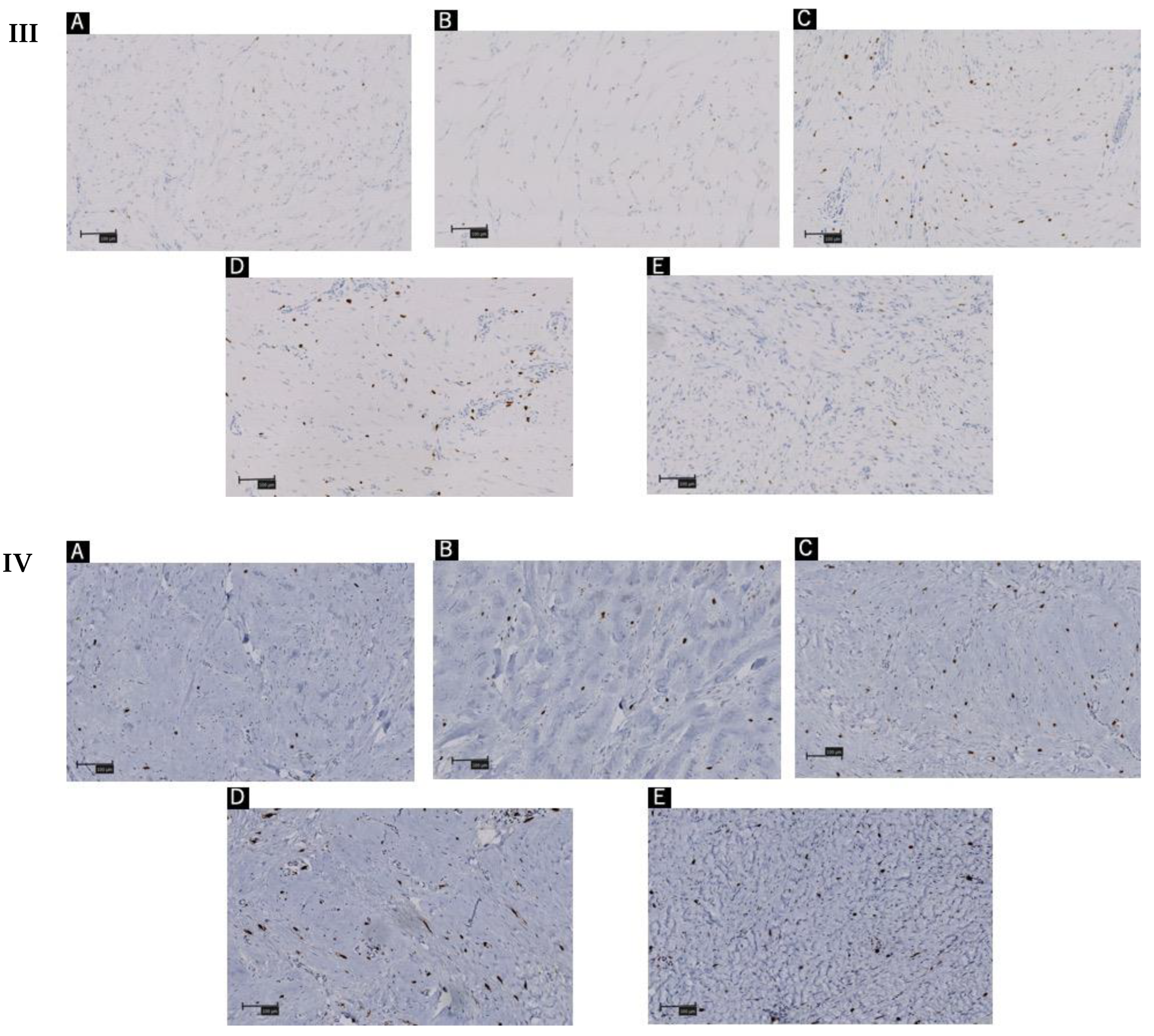
| Patient A | Patient B | Patient C | Patient D | Patient E |
|---|---|---|---|---|
| 50.68 | 48.20 | 69.12 | 58.85 | 58.34 |
| Patient A | Patient B | Patient C | Patient D | Patient E | |
|---|---|---|---|---|---|
| Tryptase + cells | 5.3 | 5 | 9 | 9 | 8.7 |
| CD117 + cells | 4.3 | 4 | 7 | 7.3 | 7.7 |
| Activated mast cells | 3 | 2 | 5 | 5.3 | 3.7 |
| No. | Age (Years) | Sex | BMI * | Lesion Side | History of Piercing | History of Pruritus | Duration of Keloid Appearance (Months) | Past Medical History |
|---|---|---|---|---|---|---|---|---|
| Patient A | 21 | Male | 20.45 | Left | + | - | 10 | N/A |
| Patient B | 22 | Male | 19.57 | Left | + | - | 18 | N/A |
| Patient C | 32 | Female | 54.11 | Right | + | + | 108 | N/A |
| Patient D | 43 | Male | 48.98 | Right | - | + | 60 | Schizophrenia |
| Patient E | 73 | Female | 22.64 | Left | + | - | 24 | Bronchiectasis |
| Risk Factor | N * | p Value |
|---|---|---|
| Lesion side | 2 | 0.1 |
| BMI (>25) | 2 | 0.1 |
| History of piercing | 1 | 0.4 |
| History of pruritus | 2 | 0.1 |
| Duration of keloid appearance (>60 months) | 2 | 0.1 |
| Number of degranulation mast cells (>5) | 2 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, Y.; Aramaki, N.; Takeuchi, N.; Yamanishi, A.; Kumagai, Y.; Okabe, K.; Yokoyama, T.; Kishi, K. Mast Cells Are Activated in the Giant Earlobe Keloids: A Case Series. Int. J. Mol. Sci. 2022, 23, 10410. https://doi.org/10.3390/ijms231810410
Nakajima Y, Aramaki N, Takeuchi N, Yamanishi A, Kumagai Y, Okabe K, Yokoyama T, Kishi K. Mast Cells Are Activated in the Giant Earlobe Keloids: A Case Series. International Journal of Molecular Sciences. 2022; 23(18):10410. https://doi.org/10.3390/ijms231810410
Chicago/Turabian StyleNakajima, Yukari, Noriko Aramaki, Nao Takeuchi, Ayumi Yamanishi, Yoshiko Kumagai, Keisuke Okabe, Tomoaki Yokoyama, and Kazuo Kishi. 2022. "Mast Cells Are Activated in the Giant Earlobe Keloids: A Case Series" International Journal of Molecular Sciences 23, no. 18: 10410. https://doi.org/10.3390/ijms231810410
APA StyleNakajima, Y., Aramaki, N., Takeuchi, N., Yamanishi, A., Kumagai, Y., Okabe, K., Yokoyama, T., & Kishi, K. (2022). Mast Cells Are Activated in the Giant Earlobe Keloids: A Case Series. International Journal of Molecular Sciences, 23(18), 10410. https://doi.org/10.3390/ijms231810410





