Hypoxia-Inducible Factors and Diabetic Kidney Disease—How Deep Can We Go?
Abstract
:1. Introduction
2. HIFs and Podocyte Dysfunction
3. HIFs and Renal Fibrosis
4. HIFs and Epigenetic Regulation
5. HIFs and Vascular Calcifications
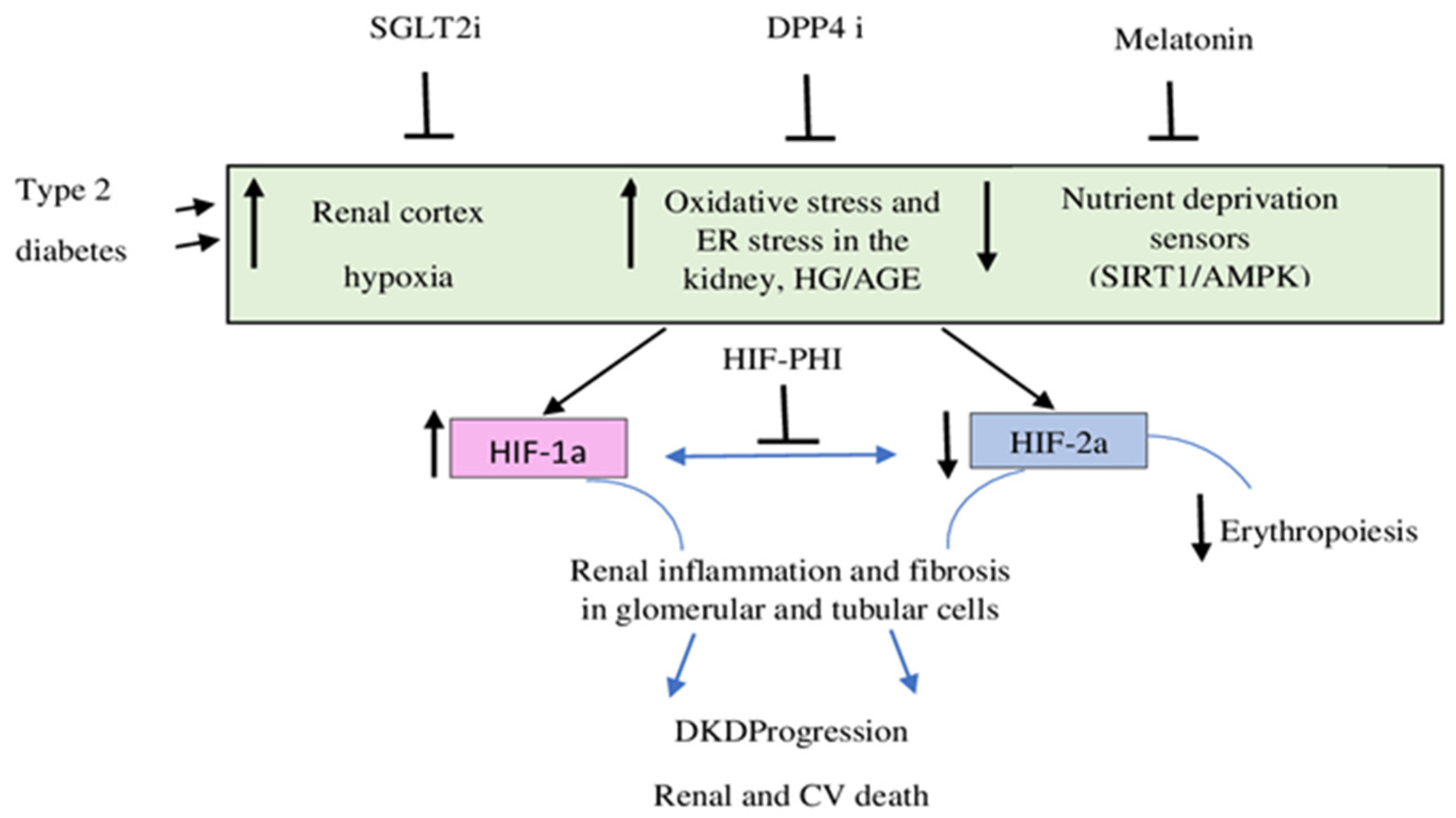
6. Melatonin, HIFs, and Diabetic Nephropathy
7. HIF-1 and Mitophagy
8. Assessment of Hypoxia in CKD
9. HIFs in the New Therapeutic Era of DKD
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Wei, Q.; Guo, C.; Dong, G.; Liu, Y.; Tang, C.; Dong, Z. Hypoxia, HIF, and Associated Signaling Networks in Chronic Kidney Disease. Int. J. Mol. Sci. 2017, 18, 950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, D.; Kang, X.; Zhou, R.; Sun, Y.; Lian, F.; Tong, X. Signaling Pathways Involved in Diabetic Renal Fibrosis. Front. Cell Dev. Biol. 2021, 9, 696542. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Colgan, S.P.; Shelley, C.S. Hypoxia: The Force that Drives Chronic Kidney Disease. Clin. Med. Res. 2016, 14, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Tanaka, T.; Nangaku, M. Renal Hypoxia in CKD.; Pathophysiology and Detecting Methods. Front. Physiol. 2017, 8, 99. [Google Scholar] [CrossRef]
- Tanaka, T. Expanding roles of the hypoxia-response network in chronic kidney disease. Clin. Exp. Nephrol. 2016, 20, 835–844. [Google Scholar] [CrossRef]
- Tanaka, T. A mechanistic link between renal ischemia and fibrosis. Med Mol. Morphol. 2017, 50, 1–8. [Google Scholar] [CrossRef]
- Mora-Gutiérrez, J.; Fernández-Seara, M.; Echeverria-Chasco, R.; Garcia-Fernandez, N. Perspectives on the Role of Magnetic Resonance Imaging (MRI) for Noninvasive Evaluation of Diabetic Kidney Disease. J. Clin. Med. 2021, 10, 2461. [Google Scholar] [CrossRef]
- Fine, L.G.; Orphanides, C.; Norman, J.T. Progressive renal disease: The chronic hypoxia hypothesis. Kidney Int. Suppl. 1998, 65, S74–S78. [Google Scholar]
- Hesp, A.C.; Schaub, J.A.; Prasad, P.V.; Vallon, V.; Laverman, G.D.; Bjornstad, P.; van Raalte, D.H. The Role of Renal Hy-poxia in the Pathogenesis of Diabetic Kidney Disease: A Promising Target for Newer Renoprotective Agents Including SGLT2 Inhibitors? Kidney Int. 2020, 98, 579–589. [Google Scholar] [CrossRef]
- Kopel, J.; Pena-Hernandez, C.; Nugent, K. Evolving spectrum of diabetic nephropathy. World J. Diabetes 2019, 10, 269–279. [Google Scholar] [CrossRef]
- Tonneijck, L.; Muskiet, M.H.; Smits, M.M.; van Bommel, E.J.; Heerspink, H.J.; van Raalte, D.H.; Joles, J.A. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J. Am. Soc. Nephrol. 2017, 28, 1023–1039. [Google Scholar] [CrossRef] [Green Version]
- Albadari, N.; Deng, S.; Li, W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin. Drug Discov. 2019, 14, 667–682. [Google Scholar] [CrossRef]
- Shu, S.; Wang, Y.; Zheng, M.; Liu, Z.; Cai, J.; Tang, C.; Dong, Z. Hypoxia and Hypoxia-Inducible Factors in Kidney Injury and Repair. Cells 2019, 8, 207. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011, 365, 537–547. [Google Scholar] [CrossRef]
- Samanta, D.; Prabhakar, N.R.; Semenza, G.L. Systems biology of oxygen homeostasis. WIREs Syst. Biol. Med. 2017, 9, e1382. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Tanaka, T.; Eto, N.; Nangaku, M. Inflammation and hypoxia linked to renal injury by CCAAT/enhancer-binding protein δ. Kidney Int. 2015, 88, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylasepathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Pugh, C.W. Modulation of the Hypoxic Response. Adv. Exp. Med. Biol. 2016, 903, 259–271. [Google Scholar] [CrossRef]
- Wiesener, M.S.; Jurgensen, J.S.; Rosenberger, C.; Scholze, C.K.; Horstrup, J.H.; Warnecke, C.; Mandriota, S.; Bechmann, I.; Frei, U.A.; Pugh, C.W.; et al. Widespread hypoxia-inducible expression of HIF-2α in distinct cell populations of different organs. FASEB J. 2003, 17, 271–273. [Google Scholar] [CrossRef]
- Bartoszewska, S.; Kochan, K.; Piotrowski, A.; Kamysz, W.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. The hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1_ expression in human endothelial cells through a negative feed-back loop. FASEB J. 2015, 29, 1467–1479. [Google Scholar] [CrossRef]
- Gunaratnam, L.; Bonventre, J.V. HIF in Kidney Disease and Development. J. Am. Soc. Nephrol. 2009, 20, 1877–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikkila, M.; Pasanen, A.; Kivirikko, K.I.; Myllyharju, J. Roles of the human hypoxia-inducible factor (HIF)-3α variants in the hypoxia response. Cell. Mol. Life Sci. 2011, 68, 3885–3901. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Mechanisms Leading to Differential Hypoxia-Inducible Factor Signaling in the Diabetic Kidney: Modulation by SGLT2 Inhibitors and Hypoxia Mimetics. Am. J. Kidney Dis. 2021, 77, 280–286. [Google Scholar] [CrossRef]
- Bessho, R.; Takiyama, Y.; Takiyama, T.; Kitsunai, H.; Takeda, Y.; Sakagami, H.; Ota, T. Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci. Rep. 2019, 9, 14754. [Google Scholar] [CrossRef]
- Macconi, D.; Remuzzi, G.; Benigni, A. Key fibrogenic mediators: Old players. Renin–angiotensin system. Kidney Int. Suppl. 2014, 4, 58–64. [Google Scholar] [CrossRef]
- Nayak, B.K.; Shanmugasundaram, K.; Friedrichs, W.E.; Cavaglierii, R.C.; Patel, M.; Barnes, J.; Block, K. HIF-1 Mediates Renal Fibrosis in OVE26 Type 1 Diabetic Mice. Diabetes 2016, 65, 1387–1397. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, Z.-F.; Wang, S.-P.; Vong, C.-T.; Yu, B.; Wang, Y.-T. HIF-1: Structure, biology and natural modulators. Chin. J. Nat. Med. 2021, 19, 521–527. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, T.; Yang, B.; Chi, Z.; Wan, Q.; Gu, H.F. Protective effect of berberine on high glucose and hypoxia-induced apoptosis via the modulation of HIF-1α in renal tubular epithelial cells. Am. J. Transl. Res. 2019, 11, 669–682. [Google Scholar]
- Chiu, C.J.; Taylor, A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog. Retin. Eye Res. 2011, 30, 18–53. [Google Scholar] [CrossRef]
- Cheng, L.; Qin, T.; Ma, J.; Duan, W.; Xu, Q.; Li, X.; Han, L.; Li, W.; Wang, Z.; Zhang, D.; et al. Hypoxia-inducible factor-1α mediates hyperglycemia-induced pancreatic cancer glycolysis. Anti-Cancer Agents Med. Chem. 2019, 19, 1503–1512. [Google Scholar] [CrossRef]
- Singh, A.K.; Kolligundla, L.P.; Francis, J.; Pasupulati, A.K. Detrimental effects of hypoxia on glomerular podocytes. J. Physiol. Biochem. 2021, 77, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Pagtalunan, M.E.; Miller, P.L.; Jumping-Eagle, S.; Nelson, R.G.; Myers, B.D.; Rennke, H.G.; Coplon, N.S.; Sun, L.; Meyer, T.W. Podocyte loss and progressive glomerular injury in type II diabetes. J. Clin. Investig. 1997, 99, 342–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaltonen, P.; Luimula, P.; Åström, E.; Palmen, T.; Grönholm, T.; Palojoki, E.; Jaakkola, I.; Ahola, H.; Tikkanen, I.; Holthöfer, H. Changes in the Expression of Nephrin Gene and Protein in Experimental Diabetic Nephropathy. Lab. Investig. 2001, 81, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Susztak, K. Podocytes: The Weakest Link in Diabetic Kidney Disease? Curr. Diabetes Rep. 2016, 16, 45. [Google Scholar] [CrossRef]
- Conti, S.; Perico, N.; Novelli, R.; Carrara, C.; Benigni, A.; Remuzzi, G. Early and late scanning electron microscopy findings in diabetic kidney disease. Sci. Rep. 2018, 8, 4909. [Google Scholar] [CrossRef]
- Zoja, C.; Xinaris, C.; Macconi, D. Diabetic Nephropathy: Novel Molecular Mechanisms and Therapeutic Targets. Front. Pharmacol. 2020, 11, 586892. [Google Scholar] [CrossRef]
- Weil, E.J.; Lemley, K.V.; Mason, C.C.; Yee, B.; Jones, L.I.; Blouch, K.; Lovato, T.; Richardson, M.; Myers, B.D.; Nelson, R.G. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012, 82, 1010–1017. [Google Scholar] [CrossRef]
- Wolf, G.; Chen, S.; Ziyadeh, F.N. From the Periphery of the Glomerular Capillary Wall Toward the Center of Disease: Podocyte injury comes of age in diabetic nephropathy. Diabetes 2005, 54, 1626–1634. [Google Scholar] [CrossRef]
- Huang, H.; Fan, Y.; Gao, Z.; Wang, W.; Shao, N.; Zhang, L.; Yang, Y.; Zhu, W.; Chen, Z.; Hu, J. HIF-1α contributes to Ang II-induced inflammatory cytokine production in podocytes. BMC Pharmacol. Toxicol. 2019, 20, 59. [Google Scholar] [CrossRef]
- Nordquist, L.; Friederich-Persson, M.; Fasching, A.; Liss, P.; Shoji, K.; Nangaku, M.; Hansell, P.; Palm, F. Activation of Hypoxia-Inducible Factors Prevents Diabetic Nephropathy. J. Am. Soc. Nephrol. 2014, 26, 328–338. [Google Scholar] [CrossRef]
- Matoba, K.; Kawanami, D.; Okada, R.; Tsukamoto, M.; Kinoshita, J.; Ito, T.; Ishizawa, S.; Kanazawa, Y.; Yokota, T.; Murai, N. Rho-kinase inhibition prevents the progression of diabetic nephropathy by downregulating hypoxia-inducible factor 1α. Kidney Int. 2013, 84, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Reidy, K.; Susztak, K. Epithelial-mesenchymal transition and podocyte loss in diabetic kidney disease. Am. J. Kidney Dis. 2009, 54, 590–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anil Kumar, P.; Welsh, G.I.; Saleem, M.A.; Menon, R.K. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front. Endocrinol. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Petermann, A.T.; Pippin, J.; Krofft, R.; Blonski, M.; Griffin, S.; Durvasula, R.; Shankland, S.J. Viable podocytes detach in ex-perimental diabetic nephropathy: Potential mechanism underlying glomerulosclerosis. Nephron Exp. Nephrol. 2004, 98, e114–e123. [Google Scholar] [CrossRef]
- Hung, T.-W.; Liou, J.-H.; Yeh, K.-T.; Tsai, J.-P.; Wu, S.-W.; Tai, H.-C.; Kao, W.-T.; Lin, S.-H.; Cheng, Y.-W.; Chang, H.-R. Renal expression of hypoxia inducible factor-1α in patients with chronic kidney disease: A clinicopathologic study from nephrectomized kidneys. Indian J. Med. Res. 2003, 137, 102–110. [Google Scholar]
- Lu, H.; Kapur, G.; Mattoo, T.K.; Lyman, W.D. Hypoxia decreases podocyte expression of slit diaphragm proteins. Int. J. Nephrol. Renov. Dis. 2013, 5, 101–107. [Google Scholar] [CrossRef]
- Chang, J.M.; Hwang, D.Y.; Chen, S.C.; Kuo, M.C.; Hung, C.C.; Hwang, S.J.; Tsai, J.C.; Chen, H.C. B7–1 expression regulates the hypoxia-driven cytoskeleton rearrangement in glomerular podocytes. Am. J. Physiol. Renal Physiol. 2013, 304, F127–F136. [Google Scholar] [CrossRef]
- Nakuluri, K.; Nishad, R.; Mukhi, D.; Kumar, S.; Nakka, V.P.; Kolligundla, L.P.; Narne, P.; Natuva, S.S.K.; Phanithi, P.B.; Pasupulati, A.K. Cerebral ischemia induces TRPC6 via HIF1α/ZEB2 axis in the glomerular podocytes and contributes to proteinuria. Sci. Rep. 2019, 9, 17897. [Google Scholar] [CrossRef]
- Higgins, D.F.; Kimura, K.; Bernhardt, W.M.; Shrimanker, N.; Akai, Y.; Hohenstein, B.; Saito, Y.; Johnson, R.S.; Kretzler, M.; Co hen, C.D.; et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Investig. 2007, 117, 3810–3820. [Google Scholar] [CrossRef]
- Jiao, Y.; Jiang, H.; Lu, H.; Yang, Y.; Zhang, Y.; Zhang, K.; Liu, H. Deficiency of hypoxia inducible factor-1α promoted progression of diabetic nephropathy with hypertension. Exp. Ther. Med. 2018, 16, 3658–3662. [Google Scholar] [CrossRef]
- Ryu, D.R.; Yu, M.R.; Kong, K.H.; Kim, H.; Kwon, S.H.; Jeon, J.S.; Han, D.C.; Noh, H. Sirt1-hypoxia-inducible factor-1α interaction is a key mediator of tubulointerstitial damage in the aged kidney. Aging Cell 2019, 18, e12904. [Google Scholar] [CrossRef]
- Owczarek, A.; Gieczewska, K.B.; Polanska, M.; Paterczyk, B.; Gruza, A.; Winiarska, K. Melatonin Lowers HIF-1α Content in Human Proximal Tubular Cells (HK-2) Due to Preventing Its Deacetylation by Sirtuin 1. Front. Physiol. 2021, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Dallatu, M.K.; Choi, M.; Oyekan, A.O. Inhibition of prolyl hydroxylase domain-containing protein on hypertension/renal injury induced by high salt diet and nitric oxide withdrawal. J. Hypertens. 2013, 31, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Zhang, W.; Zhao, C.; Zhang, Y.; Wu, H.; Jin, J.; Zhang, W.; Grenz, A.; Eltzschig, H.K.; Tao, L.; et al. Elevated endothelial hypoxia-inducible factor-1_ contributes to glomerular injury and promotes hypertensive chronic kidney disease. Hypertension 2015, 66, 75–84. [Google Scholar] [CrossRef]
- Kalucka, J.; Schley, G.; Georgescu, A.; Klanke, B.; Rössler, S.; Baumgartl, J.; Velden, J.; Amann, K.; Willam, C.; Johnson, R.S.; et al. Kidney injury is independent of endothelial HIF-1α. Klin. Wochenschr. 2015, 93, 891–904. [Google Scholar] [CrossRef]
- Gilkes, D.M.; Bajpai, S.; Chaturvedi, P.; Wirtz, D.; Semenza, G.L. Hypoxia-inducible Factor 1 (HIF-1) Promotes Extracellular Matrix Remodeling under Hypoxic Conditions by Inducing P4HA1, P4HA2, and PLOD2 Expression in Fibroblasts. J. Biol. Chem. 2013, 288, 10819–10829. [Google Scholar] [CrossRef]
- Bechtel, W.; McGoohan, S.; Zeisberg, E.M.; Müller, G.A.; Kalbacher, H.; Salant, D.J.; Müller, C.A.; Kalluri, R.; Zeisberg, M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 2010, 16, 544–550. [Google Scholar] [CrossRef]
- Han, W.; Wang, C.; Yang, Z.; Mu, L.; Wu, M.; Chen, N.; Du, C.; Duan, H.; Shi, Y. SRT1720 retards renal fibrosis via inhibition of HIF1A/GLUT1 in diabetic nephropathy. J. Endocrinol. 2019, 241, 85–98. [Google Scholar] [CrossRef]
- Deb, D.K.; Bao, R.; Li, Y.C. Critical role of the cAMP-PKA pathway in hyperglycemia-induced epigenetic activation of fibrogenic program in the kidney. FASEB J. 2017, 31, 2065–2075. [Google Scholar] [CrossRef]
- Abrass, C.K.; Hansen, K.; Popov, V.; Denisenko, O. Alterations in chromatin are associated with increases in collagen III expression in aging nephropathy. Am. J. Physiol. Renal Physiol. 2011, 300, F531–F539. [Google Scholar] [CrossRef]
- Kroening, S.; Neubauer, E.; Wullich, B.; Aten, J.; Goppelt-Struebe, M. Characterization of connective tissue growth factor expression in primary cultures of human tubular epithelial cells: Modulation by hypoxia. Am. J. Physiol. Renal Physiol. 2010, 298, F796–F806. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Chen, H.; Li, F.; Wang, S.; Guo, J. Hypoxia-induced microRNA-155 promotes fibrosis in proximal tubule cells. Mol. Med. Rep. 2015, 11, 4555–4560. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.X.; Moe, S.M. Vascular Calcification: Pathophysiology and Risk Factors. Curr. Hypertens. Rep. 2012, 14, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Mokas, S.; Larivière, R.; Lamalice, L.; Gobeil, S.; Cornfield, D.N.; Agharazii, M.; Richard, D.E. Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int. 2016, 90, 598–609. [Google Scholar] [CrossRef]
- Akahori, H.; Tsujino, T.; Naito, Y.; Sawada, H.; Sugahara, M.; Fukui, M.; Ohyanagi, M.; Mitsuno, M.; Miyamoto, Y.; Masuyama, T. Nuclear factor-κB-hypoxia-inducible factor-2 pathway in aortic valve stenosis. J. Heart Valve Dis. 2014, 23, 558–566. [Google Scholar]
- Rusdiana, R.; Widjaja, S.S.; Savira, M.; Ardinata, D. Relationship between Plasma Hypoxia Inducible Factor 1α in Type 2 Diabetes Mellitus with Malignancy and without Malignancy. Open Access Maced. J. Med. Sci. 2020, 8, 602–605. [Google Scholar] [CrossRef]
- Promsan, S.; Lungkaphin, A. The roles of melatonin on kidney injury in obese and diabetic conditions. BioFactors 2020, 46, 531–549. [Google Scholar] [CrossRef]
- Hrenak, J.; Paulis, L.; Repova, K.; Aziriova, S.; Elsbeth, E.J.; Russel, J.R.; Simko, F. Melatonin and Renal Protection: Novel Perspectives from Animal Experiments and Human Studies (Review). Curr. Pharm. Des. 2015, 21, 936–949. [Google Scholar] [CrossRef]
- Winiarska, K.; Dzik, J.M.; Labudda, M.; Focht, D.; Sierakowski, B.; Owczarek, A.; Komorowski, L.; Bielecki, W. Melatonin nephroprotective action in Zucker diabetic fatty rats involves its inhibitory effect on NADPH oxidase. J. Pineal Res. 2016, 60, 109–117. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, X.; Jiang, L.; Huang, X.; Zhang, Y.; Zhao, D.; Du, Y. Recent advances in understanding the role of hypoxia-inducible factor 1a in renal fibrosis. Int. Urol. Nephrol. 2020, 52, 1287–1295. [Google Scholar] [CrossRef]
- Honda, T.; Hirakawa, Y.; Nangaku, M. The role of oxidative stress and hypoxia in renal disease. Kidney Res. Clin. Pract. 2019, 38, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, H.L.; Gu, C.J.; Liu, Y.K.; Shao, J.; Zhu, R.; He, Y.Y.; Zhu, X.Y.; Li, M.Q. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2019, 43, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.C.; Payne, L.B.; Rathmell, W.K. Hypoxia, angiogenesis, and metabolism in the hereditary kidney cancers. J. Clin. Investig. 2019, 129, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, Y.; Guo, Y.H.; Wang, L.; Yang, Z.; Zhai, Z.H.; Tang, L. HIF-1α Alleviates High-Glucose-Induced Renal Tubular Cell Injury by Promoting Parkin/PINK1-Mediated Mitophagy. Front. Med. 2022, 8, 803874. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, J.; Li, X.; Wu, D.; Wang, Q.; Li, S.; Shi, C. Mitophagy in Diabetic Kidney Disease. Front. Cell Dev. Biol. 2021, 9, 778011. [Google Scholar] [CrossRef]
- Jiang, N.; Zhao, H.; Han, Y.; Li, L.; Xiong, S.; Zeng, L.; Xiao, Y.; Wei, L.; Xiong, X.; Gao, P.; et al. HIF-1α ameliorates tubular injury in diabetic nephropathy via HO-1–mediated control of mitochondrial dynamics. Cell Prolif. 2020, 53, e12909. [Google Scholar] [CrossRef]
- Gou, R.; Chen, J.; Sheng, S.; Wang, R.; Fang, Y.; Yang, Z.; Wang, L.; Tang, L. KIM-1 Mediates High Glucose-Induced Autophagy and Apoptosis in Renal Tubular Epithelial Cells. Cell. Physiol. Biochem. 2016, 38, 2479–2488. [Google Scholar] [CrossRef]
- Higgins, G.C.; Coughlan, M.T. Mitochondrial dysfunction and mitophagy: The beginning and end to diabetic nephropathy? Br. J. Pharmacol. 2014, 171, 1917–1942. [Google Scholar] [CrossRef]
- Hou, Y.; Li, S.; Wu, M.; Wei, J.; Ren, Y.; Du, C.; Wu, H.; Han, C.; Duan, H.; Shi, Y. Mitochondria-targeted peptide SS-31 attenuates renal injury via an antioxidant effect in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2016, 310, F547–F559. [Google Scholar] [CrossRef]
- Emans, T.W.; Janssen, B.J.; Pinkham, M.I.; Ow, C.P.C.; Evans, R.G.; Joles, J.A.; Malpas, S.C.; Krediet, C.T.P.; Koeners, M.P. Exogenous and endogenous angiotensin-II decrease renal cortical oxygen tension in conscious rats by limiting renal blood flow. J. Physiol. 2016, 594, 6287–6300. [Google Scholar] [CrossRef]
- Morelli, A.; Rocco, M.; Conti, G.; Orecchioni, A.; De Blasi, R.A.; Coluzzi, F.; Pietropaoli, P. Monitoring Renal Oxygen Supply in Critically-Ill Patients Using Urinary Oxygen Tension. Anesth. Analg. 2003, 97, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Corbin, C.; Jensen Noel, A. Warfel: Hypoxia, Chapter in Comprehensive Pharmacology; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 438–468. [Google Scholar]
- Piert, M.; Machulla, H.-J.; Picchio, M.; Reischl, G.; Ziegler, S.; Kumar, P.; Wester, H.-J.; Beck, R.; McEwan, A.J.B.; I Wiebe, L.; et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J. Nucl. Med. 2005, 46, 106–113. [Google Scholar]
- Tuckerman, J.R.; Zhao, Y.; Hewitson, K.S.; Tian, Y.-M.; Pugh, C.W.; Ratcliffe, P.; Mole, D.R. Determination and comparison of specific activity of the HIF-prolyl hydroxylases. FEBS Lett. 2004, 576, 145–150. [Google Scholar] [CrossRef]
- Heikal, L.; Ghezzi, P.; Mengozzi, M.; Ferns, G. Assessment of HIF-1α expression and release following endothelial injury in-vitro and in-vivo. Mol. Med. 2018, 24, 22. [Google Scholar] [CrossRef]
- Kondrashina, A.V.; Dmitriev, R.I.; Borisov, S.M.; Klimant, I.; O’Brien, I.; Nolan, Y.M.; Zhdanov, A.V.; Papkovsky, D.B. A Phosphorescent Nano-particle-Based Probe for Sensing and Imaging of (Intra)Cellular Oxygen in Multiple Detection Modalities. Adv. Funct. Mater. 2012, 22, 4931–4939. [Google Scholar] [CrossRef]
- Prasad, P.V.; Edelman, R.R.; Epstein, F.H. Noninvasive Evaluation of Intrarenal Oxygenation with BOLD MRI. Circulation 1996, 94, 3271–3275. [Google Scholar] [CrossRef]
- Vinovskis, C.; Li, L.-P.; Prasad, P.; Tommerdahl, K.; Pyle, L.; Nelson, R.G.; Pavkov, M.E.; van Raalte, D.; Rewers, M.; Pragnell, M.; et al. Relative Hypoxia and Early Diabetic Kidney Disease in Type 1 Diabetes. Diabetes 2020, 69, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Inoue, T.; Kozawa, E.; Ishikawa, M.; Shimada, A.; Kobayashi, N.; Tanaka, J.; Okada, H. Reduced oxygenation but not fibrosis defined by functional magnetic resonance imaging predicts the long-term progression of chronic kidney disease. Nephrol. Dial. Transplant. 2020, 35, 964–970. [Google Scholar] [CrossRef]
- Feng, Y.-Z.; Ye, Y.-J.; Cheng, Z.-Y.; Hu, J.-J.; Zhang, C.-B.; Qian, L.; Lu, X.-H.; Cai, X.-R.; Cai, X.-H. Non-invasive assessment of early stage diabetic nephropathy by DTI and BOLD MRI. Br. J. Radiol. 2020, 93, 20190562. [Google Scholar] [CrossRef]
- Kodama, Y.; Hyodo, F.; Yamato, M.; Yasukawa, K.; Minami, Y.; Sonoda, N.; Ogawa, Y.; Ichikawa, K.; Inoguchi, T. Dynamic nuclear polarization magnetic resonance imaging and the oxygen-sensitive paramagnetic agent OX63 provide a noninvasive quantitative evaluation of kidney hypoxia in diabetic mice. Kidney Int. 2019, 96, 787–792. [Google Scholar] [CrossRef]
- Gooding, K.M.; Lienczewski, C.; Papale, M.; Koivuviita, N.; Maziarz, M.; Andersson, A.-M.D.; Sharma, K.; Pontrelli, P.; Hernandez, A.G.; Bailey, J.; et al. Prognostic imaging biomarkers for diabetic kidney disease (iBEAt): Study protocol. BMC Nephrol. 2020, 21, 242. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Hao, C.; Peng, X.; Lin, H.; Yin, A.; Hao, L.; Tao, Y.; Liang, X.; Liu, Z.; Xing, C.; et al. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N. Engl. J. Med. 2019, 381, 1001–1010. [Google Scholar] [CrossRef]
- Hasegawa, S.; Tanaka, T.; Saito, T.; Fukui, K.; Wakashima, T.; Susaki, E.A.; Ueda, H.R.; Nangaku, M. The oral hypoxia-inducible factor prolyl hydroxylase inhibitor enarodustat counteracts alterations in renal energy metabolism in the early stages of diabetic kidney disease. Kidney Int. 2020, 97, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, Y.; Xing, J.; Fu, Y.-Y.; Wang, K.-Y.; Wan, P.-Z.; Zhai, X.-Y. Pretreatment with Roxadustat (FG-4592) Attenuates Folic Acid-Induced Kidney Injury through Antiferroptosis via Akt/GSK-3β/Nrf2 Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 6286984. [Google Scholar] [CrossRef]
- Provenzano, R.; Fishbane, S.; Szczech, L.; Leong, R.; Saikali, K.G.; Zhong, M.; Lee, T.T.; Houser, M.T.; Frison, L.; Houghton, J.; et al. Roxadustat Efficacy in Non-Dialysis-Dependent Through Dialysis-Dependent Chronic Kidney Disease. In Proceedings of the National Kidney Foundation 2021 Spring Clinical Meetings—POSTER, Virtual, 6–10 April 2021. [Google Scholar]
- Singh, A.K.; Carroll, K.; Perkovic, V.; Solomon, S.; Jha, V.; Johansen, K.L.; Lopes, R.D.; Macdougall, I.C.; Obrador, G.T.; Waikar, S.S.; et al. Daprodustat for the Treatment of Anemia in Patients Not Undergoing Dialysis. N. Engl. J. Med. 2021, 385, 2313–2324. [Google Scholar] [CrossRef]
- Singh, A.K.; Carroll, K.; Perkovic, V.; Solomon, S.; Jha, V.; Johansen, K.L.; Lopes, R.D.; Macdougall, I.C.; Obrador, G.T.; Waikar, S.S.; et al. Daprodustat for the Treatment of Anemia in Patients Undergoing Dialysis. N. Engl. J. Med. 2021, 385, 2325–2335. [Google Scholar] [CrossRef]
- Haase, V.H. Hypoxia-inducible factor–prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int. Suppl. 2021, 11, 8–25. [Google Scholar] [CrossRef]
- Hirota, K. HIF-α Prolyl Hydroxylase Inhibitors and Their Implications for Biomedicine: A Comprehensive Review. Biomedicines 2021, 9, 468. [Google Scholar] [CrossRef]
- Akizawa, T.; Nangaku, M.; Yamaguchi, T.; Arai, M.; Koretomo, R.; Matsui, A.; Hirakata, H. A Placebo-Controlled, Randomized Trial of Enarodustat in Patients with Chronic Kidney Disease Followed by Long-Term Trial. Am. J. Nephrol. 2019, 49, 165–174. [Google Scholar] [CrossRef]
- Yamamoto, H.; Taguchi, M.; Matsuda, Y.; Iekushi, K.; Yamada, T.; Akizawa, T. Molidustat for the treatment of renal anaemia in patients with non-dialysis-dependent chronic kidney disease: Design and rationale of two phase III studies. BMJ Open 2019, 9, e026704. [Google Scholar] [CrossRef]
- Akizawa, T.; Tanaka-Amino, K.; Otsuka, T.; Yamaguchi, Y. Clinical parameters among patients in Japan with anemia and non-dialysis-dependent chronic kidney disease with and without diabetes mellitus who received roxadustat. Clin. Exp. Nephrol. 2022, 26, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Shutov, E.; Sułowicz, W.; Esposito, C.; Tataradze, A.; Andric, B.; Reusch, M.; Valluri, U.; Dimkovic, N. Roxadustat for the treatment of anemia in chronic kidney disease patients not on dialysis: A Phase 3, randomized, double-blind, placebo-controlled study (ALPS). Nephrol. Dial. Transpl. 2021, 36, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Coyne, D.W.; Roger, S.D.; Shin, S.K.; Kim, S.G.; Cadena, A.A.; Moustafa, M.A.; Chan, T.M.; Besarab, A.; Chou, W.; Bradley, C.; et al. Roxadustat for CKD-related Anemia in Non-dialysis Patients (ANDES). Kidney Int. Rep. 2021, 6, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Barratt, J.; Andric, B.; Tataradze, A.; Schömig, M.; Reusch, M.; Valluri, U.; Mariat, C. Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: A Phase 3, randomized, open-label, active-controlled study (DOLOMITES). Nephrol. Dial. Transpl. 2021, 36, 1616–1628. [Google Scholar] [CrossRef]
- Chertow, G.M.; Pergola, P.E.; Farag, Y.M.; Agarwal, R.; Arnold, S.; Bako, G.; Block, G.A.; Burke, S.; Castillo, F.P.; Jardine, A.G.; et al. Vadadustat in Patients with Anemia and Non–Dialysis-Dependent CKD. N. Engl. J. Med. 2021, 384, 1589–1600. [Google Scholar] [CrossRef]

| Type of HIF-PH | Mechanisms [100] | Study/Main Author | Study Design: No. of Patients/ DM/DN Patients | Treatment, Duration | Primary Outcomes: Hb Response Rate |
|---|---|---|---|---|---|
| Daprodustat (GSK-1278863 | - inhibits PHD2 and PHD3 - absorption 65% - urinary excretion rate < 0.05% - half-life: 1–7 h - metabolized by CYP2C8, UGT1A9 | (NCT02791763) Kimura et al., 2019 [103] | R, OL, AC; ESA-naïve and ESA-treated; n = 299 Diabetes mellitus DAPRO: n = 64 (44%) CERA: n = 69 (46%) | DAPRO QD vs. CERA, 52 weeks | Hb at target (11–13 g/dL) during weeks 40–52: DAPRO: 92% CERA: 92% |
| ASCEND-ND (NCT02876835) Singh AK et al., 2021 [97] | R, OL, AC; ESA-naïve and ESA-treated; n = 3872 Diabetes mellitus DAPRO: n = 1076 (55.5%) DA: n = 1118 (57.8%) | DAPRO QD vs. DA, 52 weeks | Hb at target (11–13 g/dL) during weeks 28–52: DAPRO: 92% CERA: 92% | ||
| Enarodustat (JTZ-951) | - inhibits HIF-PH 1–3, but no effect on various receptors and enzymes - absorption 41.7% - urinary excretion rate: 27–61% - half-life: 11 h - less susceptible to metabolization | (Japic CTI-183870) Akizawa et al., 2021 [101] | R, OL, AC; ESA status NR; n = 216 Diabetic nephropathy: DAPRO: n = 30 (30.9%) DA: n = 32 (33.3%) | ENARO QD vs. DA, 24 weeks | Hb at target (10–12 g/dL) during weeks 1–24: ENARO: 89.6% DA: 90.6% |
| Molidustat (BAY 85-3934) | - mainly inhibits PHD2 - absorption 59% - urinary excretion rate: 4% - half-life: 4–10 h - metabolized by UGT1A1/1A9 | MIYABI ND-C (NCT03350321) Yamamoto et al., 2019 [102] | R, OL, AC; ESA-naïve; n = 162 Diabetic nephropathy MOLI: n = 34 (41.5%) DA: n = 22 (27.5%) | MOLI 5–200 mg QD vs. DA; 52 weeks | Hb at target (11–13 g/dL) during weeks 30–36: MOLI: 68.3% (exception weeks 34 and 40 in low eGFR group < 15 mL/min) DA: 80.5% |
| Roxadustat (FG -4592) | - inhibits all three PHDs - absorption 40–80% - urinary excretion rate: 1% - half-life 12–15 h - metabolized by CYP2C8/UGT1A9 | NCT02652819 Chen et al., 2019 [93] | R, OL, AC; ESA-treated; n = 152 (101 ROXA/51 EPO) Diabetes mellitus ROXA: n = 22 (22%) EPO: n = 16 (31%) | ROXA 100 or 120 mg TIW vs. EPO alfa, 26 weeks | During weeks 23–27: ROXA: 92.5% EPO alfa: 92.5% |
| NCT02964936A Akizawa et al., 2020 [103] | R, OL, NC; ESA-naïve; n = 99 Diabetes mellitus n = 28 (28.3%) | ROXA 50 or 70 mg TIW, 24 weeks | Hb at target ≥10 g/dL: ROXA (50 mg): 97.0% ROXA (70 mg): 100.0% Hb at target ≥ 10.5 g/dL: ROXA 50 mg: 94.9% ROXA 70 mg: 98.0% | ||
| NCT02988973A Akizawa et al., 2022 [103] Post hoc analysis | R, OL, AC; ESA-treated; n = 201 Diabetes mellitus n = 105 (52.2%) | ROXA 52 weeks | Maintenance of Hb at target (10–12 g/dL) | ||
| ALPS (NCT01887600) Shutov et al., 2021 [104] | R, DB, PC; ESA-naïve; n = 594 Diabetic nephropathy ROXA: n = 109 (27.9%) PCB: n = 66 (32.5%) | ROXA vs. PBO, 52–104 weeks | During weeks 1–24: ROXA: 79.2% PBO: 9.9% (p = 0.0001) | ||
| ANDES (NCT01750190) Coyne D.W. et al., 2021 [105] | R, DB, PC; ESA-naïve; n = 922 Diabetes mellitus ROXA: n= 398 (64.6%) PBO: n= 200 (65.4%) | ROXA TIWc vs. PBO, 52 weeks | During weeks 1–24: ROXA: 86.0% PBO: 6.6% (p = 0.0007) | ||
| DOLOMITES (NCT02021318) Barrat et al., 2021 [106] | R, OL, AC; ESA-naïve; n = 616 Diabetic nephropathy ROXA: n = 109 (33.7%) DA: n = 98 (33.4%) | ROXA TIW vs. DA, 104 weeks | During weeks 1–24: ROXA: 89.5% DA: 78.0% | ||
| Vadadustat (AKB-6548) | - inhibits all PHD isozymes PHD1-3 - absorption > 75% - urinary excretion rate < 1% - half-life 4–7 h - metabolized by UGT1A1/1A9 | PRO2TECT Study group (NCT02648347) Chertow et al., 2021 [107] | R, OL, AC; ESA-naïve; n = 1751 Diabetes mellitus VADA: n = 581 (66.1%) DA: n = 599 (68.7%) | VADA QD vs. DA, 52 weeks | Hb at target (10–11 g/dL) during weeks 24–36: VADA: 56.1% DA: 54.9% during weeks 40–52: VADA: 55.3% DA: 54.9% |
| PRO2TECT Study group (NCT02680574) Chertow et al., 2021 [107] | R, OL, AC; ESA-treated; n = 1725 Diabetes mellitus VADA: n = 517 (60%) DA: n = 518 (60%) | VADA QD vs. DA, 52 weeks | Hb at target (10–12 g/dL) during weeks 24–36: VADA: 65.2% DA: 64.1% Weeks 40–52: VADA: 64.5% DA: 60.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanigut, A.M.; Pana, C.; Enciu, M.; Deacu, M.; Cimpineanu, B.; Tuta, L.A. Hypoxia-Inducible Factors and Diabetic Kidney Disease—How Deep Can We Go? Int. J. Mol. Sci. 2022, 23, 10413. https://doi.org/10.3390/ijms231810413
Stanigut AM, Pana C, Enciu M, Deacu M, Cimpineanu B, Tuta LA. Hypoxia-Inducible Factors and Diabetic Kidney Disease—How Deep Can We Go? International Journal of Molecular Sciences. 2022; 23(18):10413. https://doi.org/10.3390/ijms231810413
Chicago/Turabian StyleStanigut, Alina Mihaela, Camelia Pana, Manuela Enciu, Mariana Deacu, Bogdan Cimpineanu, and Liliana Ana Tuta. 2022. "Hypoxia-Inducible Factors and Diabetic Kidney Disease—How Deep Can We Go?" International Journal of Molecular Sciences 23, no. 18: 10413. https://doi.org/10.3390/ijms231810413
APA StyleStanigut, A. M., Pana, C., Enciu, M., Deacu, M., Cimpineanu, B., & Tuta, L. A. (2022). Hypoxia-Inducible Factors and Diabetic Kidney Disease—How Deep Can We Go? International Journal of Molecular Sciences, 23(18), 10413. https://doi.org/10.3390/ijms231810413





