Plant Metabolic Engineering by Multigene Stacking: Synthesis of Diverse Mogrosides
Abstract
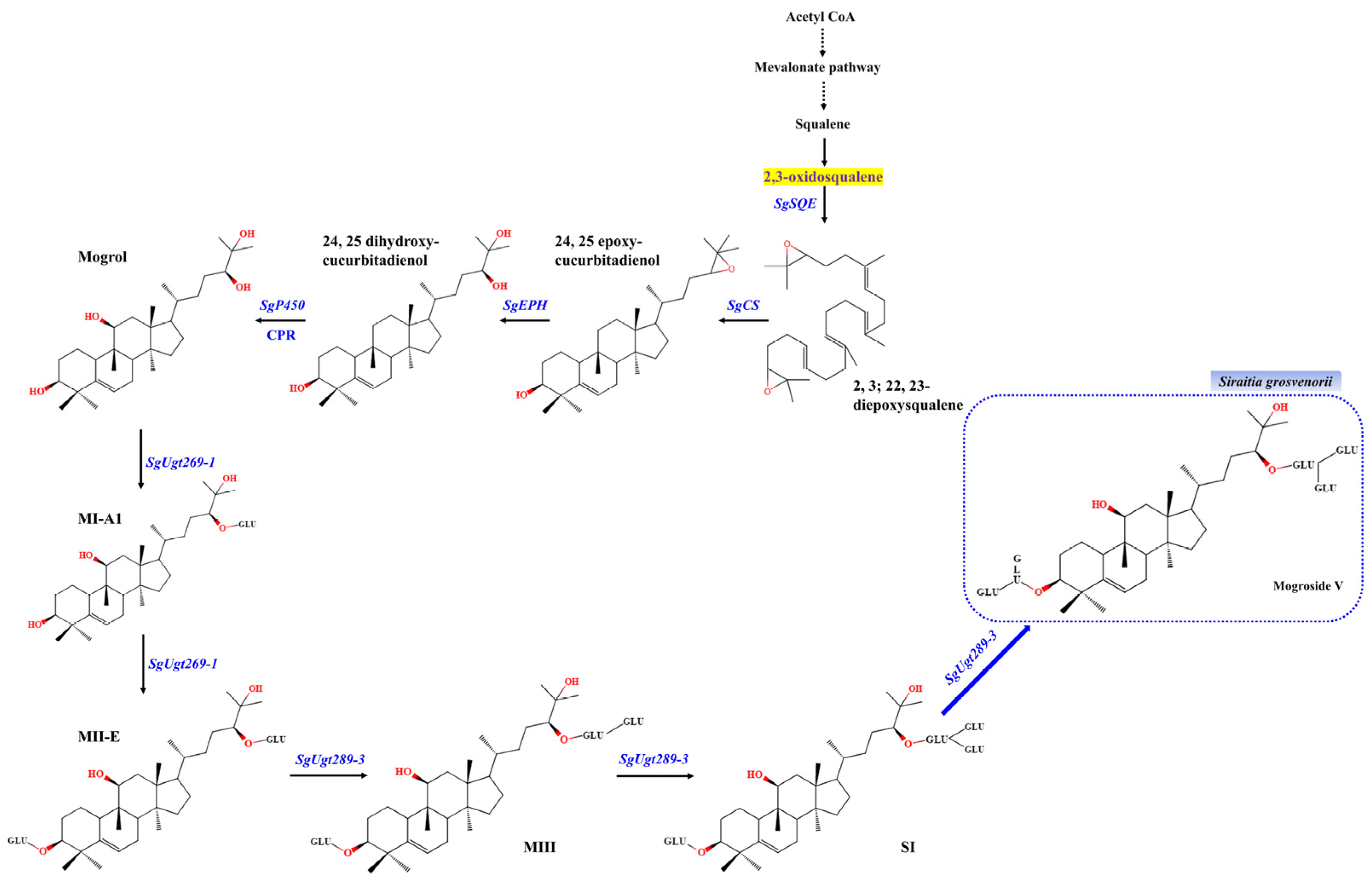
:1. Introduction
2. Results
2.1. Multigene Expression Vector Construction
2.2. Transient Expression Assays
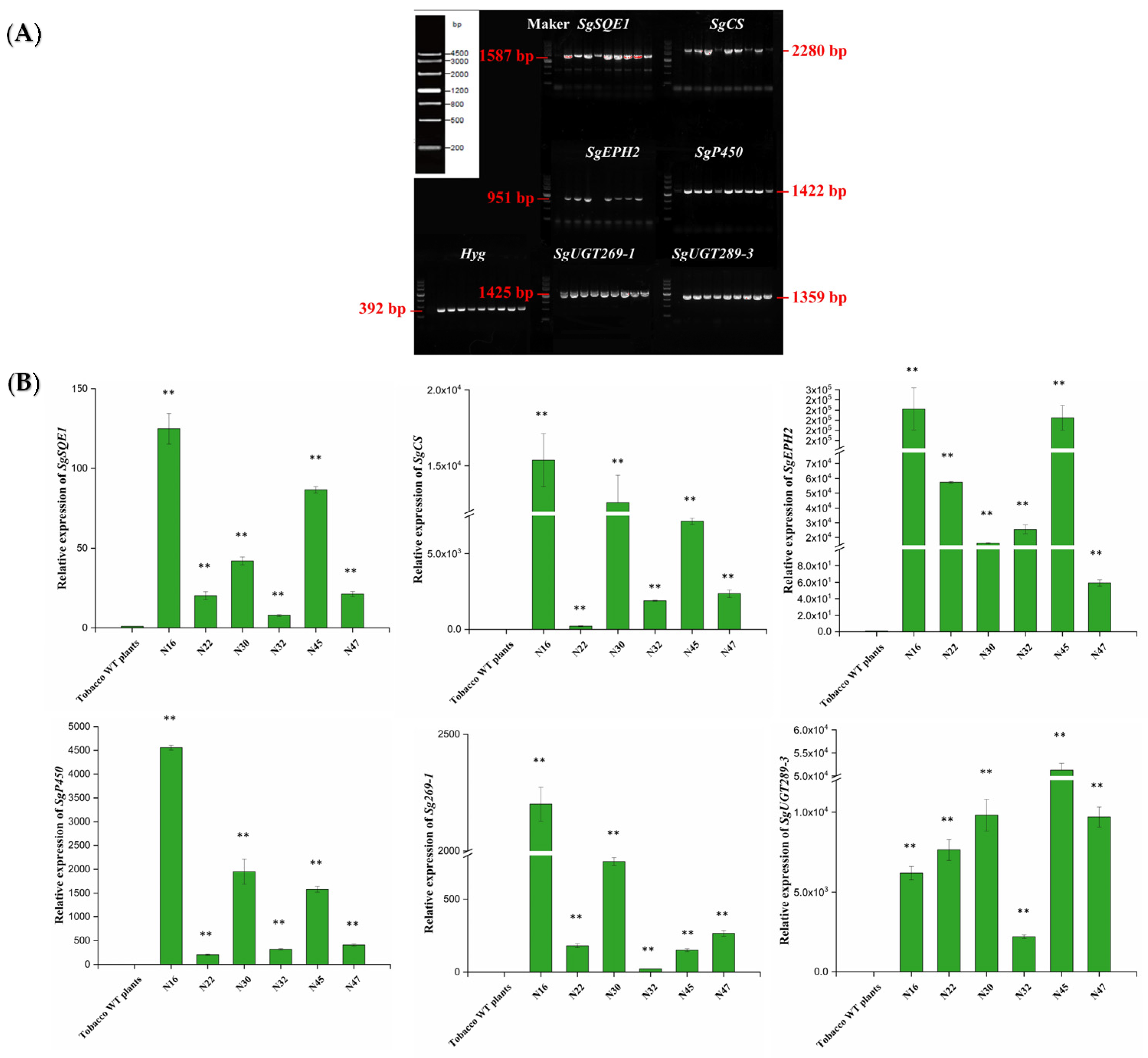
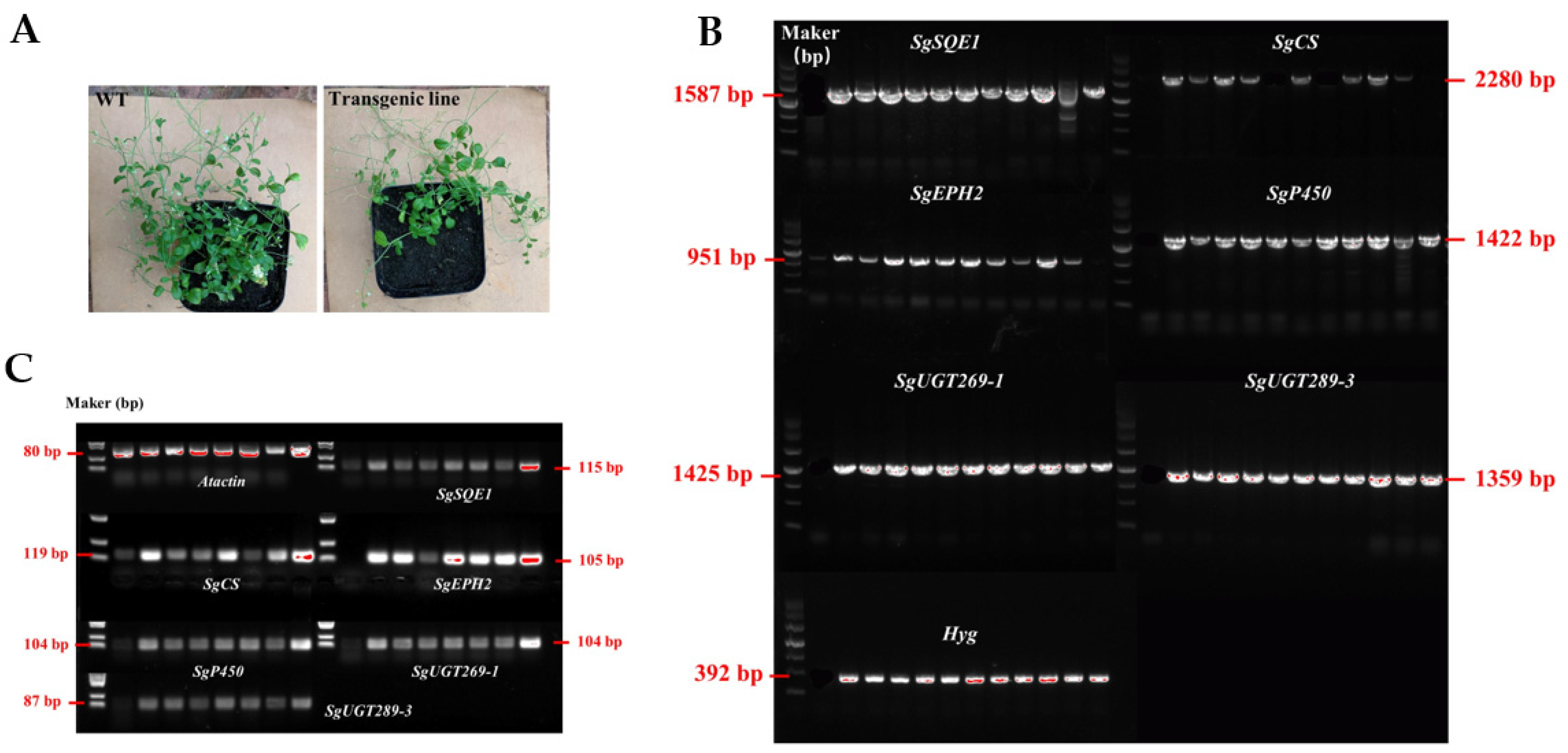
2.3. Generation of Transgenic Tobacco and Arabidopsis thaliana
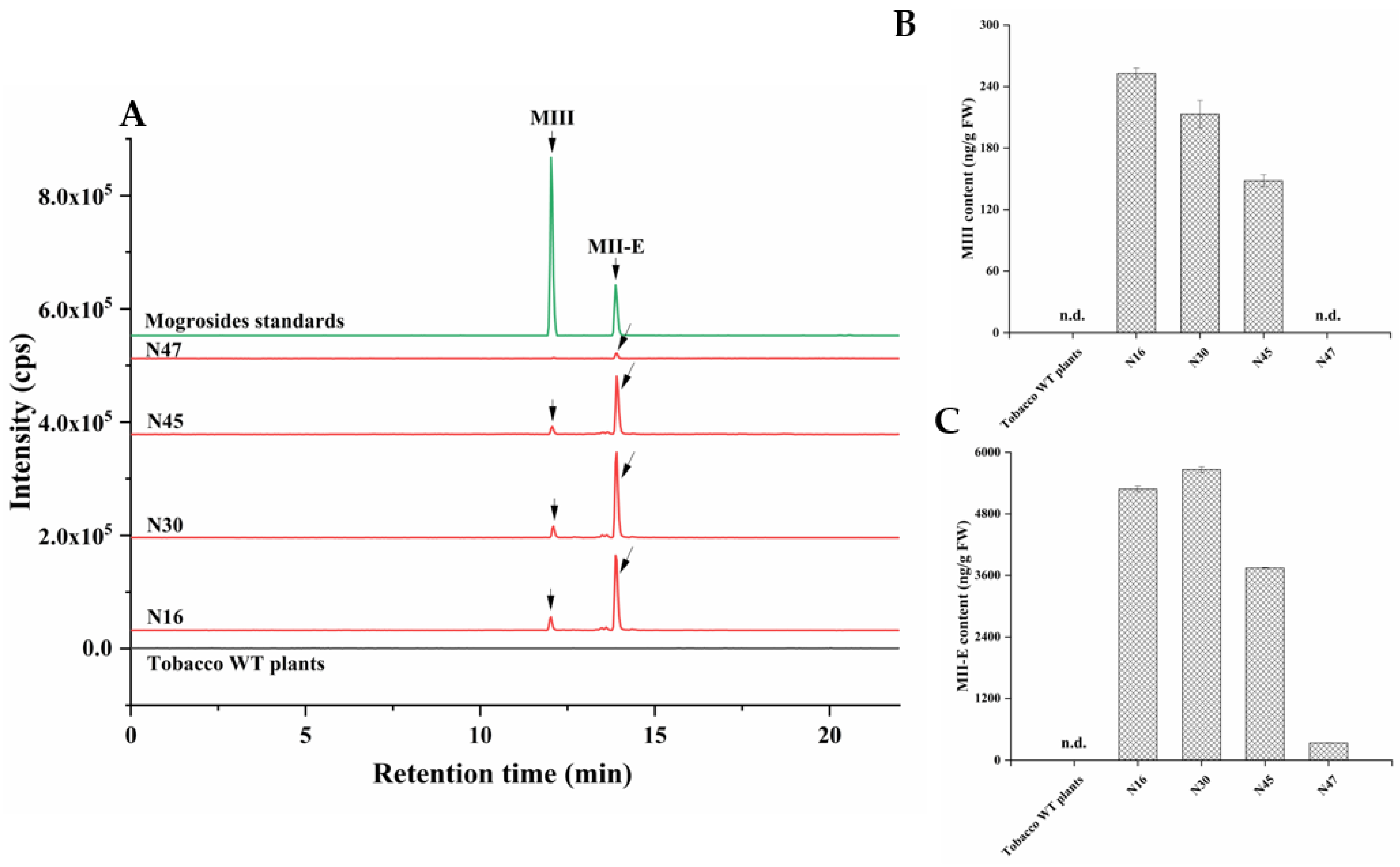
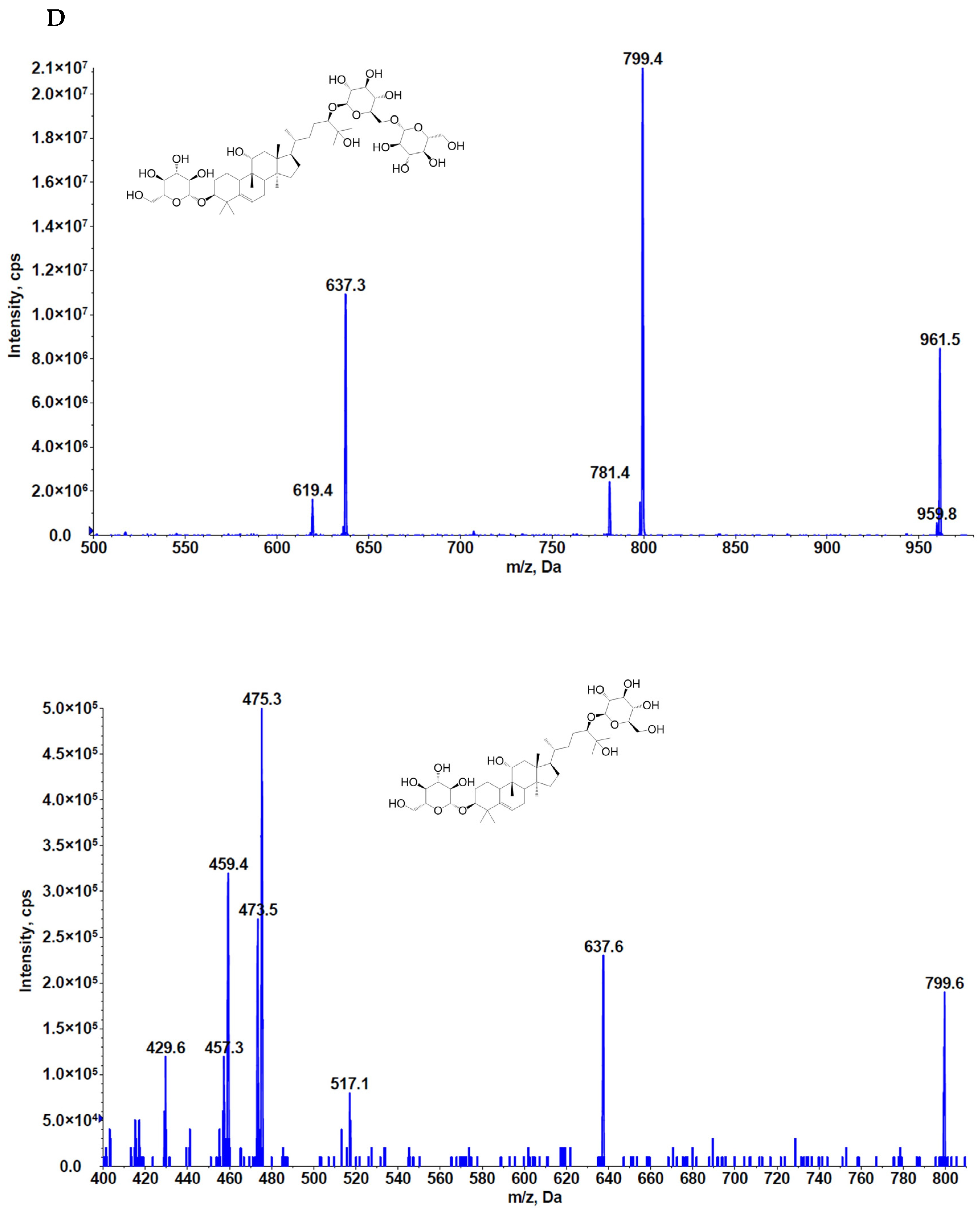
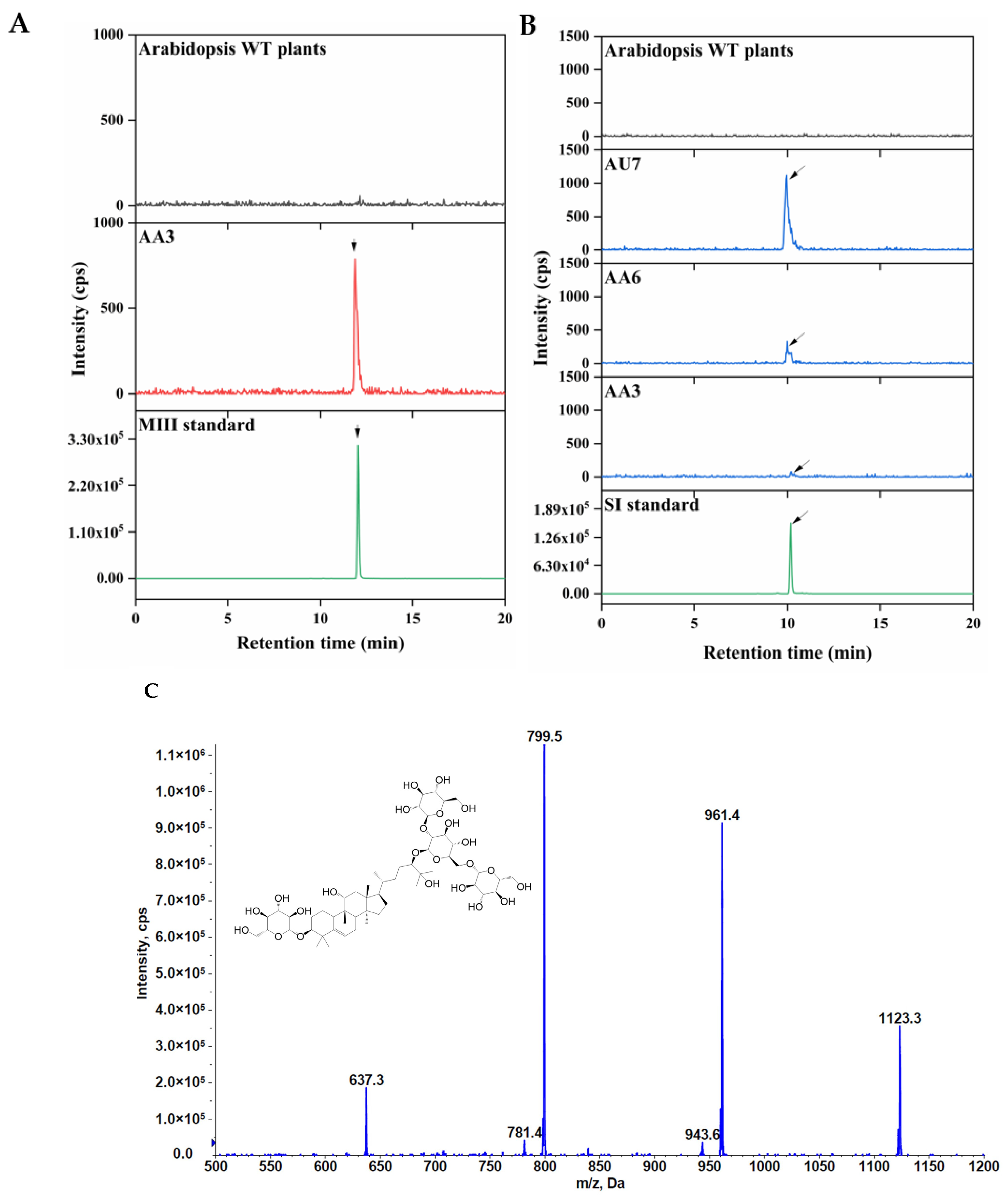
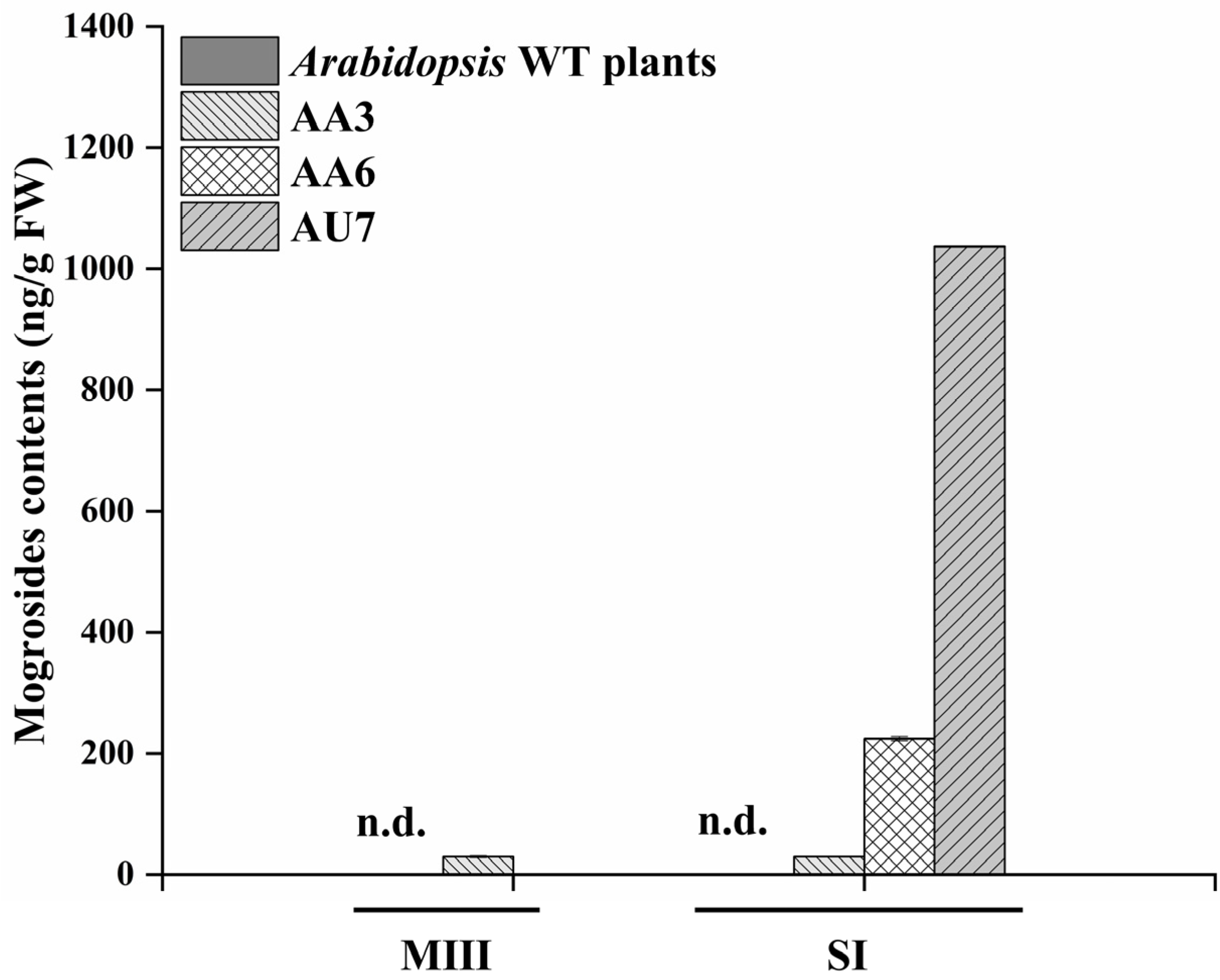
2.4. Heterologous Production of Mogrosides in Transgenic Tobacco and Arabidopsis thaliana
3. Discussion
4. Materials and Methods
4.1. Plant Material, Chemicals, and Strains
4.2. Multigene Stacking
4.3. Transient Expression Assay
4.4. Agrobacterium-Transformation in Tobacco
4.5. Arabidopsis thaliana Transformation
4.6. Gene Expression Level Analysis
4.7. HPLC-MS/MS Analysis of Mogrosides
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Food and Drug Administration, U.S.A GRAS Notice 000693: Generally Recognized as Safe (GRAS) Status of D-Allulose (D-Psicose). Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory (accessed on 2 February 2017).
- Food and Drug Administration, U.S.A GRAS Notice 000253: Rebaudioside A for Use as a General Purpose Sweetener. Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory (accessed on 14 March 2008).
- Food and Drug Administration, U.S.A GRAS Notice 000301: Luo Han Fruit. Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory (accessed on 15 January 2010).
- Sun-Waterhouse, D.; Wadhwa, S.S. Industry-relevant approaches for minimising the bitterness of bioactive compounds in functional foods: A review. Food Bioprocess Technol. 2013, 6, 607–627. [Google Scholar] [CrossRef]
- Pawar, R.S.; Krynitsky, A.J.; Rader, J.I. Sweeteners from Plants-with Emphasis on Stevia Rebaudiana (Bertoni) and Siraitia Grosvenorii (Swingle). Anal. Bioanal. Chem. 2013, 405, 4397–4407. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Jin, M.; Dewa, Y.; Nishimura, J.; Moto, M.; Murata, Y.; Shibutani, M.; Mitsumori, K. Suppressive Effect of Siraitia Grosvenorii extract on dicyclanil-promoted hepatocellular proliferative lesions in male mice. J. Toxicol. Sci. 2009, 34, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cai, T.Y.; Zhao, X.M.; Ma, L.Q.; Dou, D.Q.; Sun, Y.X. Apoptosis effect of Siraitia Grosvenorii Extracts on lung cancer cells A549 and its mechanisms. Chin. Pharm. Bull. 2015, 31, 1310–1314. [Google Scholar] [CrossRef]
- Tao, L.; Cao, F.; Xu, G.; Xie, H.; Zhang, M.; Zhang, C. Mogroside IIIE attenuates LPS-induced acute lung injury in mice partly through regulation of the TLR4/MAPK/NF-ΚB axis via AMPK activation. Phytother. Res. 2017, 31, 1097–1106. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Tomoda, M.; Muratal, Y.; Inui, H.; Sugiura, M.; Nakano, Y. Antidiabetic effect of long-term supplementation with Siraitia Grosvenori on the spontaneously diabetic goto-kakizaki rat. Brit. J. Nutr. 2007, 97, 770–775. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, Y.; Li, Y.; Wang, M.; Li, X. The metabolism of a natural product mogroside V, in healthy and type 2 diabetic rats. J. Chromatogr. B 2018, 1079, 25–33. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qi, X.; Zou, J.; Sun, Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia Grosvenorii (Swingle) fruits. J. Food Sci. Tech. 2018, 55, 1880–1888. [Google Scholar] [CrossRef]
- Makapugay, H.C.; Dhammika Nanayakkara, N.P.; Soejarto, D.D.; Kinghorn, A.D. High-performace liquid chromatographic analysis of the major sweet principle of lo han kuo fruits. J. Agric. Food Chem. 1985, 33, 348–350. [Google Scholar] [CrossRef]
- Li, D.; Ikeda, T.; Huang, Y.; Liu, J.; Nohara, T.; Sakamoto, T.; Nonaka, G.I. Seasonal variation of mogrosides in lo han kuo (Siraitia Grosvenori) fruits. J. Nat. Med. 2007, 61, 307–312. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Z.; Xin, Z.; Ma, G.; Qian, Y.; Xie, T.; Prakash, I. Analysis of mogrosides in Siraitia Grosvenorii fruits at different stages of maturity. Nat. Prod. Commun. 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Itkin, M.; Davidovich-Rikanati, R.; Cohen, S.; Portnoy, V.; Doron-Faigenboim, A.; Oren, E.; Freilich, S.; Tzuri, G.; Baranes, N.; Shen, S.; et al. The biosynthetic pathway of the nonsugar, high-intensity sweetener mogroside V from Siraitia Grosvenorii. Proc. Natl. Acad. Sci. USA 2016, 113, E7619–E7628. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Smolke, C.D. Engineering a microbial biosynthesis platform for de novo production of tropane alkaloids. Nat. Commun. 2019, 10, 3634. [Google Scholar] [CrossRef] [PubMed]
- Cravens, A.; Payne, J.; Smolke, C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat. Commun. 2019, 10, 2142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, X.; Liu, T.; Wang, Y.; Ahmed, N.; Li, Z.; Jiang, H. Synthetic biology of plant natural products: From pathway elucidation to engineered biosynthesis in plant cells. Plant Commun. 2021, 2, 100229. [Google Scholar] [CrossRef]
- Huo, L.; Hug, J.J.; Fu, C.; Bian, X.; Zhang, Y.; Müller, R. Heterologous expression of bacterial natural product biosynthetic pathways. Nat. Prod. Rep. 2019, 36, 1412–1436. [Google Scholar] [CrossRef]
- Xia, M.; Han, X.; He, H.; Yu, R.; Zhen, G.; Jia, X.; Cheng, B.; Deng, X.W. Improved de novo genome assembly and analysis of the Chinese cucurbit Siraitia Grosvenorii, also known as monk fruit or Luo-Han-Guo. Gigascience 2018, 7, giy067. [Google Scholar] [CrossRef]
- Yang, D.; Park, S.Y.; Park, Y.S.; Eun, H.; Lee, S.Y. Metabolic engineering of Escherichia Coli for natural product biosynthesis. Trends Biotechnol. 2020, 38, 745–765. [Google Scholar] [CrossRef]
- Lane, S.; Zhang, Y.; Yun, E.J.; Ziolkowski, L.; Zhang, G.; Jin, Y.S.; Avalos, J.L. Xylose assimilation enhances the production of isobutanol in engineered Saccharomyces Cerevisiae. Biotechnol. Bioeng. 2020, 117, 372–381. [Google Scholar] [CrossRef]
- Chiu, C.H.; Wang, R.; Lee, C.C.; Lo, Y.C.; Lu, T.J. Biotransformation of mogrosides from Siraitia Grosvenorii Swingle by Saccharomyces Cerevisiae. J. Agric. Food Chem. 2013, 61, 7127–7134. [Google Scholar] [CrossRef]
- Wang, R.; Chen, Y.C.; Lai, Y.J.; Lu, T.J.; Huang, S.T.; Lo, Y.C. Dekkera Bruxellensis, a beer yeast that specifically bioconverts mogroside extracts into the intense natural sweetener siamenoside I. Food Chem. 2019, 276, 43–49. [Google Scholar] [CrossRef]
- Li, J.; Mutanda, I.; Wang, K.; Yang, L.; Wang, J.; Wang, Y. Chloroplastic metabolic engineering coupled with isoprenoid pool enhancement for committed taxanes biosynthesis in Nicotiana Benthamiana. Nat. Commun. 2019, 10, 4850. [Google Scholar] [CrossRef] [PubMed]
- Farhi, M.; Marhevka, E.; Ben-Ari, J.; Algamas-Dimantov, A.; Liang, Z.; Zeevi, V.; Edelbaum, O.; Spitzer-Rimon, B.; Abeliovich, H.; Schwartz, B.; et al. Generation of the potent anti-malarial drug artemisinin in tobacco. Nat. Biotechnol. 2011, 29, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nowak, G.; Reed, D.W.; Covello, P.S. The production of artemisinin precursors in tobacco. Plant Biotechnol. J. 2011, 9, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.D.; Browse, J. The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J. 2011, 68, 387–399. [Google Scholar] [CrossRef]
- Kempinski, C.; Chappell, J. Engineering triterpene metabolism in the oilseed of Arabidopsis Thaliana. Plant Biotechnol. J. 2019, 17, 386–396. [Google Scholar] [CrossRef]
- Robert, S.S.; Singh, S.P.; Zhou, X.R.; Petrie, J.R.; Blackburn, S.I.; Mansour, P.M.; Nichols, P.D.; Liu, Q.; Green, A.G. Metabolic engineering of Arabidopsis to produce nutritionally important DHA in seed oil. Funct. Plant Biol. 2005, 32, 473–479. [Google Scholar] [CrossRef]
- Chen, Q.J.; Zhou, H.M.; Chen, J.; Wang, X.C. A Gateway-Based platform for multigene plant transformation. Plant Mol. Biol. 2006, 62, 927–936. [Google Scholar] [CrossRef]
- Gibson, D.G.; Benders, G.A.; Axelrod, K.C.; Zaveri, J.; Algire, M.A.; Moodie, M.; Montague, M.G.; Venter, J.C.; Smith, H.O.; Hutchison, C.A. One-step assembly in yeast of 25 overlapping DNA fragments to form a complete synthetic Mycoplasma genitalium genome. Proc. Natl. Acad. Sci. USA 2008, 105, 20404–20409. [Google Scholar] [CrossRef]
- Sleight, S.C.; Bartley, B.A.; Lieviant, J.A.; Sauro, H.M. In-Fusion biobrick assembly and re-engineering. Nucleic Acids. Res. 2010, 38, 2624–2636. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Yu, S.; Zeng, D.; Liu, H.; Wang, H.; Yang, Z.; Xie, X.; Shen, R.; Tan, J.; Li, H.; et al. Development of “Purple Endosperm Rice” by engineering anthocyanin biosynthesis in the endosperm with a high-efficiency transgene stacking system. Mol. Plant 2017, 10, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zeng, D.; Yu, S.; Cui, C.; Li, J.; Li, H.; Chen, J.; Zhang, R.; Zhao, X.; Chen, L.; et al. From golden rice to ASTARice: Bioengineering astaxanthin biosynthesis in rice endosperm. Mol. Plant 2018, 11, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xie, X.; Liu, W.; Huang, J.; Tan, J.; Yu, H.; Zong, W.; Tang, J.; Zhao, Y.; Xue, Y.; et al. STI PCR: An efficient method for amplification and de novo synthesis of long DNA sequences. Mol. Plant 2022, 15, 620–629. [Google Scholar] [CrossRef]
- Douin, V.; Bornes, S.; Creancier, L.; Rochaix, P.; Favre, G.; Prats, A.C.; Couderc, B. Use and comparison of different internal ribosomal entry sites (IRES) in tricistronic retroviral vectors. BMC Biotechnol. 2004, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, T.; Sun, H.; Zhan, H.; Zhao, Y. A reporter for noninvasively monitoring gene expression and plant transformation. Hortic. Res. 2020, 7, 1–6. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Mu, B.; Li, S.; Li, Y.; Zhou, X.; Zhang, C.; Fan, Y.; Chen, R. Engineering of ‘Purple Embryo Maize’ with a multigene expression system derived from a bidirectional promoter and self-cleaving 2A peptides. Plant Biotechnol. J. 2018, 16, 1107–1109. [Google Scholar] [CrossRef]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L.; et al. Improving the nutritional value of golden rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef]
- Zhu, C.; Naqvi, S.; Breitenbach, J.; Sandmann, G.; Christou, P.; Capell, T. Combinatorial genetic transformation generates a library of metabolic phenotypes for the carotenoid pathway in maize. Proc. Natl. Acad. Sci. USA 2008, 105, 18232–18237. [Google Scholar] [CrossRef] [PubMed]
- Shewmaker, C.K.; Sheehy, J.A.; Daley, M.; Colburn, S.; Ke, D.Y. Seed-specific overexpression of phytoene synthase: Increase in carotenoids and other metabolic effects. Plant J. 1999, 20, 401–412. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.K.; Kim, H.J.; Pak, J.H.; Lee, J.H.; Kim, D.H.; Choi, H.K.; Jung, H.W.; Lee, J.D.; Chung, Y.S.; et al. Genetic modification of the soybean to enhance the β-carotene content through seed-specific expression. PLoS ONE 2012, 7, e48287. [Google Scholar] [CrossRef] [Green Version]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Schijlen, E.G.W.M.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Baek, S.H.; Jo, H.J.; Yun, D.W.; Choi, Y.E. Genetically modified rice produces ginsenoside aglycone (protopanaxadiol). Planta 2019, 250, 1103–1110. [Google Scholar] [CrossRef]
- Huang, J.C.; Zhong, Y.J.; Liu, J.; Sandmann, G.; Chen, F. Metabolic engineering of tomato for high-yield production of astaxanthin. Meta Eng. 2013, 17, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Khairul Ikram, N.K.B.; Beyraghdar Kashkooli, A.; Peramuna, A.V.; van der Krol, A.R.; Bouwmeester, H.; Simonsen, H.T. Stable production of the antimalarial drug artemisinin in the moss Physcomitrella Patens. Front. Bioeng. Biotech. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Holst, J.; Vignali, K.M.; Burton, A.R.; Vignali, D.A.A. Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nat. Methods 2006, 3, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Pélissier, T.; Bourguet, P.; Becker, C.; Pouch-Pélissier, M.N.; Pogorelcnik, R.; Weingartner, M.; Weigel, D.; Deragon, J.M.; Mathieu, O. Arabidopsis proteins with a transposon-related domain act in gene silencing. Nat. Commun. 2017, 8, 15122. [Google Scholar] [CrossRef]
- Ee, S.F.; Khairunnisa, M.B.; Zeti-Azura, M.H.; Noor Azmi, S.; Zamri, Z. Effective hygromycin concentration for selection of Agrobacterium-mediated transgenic Arabidopsis Thaliana. Malays. Appl. Biol. 2014, 43, 119–123. [Google Scholar]
- Kim, N.C.; Kinghorn, A.D. Sweet-tasting and sweetness-modifying constituents of plants. Stud. Nat. Prod. Chem. 2002, 27, 3–57. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Murata, Y.; Inui, H.; Sugiura, M.; Nakano, Y. Triterpene glycosides of Siraitia Grosvenori inhibit rat intestinal maltase and suppress the rise in blood glucose level after a single oral administration of maltose in rats. J. Agric. Food Chem. 2005, 53, 2941–2946. [Google Scholar] [CrossRef]
- Wang, R.; Lin, P.Y.; Huang, S.T.; Chiu, C.H.; Lu, T.J.; Lo, Y.C. Hyperproduction of β-glucanase Exg1 promotes the bioconversion of mogrosides in Saccharomyces Cerevisiae mutants defective in mannoprotein deposition. J. Agric. Food Chem. 2015, 63, 10271–10279. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, B.; Tan, J.; Liu, T.; Li, L.; Liu, Y.G. Plant synthetic metabolic engineering for enhancing crop nutritional quality. Plant Commun. 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Dafny-Yelin, M.; Chung, S.M.; Frankman, E.L.; Tzfira, T. PSAT RNA interference vectors: A modular series for multiple gene down-regulation in plants. Plant Physiol. 2007, 145, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Halpin, C. Gene stacking in transgenic plants-The challenge for 21st century plant biotechnology. Plant Biotechnol. J. 2005, 3, 141–155. [Google Scholar] [CrossRef] [PubMed]
- De La Garza, R.I.D.; Gregory, J.F.; Hanson, A.D. Folate biofortification of tomato fruit. Proc. Natl. Acad. Sci. USA 2007, 104, 4218–4222. [Google Scholar] [CrossRef] [PubMed]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Mobilia, M.A.; Omri, B.; Santini, A. Rewiring cellular metabolism for heterologous biosynthesis of taxol. Nat. Prod. Res. 2020, 34, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Breitel, D.; Brett, P.; Alseekh, S.; Fernie, A.R.; Butelli, E.; Martin, C. Metabolic engineering of tomato fruit enriched in L-DOPA. Meta Eng. 2021, 65, 185–196. [Google Scholar] [CrossRef]
- Grützner, R.; Schubert, R.; Horn, C.; Yang, C.; Vogt, T.; Marillonnet, S. Engineering betalain biosynthesis in tomato for high level betanin production in fruits. Front. Plant Sci. 2021, 12, 682443. [Google Scholar] [CrossRef]
- Gutensohn, M.; Dudareva, N. Tomato Fruits—A Platform for Metabolic Engineering of Terpenes. Methods Enzymol. 2016, 576, 333–359. [Google Scholar] [CrossRef]
- Firsov, A.; Mitiouchkina, T.; Shaloiko, L.; Pushin, A.; Vainstein, A.; Dolgov, S. Agrobacterium-mediated transformation of chrysanthemum with artemisinin biosynthesis pathway genes. Plants 2020, 9, 537. [Google Scholar] [CrossRef]
- Gou, Y.; Zhang, F.; Tang, Y.; Jiang, C.; Bai, G.; Xie, H.; Chen, M.; Liao, Z. Engineering nootkatone biosynthesis in Artemisia Annua. ACS Synth. Biol. 2021, 10, 957–963. [Google Scholar] [CrossRef]
- Deng, B.; Zhang, P.; Ge, F.; Liu, D.Q.; Chen, C.Y. Enhancement of triterpenoid saponins biosynthesis in Panax Notoginseng cells by co-overexpressions of 3-Hydroxy-3-Methylglutaryl CoA reductase and squalene synthase genes. Biochem. Eng. J. 2017, 122, 38–46. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kasai, R.; Ohtani, K.; Tanaka, O. Minor cucurbitane-glycosides from fruits of Siraitia Grosvenori (Cucurbitaceae). Chem. Pharm. Bull. 1990, 38, 2030–2032. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral Dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis Thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Shi, H.; Zhang, K.; Qin, X.; Guo, Y.; Ma, X. Liquid chromatography with tandem mass spectrometry method for the simultaneous determination of multiple sweet mogrosides in the fruits of Siraitia Grosvenorii and its marketed sweeteners. J. Sep. Sci. 2016, 39, 4124–4135. [Google Scholar] [CrossRef] [PubMed]
| Analytes | Molecular Formula | Retention Time (min) | Production (m/z) | DP (V) | CE (eV) |
|---|---|---|---|---|---|
| SI | C54H92O24 | 9.62 | 1123.6/961.6 1123.6/799.2 | −220 | −75 |
| MIII | C48H82O19 | 11.86 | 961.6/799.4 961.6/637.3 | −170 | −70 |
| MII-E | C42H72O14 | 13.82 | 799.5/637.5 799.5/475.5 | −170 | −65 |
| Mogrol | C30H52O4 | 4.01 | 459.3/441.2 459.3/423.3 | 80 | 20 |
| MS parameters | Mogrosides | Mogrol | |||
| Ion mode | Negative | Positive | |||
| Source temperature (℃) | 550 | 550 | |||
| Ionization voltage (V) | −4500 | 5500 | |||
| GS1 (psi) | 55 | 18 | |||
| GS2 (psi) | 55 | 20 | |||
| CUR (psi) | 20 | 20 | |||
| CAD | Medium | Medium | |||
| Dwell time (ms) | 100 | 200 | |||
| EP (V) | −10 | 10 | |||
| CXP (V) | −15 | 10 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Liu, T.; Xie, L.; Mo, C.; Huang, X.; Cui, S.; Jia, X.; Lan, F.; Luo, Z.; Ma, X. Plant Metabolic Engineering by Multigene Stacking: Synthesis of Diverse Mogrosides. Int. J. Mol. Sci. 2022, 23, 10422. https://doi.org/10.3390/ijms231810422
Liao J, Liu T, Xie L, Mo C, Huang X, Cui S, Jia X, Lan F, Luo Z, Ma X. Plant Metabolic Engineering by Multigene Stacking: Synthesis of Diverse Mogrosides. International Journal of Molecular Sciences. 2022; 23(18):10422. https://doi.org/10.3390/ijms231810422
Chicago/Turabian StyleLiao, Jingjing, Tingyao Liu, Lei Xie, Changming Mo, Xiyang Huang, Shengrong Cui, Xunli Jia, Fusheng Lan, Zuliang Luo, and Xiaojun Ma. 2022. "Plant Metabolic Engineering by Multigene Stacking: Synthesis of Diverse Mogrosides" International Journal of Molecular Sciences 23, no. 18: 10422. https://doi.org/10.3390/ijms231810422






