Effects of Sterilization and Hydrolytic Degradation on the Structure, Morphology and Compressive Strength of Polylactide-Hydroxyapatite Composites
Abstract
:1. Introduction
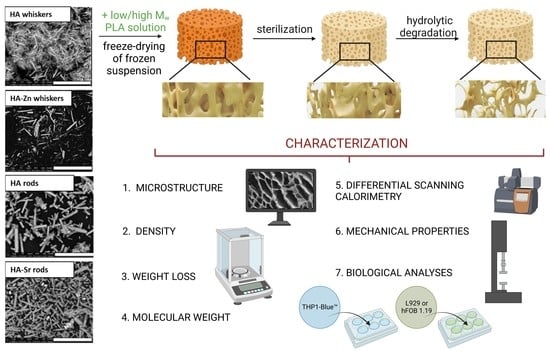
2. Results
2.1. Microstructure of HA
2.2. Density of Composites
2.3. Distribution of Pore Size in Composites
2.4. Molecular Properties
2.5. Compression Strength
2.6. Thermal Properties
2.7. Weight Loss in Composites
2.8. In Vitro Cytobiocompatibility of Produced Composites
3. Discussion
3.1. Preparation
3.2. Sterilization
3.3. Degradation
4. Materials and Methods
4.1. Synthesis and Modification of HA
4.2. Formulation of Composites
4.3. Sterilization
4.4. Hydrolytic Degradation
4.5. Density
4.6. SEM
4.7. X-ray Diffraction (XRD)
4.8. Weight Loss
4.9. Molecular Weight (Gel-Permeation Chromatography)
4.10. Mechanical Properties of Cylindrical Specimens
4.11. Differential Scanning Calorimetry
4.12. Biological Analyses
5. Conclusions
- (1)
- Sterilization led to a reduction of Mw of PLA and, furthermore, a change in the pore size distribution of composites depending on Mw and HA added.
- (2)
- Similarly, degradation reduced the Mw of PLA, but did not substantially change the compressive strength of tested materials.
- (3)
- Degradation of PLA occurred as reduction of polymer mass was greater in the control sample of high Mw, compared to HA-filled materials, indicating the lower rate of PLA degradation when HA is incorporated into the PLA matrix.
- (4)
- There was no major impact of HA morphology or metal inclusion on mechanical properties and weight loss of produced composites.
- (5)
- All produced materials should be recognized as cytocompatible when validated against L929 mouse skin fibroblasts and hFOB 1.19 human osteoblasts. The lack of cytotoxicity was accompanied by the immunocompatibility with human monocytic cells.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A















References
- Karpouzos, A.; Diamantis, E.; Farmaki, P.; Savvanis, S.; Troupis, T. Nutritional Aspects of Bone Health and Fracture Healing. J. Osteoporos. 2017, 2017, 4218472. [Google Scholar] [CrossRef] [PubMed]
- Masi, L.; Ferrari, S.; Javaid, M.K.; Papapoulos, S.; Pierroz, D.D.; Brandi, M.L. Bone fragility in patients affected by congenital diseases non skeletal in origin. Orphanet. J. Rare Dis. 2021, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Willers, C.; Borgström, F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2021, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, C.I.A.; Gabbai-Armelin, P.R.; Lopez-Perez, P.M.; Ulrich, D.J.O.; Jansen, J.A.; Renno, A.C.M.; van den Beucken, J.J.J.P. Alendronate release from calcium phosphate cement for bone regeneration in osteoporotic conditions. Sci. Rep. 2018, 8, 15398. [Google Scholar] [CrossRef]
- Murray, C.E.; Coleman, C.M. Impact of diabetes mellitus on bone health. Int. J. Mol. Sci. 2019, 20, 4873. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Herbaj, S.; Dunne, N.J. Calcium phosphate nanoparticles for therapeutic applications in bone regeneration. Nanomaterials 2019, 9, 1570. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.E.; Ciocan, L.T. Progress in Hydroxyapatite-Starch Based Sustainable Biomaterials for Biomedical Bone Substitution Applications. ACS Sustain. Chem. Eng. 2017, 5, 1788–1794. [Google Scholar] [CrossRef]
- Kolmas, J.; Krukowski, S.; Laskus, A.; Jurkitewicz, M. Synthetic hydroxyapatite in pharmaceutical applications. Ceram. Int. 2016, 42, 2472–2487. [Google Scholar] [CrossRef]
- Mathew, M.; Brown, W.E.; Schroeder, L.W.; Dickens, B. Crystal structure of octacalcium bis(hydrogenphosphate) tetrakis(phosphate)pentahydrate, Ca8(HP04)2(PO4)4·5H2O. J. Crystallogr. Spectrosc. Res. 1988, 18, 235–250. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and characterization of Zn-Doped hydroxyapatite: Scaffold application, antibacterial and bioactivity studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef]
- Gayathri, B.; Muthukumarasamy, N.; Velauthapillai, D.; Santhosh, S.B. Magnesium incorporated hydroxyapatite nanoparticles: Preparation, characterization, antibacterial and larvicidal activity. Arab. J. Chem. 2018, 11, 645–654. [Google Scholar] [CrossRef]
- Harrison, C.J.; Hatton, P.V.; Gentile, P.; Miller, C.A. Nanoscale strontium-substituted hydroxyapatite pastes and gels for bone tissue regeneration. Nanomaterials 2021, 11, 1611. [Google Scholar] [CrossRef]
- Lebre, F.; Sridharan, R.; Sawkins, M.J.; Kelly, D.J.; O’Brien, F.J.; Lavelle, E.C. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci. Rep. 2017, 7, 2922. [Google Scholar] [CrossRef]
- Domingos, M.; Gloria, A.; Coelho, J.; Bartolo, P.; Ciurana, J. Three-dimensional printed bone scaffolds: The role of nano/micro-hydroxyapatite particles on the adhesion and differentiation of human mesenchymal stem cells. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 555–564. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Lei, B.; Guo, B.; Rambhia, K.J.; Ma, P.X. Hybrid polymer biomaterials for bone tissue regeneration. Front. Med. 2019, 13, 189–201. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Qiu, Q.Q.; Sun, W.Q.; Connor, J. Sterilization of biomaterials of synthetic and biological origin. Compr. Biomater. 2011, 4, 127–144. [Google Scholar] [CrossRef]
- Mastalerz, C.; Vroman, I.; Coqueret, X.; Alix, S. Effects of electron beam irradiation on 3D-printed biopolymers for bone tissue engineering. J. Compos. Sci. 2021, 5, 182. [Google Scholar] [CrossRef]
- Bednarek, M.; Borska, K.; Kubisa, P. Crosslinking of Polylactide by High Energy Irradiation and Photo-Curing. Molecules 2020, 25, 4919. [Google Scholar] [CrossRef]
- Delabarde, C.; Plummer, C.J.G.; Bourban, P.E.; Månson, J.A.E. Accelerated ageing and degradation in poly-L-lactide/hydroxyapatite nanocomposites. Polym. Degrad. Stab. 2011, 96, 595–607. [Google Scholar] [CrossRef]
- Ignatius, A.A.; Augat, P.; Claes, L.E. Degradation behavior of composite pins made of tricalcium phosphate and poly(L,DL-lactide). J. Biomater. Sci. Polym. Ed. 2001, 12, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Ginebra, M.P.; Planell, J.A.; Barrias, C.C.; Barbosa, M.A. In vitro degradation behavior of a novel bioresorbable composite material based on PLA and a soluble CaP glass. Acta Biomater. 2005, 1, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Ito, Y.; Zhang, P.; Chen, X. A comparative study on the in vivo degradation of poly(L-lactide) based composite implants for bone fracture fixation. Sci. Rep. 2016, 6, 20770. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, T. Effect of β-tricalcium phosphate addition on the in vitro degradation of self-reinforced poly-L,D-lactide. Polym. Degrad. Stab. 2005, 89, 492–500. [Google Scholar] [CrossRef]
- Von Recum, H.A.; Cleek, R.L.; Eskin, S.G.; Mikos, A.G. Degradation of polydispersed poly(l-lactic acid) to modulate lactic acid release. Biomaterials 1995, 16, 441–447. [Google Scholar] [CrossRef]
- Törmälä, P.; Vasenius, J.; Vainionpää, S.; Laiho, J.; Pohjonen, T.; Rokkanen, P. Ultra-high-strength absorbable self-reinforced polyglycolide (SR-PGA) composite rods for internal fixation of bone fractures: In vitro and in vivo study. J. Biomed. Mater. Res. 1991, 25, 1–22. [Google Scholar] [CrossRef]
- Gorth, D.; Webster, T.J. Matrices for tissue engineering and regenerative medicine. In Biomaterials for Artificial Organs; Woodhead Publishing: Sawston, UK, 2011; pp. 270–286. [Google Scholar]
- Davila, S.P.; Rodríguez, L.G.; Chiussi, S.; Serra, J.; González, P. How to sterilize polylactic acid based medical devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef]
- Silindir, M.; Özer, A.Y. Sterilization methods and the comparison of E-beam sterilization with gamma radiation sterilization. Fabad J. Pharm. Sci. 2009, 34, 43–53. [Google Scholar]
- Soriano, I.; Martín, A.Y.; Évora, C.; Sánchez, E. Biodegradable implantable fluconazole delivery rods designed for the treatment of fungal osteomyelitis: Influence of gamma sterilization. J. Biomed. Mater. Res. Part A 2006, 77A, 632–638. [Google Scholar] [CrossRef]
- Shin, B.Y.; Han, D.H.; Narayan, R. Rheological and Thermal Properties of the PLA Modified by Electron Beam Irradiation in the Presence of Functional Monomer. J. Polym. Environ. 2010, 18, 558–566. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef]
- Türker, N.S.; Özer, A.Y.; Kutlu, B.; Nohutcu, R.; Sungur, A.; Bilgili, H.; Ekizoglu, M.; Özalp, M. The effect of gamma radiation sterilization on dental biomaterials. Tissue Eng. Regen. Med. 2014, 11, 341–349. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Gimeno-Alcañiz, J.V.; Ocio, M.J.; Lagaron, J.M. Optimization of electrospun polylactide-based ultrathin fibers for osteoconductive bone scaffolds. J. Appl. Polym. Sci. 2011, 122, 914–925. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, Y.; Tian, J. Study on the biodegradability of modified starch/polylactic acid (PLA) composite materials. RSC Adv. 2020, 10, 26298–26307. [Google Scholar] [CrossRef]
- Bleach, N.C.; Tanner, K.E.; Kellomäki, M.; Törmälä, P. Effect of filler type on the mechanical properties of self-reinforced polylactide-calcium phosphate composites. J. Mater. Sci. Mater. Med. 2001, 12, 911–915. [Google Scholar] [CrossRef]
- Araújo, A.; Botelho, G.; Oliveira, M.; Machado, A.V. Influence of clay organic modifier on the thermal-stability of PLA based nanocomposites. Appl. Clay Sci. 2014, 88–89, 144–150. [Google Scholar] [CrossRef]
- Baraúna, G.; Coraça-Huber, D.C.; Duek, E.A.D. In vitro degradation of poly-L-co-D, L-lactic acid membranes. Mater. Res. 2013, 16, 221–226. [Google Scholar] [CrossRef]
- Luo, Y.B.; Wang, X.L.; Wang, Y.Z. Effect of TiO2 nanoparticles on the long-term hydrolytic degradation behavior of PLA. Polym. Degrad. Stab. 2012, 97, 721–728. [Google Scholar] [CrossRef]
- Gazińska, M.; Krokos, A.; Kobielarz, M.; Włodarczyk, M.; Skibińska, P.; Stępak, B.; Antończak, A.; Morawiak, M.; Płociński, P.; Rudnicka, K. Influence of hydroxyapatite surface functionalization on thermal and biological properties of poly(L-lactide)-and poly(l-lactide-co-glycolide)-based composites. Int. J. Mol. Sci. 2020, 21, 6711. [Google Scholar] [CrossRef] [PubMed]
- Słota, D.; Gląb, M.; Tyliszczak, B.; Douglas, T.E.L.; Rudnicka, K.; Miernik, K.; Urbaniak, M.M.; Rusek-Wala, P.; Sobczak-Kupiec, A. Composites based on hydroxyapatite and whey protein isolate for applications in bone regeneration. Materials 2021, 14, 2317. [Google Scholar] [CrossRef] [PubMed]









| Sample ID | Preparation | Sterilization | Degradation | |||
|---|---|---|---|---|---|---|
| Mw [Dalton] | Mw/Mn | Mw [Dalton] | Mw/Mn | Mw [Dalton] | Mw/Mn | |
| Low Mw Control | 668,964 | 2.2 | 146,814 | 2.6 | 110,554 | 2.7 |
| Low Mw Whiskers | 657,872 | 2.4 | 141,291 | 2.6 | 116,469 | 3.0 |
| Low Mw Whiskers-Zn | 624,991 | 2.2 | 167,082 | 2.4 | 116,423 | 2.8 |
| Low Mw Rods | 638,310 | 2.4 | 156,717 | 2.6 | 120,735 | 3.3 |
| Low Mw Rods-Sr | 633,119 | 2.1 | 156,802 | 2.5 | 119,868 | 3.0 |
| High Mw Control | 1,034,000 | 2.3 | 164,076 | 2.5 | 123,425 | 3.5 |
| High Mw Whiskers | 1,031,000 | 2.5 | 162,828 | 2.7 | 119,578 | 3.3 |
| High Mw Whiskers-Zn | 1,059,000 | 2.5 | 170,110 | 2.5 | 122,374 | 3.0 |
| High Mw Rods | 1,142,000 | 2.3 | 167,232 | 2.7 | 121,381 | 2.7 |
| High Mw Rods-Sr | 1,061,000 | 2.9 | 166,265 | 2.7 | 118,867 | 3.4 |
| Sample ID | Concentration of Components in Composites [%Weight] | |||||
|---|---|---|---|---|---|---|
| PLA Type | HA Type | |||||
| LR706 | LR708 | Whiskers | Whiskers Zn | Hexagonal Rods | Hexagonal Rods Sr | |
| Low Mw Control | 100 | - | - | - | - | - |
| Low Mw Whiskers | 80 | - | 20 | - | - | - |
| Low Mw Whiskers-Zn | 80 | - | - | 20 | - | - |
| Low Mw Rods | 80 | - | - | - | 20 | - |
| Low Mw Rods-Sr | 80 | - | - | - | - | 20 |
| High Mw Control | - | 100 | - | - | - | - |
| High Mw Whiskers | - | 80 | 20 | - | - | - |
| High Mw Whiskers-Zn | - | 80 | - | 20 | - | - |
| High Mw Rods | - | 80 | - | - | 20 | - |
| High Mw Rods-Sr | - | 80 | - | - | - | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak, M.; Szabłowska, A.; Kurzyk, A.; Tymowicz-Grzyb, P.; Najmrodzki, A.; Woźniak, A.; Antosik, A.; Pagacz, J.; Szterner, P.; Plichta, A.; et al. Effects of Sterilization and Hydrolytic Degradation on the Structure, Morphology and Compressive Strength of Polylactide-Hydroxyapatite Composites. Int. J. Mol. Sci. 2022, 23, 10454. https://doi.org/10.3390/ijms231810454
Kasprzak M, Szabłowska A, Kurzyk A, Tymowicz-Grzyb P, Najmrodzki A, Woźniak A, Antosik A, Pagacz J, Szterner P, Plichta A, et al. Effects of Sterilization and Hydrolytic Degradation on the Structure, Morphology and Compressive Strength of Polylactide-Hydroxyapatite Composites. International Journal of Molecular Sciences. 2022; 23(18):10454. https://doi.org/10.3390/ijms231810454
Chicago/Turabian StyleKasprzak, Mirosław, Agnieszka Szabłowska, Agata Kurzyk, Paulina Tymowicz-Grzyb, Adrian Najmrodzki, Anna Woźniak, Agnieszka Antosik, Joanna Pagacz, Piotr Szterner, Andrzej Plichta, and et al. 2022. "Effects of Sterilization and Hydrolytic Degradation on the Structure, Morphology and Compressive Strength of Polylactide-Hydroxyapatite Composites" International Journal of Molecular Sciences 23, no. 18: 10454. https://doi.org/10.3390/ijms231810454
APA StyleKasprzak, M., Szabłowska, A., Kurzyk, A., Tymowicz-Grzyb, P., Najmrodzki, A., Woźniak, A., Antosik, A., Pagacz, J., Szterner, P., Plichta, A., Wieciński, P., Rusek-Wala, P., Krupa, A., Płociński, P., Rudnicka, K., & Biernat, M. (2022). Effects of Sterilization and Hydrolytic Degradation on the Structure, Morphology and Compressive Strength of Polylactide-Hydroxyapatite Composites. International Journal of Molecular Sciences, 23(18), 10454. https://doi.org/10.3390/ijms231810454