Dietary Modulation of the Human Gut Microbiota and Metabolome with Flaxseed Preparations
Abstract
:1. Introduction
2. Results
2.1. Characterization of Chemically and Enzymatically Predigested Flaxseed Press Cake and Milled Whole Flaxseeds
Sample Hydrolysis, Extraction, and Quantification
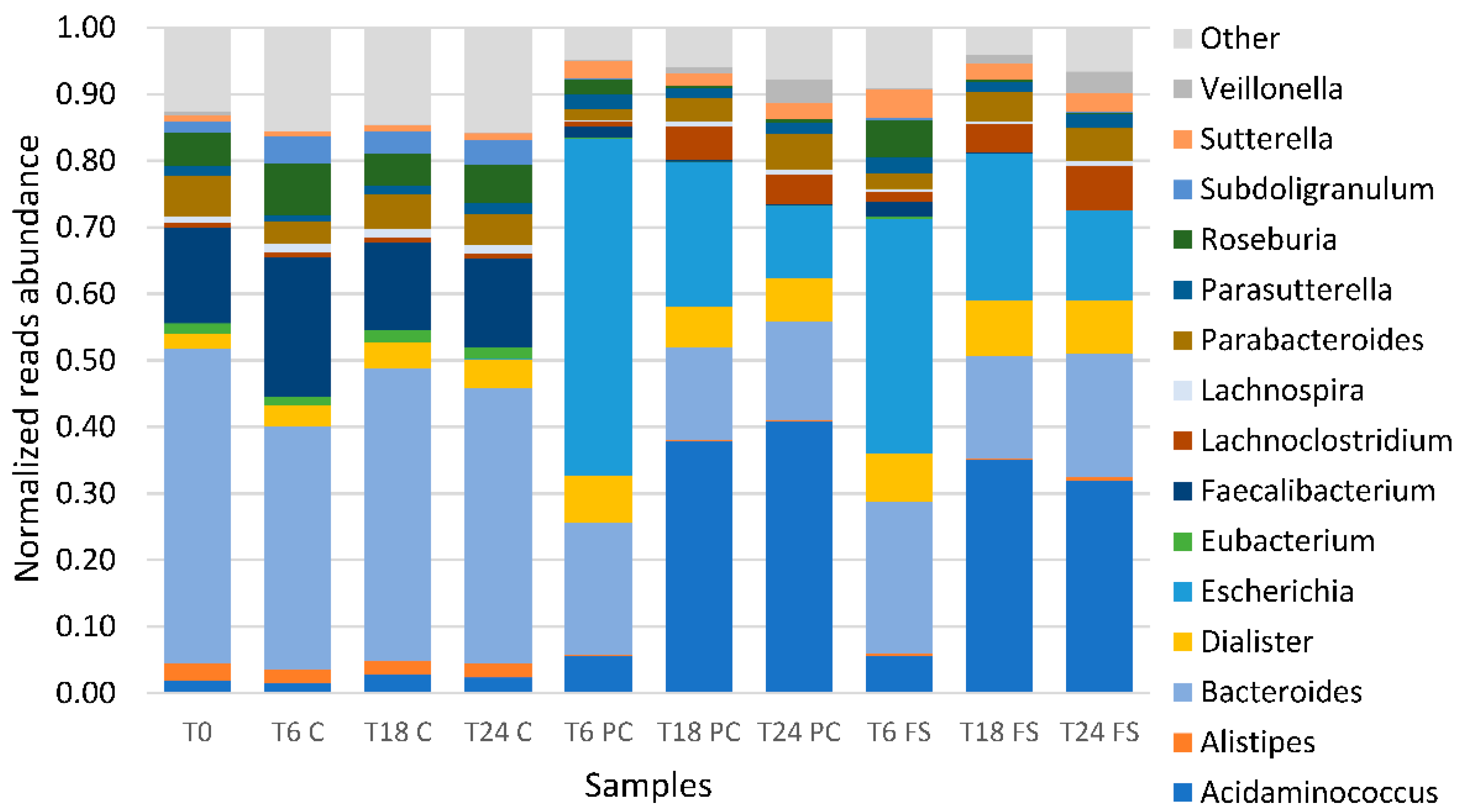
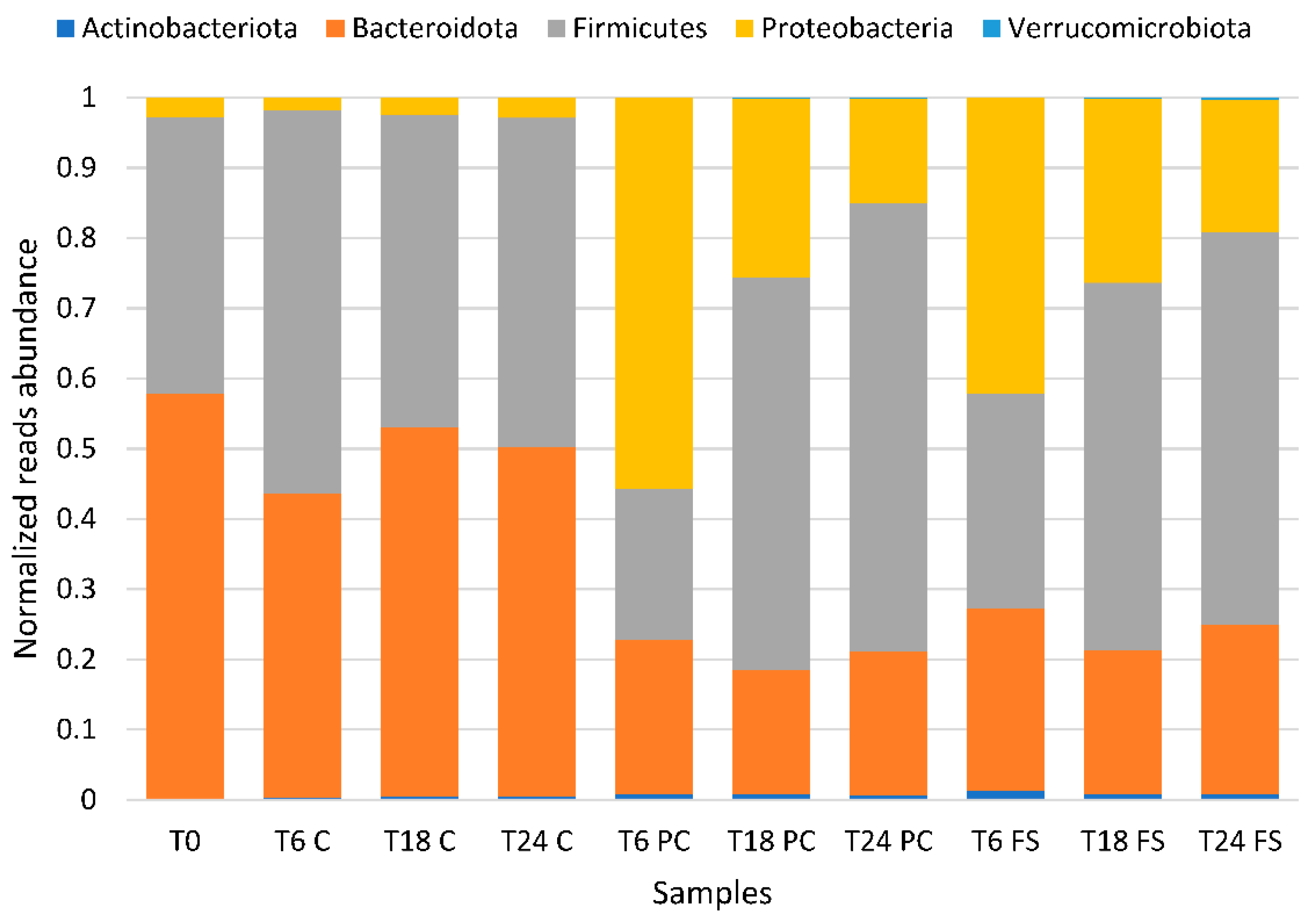
2.2. Impact of Flaxseed on Fecal-Derived Microbial Communities
2.3. Analysis of Fiber-Derived Short-Chain Fatty Acids in Fermented Samples
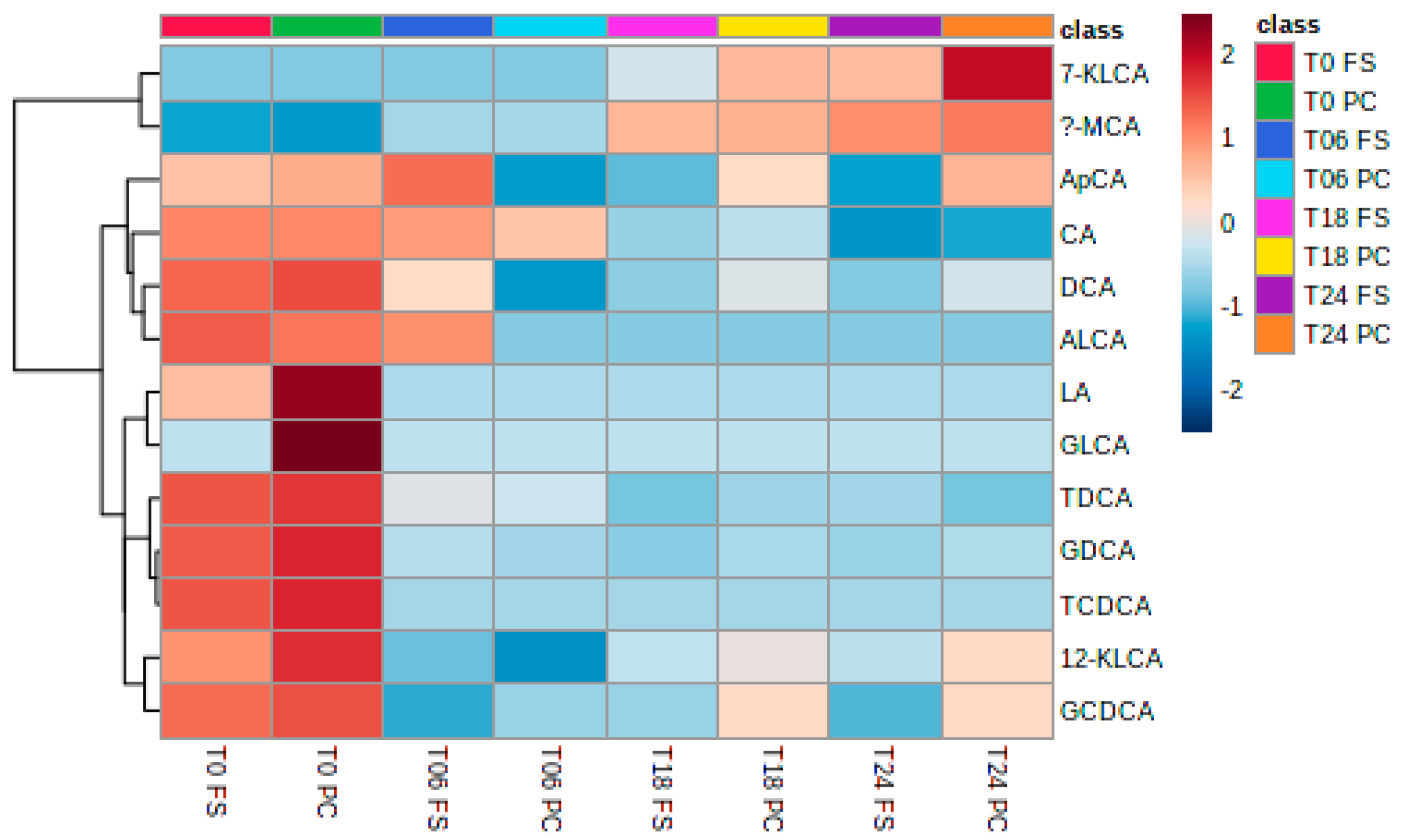
2.4. Analysis of Lignans, Cyclolinopeptides, and Bile Acids in Fermented Samples
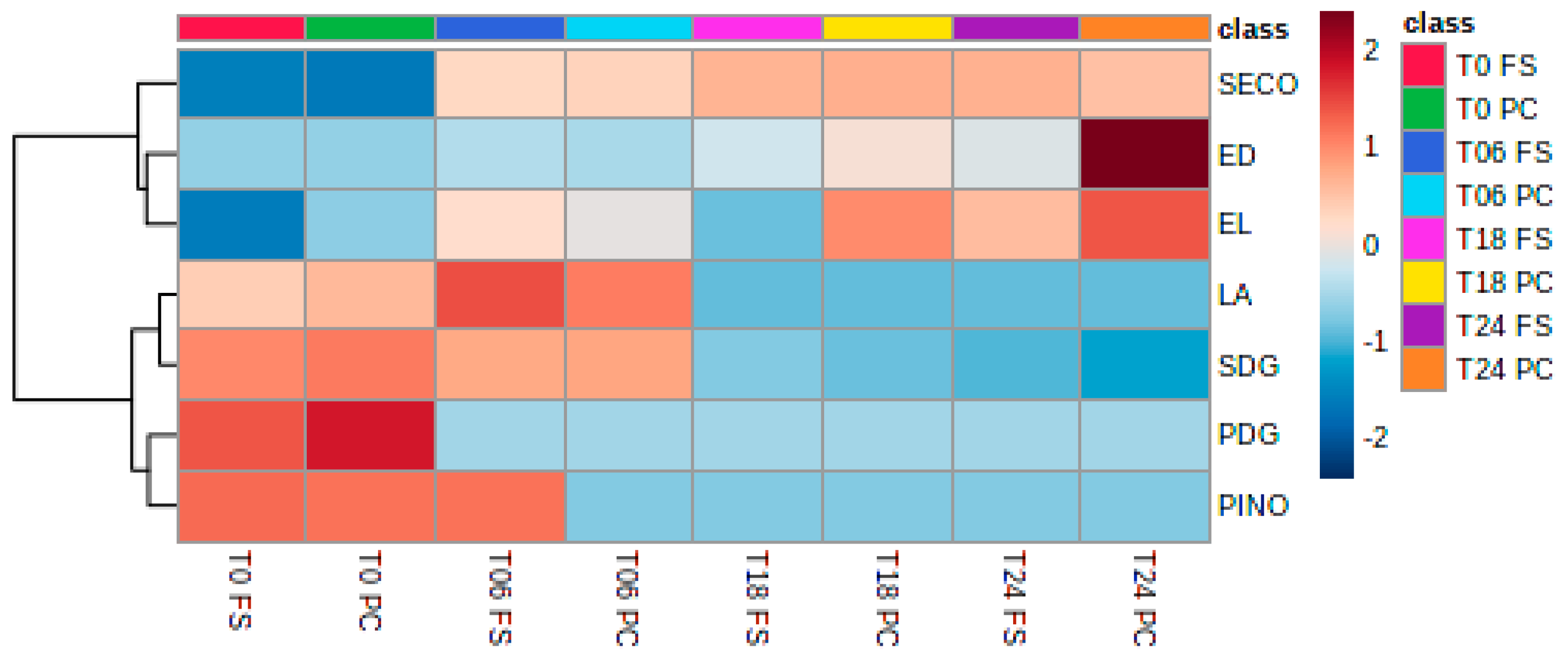
2.4.1. Lignans
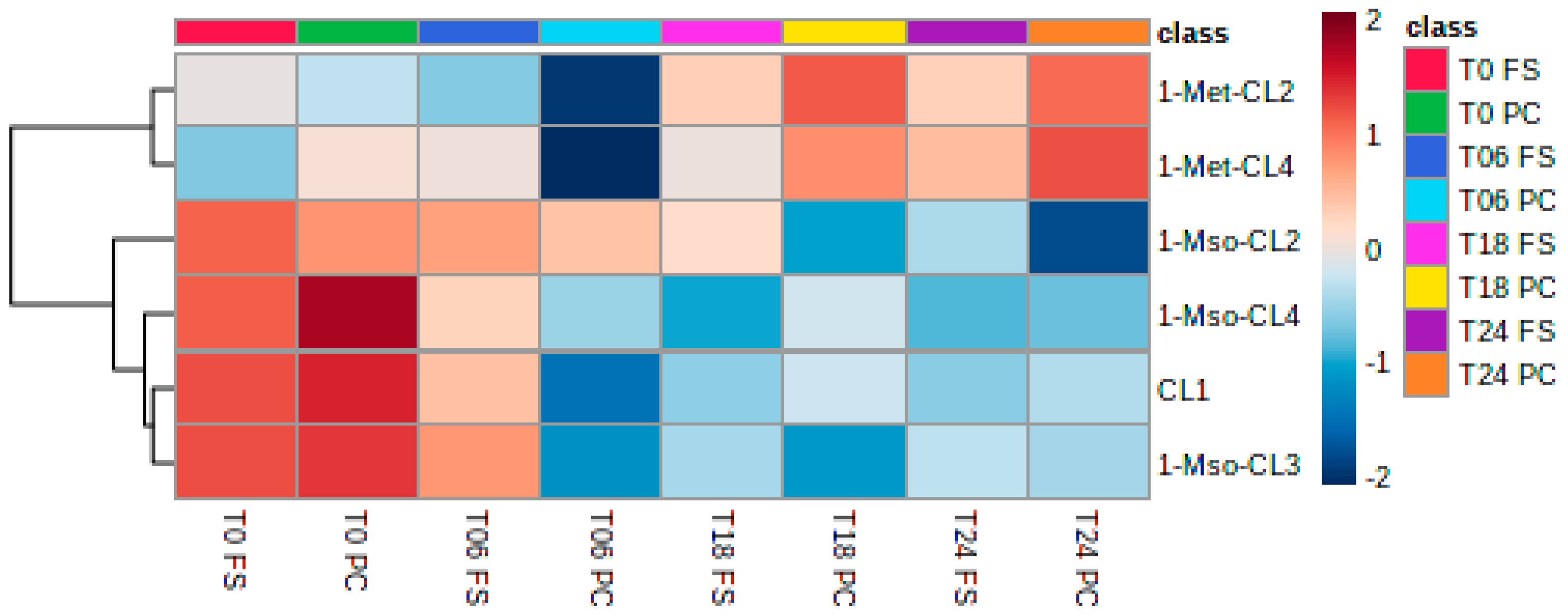
2.4.2. Cyclolinopeptides
2.4.3. Bile Acids
3. Discussion
3.1. Microbial Communities
3.2. Short-Chain Fatty Acids
3.3. Lignans
3.4. Cyclolinopeptides
3.5. Bile Acids
4. Materials and Methods
4.1. Flaxseed Processing
4.2. In Vitro Digestion
4.3. Processing of Digested Flaxseed Press Cake and Whole Flaxseed
4.4. Soluble and Insoluble Fiber Content
4.5. Dry Matter of Lyophilized Powders
4.6. Stool Sample Collection and Preparation
4.7. In Vitro Batch Culture Microbiota Model
4.8. Sequencing
Nucleotide Sequence Accession Numbers
4.9. Volatile Fatty Acid Analysis
4.10. Targeted Analysis of Lignans, Cyclolinopeptides, and Bile Acids
4.10.1. Lignans and Cyclolinopeptides
4.10.2. Bile Acids
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badole, S.L.; Zanwar, A.A.; Bodhankar, S.L. Chapter 5—Antihyperglycemic potential of secoisolaricinol diglucoside. In Bioactive Food as Dietary Interventions for Diabetes; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 53–57. [Google Scholar] [CrossRef]
- Agricultural Marketing Resource Center. Flax Profile. 2021. Available online: https://www.agmrc.org/commodities-products/grains-oilseeds/flax-profile (accessed on 4 November 2021).
- Muir, A.D.; Westcott, N.D. (Eds.) Flaxseed constituents and human health. In Flax: The Genus Linum; Taylor & Francis: London, UK, 2003; pp. 243–251. [Google Scholar]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary flaxseed as a strategy for improving human health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Netticadan, T.; Pierce, G.N. Flaxseed: Its bioactive components and their cardiovascular benefits. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H146–H159. [Google Scholar] [CrossRef] [PubMed]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed-a potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Dzuvor, C.K.O.; Taylor, J.T.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of functional ingredients from flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef]
- Brühl, L.; Matthäus, B.; Fehling, E.; Wiege, B.; Lehmann, B.; Luftmann, H.; Bergander, K.; Quiroga, K.; Scheipers, A.; Frank, O.; et al. Identification of bitter off-taste compounds in the stored cold pressed linseed oil. J. Agric. Food Chem. 2007, 55, 7864–7868. [Google Scholar] [CrossRef]
- Lang, T.; Frank, O.; Lang, R.; Hofmann, T.; Behrens, M. Activation spectra of human bitter taste receptors stimulated with cyclolinopeptides corresponding to fresh and aged linseed oil. J. Agric. Food Chem. 2022, 70, 4382–4390. [Google Scholar] [CrossRef]
- Bernacchia, R.; Preti, R.; Vinci, G. Chemical composition and health benefits of flaxseed. Austin J. Nutr. Food Sci. 2014, 2, 1045. [Google Scholar]
- Qian, K.Y.; Cui, S.W.; Goff, H.D. Flaxseed gum from flaxseed hulls: Extraction, fractionation, and characterization. Food Hydrocoll. 2012, 28, 275–283. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Huang, F.; Yang, C.; Huang, Q. In vitro digestion and fermentation by human fecal microbiota of polysaccharides from flaxseed. Molecules 2020, 25, 4354. [Google Scholar] [CrossRef]
- Vieira, J.M.; Andrade, C.C.P.; Santos, T.P.; Okuro, P.K.; Garcia, S.T.; Rodrigues, M.I.; Vicente, A.A.; Cunha, R.L. Flaxseed gum-biopolymers interactions driving rheological behaviour of oropharyngeal dysphagia-oriented products. Food Hydrocoll. 2020, 111, 132–144. [Google Scholar] [CrossRef]
- Arora, T.; Rudenko, O.; Egerod, K.L.; Husted, A.S.; Kovatcheva-Datchary, P.; Akrami, R.; Kristensen, M.; Schwartz, T.W.; Bäckhed, F. Microbial fermentation of flaxseed fibers modulates the transcriptome of GPR41-expressing enteroendocrine cells and protects mice against diet-induced obesity. Am. J. Physiol. Endocrinol. Metabol. 2019, 316, E453–E463. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.H.; Cui, S.W.; Douglas, H.G.; Gong, J. Short-chain fatty acid profiles from flaxseed dietary fibres after in vitro fermentation of pig colonic digesta: Structure–function relationship. Bioact. Carbohydr. Diet. Fibre 2015, 6, 62–68. [Google Scholar] [CrossRef]
- Sok, D.E.; Cui, H.S.; Kim, M.R. Isolation and bioactivities of furfuran type lignan compounds from edible plants. Recent Pat. Food Nutr. Agric. 2009, 1, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary lignans: Definition, description and research trends in databases development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, B.; Hoikkala, A.; Leppälä, E.; Wähälä, K. Enterolignans. J. Chromatogr. B 2002, 777, 29–43. [Google Scholar] [CrossRef]
- Johnsson, P.; Kamal-Eldin, A.; Lundgren, L.N.; Aman, P. HPLC method for analysis of secoisolariciresinol diglucoside in flaxseeds. J. Agric. Food Chem. 2000, 48, 5216–5219. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.A. Lignans, neolignans and related compounds. Nat. Prod. Rep. 1987, 4, 499–525. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Lampe, J.W.; Kim, E.; Levy, L.; Davidson, L.A.; Goldsby, J.S.; Miles, F.L.; Navarro, S.L.; Randolph, T.W.; Zhao, N.; Ivanov, I.; et al. Colonic mucosal and exfoliome transcriptomic profiling and fecal microbiome response to a flaxseed lignan extract intervention in humans. Am. J. Clin. Nutr. 2019, 110, 377–390. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Bannwart, C.; Wähälä, K.; Mäkelä, T.; Brunow, G.; Hase, T.; Arosemena, P.J.; Kellis, J.T.; Vickery, L.E. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J. Steroid Biochem. Mol. Biol. 1993, 44, 147–153. [Google Scholar] [CrossRef]
- Mousavi, Y.; Adlercreutz, H. Enterolactone and estradiol inhibit each other’s proliferative effect on MCF-7 breast cancer cells in culture. J. Steroid Biochem. Mol. Biol. 1992, 41, 615–619. [Google Scholar] [CrossRef]
- Yuan, J.P.; Li, X.; Xu, S.P.; Wang, J.H.; Liu, X. Hydrolysis kinetics of secoisolariciresinol diglucoside oligomers from flaxseed. J. Agric. Food Chem. 2008, 56, 10041–10047. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Henderson, G.; Engst, W.; Doré, J.; Blaut, M. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol. Ecol. 2006, 55, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Roncaglia, L.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M. Role of bifidobacteria in the activation of the lignan secoisolariciresinol diglucoside. Appl. Microbiol. Biotechnol. 2011, 92, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Kläring, K.; Heinzmann, S.S.; Platz, S.; Scholz, B.; Engel, K.; Schmitt-Kopplin, P.; Haller, D.; Rohn, S.; Skurk, T. Gut metabolites and bacterial community networks during a pilot intervention study with flaxseeds in healthy adult men. Mol. Nutr. Food Res. 2015, 59, 1614–1628. [Google Scholar] [CrossRef]
- Corona, G.; Kreimes, A.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Costabile, A. Impact of lignans in oilseed mix on gut microbiome composition and enterolignan production in younger healthy and premenopausal women: An in vitro pilot study. Microb. Cell Fact. 2020, 19, 82. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Yin, D.; Suhaib, M.; Yuan, S.; Yuan, J. Flaxseed diet caused inflammation by altering the gut microbiota of Peking ducks. Anim. Biotechnol. 2020, 31, 520–531. [Google Scholar] [CrossRef]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Mainali, R.; Soleimanian-Zad, S.; Kitzman, D.; Yadav, H. An In Vitro batch-culture model to estimate the effects of interventional regimens on human fecal microbiota. J. Vis. Exp. 2019, 31, 149. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Shi, X.; Zhou, B.; Sui, J.; Yang, C.; Liu, H.; Yang, L.; Wang, S.; Sun, G. Milled flaxseed-added diets ameliorated hepatic inflammation by reducing gene expression of TLR4/NF-κB pathway and altered gut microbiota in STZ-induced type 1 diabetic mice. Food Sci. Hum. Wellness 2022, 11, 32–40. [Google Scholar] [CrossRef]
- Thumann, T.A.; Pferschy-Wenzig, E.-M.; Moissl-Eichinger, C.; Bauer, R. The role of gut microbiota for the activity of medicinal plants traditionally used in the European Union for gastrointestinal disorders. J. Ethnopharmacol. 2019, 245, 112153. [Google Scholar] [CrossRef]
- Määttänen, P.; Lurz, E.; Botts, S.R.; Wu, R.Y.; Yeung, C.W.; Li, B.; Abiff, S.; Johnson-Henry, K.C.; Lepp, D.; Power, K.A.; et al. Ground flaxseed reverses protection of a reduced-fat diet against Citrobacter rodentium-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G788–G798. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, T.; Zhang, X.; Ning, Y.; Zhang, G.; Yan, R.; Li, Y.; Yu, J.; He, J.; Jia, S.; et al. Exosomes derived from human placental mesenchymal stem cells ameliorate myocardial infarction via anti-inflammation and restoring gut dysbiosis. BMC Cardiovasc. Disord. 2022, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.; Boland, T.; Storey, S.; Doyle, E. Linseed oil supplementation of lambs’ diet in early life leads to persistent changes in rumen microbiome structure. Front. Microbiol. 2017, 8, 1656. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gong, Z.; Sun, G.; Xu, J.; Qi, C.; Sun, W.; Jiang, H.; Cao, P.; Ju, H. Dysbiosis of gut microbiota in patients with acute myocardial infarction. Front. Microbiol. 2021, 12, 680101. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Leonel, A.J.; Alvarez-Leite, J.I. Butyrate: Implications for intestinal function. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 474–479. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Fuentealba, C.; Figuerola, F.; Estévez, A.M.; Bastías, J.M.; Muñoz, O. Bioaccessibility of lignans from flaxseed (Linum usitatissimum L.) determined by single-batch in vitro simulation of the digestive process. J. Sci. Food Agric. 2014, 94, 1729–1738. [Google Scholar] [CrossRef]
- Zarepoor, L.; Lu, J.T.; Zhang, C.; Wu, W.; Lepp, D.; Robinson, L.; Wanasundara, J.; Cui, S.; Villeneuve, S.; Fofana, B.; et al. Dietary flaxseed intake exacerbates acute colonic mucosal injury and inflammation induced by dextran sodium sulfate. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G1042–G1055. [Google Scholar] [CrossRef] [PubMed]
- Bourriaud, C.; Robins, R.J.; Martin, L.; Kozlowski, F.; Tenailleau, E.; Cherbut, C.; Michel, C. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J. Appl. Microbiol. 2005, 99, 201–212. [Google Scholar] [CrossRef]
- Jumas-Bilak, E.; Carlier, J.-P.; Jean-Pierre, H.; Mory, F.; Teyssier, C.; Gay, B.; Campos, J.; Marchandin, H. Acidaminococcus intestini sp. nov., isolated from human clinical samples. Int. J. Syst. Evol. 2007, 57, 2314–2319. [Google Scholar] [CrossRef] [PubMed]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and gut microbiota: An interplay revealing potential health implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef]
- Taibi, A.; Ku, M.; Lin, Z.; Gargari, G.; Kubant, A.; Lepp, D.; Power, K.A.; Guglielmetti, S.; Thompson, L.U.; Comelli, E.M. Discriminatory and cooperative effects within the mouse gut microbiota in response to flaxseed and its oil and lignan components. J. Nutr. Biochem. 2021, 98, 108818. [Google Scholar] [CrossRef]
- Taibi, A.; Ku, M.; Lin, Z.; Gargari, G.; Kubant, A.; Lepp, D.; Power, K.A.; Guglielmetti, S.; Thompson, L.U.; Comelli, E.M. Data on cecal and fecal microbiota and predicted metagenomes profiles of female mice receiving whole flaxseed or its oil and secoisolariciresinol diglucoside components. Data Brief 2021, 38, 107409. [Google Scholar] [CrossRef] [PubMed]
- Resch, C.; Parikh, M.; Austria, J.A.; Proctor, S.D.; Netticadan, T.; Blewett, H.; Pierce, G.N. The Influence of Diet and Sex on the Gut Microbiota of Lean and Obese JCR:LA-cp Rats. Microorganisms 2021, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef]
- Gaya, P.; Medina, M.; Sánchez-Jiménez, A.; Landete, J.M. Phytoestrogen metabolism by adult human gut microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef]
- Xie, L.H.; Akao, T.; Hamasaki, K.; Deyama, T.; Hattori, M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol. Chem. Pharm. Bull. 2003, 51, 508–515. [Google Scholar] [CrossRef]
- Behsaz, B.; Mohimani, H.; Gurevich, A.; Prjibelski, A.; Fisher, M.F.; Smarr, L.; Dorrestein, P.C.; Mylne, J.S.; Pevzner, P.A. De novo peptide sequencing reveals a vast cyclopeptidome in human gut and other environments. Cell Syst. 2020, 10, 99–108.e5. [Google Scholar] [CrossRef]
- Navarro, S.L.; Levy, L.; Curtis, K.R.; Elkon, I.; Kahsai, O.J.; Ammar, H.S.; Randolph, T.W.; Hong, N.N.; Carnevale Neto, F.; Raftery, D.; et al. Effect of a flaxseed lignan intervention on circulating bile acids in a placebo-controlled randomized, crossover trial. Nutrients 2020, 12, 1837. [Google Scholar] [CrossRef]
- Pushpass, R.; Alzoufairi, S.; Jackson, K.; Lovegrove, J. Circulating bile acids as a link between the gut microbiota and cardiovascular health: Impact of prebiotics, probiotics and polyphenol-rich foods. Nutr. Res. Rev. 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Pang, S.; Sun, Y.; Tian, Y.; Yu, L.; Dang, N. Coordinated actions of FXR and LXR in metabolism: From pathogenesis to pharmacological targets for Type 2 Diabetes. Int. J. Endocrinol. 2014, 2014, 751859. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Huang, F.; Zhao, A.; Lei, S.; Zhang, Y.; Xie, G.; Chen, T.; Qu, C.; Rajani, C.; Dong, B.; et al. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 2017, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kosinska, A.; Xu, H.; Andlauer, W. Milk enhances intestinal absorption of green tea catechins in in vitro digestion/Caco-2 cells model. Food Res. Int. 2013, 53, 793–800. [Google Scholar] [CrossRef]
- Gibson, G.R.; Fuller, R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J. Nutr. 2020, 130, 391S–395S. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.T.; Moughan, P.J.; Darraugh, A.J. In vitro digestion and fermentation methods, including gas production techniques, as applied to nutritive evaluation of foods in the hindgut of humans and other simple-stomached animals. Anim. Feed Sci. Technol. 2005, 123–124, 421–444. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S.; Gibson, G.R. Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb. Ecol. 1998, 33, 180–187. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 4 November 2021).
- Lagkouvardos, I.; Fischer, S.; Kumar, N.; Clavel, T. Rhea: A transparent and modular R pipeline for microbial profiling based on 16S rRNA gene amplicons. PeerJ 2017, 5, e2836. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bittinger, K.; Charlson, E.S.; Hoffmann, C.; Lewis, J.; Wu, G.D.; Collman, R.G.; Bushman, F.D.; Li, H. Associating microbiome composition with environmental covariates using generalized UniFrac distances. Bioinformatics 2012, 28, 2106–2113. [Google Scholar] [CrossRef] [Green Version]
- Murtagh, F.; Legendre, P. Ward’s hierarchical agglomerative clustering method: Which algorithms implement Ward’s criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Leinonen, R.; Sugawara, H.; Shumway, M. The sequence read archive. Nucleic Acids Res. 2011, 39, D19–D21. [Google Scholar] [CrossRef] [PubMed]
- Minchin, P.R. An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio 1987, 69, 89–107. [Google Scholar] [CrossRef]
- Frank, O.; Kreissl, J.K.; Daschner, A.; Hofmann, T. Accurate determination of reference materials and natural isolates by means of quantitative (1)h NMR spectroscopy. J. Agric. Food Chem. 2014, 62, 2506–25015. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; Morais, D.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Reiter, S.; Dunkel, A.; Metwaly, A.; Panes, J.; Salas, A.; Haller, D.; Hofmann, T. Development of a highly sensitive ultra-high-performance liquid chromatography coupled to eectrospray ionization tandem mass spectrometry quantitation method for fecal bile acids and application on Crohn’s disease studies. J. Agric. Food Chem. 2021, 69, 5238–5251. [Google Scholar] [CrossRef] [PubMed]


| Richness | Normalized Richness | Effective Richness | Shannon Index | Shannon Effective | Simpson Index | Simpson Effective | Evenness | |
|---|---|---|---|---|---|---|---|---|
| T0 | 78 | 70 | 51 | 3.28 | 26.58 | 0.08 | 13.07 | 0.52 |
| C T6 | 77 | 71 | 56 | 3.35 | 28.38 | 0.07 | 14.22 | 0.53 |
| C T18 | 80 | 74 | 55 | 3.29 | 26.74 | 0.08 | 12.53 | 0.52 |
| C T24 | 80 | 72 | 54 | 3.28 | 26.05 | 0.08 | 12.36 | 0.52 |
| T6 PC | 71 | 57 | 29 | 2.21 | 9.98 | 0.28 | 4.27 | 0.36 |
| T18 PC | 73 | 50 | 34 | 2.69 | 15.99 | 0.14 | 8.27 | 0.43 |
| T24 PC | 75 | 52 | 36 | 2.75 | 16.58 | 0.15 | 7.68 | 0.44 |
| T6 FS | 79 | 66 | 37 | 2.65 | 16.82 | 0.20 | 7.95 | 0.42 |
| T18 FS | 75 | 51 | 33 | 2.59 | 14.60 | 0.16 | 7.70 | 0.42 |
| T24 FS | 73 | 50 | 33 | 2.78 | 16.57 | 0.12 | 9.39 | 0.45 |
| Component | g × L−1 |
|---|---|
| Starch (BDH Ltd.) | 5.0 |
| Pectin (citrus) | 2.0 |
| Guar gum | 1.0 |
| Mucin (porcine gastric type III) | 4.0 |
| Xylan (oat spelt) | 2.0 |
| Arabinogalactan (larch wood) | 2.0 |
| Inulin | 1.0 |
| Casein (BDH Ltd.) | 3.0 |
| Peptone water | 5.0 |
| Tryptone | 5.0 |
| Bile salts No. 3 | 0.4 |
| Yeast extract | 4.5 |
| FeSO4 × 7H2O | 0.005 |
| NaCl | 4.5 |
| KCl | 4.5 |
| KH2PO4 | 0.5 |
| SO4 × 7H2O | 1.25 |
| CaCl2 × 6H2O | 0.15 |
| NaHCO3 | 1.5 |
| Cysteine | 0.8 |
| Hemin | 0.01 |
| Tween 80 | 1.0 |
| Sample Name | Polarity | Q1 (Da) | Q3 (Da) | Dwell Time (ms) | DP (V) | EP (volts) | CE (volts) | CXP (volts) |
|---|---|---|---|---|---|---|---|---|
| (+)-Lariciresinol Q | - | 359.027 | 175.000 | 25 | −115 | −10 | −32 | −9 |
| (+)-Lariciresinol ID | - | 359.027 | 160.400 | 25 | −115 | −10 | −40 | −7 |
| Enterodiol Q | - | 301.031 | 106.500 | 25 | −140 | −10 | −42 | −15 |
| Enterodiol ID | - | 301.031 | 270.900 | 25 | −140 | −10 | −34 | −11 |
| Enterolactone Q | - | 296.998 | 106.500 | 25 | −100 | −10 | −40 | −5 |
| Enterolactone ID | - | 296.998 | 121.100 | 25 | −100 | −10 | −32 | −1 |
| Secoisolariciresinol diglucoside ID | - | 685.153 | 361.000 | 25 | −290 | −10 | −56 | −15 |
| Secoisolariciresinol diglucoside Q | - | 685.153 | 58.900 | 25 | −290 | −10 | −112 | −9 |
| Secoisolariciresinol Q | - | 361.041 | 165.100 | 25 | −40 | −10 | −34 | −5 |
| Secoisolariciresinol ID | - | 361.041 | 120.900 | 25 | −40 | −10 | −58 | −7 |
| Pinoresinol diglucoside Q | - | 682.108 | 520.100 | 25 | −210 | −10 | −22 | −21 |
| Pinoresinol diglucoside ID | - | 682.108 | 151.100 | 25 | −210 | −10 | −66 | −5 |
| Pinoresinol Q | + | 359.067 | 137.100 | 25 | +96 | +10 | +33 | +6 |
| Pinoresinol ID | + | 359.067 | 175.100 | 25 | +96 | +10 | +23 | +6 |
| CL1 Q | + | 1040.600 | 941.4 | 25 | +296 | +10 | +41 | +36 |
| CL1 ID | + | 1040.600 | 828.4 | 25 | +296 | +10 | +49 | +30 |
| 1-Met-CL2 Q | + | 1058.6 | 945.4 | 25 | +296 | +10 | +45 | +36 |
| 1-Met-CL2 ID | + | 1058.6 | 588.2 | 25 | +296 | +10 | +61 | +20 |
| 1-Mso-CL2 Q | + | 1074.5 | 961.3 | 25 | +286 | +10 | +49 | +38 |
| 1-Mso-CL2 ID | + | 1074.5 | 489.1 | 25 | +286 | +10 | +73 | +18 |
| 1-Met-CL3 Q | + | 1048.5 | 935.3 | 25 | +241 | +10 | +35 | +34 |
| 1-Met-CL3 ID | + | 1048.5 | 822.2 | 25 | +241 | +10 | +45 | +30 |
| 1-Mso-CL3 Q | + | 1064.5 | 951.3 | 25 | +266 | +10 | +41 | +36 |
| 1-Mso-CL3 ID | + | 1064.5 | 1000.4 | 25 | +266 | +10 | +37 | +36 |
| 1-Met-CL4 Q | + | 961.5 | 814.3 | 25 | +296 | +10 | +35 | +30 |
| 1-Met-CL4 ID | + | 961.5 | 358.1 | 25 | +296 | +10 | +57 | +40 |
| 1-Mso-CL4 Q | + | 977.5 | 358.1 | 25 | +296 | +10 | +59 | +12 |
| 1-Mso-CL4 ID | + | 977.5 | 211.1 | 25 | +296 | +10 | +67 | +24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleigrewe, K.; Haack, M.; Baudin, M.; Ménabréaz, T.; Crovadore, J.; Masri, M.; Beyrer, M.; Andlauer, W.; Lefort, F.; Dawid, C.; et al. Dietary Modulation of the Human Gut Microbiota and Metabolome with Flaxseed Preparations. Int. J. Mol. Sci. 2022, 23, 10473. https://doi.org/10.3390/ijms231810473
Kleigrewe K, Haack M, Baudin M, Ménabréaz T, Crovadore J, Masri M, Beyrer M, Andlauer W, Lefort F, Dawid C, et al. Dietary Modulation of the Human Gut Microbiota and Metabolome with Flaxseed Preparations. International Journal of Molecular Sciences. 2022; 23(18):10473. https://doi.org/10.3390/ijms231810473
Chicago/Turabian StyleKleigrewe, Karin, Martina Haack, Martine Baudin, Thomas Ménabréaz, Julien Crovadore, Mahmoud Masri, Michael Beyrer, Wilfried Andlauer, François Lefort, Corinna Dawid, and et al. 2022. "Dietary Modulation of the Human Gut Microbiota and Metabolome with Flaxseed Preparations" International Journal of Molecular Sciences 23, no. 18: 10473. https://doi.org/10.3390/ijms231810473







