Effect of Dried Apple Pomace (DAP) as a Feed Additive on Antioxidant System in the Rumen Fluid
Abstract
:1. Introduction
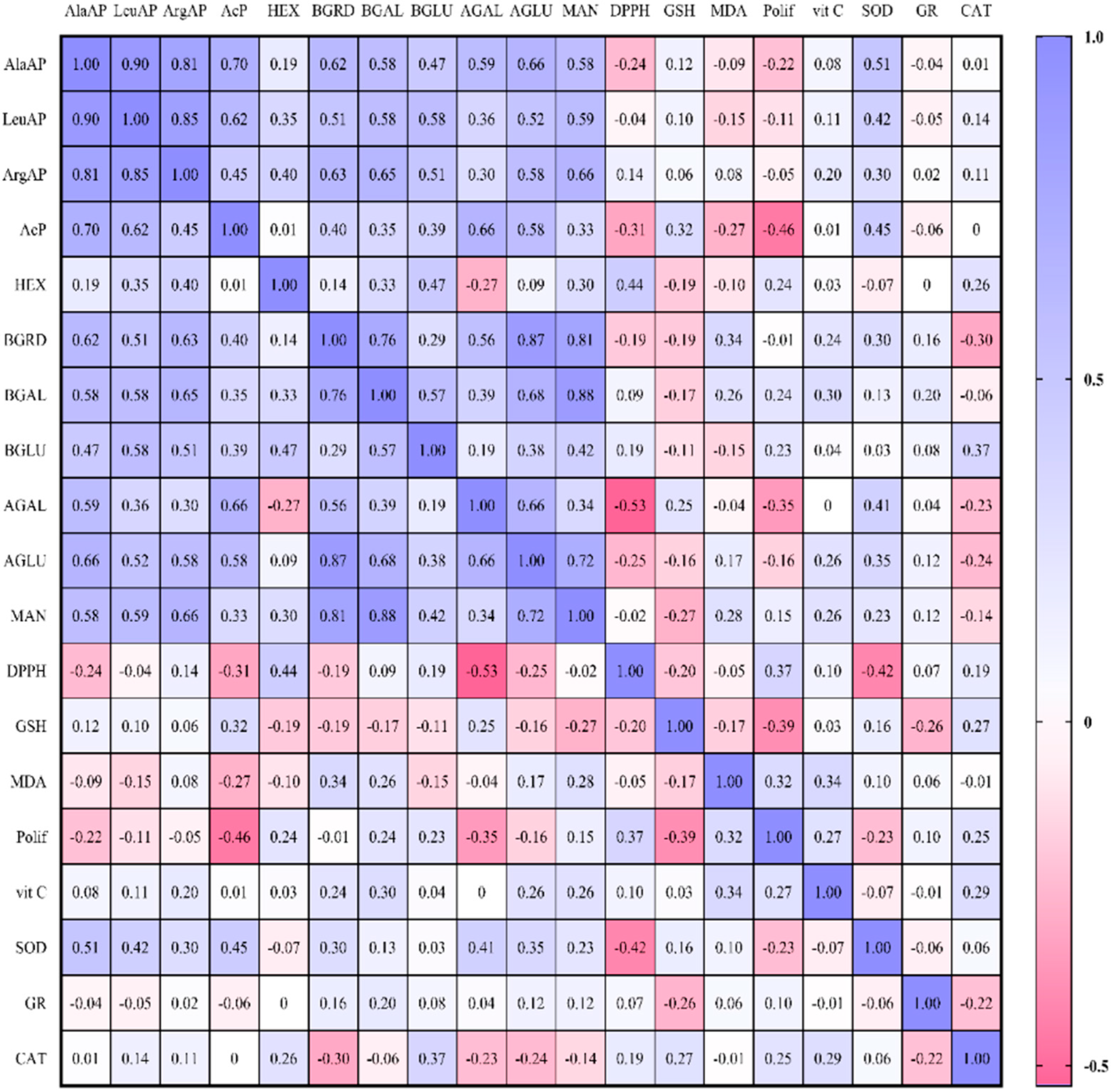
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Plan/Design
4.3. Collecting of Rumen Fluid
4.4. Measurement of Lysosomal Enzymes Activity
4.5. Superoxide Dismutase (SOD E.C 1.15.1.1) Activity
4.6. Glutathione Reductase (GR E.C 1.8.1.7) Activity
4.7. Catalase (CAT E.C 1.11.1.6) Activity
4.8. Measurement of Malondialdehyde (MDA) Level
4.9. Reduced Glutathione (GSH) Content
4.10. Level of Vitamin C (Ascorbic Acid)
4.11. Total Polyphenols Content
4.12. Potential to Scavenge the Free DPPH Radical
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew Sust. Energ. Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Rosend, J.; Kaleda, A.; Kuldjärv, R.; Arju, G.; Nisamedtinov, I. The effect of apple juice concentration on cider fermentation and properties of the final product. Foods 2020, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Ferreira, J.A.; Sirohi, R.; Sarsaiya, S.; Khoshnevisan, B.; Baladi, S.; Sindhu, R.; Binod, P.; Pandey, A.; Kumar, D.; et al. A critical review on the development stage of biorefinery systems towards the management of apple processing-derived waste. Renew. Sust. Energ. Rev. 2021, 143, 110972. [Google Scholar] [CrossRef]
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788. [Google Scholar] [CrossRef]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple pomace as a functional and healthy ingredient in food products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, T.; Wang, X.; Lü, X. Apple pomace as a potential valuable resource for full-components utilization: A review. J. Clean. Prod. 2021, 329, 129676. [Google Scholar] [CrossRef]
- Beigh, Y.A.; Ganai, A.M.; Ahmad, H.A. Utilization of apple pomace as livestock feed: A review. Ind. J. Small Rum. 2015, 21, 165–179. [Google Scholar] [CrossRef]
- Bava, L.; Sandrucci, A.; Zucali, M.; Guerci, M.; Tamburini, A. How can farming intensification affect the environmental impact of milk production? Int. J. Dairy Sci. 2014, 97, 4579–4593. [Google Scholar] [CrossRef]
- Marwicka, J.; Zięba, A. Antioxidants as a defence against reactive oxygen species. Aesthetic Cosmetol. Med. 2021, 10, 271–276. [Google Scholar] [CrossRef]
- Safa, S.; Kargar, S.; Moghaddam, G.A.; Ciliberti, M.G.; Caroprese, M. Heat stress abatement during the postpartum period: Effects on whole lactation milk yield, indicators of metabolic status, inflammatory cytokines, and biomarkers of the oxidative stress. J. Anim. Sci. 2019, 97, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.K.; Naqshbandi, A.; Fareed, M.; Mahmood, R. Oral administration of a nephrotoxic dose of potassium bromate, a food additive, alters renal redox and metabolic status and inhibits brush border membrane enzymes in rats. Food Chem. 2012, 134, 980–985. [Google Scholar] [CrossRef]
- Jóźwik, A.; Śliwa-Jóźwik, A.K. The effect of administration of high doses of selected antioxidants upon the activity of beta-glucuronidase (β-GL) in liver, kidney and blood plasma of mice. Anim. Sci. Pap. Rep. 2005, 23, 219–227. [Google Scholar]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes and oxidative stress in aging and apoptosis. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Całyniuk, B.; Grochowska-Niedworok, E.; Walkiewicz, K.W.; Kawecka, S.; Popiołek, E.; Fatyga, E. Malondialdehyde (MDA)–product of lipid peroxidation as marker of homeostasis disorders and aging. Ann. Acad. Med. Siles. 2016, 70, 224–228. [Google Scholar] [CrossRef]
- Gharehbagh, A.G.; Pirmohammadi, R.; Alijoo, Y.A.; Behroozyar, H.K. Investigating the effect of apple pomac silage as fodder source on performance and residues of its toxins in milk and some rumen fermentation in Mahabadi lactation goats in early lactation period. Ukr. J. Ecol. 2020, 10, 241–245. [Google Scholar] [CrossRef]
- Rodríguez-Muela, C.; Rodríguez, H.E.; Arzola, C.; Díaz-Plascencia, D.; Ramírez-Godíne, J.A.; Flores-Mariñelarena, A.; Mancillas-Flores, P.F.; Corral, G. Antioxidant activity in plasma and rumen papillae development in lambs fed fermented apple pomace. J. Anim. Sci. 2015, 93, 2357–2362. [Google Scholar] [CrossRef]
- De Godoy, M.R.; Kerr, K.R.; Fahey, G.C., Jr. Alternative dietary fiber sources in companion animal nutrition. Nutrients 2013, 5, 3099–3117. [Google Scholar] [CrossRef]
- Colombino, E.; Ferrocino, I.; Biasato, I.; Cocolin, L.S.; Prieto-Botella, D.; Zduńczyk, Z.; Janowski, J.; Milala, J.; Kosmala, M.; Fotschki, B.; et al. Dried fruit pomace inclusion in poultry diet: Growth performance, intestinal morphology and physiology. J. Anim. Sci. Biotech. 2020, 11, 63. [Google Scholar] [CrossRef]
- Tayengwa, T.; Mapiye, C. Citrus and winery wastes: Promising dietary supplements for sustainable ruminant animal nutrition, health, production, and meat quality. Sustainability 2018, 10, 3718. [Google Scholar] [CrossRef]
- Pauletto, M.; Elgendy, R.; Ianni, A.; Marone, E.; Giantin, M.; Grotta, L.; Ramazzotti, S.; Bennato, F.; Martino, G. Nutrigenomic effects of long-term grape pomace supplementation in dairy cows. Animals 2020, 10, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, A.W.K.; Aggarwal, B.B.; Orhan, I.E.; Horbańczuk, O.K.; Barreca, D.; Battino, M.; Belwal, T.; Bishayee, A.; Daglia, M.; Devkota, H.P.; et al. Resveratrol, a popular dietary supplement for human and animal health: Quantitative research literature analysis-a review. Anim. Sci. Pap. Rep. 2019, 37, 103–118. [Google Scholar]
- Yeung, A.W.K.; Choudhary, N.; Tewari, D.; El-Demerdash, A.; Tomczyk, M.; Das, N.; Pirgozliev, V.; Lucarini, M.; Durazzo, A.; Souto, E.B.; et al. Lycopene: Total-scale literature landscape analysis of a valuable nutraceutical with numerous potential applications in the promotion of human and animal health. Anim. Sci. Pap. Rep. 2022, 40, 119–134. [Google Scholar]
- Abdollahzadeh, F.; Pirmohammadi, R.; Farhoomand, P.; Fatehi, F.; Pazhoh, F.F. The effect of ensiled mixed tomato and apple pomace on Holstein dairy cow. Ital. J. Anim. Sci. 2010, 9, e41. [Google Scholar]
- Abdollahzadeh, F.; Abdulkarimi, R. The effects of some agricultural by-products on ruminal fermentation and apparent digestibility of Holstein dairy cow. Life Sci. J. 2012, 9, 81–85. [Google Scholar]
- Steyn, L.; Meeske, R.; Cruywagen, C.W. The effect of dried apple pomace as a replacer for maize in the concentrate for Jersey cows grazing ryegrass pasture on production and rumen metabolism. Anim. Feed Sci. Technol. 2017, 234, 264–273. [Google Scholar] [CrossRef]
- Nandan, A.; Nampoothiri, K.M. Molecular advances in microbial aminopeptidases. Bioresour. Technol. 2017, 245, 1757–1765. [Google Scholar] [CrossRef]
- Solomon, M.; Muro, S. Lysosomal enzyme replacement therapies: Historical development, clinical outcomes, and future perspectives. Adv. Drug Deliv. Rev. 2017, 118, 109–134. [Google Scholar] [CrossRef]
- Górka, P.; Śliwiński, B.; Miltko, R.; Flaga, J.; Barć, J.; Godlewski, M.M.; Zabielski, R.; Kowalski, Z.M. 642 Effect of supplemental sodium butyrate on the activity of carbohydrate-digesting enzymes in the reticulo-ru.minal digesta and brush border enzymes in sheep. J. Anim. Sci. 2017, 95, 314–315. [Google Scholar] [CrossRef]
- Jóźwik, A.; Marchewka, J.; Strzałkowska, N.; Horbańczuk, J.O.; Szumacher-Strabel, M.; Cieślak, A.; Lipińska-Palka, P.; Józefiak, D.; Kamińska, A.; Atanasov, A.G. The effect of different levels of Cu, Zn and Mn nanoparticles in hen turkey diet on the activity of aminopeptidases. Molecules 2018, 23, 1150. [Google Scholar] [CrossRef]
- Bagnicka, E.; Kościuczuk, E.M.; Jarczak, J.; Jóźwik, A.; Strzałkowska, N.; Słoniewska, D.; Krzyżewski, J. The effect of inorganic and organic selenium added to diets on milk yield, milk chemical and mineral composition and the blood serum metabolic profile of dairy cows. Anim. Sci. Pap. Rep. 2017, 35, 17–33. [Google Scholar]
- Jóźwik, A.; Bagnicka, A.Ś.J.; Strzałkowska, K.S.; Krzyżewski, A.K. Activity of selected aminopeptidases of whole milk in cows as related to feeding season (autumn/winter vs. spring/summer). Anim. Sci. Pap. Rep. 2004, 22, 667–672. [Google Scholar]
- Montoro-Molina, S.; Quesada, A.; Zafra-Ruiz, P.V.; O’Vall, E.F.; Vargas, F.; de Gracia, M.C.; Osuna, A. Immunological detection of glutamyl aminopeptidase in urine samples from cisplatin-treated rats. Proteomics Clin. Appl. 2015, 9, 630–635. [Google Scholar] [CrossRef]
- Larsen, L.B.; Rasmussen, M.D.; Bjerring, M.; Nielsen, J.H. Proteases and protein degradation in milk from cows infected with Streptococcus uberis. Int. Dairy J. 2004, 14, 899–907.15. [Google Scholar] [CrossRef]
- Vargas, F.; Wangesteen, R.; Rodríguez-Gómez, I.; García-Estañ, J. Aminopeptidases in cardiovascular and renal function. Role as predictive renal injury biomarkers. Int. J. Mol. Sci. 2020, 21, 5615. [Google Scholar] [CrossRef]
- Pirmohammadi, R.; Rouzbehan, Y.; Rezayazdi, K.; Zahedifar, M. Chemical composition, digestibility and in situ degradability of dried and ensiled apple pomace and maize silage. Small Rum. 2006, 66, 150–155. [Google Scholar] [CrossRef]
- Metcalf, J.A.; Mansbridge, R.J.; Blake, J.S.; Oldham, J.D.; Newbold, J.R. The efficiency of conversion of metabolisable protein into milk true protein over a range of metabolisable protein intakes. Animals 2008, 2, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Newbold, C.J.; De la Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The role of ciliate protozoa in the rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef]
- Kötzler, M.P.; Hancock, S.M.; Withers, S.G. Glycosidases: Functions, families and folds. eLS 2014. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Sato, M.F.; Vieira, R.G.; Zardo, D.M.; Falcão, L.D.; Nogueira, A.; Wosiacki, G. Apple pomace from eleven cultivars: An approach to identify sources of bioactive compounds. Acta Sci. Agron. 2010, 32, 29–35. [Google Scholar] [CrossRef]
- Wendeler, M.; Sandhoff, K. Hexosaminidase assays. Glycoconj. J. 2009, 26, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.R.K.; Esen, A. β-Glucosidases. Cell. Mol. Life Sci. 2010, 67, 3389–3405. [Google Scholar] [CrossRef]
- Ajila, C.M.; Gassara, F.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Polyphenolic antioxidant mobilization in apple pomace by different methods of solid-state fermentation and evaluation of its antioxidant activity. Food Bioproc. Tech. 2012, 5, 2697–2707. [Google Scholar] [CrossRef]
- Ferraresi, R.; Troiano, L.; Roat, E.; Lugli, E.; Nemes, E.; Nasi, M.; Pinti, M.; Garcia Fernandez, M.; Cooper, E.; Cossarizza, A. Essential requirement of reduced glutathione (GSH) for the anti-oxidant effect of the flavonoid quercetin. Free Radic. Res. 2005, 39, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Śliwa-Jóźwik, A.; Jóźwik, A.; Kołataj, A. Influence of exogenous glutathione (GSH), as stressfactor, on the activity of lysosome enzymes in some organs of mice. Arch. Anim. Breed. 2002, 45, 307–314. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Shang, F.; Lu, M.; Dudek, E.; Reddan, J.; Taylor, A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free Radic. Biol. Med. 2003, 34, 521–530. [Google Scholar] [CrossRef]
- Heck, D.E.; Shakarjian, M.; Kim, H.D.; Laskin, J.D.; Vetrano, A.M. Mechanisms of oxidant generation by catalase. Ann. N. Y. Acad. Sci. 2010, 1203, 120–125. [Google Scholar] [CrossRef]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple pomace as potential source of natural active compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; El-Demerdash, A.; Horbanczuk, O.K.; Das, N.; Pirgozliev, V.; Lucarini, M.; Durazzo, A.; Souto, E.B.; Santini, A.; et al. Apple polyphenols in human and animal health. Anim. Sci. Pap. Rep. 2021, 39, 105–118. [Google Scholar]
- Fernandes, P.A.; Le Bourvellec, C.; Renard, C.M.; Nunes, F.M.; Bastos, R.; Coelho, E.; Wessel, D.F.; Coimbra, M.; Cardoso, S.M. Revisiting the chemistry of apple pomace polyphenols. Food Chem. 2019, 294, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Will, F.; Olk, M.; Hopf, I.; Dietrich, H. Characterization of Polyphenol Extracts from Apple Juice. Dtsch. Dtsch. Lebensm.-Rundsch. 2006, 102, 297–302. [Google Scholar]
- Xu, X.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; He, J.; Luo, Y.; Zheng, P.; Yu, J.; et al. Dietary apple polyphenols supplementation enhances antioxidant capacity and improves lipid metabolism in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1512–1520. [Google Scholar] [CrossRef]
- De Paula, E.M.; Samensari, R.B.; Machado, E.; Pereira, L.M.; Maia, F.J.; Yoshimura, E.H.; Franzolin, R.; Faciola, A.P.; Zeoula, L.M. Effects of phenolic compounds on ruminal protozoa population, ruminal fermentation, and digestion in water buffaloes. Livest. Sci. 2016, 185, 136–141. [Google Scholar] [CrossRef]
- Kozłowska, M.; Cieślak, A.; Jóźwik, A.; El-Sherbiny, M.; Stochmal, A.; Oleszek, W.; Kowalczyk, M.; Filipiak, W.; Szumacher-Strabel, M. The effect of total and individual alfalfa saponins on rumen methane production. J. Sci. Food Agric. 2020, 100, 1922–1930. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A.; Simal-Gandara, J. Polyphenols in Farm Animals: Source of Reproductive Gain or Waste? Antioxidants 2020, 9, 1023. [Google Scholar] [CrossRef] [PubMed]
- Bešlo, D.; Došlić, G.; Agić, D.; Rastija, V.; Šperanda, M.; Gantner, V.; Lučić, B. Polyphenols in Ruminant Nutrition and Their Effects on Reproduction. Antioxidants 2022, 11, 970. [Google Scholar] [CrossRef]
- Yasui, K.; Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006, 55, 359–363. [Google Scholar] [CrossRef]
- Grotto, D.; Maria, L.S.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim. Nova 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Gabai, G.; Testoni, S.; Piccinini, R.; Marinelli, L.; Howard, C.; Stradaioli, G. Oxidative stress in primiparous cows in relations to dietary starch and the progress of lactation. Anim. Sci. 2004, 79, 99–108. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, X.; Zou, Y.; Yang, Z.; Li, S. Changes in feed intake, nutrient digestion, plasma metabolites, and oxidative stress parameters in dairy cows with subacute ruminal acidosis and its regulation with pelleted beet pulp. J. Anim. Sci. Biotechnol. 2013, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.; van Gastelen, S.; Dieho, K.; Nichols, K.; Bannink, A. Review: Rumen sensors: Data and interpretation for key rumen metabolic processes. Animal 2020, 14, s176–s186. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Batbekh, B.; Fukuma, N.; Kand, D.; Hanada, M.; Nishida, T. A garlic and citrus extract: Impacts on behavior, feed intake, rumen fermentation, and digestibility in sheep. Anim. Feed Sci. Technol. 2021, 278, 115007. [Google Scholar] [CrossRef]
- Netto, A.J.; Gama, M.A.S.; Guido, S.L.; Bessa, W.J.B.; Inácio, J.G.; Monteiro, C.C.F.; Melo, E.F.; Ribeiro, M.; Ferreira, M.A. Replacing corn with full-fat corn germ in a basal diet containing cactus (Opuntia stricta) cladodes and sugarcane as forage sources induces milk fat depression associated with the trans−10 shift in dairy cows. Anim. Feed Sci. Technol. 2022, 288, 115289. [Google Scholar] [CrossRef]
- Silva, S.N.S.; Chabrillat, T.; Kerros, S.; Guillaume, S.; Gandra, J.R.; de Carvalho, G.G.P.; da Silva, F.F.; Mesquita, L.G.; Gordiano, L.A.; Camargo, G.M.F.; et al. Effects of plant extract supplementations or monensin on nutrient intake, digestibility, ruminal fermentation and metabolism in dairy cows. Anim. Feed Sci. Technol. 2021, 275, 114886. [Google Scholar] [CrossRef]
- Huang, H.; Lechniak, D.; Szumacher-Strabel, M.; Patra, K.A.; Kozłowska, M.; Kołodziejski, P.; Gao, M.; Ślusarczyk, S.; Petrič, D.; Cieślak, A. The effect of ensiled paulownia leaves in a high-forage diet on ruminal fermentation, methane production, fatty acid composition, and milk production performance of dairy cows. J. Anim. Sci. Biotechnol. 2022, 13, 104. [Google Scholar] [CrossRef]
- Naseri, V.; Kafilzadeh, F.; Jahani-Azizabadi, H. Effects of Pistacia atlantica gum essential oil on ruminal methanogen, protozoa, selected bacteria species and fermentation characteristics in sheep. Small Rumin. Res. 2022, 209, 106650. [Google Scholar] [CrossRef]
- Belverdy, M.S.; Khadem, A.A.; Alamouti, A.A.; Khani, J.; Casmaligia, S. Use of fat-coated or heat-treated soybean meal for partial replacement of solvent-extracted soybean meal in the diets of early lactation dairy cows. Anim. Prod. Sci. 2021, 62, 783–791. [Google Scholar] [CrossRef]
- Kozłowska, M.; Cieślak, A.; Jóźwik, A.; El-Sherbiny, M.; Gogulski, M.; Lechniak, D.; Gao, M.; Yanza, Y.R.; Vazirigohar, M.; Szumacher-Strabel, M. Effects of partially replacing grass silage by lucerne silage cultivars in a high-forage diet on ruminal fermentation, methane production, and fatty acid composition in the rumen and milk of dairy cows. Anim. Feed Sci. Technol. 2021, 277, 114959. [Google Scholar] [CrossRef]
- McDonald, J.K.; Barrett, A.J. Mammalian Proteases, a Glossary and Bibliography. In Exopeptidases; Academic Press: London, UK, 1986; Volume 2, p. 48. [Google Scholar]
- Barrett, A.J.; Heath, M.F. Lysosomal enzymes. In Lysosomes; A Laboratory Handbook; Dingle, J.T., Ed.; North-Holland Publ. Co.: Amsterdam, The Netherlands, 1972; pp. 19–135. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
| CON | EXP | SEM | p-Value * | |
|---|---|---|---|---|
| AlaAP | 154.90 | 140.90 | 3.61 | 0.10 |
| LeuAP | 163.70 | 143.10 | 3.48 | <0.01 ** |
| ArgAP | 157.60 | 159.10 | 2.91 | 0.15 |
| AcP | 1618.00 | 1412.00 | 41.50 | 0.11 |
| HEX | 231.00 | 194.00 | 6.11 | <0.01 ** |
| BGRD | 31.10 | 42.40 | 1.48 | <0.01 ** |
| β-GAL | 163.00 | 186.00 | 6.31 | 0.07 |
| β-GLU | 1294.00 | 1136.00 | 25.60 | <0.01 ** |
| α-GAL | 368.00 | 357.00 | 17.20 | 0.89 |
| α-GLU | 245.00 | 327.00 | 13.20 | <0.01 ** |
| MAN | 29.70 | 36.70 | 1.17 | <0.01 ** |
| Unit | CON | EXP | SEM | p-Value * | |
|---|---|---|---|---|---|
| SOD | U/mL | 2.19 | 2.15 | 0.06 | 0.52 |
| GR | nmol/min/mL | 16.10 | 20.80 | 1.19 | 0.06 |
| CAT | nmol/min/mL | 93.20 | 68.80 | 3.72 | <0.01 ** |
| Unit | CON | EXP | SEM | p-Value * | |
|---|---|---|---|---|---|
| DPPH | % of remaining DPPH | 73.20 | 72.80 | 0.39 | 0.95 |
| GSH | µM | 140.00 | 118.00 | 2.65 | <0.01 ** |
| MDA | µM | 0.36 | 0.45 | 0.01 | <0.01 ** |
| Polyphenols | mg/GAE/mL | 1.38 | 1.40 | 0.01 | 0.16 |
| Vit C | mg/100 | 22.90 | 24.10 | 0.25 | <0.01 ** |
| Items | Unit | Control Diet | Experimental Diet |
|---|---|---|---|
| Dry matter (DM), kg/kg as fed | Kg | 23.80 | 25.40 |
| Metabolizable Energy (ME) | MJ | 255.00 | 245.00 |
| Net Energy Lactation (NEL) | MJ | 157.00 | 151.00 |
| Crude protein (CP), g/kg DM | G | 3505.00 | 3515.00 |
| Utilizable protein, g/kg DM | G | 3310.00 | 3174.00 |
| Total undegradable protein in rumen, g/kg DM | G | 794.00 | 661.00 |
| Total fiber, g/kg DM | G | 3285.00 | 4013.00 |
| Neutral detergent fiber (aNDF), g/kg DM | % | 14.09 | 13.52 |
| Digestibility of the dry matter dose (IVDMD, %) | % | 78.08 | 78.82 |
| Percentage of dry matter intake/cow weight | % | 4.33 | 4.65 |
| Relative Feedstuff Value (RFV) index | 262.00 | 284.00 | |
| Starch, g/kg DM | G | 7963 | 7211 |
| Calcium, g/kg of DM | G | 69.90 | 67.20 |
| Phosphorus, g/kg of DM | G | 72.60 | 67.70 |
| Calcium: Phosphorus | 01:01 | 01:01 | |
| Magnesium, g/kg of DM | G | 38.40 | 35.00 |
| Sodium, g/kg of DM | G | 15.00 | 13.60 |
| Zinc, g/kg of DM | Mg | 189.00 | 157.00 |
| Copper, g/kg of DM | Mg | 39.90 | 33.20 |
| Manganese, g/kg of DM | Mg | 157.00 | 131.00 |
| Cobalt, g/kg of DM | Mg | 0.63 | 0.49 |
| Iodine, g/kg of DM | Mg | 105.00 | 87.50 |
| Selenium, g/kg of DM | Mg | 0.28 | 0.21 |
| Vitamin A, IU/kg | j.m. | 18,900.00 | 15,750.00 |
| Vitamin D, IU/kg | j.m. | 5906.00 | 4922.00 |
| Vitamin E, IU/kg | Mg | 77.70 | 64.70 |
| Niacin, g/kg of DM | Mg | 126.00 | 105.00 |
| Biotin, g/kg of DM | µg | 550.00 | 458.00 |
| Total polyphenols | mgGEA/g | 6.67 | 6.85 |
| Dried Apple Pomace Composition | Values | SEM | |
|---|---|---|---|
| Metabolic Energy | MJ/kg | 11.50 | 1.01 |
| Protein | % | 11.60 | 1.21 |
| Fat | % | 5.10 | 0.48 |
| Fiber | % | 60.00 | 5.56 |
| Total Sugars | % | 16.90 | 2.02 |
| Ash | % | 2.20 | 0.18 |
| Moisture content | % | 4.30 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartel, I.; Koszarska, M.; Wysocki, K.; Kozłowska, M.; Szumacher-Strabel, M.; Cieślak, A.; Wyrwał, B.; Szejner, A.; Strzałkowska, N.; Horbańczuk, J.O.; et al. Effect of Dried Apple Pomace (DAP) as a Feed Additive on Antioxidant System in the Rumen Fluid. Int. J. Mol. Sci. 2022, 23, 10475. https://doi.org/10.3390/ijms231810475
Bartel I, Koszarska M, Wysocki K, Kozłowska M, Szumacher-Strabel M, Cieślak A, Wyrwał B, Szejner A, Strzałkowska N, Horbańczuk JO, et al. Effect of Dried Apple Pomace (DAP) as a Feed Additive on Antioxidant System in the Rumen Fluid. International Journal of Molecular Sciences. 2022; 23(18):10475. https://doi.org/10.3390/ijms231810475
Chicago/Turabian StyleBartel, Iga, Magdalena Koszarska, Kamil Wysocki, Martyna Kozłowska, Małgorzata Szumacher-Strabel, Adam Cieślak, Beata Wyrwał, Aleksandra Szejner, Nina Strzałkowska, Jarosław Olav Horbańczuk, and et al. 2022. "Effect of Dried Apple Pomace (DAP) as a Feed Additive on Antioxidant System in the Rumen Fluid" International Journal of Molecular Sciences 23, no. 18: 10475. https://doi.org/10.3390/ijms231810475






