Investigation of the Properties of Linen Fibers and Dressings
Abstract
:1. Introduction
2. Results
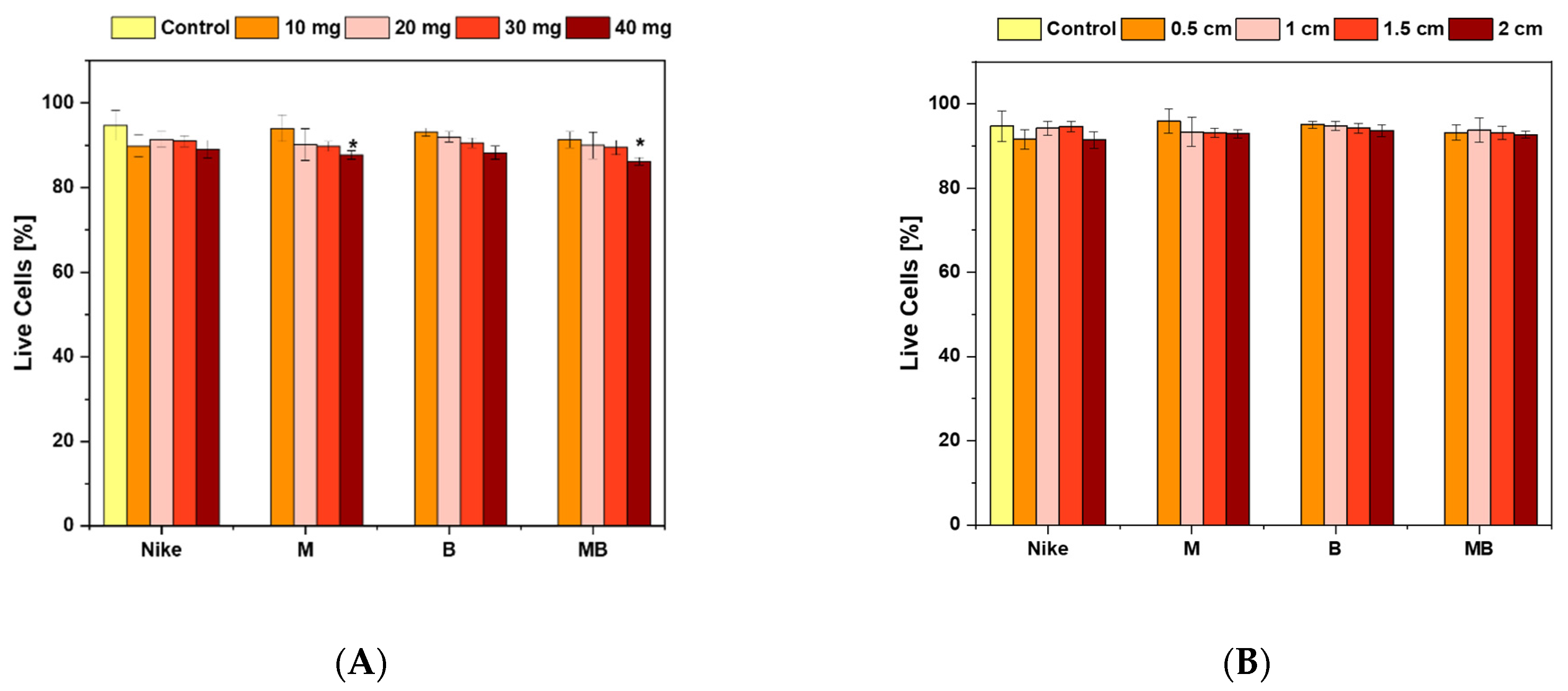
2.1. Cell Viability
2.2. Cell Proliferation
2.3. Evaluation of the Intracellular Free Radical Level
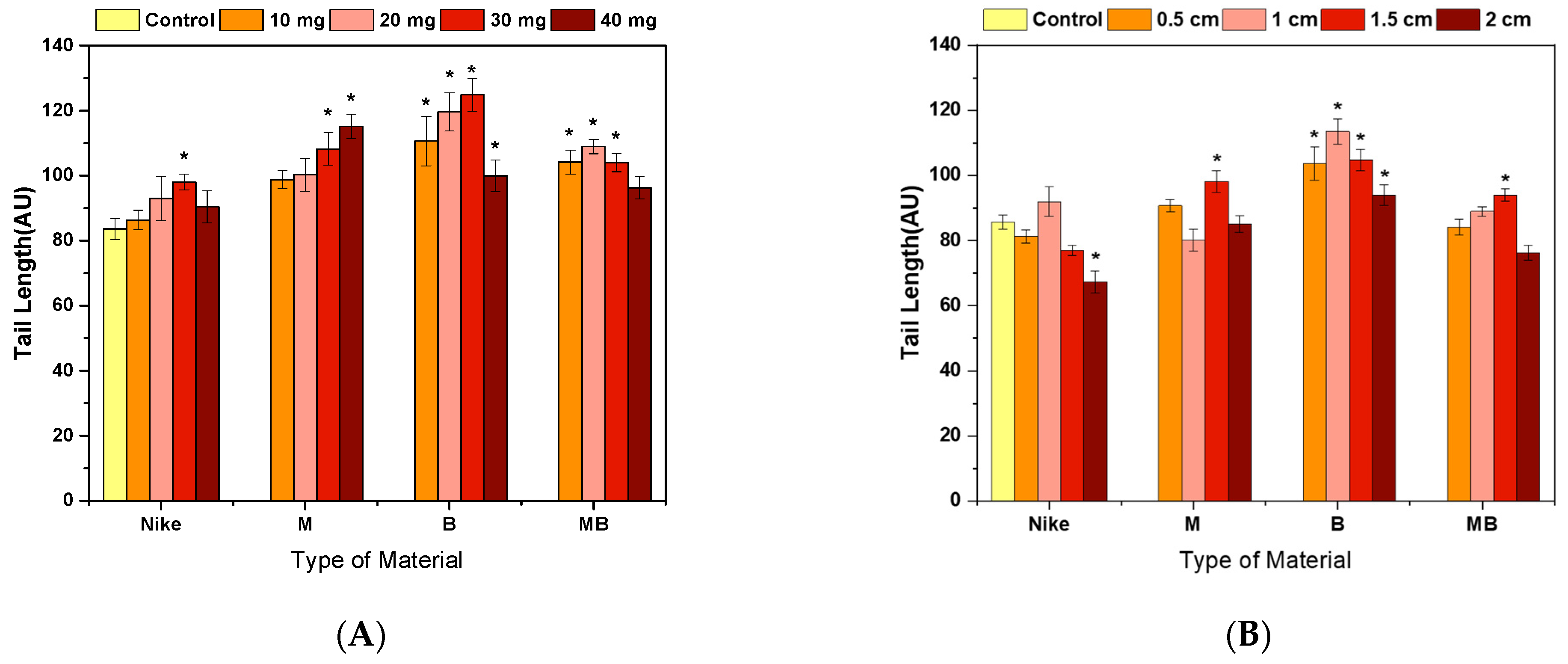
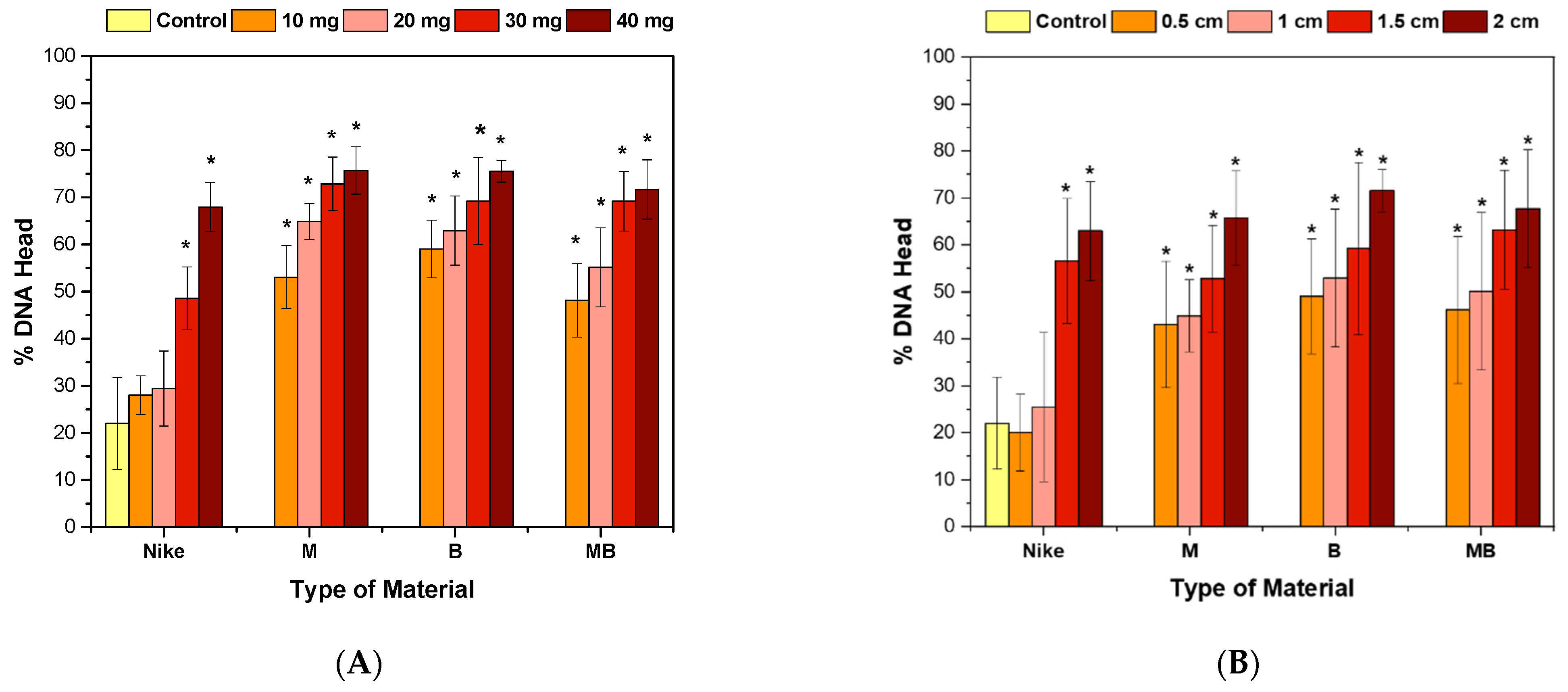
2.4. Genotoxicity Assessment
2.5. Potential Wound Environment Response to Oxidative Stress
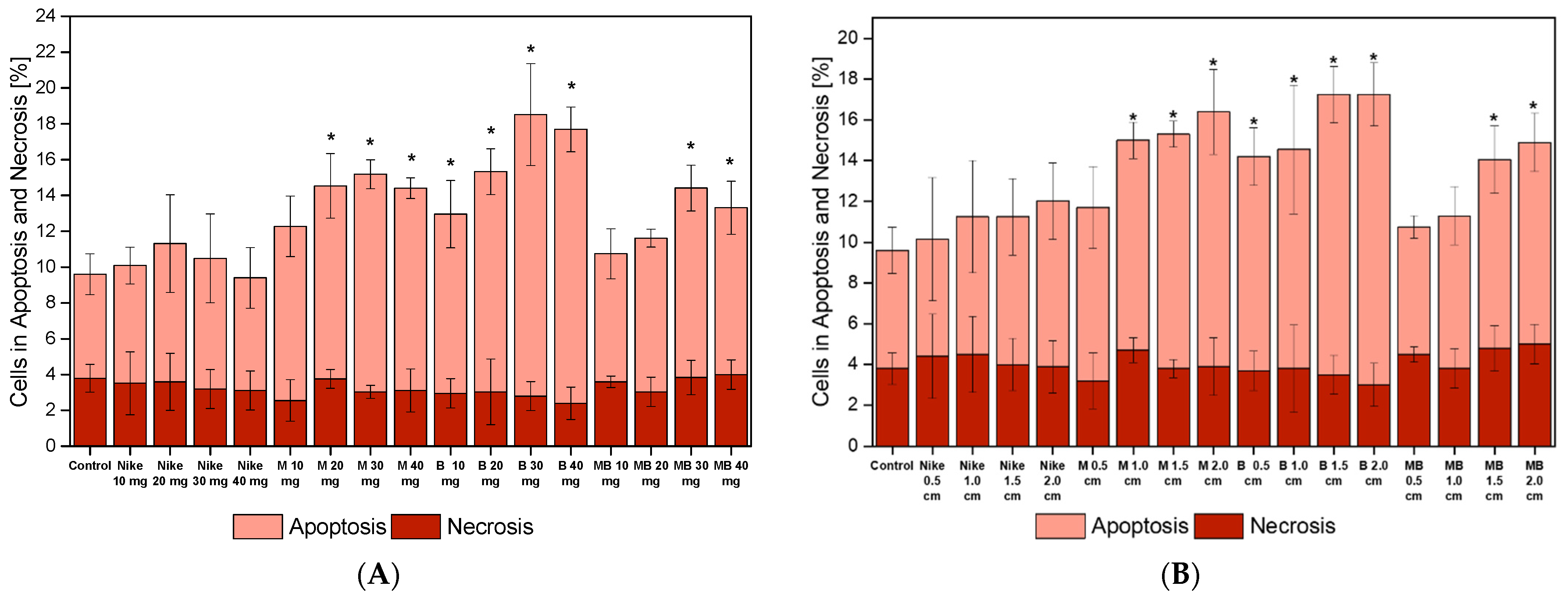
2.6. Apoptotic and Necrotic Cells
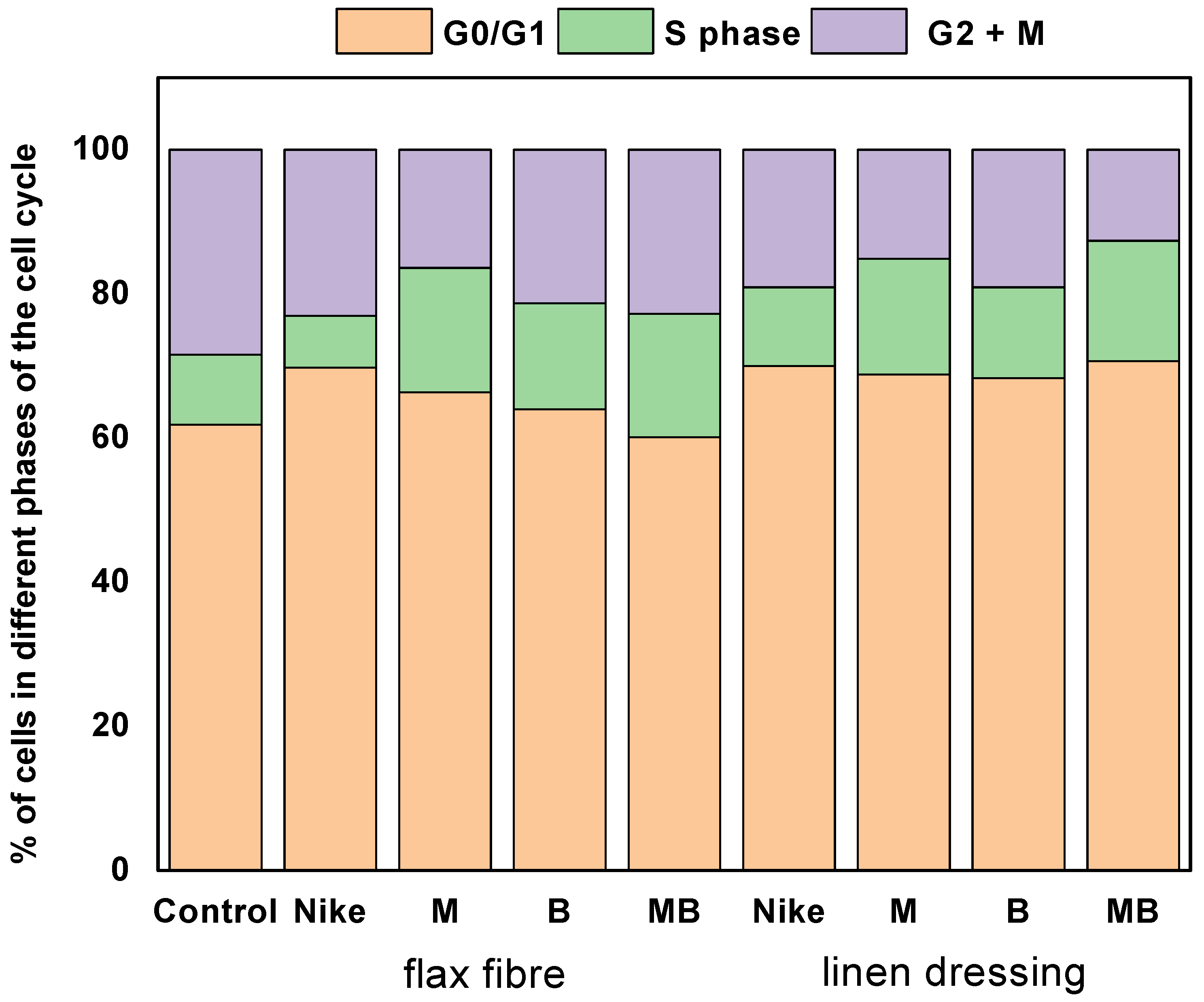
2.7. Cell Cycle
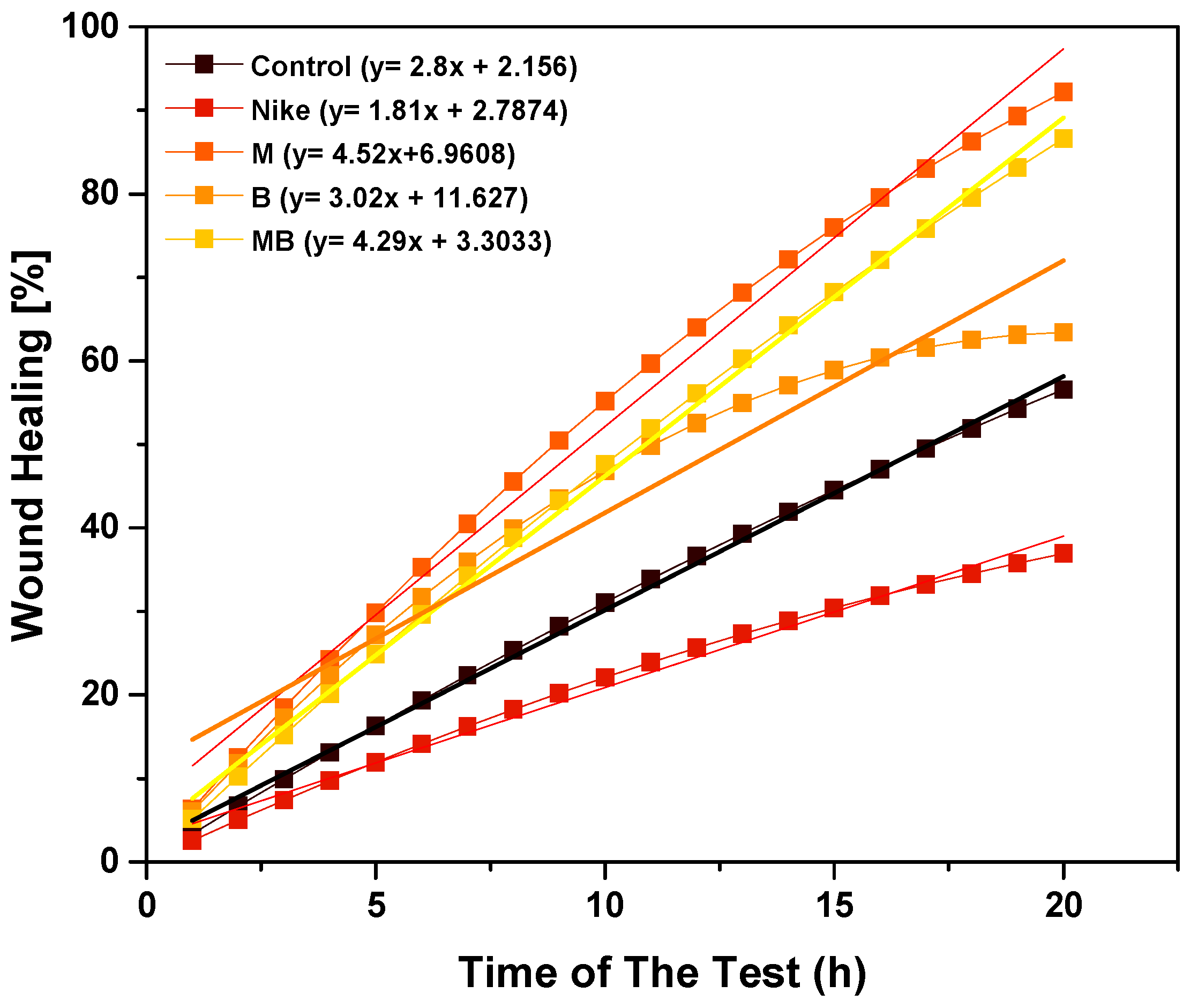
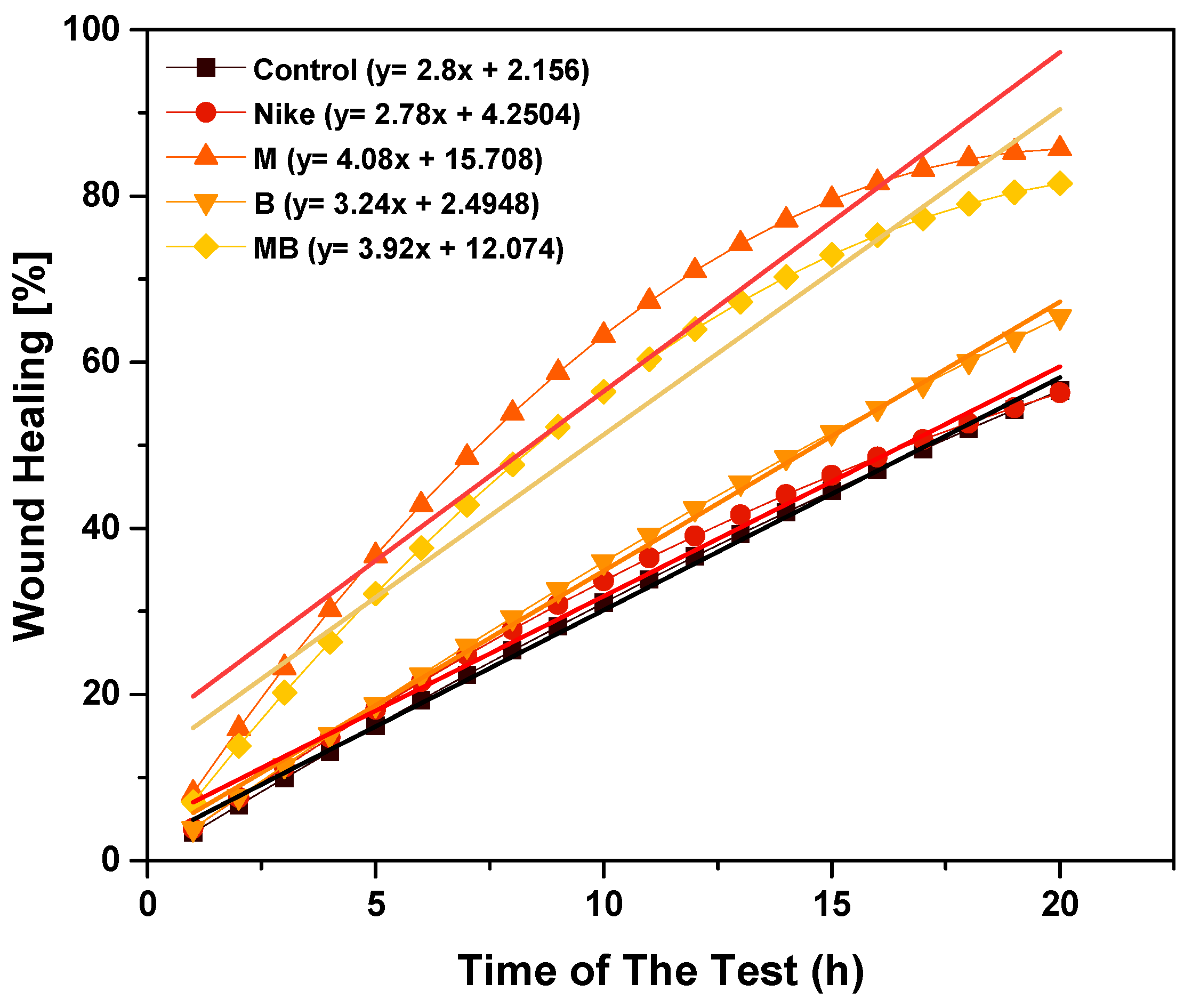
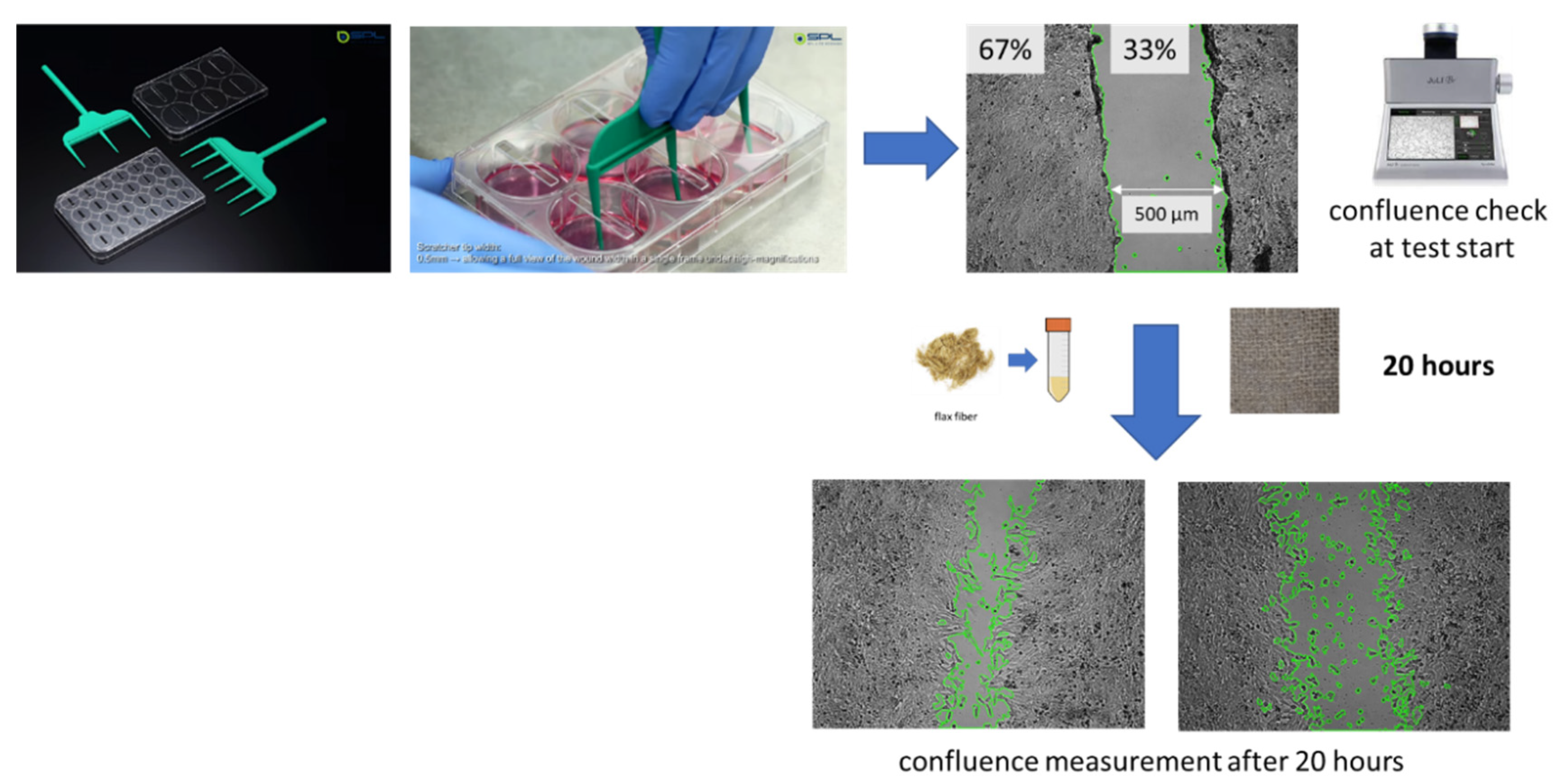
2.8. Scratch Assay
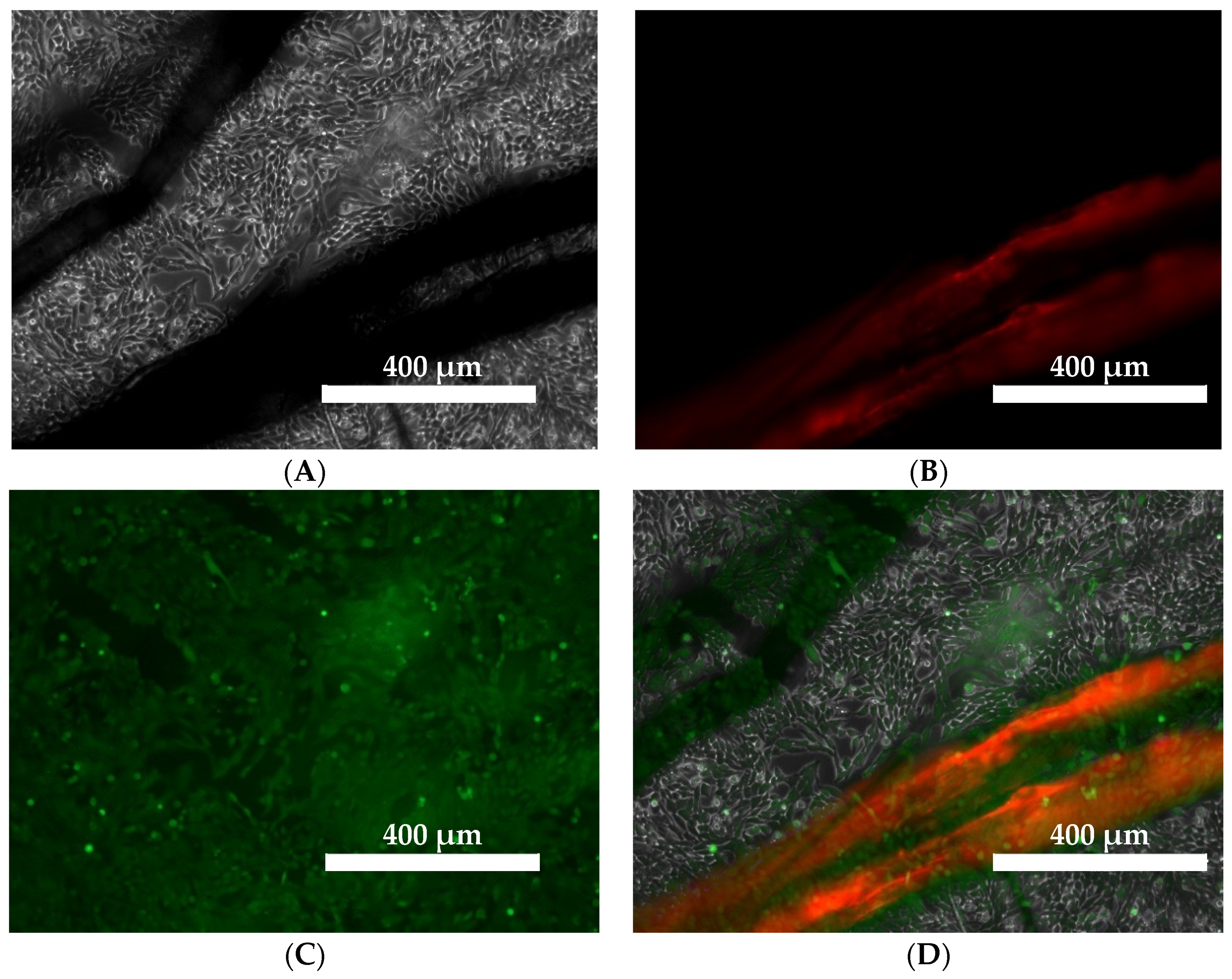
2.9. The Effect of Linen Fiber and Linen Dressing on Wound Healing in the V79 Cell Model
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Reagents
4.3. Preparation of the Flax Fabric for Biological Tests
4.4. Cell Line and Cell Culture Conditions
4.5. Cell Viability
4.6. Cell Proliferation
4.7. Evaluation of the Intracellular Free Radical Level
4.8. Comet Assay
4.9. Apoptotic and Necrotic Cells
4.10. Cell Cycle
4.11. Scratch Test
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rasouli, M.; Rahimi, A.; Soleimani, M.; Keshel, S.H. The Interplay between Extracellular Matrix and Progenitor/Stem Cells during Wound Healing: Opportunities and Future Directions. Acta Histochem. 2021, 123, 151785. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Cappadone, C.; Sallustio, V.; Picone, G.; Rossi, M.; Nicoletta, F.P.; Luppi, B.; Bigucci, F.; Cerchiara, T. Development of Spanish Broom and Flax Dressings with Glycyrrhetinic Acid-Loaded Films for Wound Healing: Characterization and Evaluation of Biological Properties. Pharmaceutics 2021, 13, 1192. [Google Scholar] [CrossRef] [PubMed]
- Czemplik, M.; Kulma, A.; Bazela, K.; Szopa, J. The Biomedical Potential of Genetically Modified Flax Seeds Overexpressing the Glucosyltransferase Gene. BMC Complement. Altern. Med. 2012, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Draganescu, D.; Ibanescu, C.; Tamba, B.I.; Andritoiu, C.V.; Dodi, G.; Popa, M.I. Flaxseed Lignan Wound Healing Formulation: Characterization and in Vivo Therapeutic Evaluation. Int. J. Biol. Macromol. 2015, 72, 614–623. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Mierziak-Darecka, J.; Wrobel-Kwiatkowska, M.; Gebarowski, T.; Szopa, J.; Zuk, M. Wound Coverage by the Linen Dressing Accelerates Ulcer Healing. Postep. Dermatol. Alergol. 2021, 38, 827–841. [Google Scholar] [CrossRef]
- Paul-Victor, C.; Dalle Vacche, S.; Sordo, F.; Fink, S.; Speck, T.; Michaud, V.; Speck, O. Effect of Mechanical Damage and Wound Healing on the Viscoelastic Properties of Stems of Flax Cultivars (Linum Usitatissimum L. Cv. Eden and Cv. Drakkar). PLoS ONE 2017, 12, e0185958. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Arizaga, G.G.C.; Wypych, F. Biodegradable Composites Based on Lignocellulosic Fibers-An Overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- Charlet, K.; Eve, S.; Jernot, J.P.; Gomina, M.; Breard, J. Tensile Deformation of a Flax Fiber. Procedia Eng. 2009, 1, 233–236. [Google Scholar] [CrossRef]
- Morvan, C.; Andème-Onzighi, C.; Girault, R.; Himmelsbach, D.S.; Driouich, A.; Akin, D.E. Building Flax Fibres: More than One Brick in the Walls. Plant Physiol. Biochem. 2003, 41, 935–944. [Google Scholar] [CrossRef]
- Hughes, M. Defects in Natural Fibres: Their Origin, Characteristics and Implications for Natural Fibre-Reinforced Composites. J. Mater. Sci. 2012, 47, 599–609. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Zuk, M.; Kulma, A.; Bugajska-Prusak, A.; Ratajczak, K.; Gasiorowski, K.; Kostyn, K.; Szopa, J. New Dressing Materials Derived from Transgenic Flax Products to Treat Long-Standing Venous Ulcers—A Pilot Study. Wound Repair Regen. 2010, 18, 168–179. [Google Scholar] [CrossRef]
- Paladini, F.; Picca, R.A.; Sportelli, M.C.; Cioffi, N.; Sannino, A.; Pollini, M. Surface Chemical and Biological Characterization of Flax Fabrics Modified with Silver Nanoparticles for Biomedical Applications. Mater. Sci. Eng. C 2015, 52, 1–10. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Kulma, A.; Gębarowski, T.; Wojtasik, W.; Kostyn, K.; Moreira, H.; Szyjka, A.; Boba, A.; Preisner, M.; Mierziak, J.; et al. V79 Fibroblasts Are Protected Against Reactive Oxygen Species by Flax Fabric. Appl. Biochem. Biotechnol. 2018, 184, 366–385. [Google Scholar] [CrossRef]
- Gębarowski, T.; Moreira, H.; Szyjka, A.; Wiatrak, B.; Wojtasik, W.; Kulma, A.; Szopa, J.; Gasiorowski, K. Impact of Fabrics from Transgenic Flax Plant on Human Dermal Fibroblasts in Vitro Proliferation. Acta Pol. Pharm. -Drug Res. 2017, 74, 642–652. [Google Scholar]
- Gąsiorowski, K.; Gębarowski, T.; Moreira, H.; Kulma, A.; Szatkowski, M.; Szopa, J. Impact of Fabrics from Transgenic Flax on Cultures of Skin Cells. Adv. Clin. Exp. Med. 2019, 28, 431–438. [Google Scholar] [CrossRef]
- Gębarowski, T.; Wiatrak, B.; Janeczek, M.; Żuk, M.; Pistor, P.; Gąsiorowski, K. Were Our Ancestors Right in Using Flax Dressings? Research on the Properties of Flax Fibre and Its Usefulness in Wound Healing. Oxid. Med. Cell. Longev. 2020, 2020, 1682317. [Google Scholar] [CrossRef]
- Gębarowski, T.; Jęśkowiak, I.; Janeczek, M.; Żuk, M.; Dobosz, A.; Wiatrak, B. The Technological Process of Obtaining New Linen Dressings Did Not Cause the Loss of Their Wound-Healing Properties. Materials 2021, 14, 7736. [Google Scholar] [CrossRef]
- Chaung, W.; Mi, L.J.; Boorstein, R.J. The P53 Status of Chinese Hamster V79 Cells Frequently Used for Studies on DNA Damage and DNA Repair. Nucleic Acids Res. 1996, 25, 992–994. [Google Scholar] [CrossRef]
- Sannino, A.; Zeni, O.; Romeo, S.; Massa, R.; Scarfi, M.R. Adverse and Beneficial Effects in Chinese Hamster Lung Fibroblast Cells Following Radiofrequency Exposure. Bioelectromagnetics 2017, 38, 245–254. [Google Scholar] [CrossRef]
- Miao, F.; Li, Y.; Tai, Z.; Zhang, Y.; Gao, Y.; Hu, M.; Zhu, Q. Antimicrobial Peptides: The Promising Therapeutics for Cutaneous Wound Healing. Macromol. Biosci. 2021, 21, 2100103. [Google Scholar] [CrossRef]
- Gould, L.J.; Orgill, D.P.; Armstrong, D.G.; Galiano, R.D.; Glat, P.M.; Zelen, C.M.; DiDomenico, L.A.; Carter, M.J.; Li, W.W. Improved Healing of Chronic Diabetic Foot Wounds in a Prospective Randomised Controlled Multi-Centre Clinical Trial with a Microvascular Tissue Allograft. Int. Wound J. 2022, 19, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Brega, C.; Calvi, S.; Albertini, A. Use of a Negative Pressure Wound Therapy System over Closed Incisions Option in Preventing Post-Sternotomy Wound Complications. Wound Repair Regen. 2021, 29, 848–852. [Google Scholar] [CrossRef]
- Bieniek, E.; Skołucka-Szary, K.; Brzeziński, J.; Piaskowski, S.; Lewiński, A. Innovative Biodegradable Dibutyrylchitin Dressing for the Treatment of Ulcers Occurring during Chronic Venous Insufficiency in Patients with Type 2 Diabetes. Int. J. Occup. Med. Environ. Health 2021, 34, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic Wounds: Treatment Consensus. Wound Repair Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.; Wächter, J.; Windbergs, M. Therapy of Infected Wounds: Overcoming Clinical Challenges by Advanced Drug Delivery Systems. Drug Deliv. Transl. Res. 2021, 11, 1545–1567. [Google Scholar] [CrossRef] [PubMed]
- Zahel, P.; Beekmann, U.; Eberlein, T.; Schmitz, M.; Werz, O.; Kralisch, D. Bacterial Cellulose—Adaptation of a Nature-Identical Material to the Needs of Advanced Chronic Wound Care. Pharmaceuticals 2022, 15, 683. [Google Scholar] [CrossRef]
- Verdolino, D.V.; Thomason, H.A.; Fotticchia, A.; Cartmell, S. Wound Dressings: Curbing Inflammation in Chronic Wound Healing. Emerg. Top. Life Sci. 2021, 5, 523–537. [Google Scholar] [CrossRef]
- Barrigah-Benissan, K.; Ory, J.; Sotto, A.; Salipante, F.; Lavigne, J.P.; Loubet, P. Antiseptic Agents for Chronic Wounds: A Systematic Review. Antibiotics 2022, 11, 350. [Google Scholar] [CrossRef]
- Agostinis, C.; Spazzapan, M.; Vuerich, R.; Balduit, A.; Stocco, C.; Mangogna, A.; Ricci, G.; Papa, G.; Zacchigna, S.; Bulla, R. Differential Capability of Clinically Employed Dermal Regeneration Scaffolds to Support Vascularization for Tissue Bioengineering. Biomedicines 2021, 9, 1458. [Google Scholar] [CrossRef]
- Del Amo, C.; Fern, X.; Cascajo-castresana, M.; Perez-valle, A.; Madarieta, I.; Olalde, B.; Andia, I. Wound-Microenvironment Engineering through Advanced-Dressing Bioprinting. Int. J. Mol. Sci. 2022, 23, 2836. [Google Scholar] [CrossRef]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef]
- Bray, E.R.; Oropallo, A.R.; Grande, D.A.; Kirsner, R.S.; Badiavas, E.V. Extracellular Vesicles as Therapeutic Tools for the Treatment of Chronic Wounds. Pharmaceutics 2021, 13, 1543. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Cao, X.; Jiang, H.; Wu, Z.; Zeng, Z.; Chen, H.; Zhang, J. The Role of Gel Wound Dressings Loaded with Stem Cells in the Treatment of Diabetic Foot Ulcers. Am. J. Transl Res. 2021, 13, 13261–13272. [Google Scholar]
- Derwin, R.; Patton, D.; Avsar, P.; Strapp, H.; Moore, Z. The Impact of Topical Agents and Dressing on PH and Temperature on Wound Healing: A Systematic, Narrative Review. Int. Wound J. 2021. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Li, H.; Chen, X.; Feng, S.; Mei, Z. How Effective Are Nano-Based Dressings in Diabetic Wound Healing? A Comprehensive Review of Literature. Int. J. Nanomed. 2022, 17, 2097–2119. [Google Scholar] [CrossRef]
- Aitcheson, S.M.; Frentiu, F.D.; Hurn, S.E.; Edwards, K.; Murray, R.Z. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules 2021, 26, 4917. [Google Scholar] [CrossRef]
- Li, Q.; Liu, K.; Jiang, T.; Ren, S.; Kang, Y.; Li, W.; Yao, H.; Yang, X.; Dai, H.; Chen, Z. Injectable and Self-Healing Chitosan-Based Hydrogel with MOF-Loaded α-Lipoic Acid Promotes Diabetic Wound Healing. Mater. Sci. Eng. C 2021, 131, 112519. [Google Scholar] [CrossRef]
- Konop, M.; Rybka, M.; Drapała, A. Keratin Biomaterials in Skin Wound Healing, an Old Player in Modern Medicine: A Mini Review. Pharmaceutics 2021, 13, 2029. [Google Scholar] [CrossRef]
- Styrczewska, M.; Kostyn, A.; Kulma, A.; Majkowska-Skrobek, G.; Augustyniak, D.; Prescha, A.; Czuj, T.; Szopa, J. Flax Fiber Hydrophobic Extract Inhibits Human Skin Cells Inflammation and Causes Remodeling of Extracellular Matrix and Wound Closure Activation. Biomed. Res. Int. 2015, 2015, 862391. [Google Scholar] [CrossRef]
- He, C.; Liu, X.; Zhou, Z.; Liu, N.; Ning, X.; Miao, Y.; Long, Y.; Wu, T.; Leng, X. Harnessing Biocompatible Nanofibers and Silver Nanoparticles for Wound Healing: Sandwich Wound Dressing versus Commercial Silver Sulfadiazine Dressing. Mater. Sci. Eng. C 2021, 128, 112342. [Google Scholar] [CrossRef]
- Shahzad, F. Management of Skin Graft Donor Site in Pediatric Patients with Tumescent Technique and AQUACEL® Ag Foam Dressing. J. Plast. Surg. Hand Surg. 2021, 55, 309–314. [Google Scholar] [CrossRef]
- Yang, W.; Xu, F.; Ma, X.; Guo, J.; Li, C.; Shen, S.; Puglia, D.; Chen, J.; Xu, P.; Kenny, J.; et al. Highly-Toughened PVA/Nanocellulose Hydrogels with Anti-Oxidative and Antibacterial Properties Triggered by Lignin-Ag Nanoparticles. Mater. Sci. Eng. C 2021, 129, 112385. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Zayed, M.A.; El-dek, S.I.; Hady, M.A.; El Sherbiny, D.H.; Uskoković, V. Nanofibrous ε-Polycaprolactone Scaffolds Containing Ag-Doped Magnetite Nanoparticles: Physicochemical Characterization and Biological Testing for Wound Dressing Applications in Vitro and in Vivo. Bioact. Mater. 2021, 6, 2070–2088. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Ji, N.; Lee, S.; Wang, G.; Zheng, Y.; Zhang, X.; Yang, L.; Qin, Z.; Yang, Y. Bioinspired Design of Sericin/Chitosan/Ag@mof/Go Hydrogels for Efficiently Combating Resistant Bacteria, Rapid Hemostasis, and Wound Healing. Polymers 2021, 13, 2812. [Google Scholar] [CrossRef] [PubMed]
- Farazin, A.; Mohammadimehr, M.; Ghasemi, A.H.; Naeimi, H. Design, Preparation, and Characterization of CS/PVA/SA Hydrogels Modified with Mesoporous Ag2O/SiO2and Curcumin Nanoparticles for Green, Biocompatible, and Antibacterial Biopolymer Film. RSC Adv. 2021, 11, 32775–32791. [Google Scholar] [CrossRef] [PubMed]
- Kulma, A.; Skórkowska-Telichowska, K.; Kostyn, K.; Szatkowski, M.; Skała, J.; Drulis-Kawa, Z.; Preisner, M.; Zuk, M.; Szperlik, J.; Wang, Y.F.; et al. New Flax Producing Bioplastic Fibers for Medical Purposes. Ind. Crops Prod. 2015, 68, 80–89. [Google Scholar] [CrossRef]
- Wróbel-Kwiatkowska, M.; Turnau, K.; Góralska, K.; Anielska, T.; Szopa, J. Effects of Genetic Modifications to Flax (Linum Usitatissimum) on Arbuscular Mycorrhiza and Plant Performance. Mycorrhiza 2012, 22, 493–499. [Google Scholar] [CrossRef]
- Lukiw, W.J. Bacteroides Fragilis Lipopolysaccharide and Inflammatory Signaling in Alzheimer’s Disease. Front. Microbiol. 2016, 7, 1544. [Google Scholar] [CrossRef]
- Wróbel-Kwiatkowska, M.; Lorenz-Kukula, K.; Starzycki, M.; Oszmiański, J.; Kepczyńska, E.; Szopa, J. Expression of β-1,3-Glucanase in Flax Causes Increased Resistance to Fungi. Physiol. Mol. Plant Pathol. 2004, 65, 245–256. [Google Scholar] [CrossRef]
- Arendt-Pindel, A.; Marszałek-Harych, A.; Gȩbarowska, E.; Gȩbarowski, T.; Jȩdrzkiewicz, D.; Plaskowska, E.; Zalewski, D.; Gulia, N.; Szafert, S.; Ejfler, J. Design and Functionalization of Bioactive Benzoxazines. An Unexpected Ortho-Substitution Effect. New J. Chem. 2019, 43, 12042–12053. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chang, T.S.; Jeong, W.; Kang, D. Methods for Detection and Measurement of Hydrogen Peroxide inside and Outside of Cells. Mol. Cells 2010, 29, 539–549. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Appl. Biochem. Biotechnol. Part B Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]





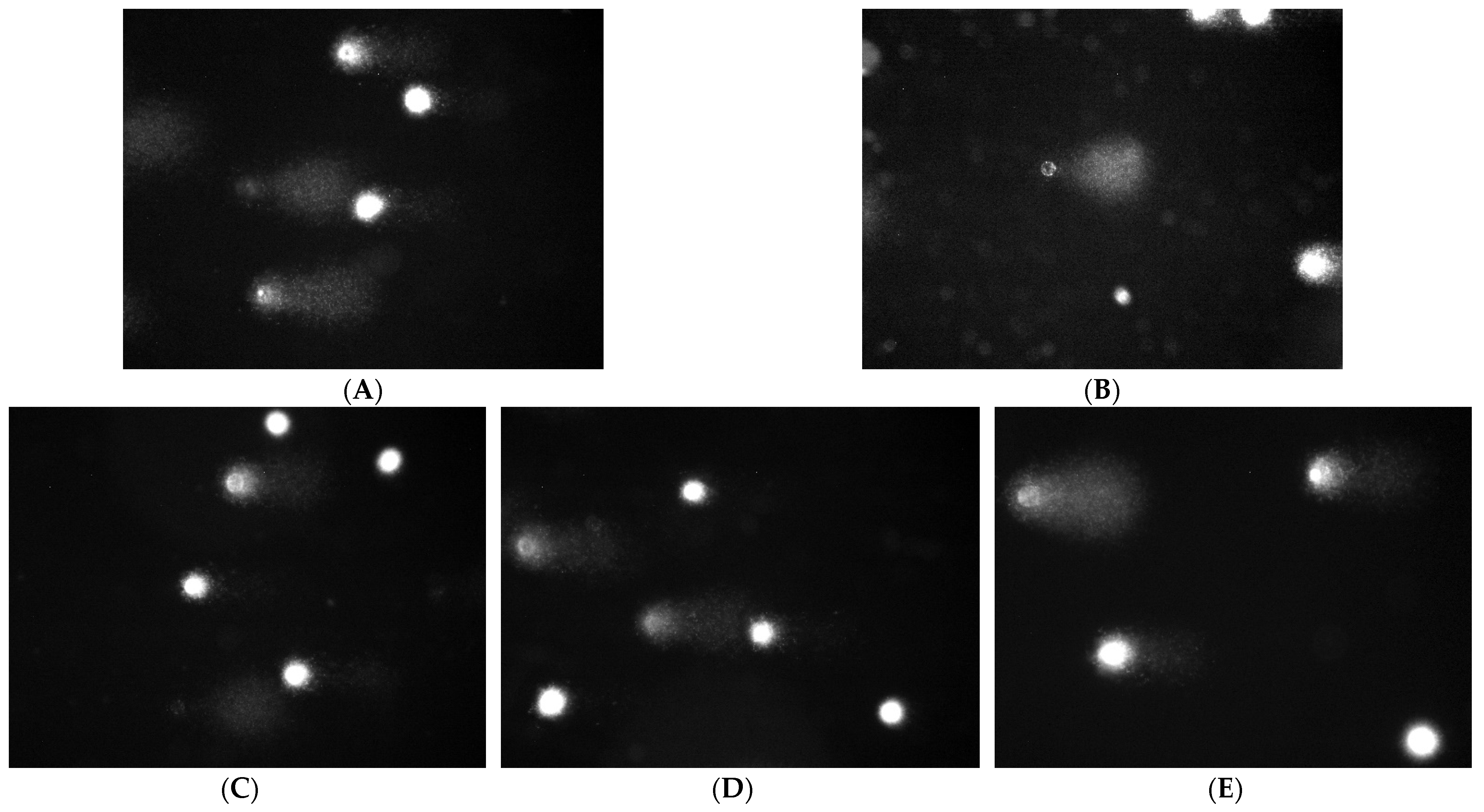





| Research Group | Material | Results |
|---|---|---|
| Skórkowska-Telichowska Katarzyna et al. [11] | Balb/3T3 cell line 30 patients with wounds that had lasted at least 2 years |
|
| Paladini et al. [12] | Balb/3T3 cell line |
|
| Skórkowska-Telichowska Katarzyna et al. [13] | V79 cell line |
|
| Gębarowski Tomasz et al. [14] | NHDF cell line |
|
| Gąsiorowski Kazimierz et al. [15] | NHEK, NHDF, HUVEC, THP-1 cell lines |
|
| Gębarowski Tomasz et al. [16] | Balb/3T3, NHDF, THP-1, NHEK, HMVEK, A431 cell lines |
|
| Skórkowska-Telichowska Katarztna et al. [5] | NHDF cell line 22 patients suffered from chronic non-healing ulcerations |
|
| Gębarowski Tomasz et al. [17] | NHEK, Balb 3T3, HMCEV, THP-1 cell lines |
|
| Test | Fiber | Fabric |
|---|---|---|
| Cell viability | B | B |
| Cell proliferation | MB | MB |
| Free Radical Level | NIKE | NIKE |
| Genotoxic in the comet assay | MB | MB |
| Potential wound environment response to oxidative stress | M, B | MB |
| Apoptosis | B | B |
| Cell cycle | MB | MB |
| Scratch test—migration assay | M, MB | M, MB |
| The effect on wound healing in the V79 cell model | The first M, then MB | The first B, then M |
| * DCFDA (H2O2)/** Tail (H2O2) | ||||
|---|---|---|---|---|
| *** NIKE | **** M | **** B | ***** MB | |
| fiber | 0.699 | −0.084 | 0.892 | 0.879 |
| fabric | 0.938 | −0.686 | 0.452 | 0.874 |
| apoptosis/tail | ||||
| fiber | 0.622095 | 0.678914 | 0.04192 | −0.22859 |
| fabric | −0.64786 | 0.00032 | −0.68978 | −0.17688 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gębarowski, T.; Jęśkowiak, I.; Wiatrak, B. Investigation of the Properties of Linen Fibers and Dressings. Int. J. Mol. Sci. 2022, 23, 10480. https://doi.org/10.3390/ijms231810480
Gębarowski T, Jęśkowiak I, Wiatrak B. Investigation of the Properties of Linen Fibers and Dressings. International Journal of Molecular Sciences. 2022; 23(18):10480. https://doi.org/10.3390/ijms231810480
Chicago/Turabian StyleGębarowski, Tomasz, Izabela Jęśkowiak, and Benita Wiatrak. 2022. "Investigation of the Properties of Linen Fibers and Dressings" International Journal of Molecular Sciences 23, no. 18: 10480. https://doi.org/10.3390/ijms231810480







