Identification of Adipose Tissue as a Reservoir of Macrophages after Acute Myocardial Infarction
Abstract
:1. Introduction
2. Results
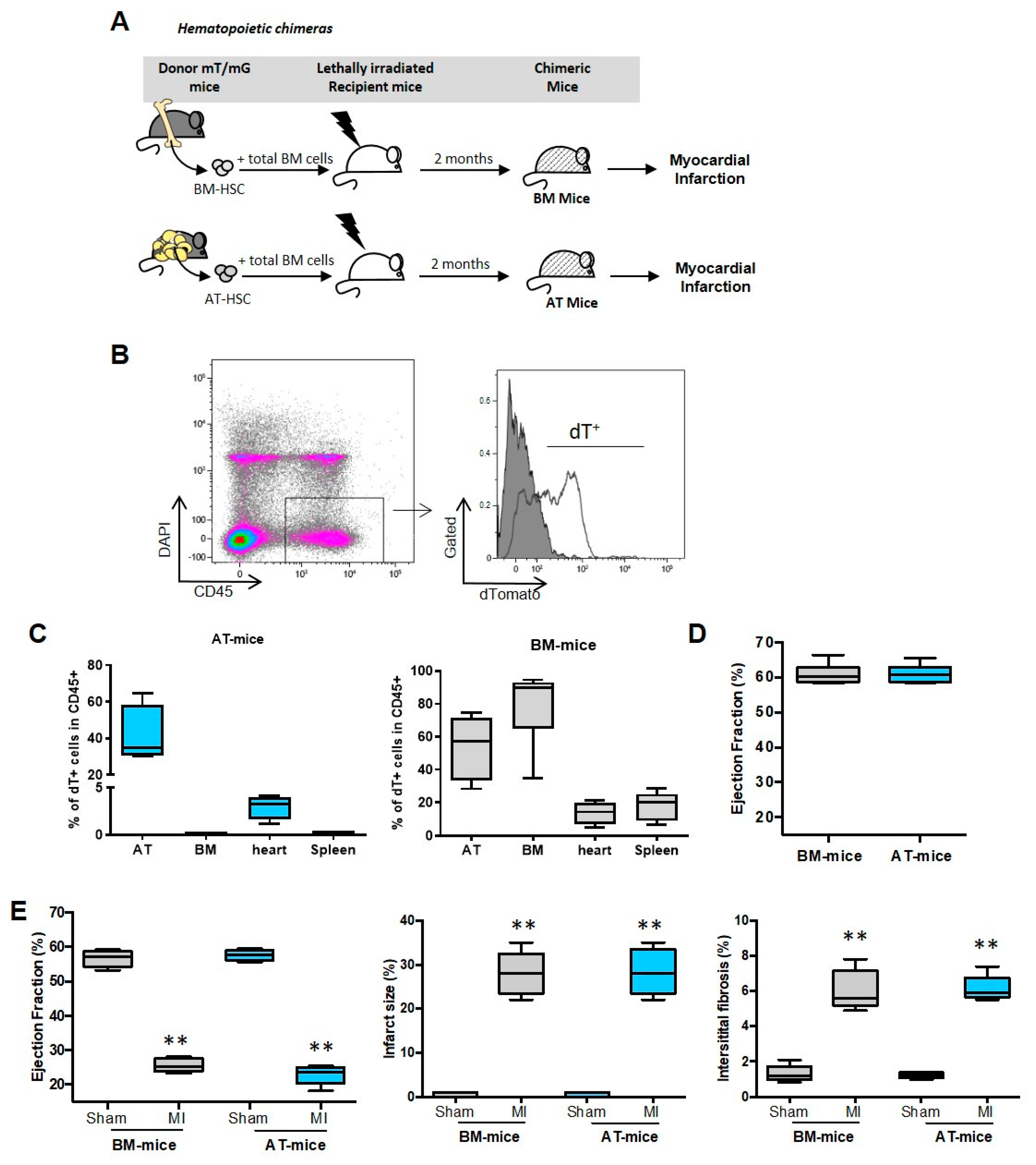
2.1. Establishment of Experimental Models
2.2. A Subset of Cardiac Inflammatory Myeloid Cells Emerges from AT-Endogenous Hematopoiesis
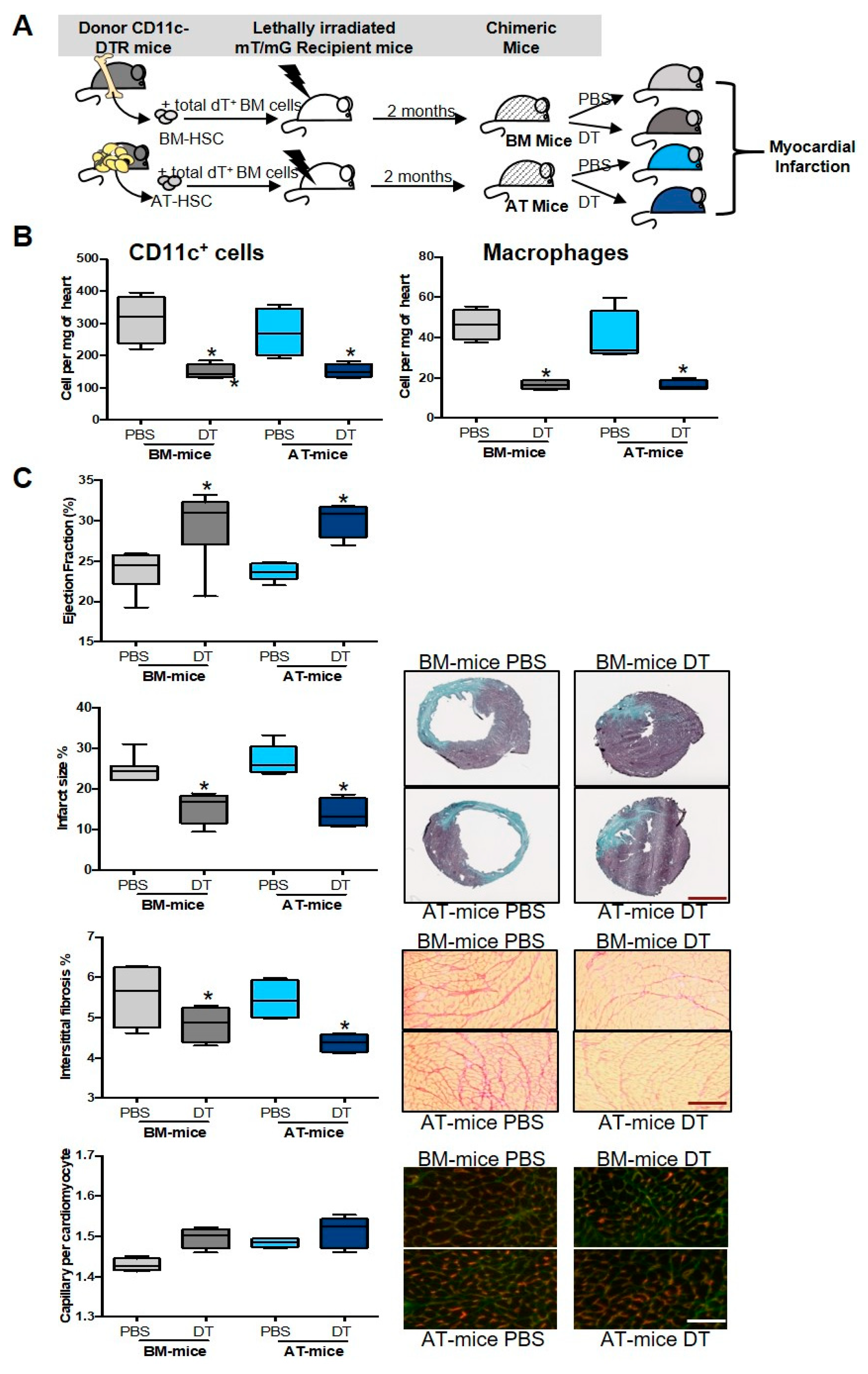
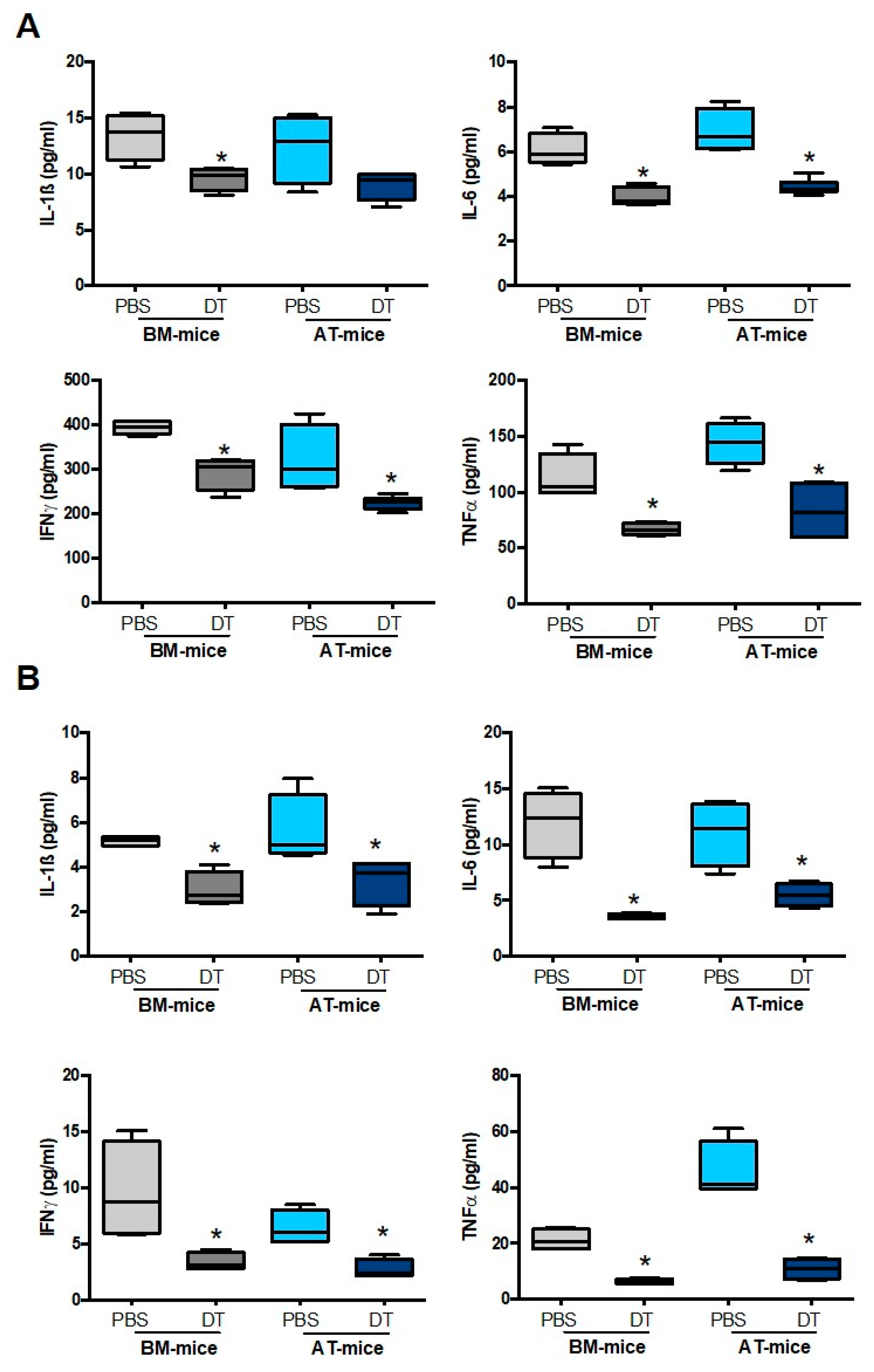
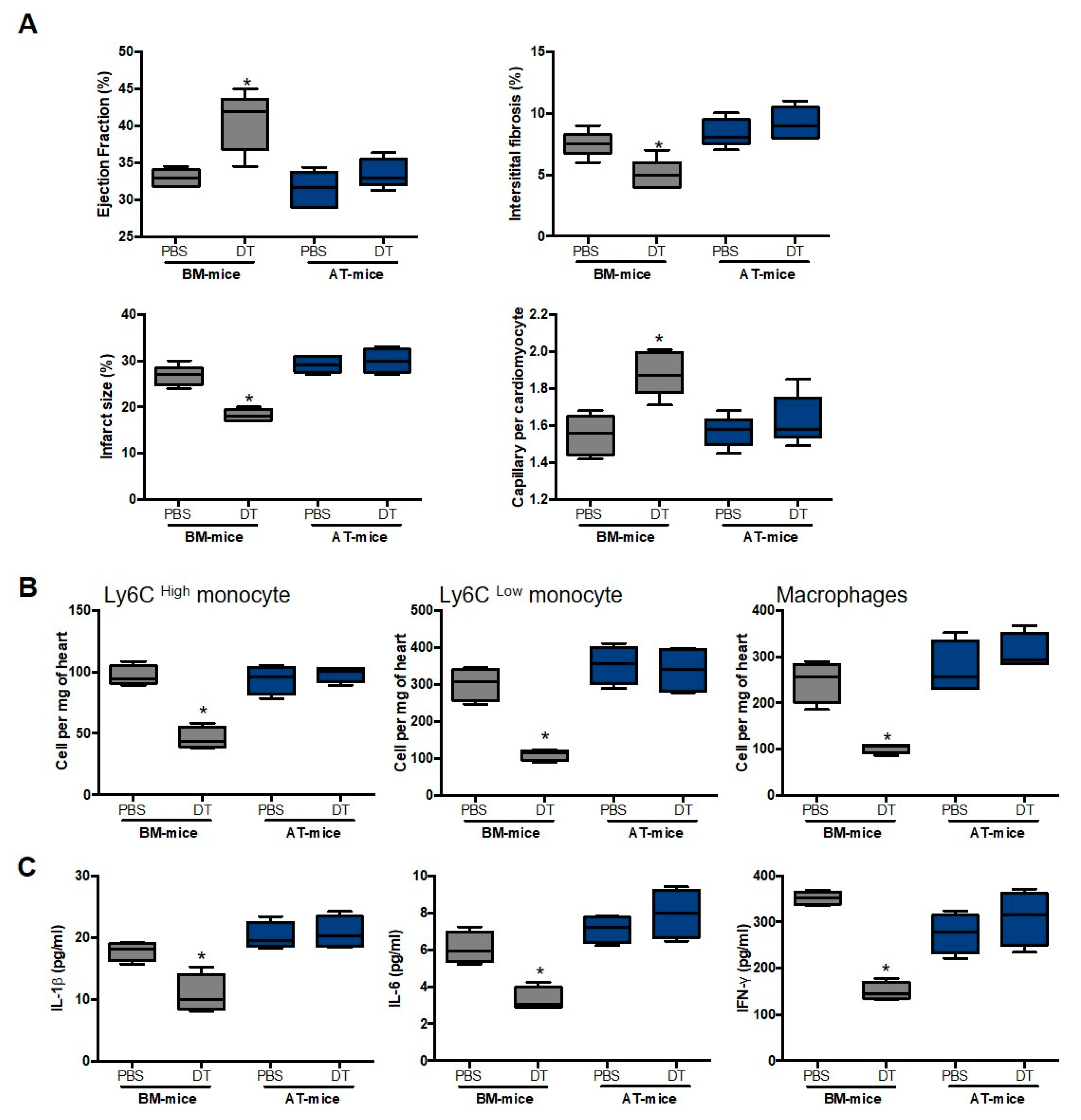
2.3. Specific Depletion of AT-Derived Myeloid Cells Improves Cardiac Remodeling after MI
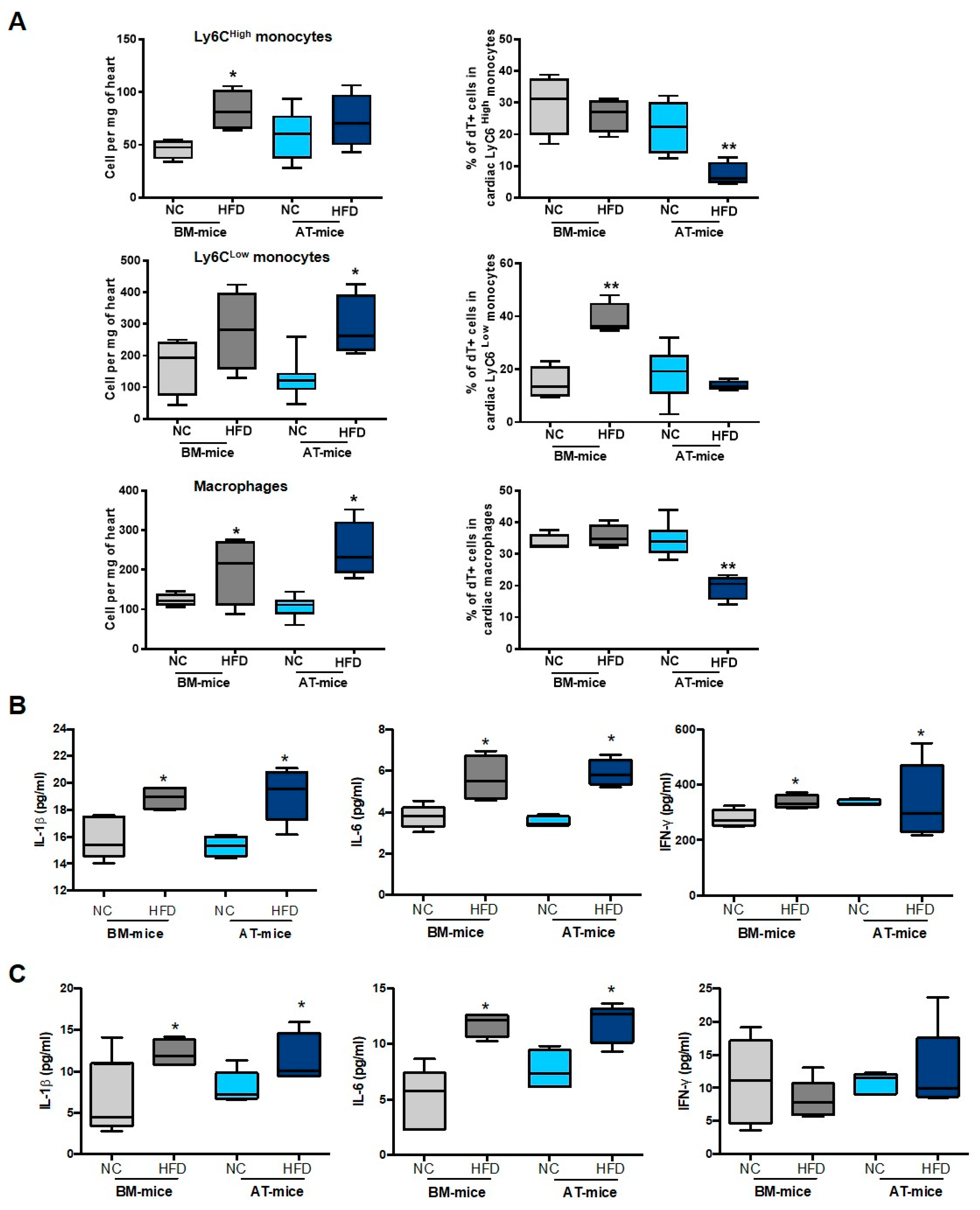
2.4. Diabetes Abrogates AT-Derived Cell Supply in the Heart
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, J.; Koh, Y.J.; Moon, H.R.; Ryoo, H.G.; Cho, C.H.; Kim, I.; Koh, G.Y. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood 2010, 115, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Poglio, S.; De Toni, F.; Lewandowski, D.; Minot, A.; Arnaud, E.; Barroca, V.; Laharrague, P.; Casteilla, L.; Cousin, B. In situ production of innate immune cells in murine white adipose tissue. Blood 2012, 120, 4952–4962. [Google Scholar] [CrossRef] [PubMed]
- Cousin, B.; Casteilla, L.; Laharrague, P.; Luche, E.; Lorsignol, A.; Cuminetti, V.; Paupert, J. Immuno-metabolism and adipose tissue: The key role of hematopoietic stem cells. Biochimie 2016, 124, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Rabiller, L.; Robert, V.; Arlat, A.; Labit, E.; Ousset, M.; Salon, M.; Coste, A.; Costa-Fernandes, D.; Monsarrat, P.; Ségui, B.; et al. Driving regeneration, instead of healing, in adult mammals: The decisive role of resident macrophages through efferocytosis. NPJ Regen. Med. 2021, 6, 1–12. [Google Scholar] [CrossRef]
- Poglio, S.; De Toni-Costes, F.; Arnaud, E.; Laharrague, P.; Espinosa, E.; Casteilla, L.; Cousin, B. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells 2010, 28, 2065–2072. [Google Scholar] [CrossRef]
- Ngkelo, A.; Richart, A.; Kirk, J.A.; Bonnin, P.; Vilar, J.; Lemitre, M.; Marck, P.; Branchereau, M.; Le Gall, S.; Renault, N.; et al. Mast cells regulate myofilament calcium sensitization and heart function after myocardial infarction. J. Exp. Med. 2016, 213, 1353–1374. [Google Scholar] [CrossRef]
- Luche, E.; Robert, V.; Cuminetti, V.; Pomie, C.; Sastourne-Arrey, Q.; Waget, A.; Arnaud, E.; Varin, A.; Labit, E.; Laharrague, P.; et al. Corrupted adipose tissue endogenous myelopoiesis initiates diet-induced metabolic disease. eLife 2017, 6, e23194. [Google Scholar] [CrossRef]
- Peet, C.; Ivetic, A.; Bromage, D.I.; Shah, A.M. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc. Res. 2020, 116, 1101–1112. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.; Stangenberg, L.; Wurdinger, T.; Figueiredo, J.L.; Libby, P.; Weissleder, R.; Pittet, M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Leuschner, F.; Rauch, P.J.; Ueno, T.; Gorbatov, R.; Marinelli, B.; Lee, W.W.; Dutta, P.; Wei, Y.; Robbins, C.; Iwamoto, Y.; et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 2012, 209, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Howangyin, K.Y.; Zlatanova, I.; Pinto, C.; Ngkelo, A.; Cochain, C.; Rouanet, M.; Vilar, J.; Lemitre, M.; Stockmann, C.; Fleischmann, B.K.; et al. Myeloid-Epithelial-Reproductive Receptor Tyrosine Kinase and Milk Fat Globule Epidermal Growth Factor 8 Coordinately Improve Remodeling After Myocardial Infarction via Local Delivery of Vascular Endothelial Growth Factor. Circulation 2016, 133, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K.; Singla, R.D.; Abdelli, L.S.; Glass, C. Fibroblast growth factor-9 enhances M2 macrophage differentiation and attenuates adverse cardiac remodeling in the infarcted diabetic heart. PLoS ONE 2015, 10, e0120739. [Google Scholar] [CrossRef]
- Fadini, G.P.; de Kreutzenberg, S.V.; Boscaro, E.; Albiero, M.; Cappellari, R.; Krankel, N.; Landmesser, U.; Toniolo, A.; Bolego, C.; Cignarella, A.; et al. An unbalanced monocyte polarisation in peripheral blood and bone marrow of patients with type 2 diabetes has an impact on microangiopathy. Diabetologia 2013, 56, 1856–1866. [Google Scholar] [CrossRef]
- Jadhav, A.; Tiwari, S.; Lee, P.; Ndisang, J.F. The heme oxygenase system selectively enhances the anti-inflammatory macrophage-M2 phenotype, reduces pericardial adiposity, and ameliorated cardiac injury in diabetic cardiomyopathy in Zucker diabetic fatty rats. J. Pharmacol. Exp. Ther. 2013, 345, 239–249. [Google Scholar] [CrossRef]
- Nagareddy, P.R.; Murphy, A.J.; Stirzaker, R.A.; Hu, Y.; Yu, S.; Miller, R.G.; Ramkhelawon, B.; Distel, E.; Westerterp, M.; Huang, L.S.; et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013, 17, 695–708. [Google Scholar] [CrossRef]
- Miller, C.L.; Dykstra, B.; Eaves, C.J. Characterization of mouse hematopoietic stem and progenitor cells. Curr. Protoc. Immunol. 2008, 22, 22B.2. [Google Scholar] [CrossRef]
- Burcelin, R.; Crivelli, V.; Dacosta, A.; Roy-Tirelli, A.; Thorens, B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E834–E842. [Google Scholar] [CrossRef]
- Zaman, R.; Hamidzada, H.; Epelman, S. Exploring cardiac macrophage heterogeneity in the healthy and diseased myocardium. Curr. Opin. Immunol. 2021, 68, 54–63. [Google Scholar] [CrossRef]
- Guilliams, M.; Thierry, G.R.; Bonnardel, J.; Bajenoff, M. Establishment and Maintenance of the Macrophage Niche. Immunity 2020, 52, 434–451. [Google Scholar] [CrossRef] [PubMed]
- Frantz, S.; Nahrendorf, M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc. Res. 2014, 102, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, G.; Schneider, C.; Wong, N.; Bredemeyer, A.; Hulsmans, M.; Nahrendorf, M.; Epelman, S.; Kreisel, D.; Liu, Y.; Itoh, A.; et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat. Med. 2018, 24, 1234–1245. [Google Scholar] [CrossRef]
- Dick, S.A.; Wong, A.; Hamidzada, H.; Nejat, S.; Nechanitzky, R.; Vohra, S.; Mueller, B.; Zaman, R.; Kantores, C.; Aronoff, L.; et al. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci. Immunol. 2022, 7, eabf777. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Kuppe, C.; Ramirez Flores, R.O.; Li, Z.; Hayat, S.; Levinson, R.T.; Liao, X.; Hannani, M.T.; Tanevski, J.; Wunnemann, F.; Nagai, J.S.; et al. Spatial multi-omic map of human myocardial infarction. Nature 2022, 608, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Tie, G.; Wang, S.; Tutto, A.; DeMarco, N.; Khair, L.; Fazzio, T.G.; Messina, L.M. Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages. Nat. Commun. 2018, 9, 33. [Google Scholar] [CrossRef]
- Barman, P.K.; Koh, T.J. Macrophage Dysregulation and Impaired Skin Wound Healing in Diabetes. Front. Cell Dev. Biol. 2020, 8, 528. [Google Scholar] [CrossRef]
- Ritchie, R.H.; Abel, E.D. Basic Mechanisms of Diabetic Heart Disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef]
- Lopaschuk, G.D. Metabolic Abnormalities in the Diabetic Heart. Heart Fail. Rev. 2002, 7, 149. [Google Scholar] [CrossRef]
- Zlatanova, I.; Pinto, C.; Bonnin, P.; Mathieu, J.R.; Bakker, W.; Vilar, J.; Lemitre, M.; Voehringer, D.; Vaulont, S.; Peyssonnaux, C.; et al. Iron Regulator Hepcidin Impairs Macrophage-Dependent Cardiac Repair After Injury. Circulation 2019, 139, 1530–1547. [Google Scholar] [CrossRef] [PubMed]
- Lima Correa, B.; El Harane, N.; Gomez, I.; Rachid Hocine, H.; Vilar, J.; Desgres, M.; Bellamy, V.; Keirththana, K.; Guillas, C.; Perotto, M.; et al. Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovasc. Res. 2021, 117, 292–307. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, I.; Robert, V.; Alayrac, P.; Arlat, A.; Duval, V.; Renoud, M.-L.; Vilar, J.; Lemitre, M.; Silvestre, J.-S.; Cousin, B. Identification of Adipose Tissue as a Reservoir of Macrophages after Acute Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 10498. https://doi.org/10.3390/ijms231810498
Gomez I, Robert V, Alayrac P, Arlat A, Duval V, Renoud M-L, Vilar J, Lemitre M, Silvestre J-S, Cousin B. Identification of Adipose Tissue as a Reservoir of Macrophages after Acute Myocardial Infarction. International Journal of Molecular Sciences. 2022; 23(18):10498. https://doi.org/10.3390/ijms231810498
Chicago/Turabian StyleGomez, Ingrid, Virginie Robert, Paul Alayrac, Adèle Arlat, Vincent Duval, Marie-Laure Renoud, José Vilar, Mathilde Lemitre, Jean-Sébastien Silvestre, and Béatrice Cousin. 2022. "Identification of Adipose Tissue as a Reservoir of Macrophages after Acute Myocardial Infarction" International Journal of Molecular Sciences 23, no. 18: 10498. https://doi.org/10.3390/ijms231810498
APA StyleGomez, I., Robert, V., Alayrac, P., Arlat, A., Duval, V., Renoud, M.-L., Vilar, J., Lemitre, M., Silvestre, J.-S., & Cousin, B. (2022). Identification of Adipose Tissue as a Reservoir of Macrophages after Acute Myocardial Infarction. International Journal of Molecular Sciences, 23(18), 10498. https://doi.org/10.3390/ijms231810498





