Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine
Abstract
:1. Introduction
2. Albumin as a Biomaterial
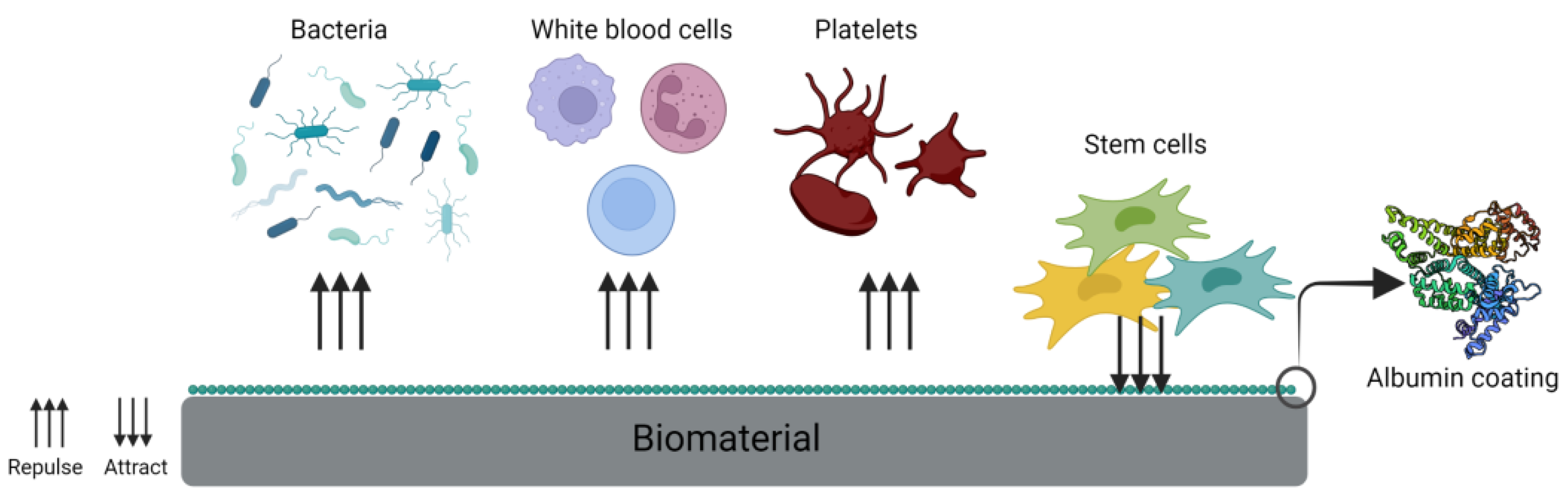
2.1. Coating
2.2. Anticoagulant Properties
2.3. Antibacterial Properties
2.4. Albumin as Cell Adhesion Protein
2.5. Scaffolds
2.5.1. Albumin-Only Scaffold
2.5.2. Albumin Hybrid Scaffolds and Hydrogels
3. Albumin as a Local Therapeutic Agent
3.1. Bioactive Sites on the Albumin Chain
3.2. Clinical Applications
3.3. Albumin and Blood-Derived Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, T.W. Review article: Albumin as a drug—Biological effects of albumin unrelated to oncotic pressure. Aliment. Pharmacol. Ther. 2002, 16, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, K.; Kogan, M.J.; Araya, E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int. J. Nanomed. 2019, 14, 6387. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.; Fries, M.R.; Buchholz, C.; Zhang, F.; Schreiber, F. Human versus Bovine Serum Albumin: A Subtle Difference in Hydrophobicity Leads to Large Differences in Bulk and Interface Behavior. Cryst. Growth Des. 2021, 21, 5451–5459. [Google Scholar] [CrossRef]
- Raoufinia, R.; Mota, A.; Keyhanvar, N.; Safari, F.; Shamekhi, S.; Abdolalizadeh, J. Overview of Albumin and Its Purification Methods. Adv. Pharm. Bull. 2016, 6, 495. [Google Scholar] [CrossRef]
- Mishra, V.; Heath, R. Structural and Biochemical Features of Human Serum Albumin Essential for Eukaryotic Cell Culture. Int. J. Mol. Sci. 2021, 22, 8411. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.; Singh, S.; Mishra, N.; Pal, R.; Singh, N.; Parashar, P.; Saraf, S.A. Albumin-based nanomaterials in drug delivery and biomedical applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Academic Press: Cambridge, MA, USA, 2021; pp. 465–496. [Google Scholar] [CrossRef]
- Pulimood, T.B.; Park, G.R. Debate: Albumin administration should be avoided in the critically ill. Crit. Care 2000, 4, 151–155. [Google Scholar] [CrossRef]
- Huang, B.X.; Kim, H.-Y.; Dass, C. Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 1237–1247. [Google Scholar] [CrossRef]
- Gonzalez, U.A.; Menendez, C.; Saitua, H.A.; Rigau, J. Multiple response optimization of heat shock process for separation of bovine serum albumin from plasma. Sep. Sci. Technol. 2017, 52, 1992–2001. [Google Scholar] [CrossRef]
- Bovine Serum Albumin—A Help or Hindrance in Immunoassays [Internet]. Available online: https://cellculturedish.com/bovine-serum-albumin-a-help-or-hindrance-in-immunoassays/ (accessed on 24 August 2022).
- Strixner, T.; Kulozik, U. Chapter 7: Egg proteins. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 445–459. [Google Scholar]
- Pereira, M.M.; Cruz, R.A.; Almeida, M.; Lima, A.; Coutinho, J.; Freire, M.G. Single-step purification of ovalbumin from egg white using aqueous biphasic systems. Process Biochem. 2016, 51, 781–791. [Google Scholar] [CrossRef]
- Alenius, H.; Shurin, M.R.; Shurin, G.V.; Beezhold, D.; Shvedova, A.A. Respiratory System, Part Two: Allergy and Asthma. In Adverse Effects of Engineered Nanomaterials, 2nd; Academic Press: Cambridge, MA, USA, 2017; pp. 243–253. [Google Scholar]
- Sánchez, L.; Pérez, M.D.; Parrón, J.A. HPP in dairy products: Impact on quality and applications. In Present and Future of High Pressure Processing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–272. [Google Scholar]
- Kamau, S.M.; Cheison, S.C.; Chen, W.; Liu, X.-M.; Lu, R.-R. Alpha-Lactalbumin: Its Production Technologies and Bioactive Peptides. Compr. Rev. Food Sci. Food Saf. 2010, 9, 197–212. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Ramos, O.; Pereira, R.; Rodrigues, R.; Teixeira, J.; Vicente, A.; Malcata, F. Whey and Whey Powders: Production and Uses; Elsevier: Amsterdam, The Netherlands, 2015; pp. 498–505. [Google Scholar] [CrossRef]
- Ito, M.; Kato, T.; Matsuda, T. Rice Allergenic Proteins, 14–16 kDa Albumin and α-Globulin, Remain Insoluble in Rice Grains Recovered from Rice Miso (Rice-Containing Fermented Soybean Paste). Biosci. Biotechnol. Biochem. 2005, 69, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Radovic, R.S.; Maksimovic, R.V.; Brkljacic, M.J.; Gasic, I.E.V.; Savic, P.A. 2S Albumin from Buckwheat (Fagopyrum esculentum Moench) Seeds. J. Agric. Food Chem. 1999, 47, 1467–1470. [Google Scholar] [CrossRef]
- Souza, P.F. The forgotten 2S albumin proteins: Importance, structure, and biotechnological application in agriculture and human health. Int. J. Biol. Macromol. 2020, 164, 4638–4649. [Google Scholar] [CrossRef]
- Yang, J.; Kornet, R.; Diedericks, C.F.; Yang, Q.; Berton-Carabin, C.C.; Nikiforidis, C.V.; Venema, P.; van der Linden, E.; Sagis, L.M. Rethinking plant protein extraction: Albumin—From side stream to an excellent foaming ingredient. Food Struct. 2022, 31, 100254. [Google Scholar] [CrossRef]
- Mylne, J.S.; Hara-Nishimura, I.; Rosengren, K.J. Seed storage albumins: Biosynthesis, trafficking and structures. Funct. Plant Biol. 2014, 41, 671–677. [Google Scholar] [CrossRef]
- Bosse, D.; Praus, M.; Kiessling, P.; Nyman, L.; Andresen, C.; Waters, J.; Schindel, F. Phase I Comparability of Recombinant Human Albumin and Human Serum Albumin. J. Clin. Pharmacol. 2005, 45, 57–67. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, H.J.; Ham, S.; Jeong, D.; Lee, M.; Lee, U.; Lee, M.; Kwon, T.; Ko, K. Plant-derived human recombinant growth factors and serum albumin maintain stemness of human-induced pluripotent stem cells. Cell Biol. Int. 2021, 46, 139–147. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Simon, M.; Schwarz, C.M.; Masteling, M.; Vácz, G.; Hornyák, I.; Lacza, Z. Serum albumin as a local therapeutic agent in cell therapy and tissue engineering. BioFactors 2016, 43, 315–330. [Google Scholar] [CrossRef]
- Bteich, M. An overview of albumin and alpha-1-acid glycoprotein main characteristics: Highlighting the roles of amino acids in binding kinetics and molecular interactions. Heliyon 2019, 5, e02879. [Google Scholar] [CrossRef] [PubMed]
- Peters, T. The Albumin Molecule: Its Structure and Chemical Properties. In All About Albumin; Elsevier: Amsterdam, The Netherlands, 1995; pp. 9–13. [Google Scholar]
- Borrelli, M.R.; Hu, M.S.; Longaker, M.T.; Lorenz, H.P. Tissue Engineering and Regenerative Medicine in Craniofacial Reconstruction and Facial Aesthetics. J. Craniofacial Surg. 2020, 31, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Cvrček, L.; Horáková, M. Plasma Modified Polymeric Materials for Implant Applications. In Non-Thermal Plasma Technology for Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 367–407. [Google Scholar]
- Raut, H.K.; Das, R.; Ziqian, L.; Xiaoling, L.; Ramakrishna, S. Biocompatibility of Biomaterials for Tissue Regeneration or Replacement. Biotechnol. J. 2020, 15, 2000160. [Google Scholar] [CrossRef] [PubMed]
- Nikzamir, M.; Akbarzadeh, A.; Panahi, Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. J. Drug Deliv. Sci. Technol. 2020, 61, 102316. [Google Scholar] [CrossRef]
- Hermawan, H.; Ramdan, D.; Djuansjah, J.R.P. Metals for Biomedical Applications. Biomed. Eng. -Theory Appl. 2011, 1, 411–430. [Google Scholar]
- Punj, S.; Singh, J.; Singh, K. Ceramic biomaterials: Properties, state of the art and future prospectives. Ceram Int. 2021, 47, 28059–28074. [Google Scholar] [CrossRef]
- Xia, H.; Zhao, D.; Zhu, H.; Hua, Y.; Xiao, K.; Xu, Y.; Liu, Y.; Chen, W.; Liu, Y.; Zhang, W.; et al. Lyophilized Scaffolds Fabricated from 3D-Printed Photocurable Natural Hydrogel for Cartilage Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 31704–31715. [Google Scholar] [CrossRef]
- Nseir, N.; Regev, O.; Kaully, T.; Blumenthal, J.; Levenberg, S.; Zussman, E. Biodegradable Scaffold Fabricated of Electrospun Albumin Fibers: Mechanical and Biological Characterization. Tissue Eng. Part C Methods 2013, 19, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Takashi, U.; Tateishi, T. Scaffold Design for Tissue Engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Azevedo, H.S.; Santos, T.C.; Reis, R.L. Controlling the degradation of natural polymers for biomedical applications. In Natural-Based Polymers for Biomedical Applications; Woodhead Publishing Series in Biomaterials: Sawston, UK, 2008; pp. 106–128. [Google Scholar]
- Hinsenkamp, A.; Kun, K.; Gajnut, F.; Majer, A.; Lacza, Z.; Hornyák, I. Cell Attachment Capacity and Compounds of Fibrin Membranes Isolated from Fresh Frozen Plasma and Cryoprecipitate. Membranes 2021, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Li, P.S.; Liang Lee, I.; Yu, W.L.; Sun, J.S.; Jane, W.N.; Shen, H.H. A Novel Albumin-Based Tissue Scaffold for Autogenic Tissue Engineering Applications. Sci. Rep. 2014, 4, 5600. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Sanganeria, P.; Sachar, S.; Chandra, S.; Bahadur, D.; Ray, P.; Khanna, A. Cellular internalization and detailed toxicity analysis of protein-immobilized iron oxide nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ostroverkhov, P.; Semkina, A.; Nikitin, A.; Smirnov, A.; Vedenyapina, D.; Vlasova, K.; Kireev, I.; Grin, M.; Chekhonin, V.; Majouga, A.; et al. Human serum albumin as an effective coating for hydrophobic photosensitizes immobilization on magnetic nanoparticles. J. Magn. Magn. Mater. 2019, 475, 108–114. [Google Scholar] [CrossRef]
- Othman, Z.; Cillero Pastor, B.; van Rijt, S.; Habibovic, P. Understanding interactions between biomaterials and biological systems using proteomics. Biomaterials 2018, 167, 191–204. [Google Scholar] [CrossRef]
- Amiji, M.; Park, H.; Park, K. Study on the prevention of surface-induced platelet activation by albumin coating. J. Biomater. Sci. Polym. Ed. 1992, 3, 375–388. [Google Scholar] [CrossRef]
- Özliseli, E.; Ṣen Karaman, D.; Chakraborti, S.; Slita, A.; Parikainen, M.; Sahlgren, C.M.; Rosenholm, J.M. Rational evaluation of human serum albumin coated mesoporous silica nanoparticles for xenogenic-free stem cell therapies. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124945. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Schandl, K.; Schwarz, C.M.; Renner, K.; Hornyák, I.; Szabó, B.T.; Niculescu-Morzsa, E.; Nehrer, S.; Dobó-Nagy, C.; Doros, A.; et al. Serum albumin–coated bone allograft (BoneAlbumin) results in faster bone formation and mechanically stronger bone in aging rats. J. Tissue Eng. Regen. Med. 2019, 13, 416–422. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Vácz, G.; Cselenyák, A.; Weszl, M.; Kiss, L.; Lacza, Z. Albumin-coated bioactive suture for cell transplantation. Surg Innov. 2013, 20, 249–255. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Vácz, G.; Szabó, T.; Szigyártó, I.C.; Toró, I.; Vámos, B.; Hornyák, I.; Renner, K.; Klára, T.; Szabó, B.T.; et al. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 126–132. [Google Scholar] [CrossRef]
- Wang, J.; Cui, L.; Ren, Y.; Zou, Y.; Ma, J.; Wang, C.; Zheng, Z.; Chen, X.-B.; Zeng, R.; Zheng, Y. In vitro and in vivo biodegradation and biocompatibility of an MMT/BSA composite coating upon magnesium alloy AZ31. J. Mater. Sci. Technol. 2020, 47, 52–67. [Google Scholar] [CrossRef]
- Yamazoe, H.; Tanabe, T. Preparation of water-insoluble albumin film possessing nonadherent surface for cells and ligand binding ability. J. Biomed. Mater. Res. Part A 2008, 86, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, H.; Uemura, T.; Tanabe, T. Facile Cell Patterning on an Albumin-Coated Surface. Langmuir 2008, 24, 8402–8404. [Google Scholar] [CrossRef]
- Wei, J.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Liu, B.; Oda, Y. Adhesion of mouse fibroblasts on hexamethyldisiloxane surfaces with wide range of wettability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81B, 66–75. [Google Scholar] [CrossRef]
- Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Surface Characterisation of Human Serum Albumin Layers on Activated Ti6Al4V. Materials 2021, 14, 7416. [Google Scholar] [CrossRef] [PubMed]
- Nugud, A.; Alghfeli, L.; Elmasry, M.; El-Serafi, I.; El-Serafi, A.T. Biomaterials as a Vital Frontier for Stem Cell-Based Tissue Regeneration. Front. Cell Dev. Biol. 2022, 10, 713934. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef]
- Mulvihill, J.N.; Faradji, A.; Oberling, F.; Cazenave, J.-P. Surface passivation by human albumin of plasmapheresis circuits reduces platelet accumulation and thrombus formation. Experimental and clinical studies. J. Biomed. Mater. Res. 1990, 24, 155–163. [Google Scholar] [CrossRef]
- Maul, T.M.; Massicotte, M.P.; Wearden, P.D. ECMO Biocompatibility: Surface Coatings, Anticoagulation, and Coagulation Monitoring. In Extracorporeal Membrane Oxygenation-Advances in Therapy; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Pereda, P.; Vilhena, J.G.; Takeuchi, N.; Serena, P.A.; Pérez, R. Albumin (BSA) adsorption onto graphite stepped surfaces. J. Chem. Phys. 2017, 146, 214704. [Google Scholar] [CrossRef]
- Sivaraman, B.; Latour, R.A. Time-Dependent Conformational Changes in Adsorbed Albumin and Its Effect on Platelet Adhesion. Langmuir 2012, 28, 2745–2752. [Google Scholar] [CrossRef]
- Kottke-Marchant, K.; Anderson, J.M.; Umemura, Y.; E Marchant, R. Effect of albumin coating on the in vitro blood compatibility of Dacron® arterial prostheses. Biomaterials 1989, 10, 147–155. [Google Scholar] [CrossRef]
- Preston, T.J.; Ratliff, T.M.; Gomez, D.; E Olshove, V.; Nicol, K.K.; Sargel, C.L.; Chicoine, L.G. Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J. Extra-Corpor. Technol. 2010, 42, 199. [Google Scholar]
- Mijiritsky, E.; Gardin, C.; Ferroni, L.; Lacza, Z.; Zavan, B. Albumin-impregnated bone granules modulate the interactions between mesenchymal stem cells and monocytes under in vitro inflammatory conditions. Mater. Sci. Eng. C 2020, 110, 110678. [Google Scholar] [CrossRef] [PubMed]
- Skaliczki, G.; Schandl, K.; Weszl, M.; Major, T.; Kovács, M.; Skaliczki, J.; Szendrői, M.; Dobó-Nagy, C.; Lacza, Z. Serum albumin enhances bone healing in a nonunion femoral defect model in rats: A computer tomography micromorphometry study. Int. Orthop. 2013, 37, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Budán, F.; Szigeti, K.; Weszl, M.; Horváth, I.; Balogh, E.; Kanaan, R.; Berényi, K.; Lacza, Z.; Máthé, D.; Gyöngyi, Z. Novel radiomics evaluation of bone formation utilizing multimodal (SPECT/X-ray CT) in vivo imaging. PLoS ONE 2018, 13, e0204423. [Google Scholar] [CrossRef]
- Weszl, M.; Skaliczki, G.; Cselenyák, A.; Kiss, L.; Major, T.; Schandl, K.; Bognar, E.; Stadler, G.; Peterbauer, A.; Csonge, L.; et al. Freeze-dried human serum albumin improves the adherence and proliferation of mesenchymal stem cells on mineralized human bone allografts. J. Orthop. Res. 2012, 30, 489–496. [Google Scholar] [CrossRef]
- Schandl, K.; Horváthy, D.B.; Doros, A.; Majzik, E.; Schwarz, C.M.; Csönge, L.; Abkarovits, G.; Bucsi, L.; Lacza, Z. Bone-Albumin filling decreases donor site morbidity and enhances bone formation after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autografts. Int. Orthop. 2016, 40, 2097–2104. [Google Scholar] [CrossRef]
- Klára, T.; Csönge, L.; Janositz, G.; Pap, K.; Lacza, Z. The use of structural proximal tibial allografts coated with human albumin in treating extensive periprosthetic knee-joint bone deficiency and averting late complications. Case Rep. Orv. Hetil. 2015, 156, 67–70. [Google Scholar] [CrossRef]
- Simonffy, L.; Minya, F.; Trimmel, B.; Lacza, Z.; Dobo-Nagy, C. Albumin-Impregnated Allograft Filling of Surgical Extraction Sockets Achieves Better Bone Remodeling Than Filling with Either Blood Clot or Bovine Xenograft. Int. J. Oral Maxillofac. Implant. 2020, 35, 297–304. [Google Scholar] [CrossRef]
- Abraham, M.-K.; Jost, E.; Hohmann, J.D.; Searle, A.K.; Bongcaron, V.; Song, Y.; Wendel, H.P.; Peter, K.; Krajewski, S.; Wang, X. A Recombinant Fusion Construct between Human Serum Albumin and NTPDase CD39 Allows Anti-Inflammatory and Anti-Thrombotic Coating of Medical Devices. Pharmaceutics 2021, 13, 1504. [Google Scholar] [CrossRef]
- An, Y.H.; Bradley, J.; Powers, D.L.; Friedman, R.J. The prevention of prosthetic infection using a cross-linked albumin coating in a rabbit model. J. Bone Jt. Surgery. Br. Vol. 1997, 79, 816–819. [Google Scholar] [CrossRef]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Kinnari, T.J.; I Peltonen, L.; Kuusela, P.; Kivilahti, J.; Könönen, M.; Jero, J. Bacterial Adherence to Titanium Surface Coated with Human Serum Albumin. Otol. Neurotol. 2005, 26, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Post, J.C. Candidate??s Thesis: Direct Evidence of Bacterial Biofilms in Otitis Media. Laryngoscope 2001, 111, 2083–2094. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, W.; Zhu, G.; Lian, J.; Wang, J.; Hui, P.; He, S.; Chen, W.; Jiang, X. Albumin Broadens the Antibacterial Capabilities of Nonantibiotic Small Molecule-Capped Gold Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 45381–45389. [Google Scholar] [CrossRef]
- Cometta, S.; Bock, N.; Suresh, S.; Dargaville, T.R.; Hutmacher, D.W. Antibacterial Albumin-Tannic Acid Coatings for Scaffold-Guided Breast Reconstruction. Front. Bioeng. Biotechnol. 2021, 9, 638577. [Google Scholar] [CrossRef] [PubMed]
- Klára, T.; Csönge, L.; Janositz, G.; Csernátony, Z.; Lacza, Z. Albumin-coated structural lyophilized bone allografts: A clinical report of 10 cases. Cell Tissue Bank 2014, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; David, A.E.; Choi, Y.-S.; Wu, Y.; Buschle-Diller, G. Scaffold materials from glycosylated and PEGylated bovine serum albumin. J. Biomed. Mater. Res. Part A 2015, 103, 2839–2846. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Ser. B Chem. 2014, 57, 490. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Doillon, C. Skin replacement using collagen extracted from bovine hide. Clin. Mater. 1992, 9, 189–193. [Google Scholar] [CrossRef]
- Dror, Y.; Ziv, T.; Makarov, V.; Wolf, H.; Admon, A.; Zussman, E. Nanofibers Made of Globular Proteins. Biomacromolecules 2008, 9, 2749–2754. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 379–386. [Google Scholar] [CrossRef]
- Fleischer, S.; Shapira, A.; Regev, O.; Nseir, N.; Zussman, E.; Dvir, T. Albumin fiber scaffolds for engineering functional cardiac tissues. Biotechnol. Bioeng. 2014, 111, 1246–1257. [Google Scholar] [CrossRef]
- Sanches, M.; D’Angelo, I.; Jaramillo, M.; Baardsnes, J.; Zwaagstra, J.; Schrag, J.; Schoenhofen, I.; Acchione, M.; Lawn, S.; Wickman, G.; et al. AlbuCORE: An albumin-based molecular scaffold for multivalent biologics design. mAbs 2020, 12, 1802188. [Google Scholar] [CrossRef]
- An, F.-F.; Zhang, X.-H. Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667. [Google Scholar] [CrossRef]
- Peppas, A.N. Hydrogels in Medicine and Pharmacy. In Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, USA, 1986; Volume 1–3. [Google Scholar]
- Gayet, J.-C.; Fortier, G. High water content BSA-PEG hydrogel for controlled release device: Evaluation of the drug release properties. J. Control Release 1996, 38, 177–184. [Google Scholar] [CrossRef]
- Oss-Ronen, L.; Seliktar, D. Polymer-conjugated albumin and fibrinogen composite hydrogels as cell scaffolds designed for affinity-based drug delivery. Acta Biomater. 2011, 7, 163–170. [Google Scholar] [CrossRef]
- Gonen-Wadmany, M.; Oss-Ronen, L.; Seliktar, D. Protein–polymer conjugates for forming photopolymerizable biomimetic hydrogels for tissue engineering. Biomaterials 2007, 28, 3876–3886. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef]
- Fu, Q.-W.; Zi, Y.-P.; Xu, W.; Zhou, R.; Cai, Z.-Y.; Zheng, W.-J.; Chen, F.; Qian, Q.-R. Electrospinning of calcium phosphate-poly(D,L-lactic acid) nanofibers for sustained release of water-soluble drug and fast mineralization. Int. J. Nanomed. 2016, 11, 5087–5097. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Haag, S.L.; Patel, J.S.; Ytreberg, F.M.; Bernards, M.T. Paired Simulations and Experimental Investigations into the Calcium-Dependent Conformation of Albumin. J. Chem. Inf. Model. 2022, 62, 1282–1293. [Google Scholar] [CrossRef]
- Haag, S.L.; Schiele, N.R.; Bernards, M.T. Enhancement and mechanisms of MC3T3-E1 osteoblast-like cell adhesion to albumin through calcium exposure. Biotechnol. Appl. Biochem. 2022, 69, 492–502. [Google Scholar] [CrossRef]
- Haag, S.L.; Martinez-Alvarez, J.; Schiele, N.R.; Bernards, M.T. Delivery of bioactive albumin from multi-functional polyampholyte hydrogels. J. Appl. Polym. Sci. 2022, 139, e52846. [Google Scholar] [CrossRef]
- Tam, R.Y.; Fuehrmann, T.; Mitrousis, N.; Shoichet, M.S. Regenerative Therapies for Central Nervous System Diseases: A Biomaterials Approach. Neuropsychopharmacology 2014, 39, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Sayad, S.; Zaminy, A. Stem cell therapy for nerve injury. World J. Stem. Cells 2017, 9, 144. [Google Scholar]
- Tsiftsoglou, A.S.; Tsamadou, A.I.; Papadopoulou, L.C. Heme as key regulator of major mammalian cellular functions: Molecular, cellular, and pharmacological aspects. Pharmacol. Ther. 2006, 111, 327–345. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Serio, A.; Amdursky, N.; Besnard, C.; Stevens, M.M. Fabrication of Hemin-Doped Serum Albumin-Based Fibrous Scaffolds for Neural Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 5305–5317. [Google Scholar] [CrossRef]
- Prasopdee, T.; Sinthuvanich, C.; Chollakup, R.; Uttayarat, P.; Smitthipong, W. The albumin/starch scaffold and its biocompatibility with living cells. Mater. Today Commun. 2021, 27, 102164. [Google Scholar] [CrossRef]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Starch-based biomaterials for wound-dressing applications. Starch–Stärke 2013, 65, 543–551. [Google Scholar] [CrossRef]
- Garcia, J.; Felix, M.; Cordobés, F.; Guerrero, A. Effect of solvent and additives on the electrospinnability of BSA solutions. Colloids Surf. B Biointerfaces 2022, 217, 112683. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, M.R.; Delfanian, S.; Aghakhani, P.; Homaeigohar, S.; Alipour, A.; Shahsavarani, H. Recent Advances in Development of Natural Cellulosic Non-Woven Scaffolds for Tissue Engineering. Polymers 2022, 14, 1531. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Caironi, P.; Gattinoni, L. The clinical use of albumin: The point of view of a specialist in intensive care. Blood Transfus. 2009, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural Basis of the Drug-binding Specificity of Human Serum Albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F. Subdomain IB Is the Third Major Drug Binding Region of Human Serum Albumin: Toward the Three-Sites Model. Mol. Pharm. 2013, 10, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef]
- Oettl, K.; E Stauber, R. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. J. Cereb. Blood Flow Metab. 2007, 151, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Marsche, G. Redox State of Human Serum Albumin in Terms of Cysteine-34 in Health and Disease. Methods Enzymol. 2010, 474, 181–195. [Google Scholar] [CrossRef]
- Uchida, K.; Stadtman, E.R. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc. Natl. Acad. Sci. USA 1992, 89, 4544–4548. [Google Scholar] [CrossRef] [Green Version]
- Negre-Salvayre, A.; Augé, N.; Ayala, V.; Basaga, H.; Boada, J.; Brenke, R.; Chapple, S.; Cohen, G.; Feher, J.; Grune, T.; et al. Pathological aspects of lipid peroxidation. Free Radic. Res. 2010, 44, 1125–1171. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.; Ahn, S.N. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int. J. Biol. Macromol. 2019, 123, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Melder, R.J.; Osborn, B.L.; Riccobene, T.; Kanakaraj, P.; Wei, P.; Chen, G.; Stolow, D.; Halpern, W.G.; Migone, T.-S.; Wang, Q.; et al. Pharmacokinetics and in vitro and in vivo anti-tumor response of an interleukin-2-human serum albumin fusion protein in mice. Cancer Immunol. Immunother. 2005, 54, 535–5475. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Karle, A.; Meißburger, B.; Höfig, I.; Stork, R.; Kontermann, R.E. Improved Pharmacokinetics of Recombinant Bispecific Antibody Molecules by Fusion to Human Serum Albumin. J. Biol. Chem. 2007, 282, 12650–12660. [Google Scholar] [CrossRef]
- Ciaccio, M. Introduction of glycated albumin in clinical practice. J. Lab. Precis. Med. 2019, 4, 28. [Google Scholar] [CrossRef]
- Mendez, C.M.; McClain, C.J.; Marsano, L.S. Albumin Therapy in Clinical Practice. Nutr. Clin. Pract. 2005, 20, 314–320. [Google Scholar] [CrossRef]
- Vincent, J.L.; Russell, J.A.; Jacob, M.; Martin, G.; Guidet, B.; Wernerman, J.; Roca, R.F.; McCluskey, S.A.; Gattinoni, L. Albumin administration in the acutely ill: What is new and where next? Crit. Care 2014, 18, 213. [Google Scholar]
- Coyle, T.; John, S.M. Evaluation of albumin use in a community hospital setting: A retrospective study looking at appropriate use and prescribing patterns. PLoS ONE 2021, 16, e0257858. [Google Scholar] [CrossRef]
- Reviewers, C.I.G.A.; Berger, A. Human albumin administration in critically ill patients: Systematic review of randomised controlled trials. Cochrane Inj. Group Albumin Rev. 1998, 317, 235–240. [Google Scholar]
- Blood Transfusion: Albumin Administration. Available online: https://www.rch.org.au/bloodtrans/blood_administration/Albumin_Administration/ (accessed on 23 August 2022).
- Bielecki, T. Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF): Surgical Adjuvants, Preparations for In Situ Regenerative Medicine and Tools for Tissue Engineering. Curr. Pharm. Biotechnol. 2012, 13, 1121–1130. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Pietrzak, W.S.; Eppley, B.L. Platelet Rich Plasma: Biology and New Technology. J. Craniofacial Surg. 2005, 16, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M. Platelet-Rich Plasma: A Review of Biology and Applications in Plastic Surgery. Plast. Reconstr. Surg. 2006, 118, 147e–159e. [Google Scholar] [CrossRef] [PubMed]
- Salemi, S.; Rinaldi, C.; Manna, F.; Guarneri, G.F.; Parodi, P.C. Reconstruction of lower leg skin ulcer with autologous adipose tissue and platelet-rich plasma. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Lindeboom, J.A.H.; Mathura, K.R.; Aartman, I.H.A.; Kroon, F.H.M.; Milstein, D.M.J.; Ince, C. Influence of the application of platelet-enriched plasma in oral mucosal wound healing. Clin. Oral. Implant. Res. 2007, 18, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Nikolidakis, D.; Jansen, J.A. The Biology of Platelet-Rich Plasma and Its Application in Oral Surgery: Literature Review. Tissue Eng. Part B Rev. 2008, 14, 249–258. [Google Scholar] [CrossRef]
- Shashikiran, N.; Reddy, V.S.; Yavagal, C.; Zakirulla, M. Applications of platelet- rich plasma (prp) in contemporary pediatric dentistry. J. Clin. Pediatr. Dent. 2006, 30, 283–286. [Google Scholar] [CrossRef]
- Wrotniak, M.; Bielecki, T.; Gaździk, T.S. Current opinion about using the platelet-rich gel in orthopaedics and trauma surgery. Ortop. Traumatol. Rehabil. 2007, 9, 227–238. [Google Scholar]
- Mishra, A.; Woodall, J., Jr.; Vieira, A. Treatment of Tendon and Muscle Using Platelet-Rich Plasma. Clin. Sports Med. 2009, 28, 113–125. [Google Scholar] [CrossRef]
- Fréchette, J.-P.; Martineau, I.; Gagnon, G. Platelet-rich Plasmas: Growth Factor Content and Roles in Wound Healing. J. Dent. Res. 2005, 84, 434–439. [Google Scholar] [CrossRef]
- Keene, D.J.; Alsousou, J. How effective are platelet rich plasma injections in treating musculoskeletal soft tissue injuries? BMJ 2016, 517, i157. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.A. Autologous platelet rich plasma for neck and lower back pain secondary to spinal disc herniation: Midterm results. Arch. Med. 2017, 3, 10. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Roffi, A.; Di Matteo, B.; Merli, M.L.; Marcacci, M. Platelet-rich plasma: Why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2459–2474. [Google Scholar] [CrossRef] [Green Version]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc. Med. 2016, 2, e000060. [Google Scholar] [CrossRef] [PubMed]
- Meijer, H.; Reinecke, J.; Becker, C.; Tholen, G.; Wehling, P. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Agents Actions 2003, 52, 404–407. [Google Scholar] [CrossRef]
- Kardos, D.; Marschall, B.; Simon, M.; Hornyák, I.; Hinsenkamp, A.; Kuten, O.; Gyevnár, Z.; Erdélyi, G.; Bárdos, T.; Paukovits, T.M.; et al. Investigation of Cytokine Changes in Osteoarthritic Knee Joint Tissues in Response to Hyperacute Serum Treatment. Cells 2019, 8, 824. [Google Scholar] [CrossRef]
- Kardos, D.; Simon, M.; Vácz, G.; Hinsenkamp, A.; Holczer, T.; Cseh, D.; Sárközi, A.; Szenthe, K.; Bánáti, F.; Szathmary, S.; et al. The Composition of Hyperacute Serum and Platelet-Rich Plasma Is Markedly Different despite the Similar Production Method. Int. J. Mol. Sci. 2019, 20, 721. [Google Scholar] [CrossRef]
- Neubauer, M.; Kuten, O.; Stotter, C.; Kramer, K.; De Luna, A.; Muellner, T.; Lacza, Z.; Nehrer, S. The Effect of Blood-Derived Products on the Chondrogenic and Osteogenic Differentiation Potential of Adipose-Derived Mesenchymal Stem Cells Originated from Three Different Locations. Stem Cells Int. 2019, 2019, 1358267. [Google Scholar] [CrossRef]
- Kuten, O.; Simon, M.; Hornyák, I.; De Luna-Preitschopf, A.; Nehrer, S.; Lacza, Z. The Effects of Hyperacute Serum on Adipogenesis and Cell Proliferation of Mesenchymal Stromal Cells. Tissue Eng. Part A 2018, 24, 1011–1021. [Google Scholar] [CrossRef]
- Simon, M.; Major, B.; Vácz, G.; Kuten, O.; Hornyak, I.; Hinsenkamp, A.; Kardos, D.; Bagó, M.; Cseh, D.; Sarkozi, A.; et al. The Effects of Hyperacute Serum on the Elements of the Human Subchondral Bone Marrow Niche. Stem Cells Int. 2018, 2018, 4854619. [Google Scholar] [CrossRef]
- Calvo, I.O.; Kuten-Pella, O.; Kramer, K.; Madár, Á.; Takács, S.; Kardos, D.; Simon, D.; Erdö-Bonyár, S.; Berki, T.; De Luna, A.; et al. Optimization of Lyophilized Hyperacute Serum (HAS) as a Regenerative Therapeutic in Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 7496. [Google Scholar] [CrossRef]
- Calvo, I.O.; Fodor, E.; Kardos, D.; Hornyák, I.; Hinsenkamp, A.; Kuten-Pella, O.; Gyevnar, Z.; Erdelyi, G.; Bardos, T.; Paukovits, T.M.; et al. A Pilot Clinical Study of Hyperacute Serum Treatment in Osteoarthritic Knee Joint: Cytokine Changes and Clinical Effects. Curr Issues Mol. Biol. 2021, 43, 637–649. [Google Scholar] [CrossRef]
- Kuten-Pella, O.; De Luna, A.; Kramer, K.; Neubauer, M.; Nehrer, S.; Lacza, Z. Regenerative Potential of Blood-Derived Products in 3D Osteoarthritic Chondrocyte Culture System. Curr. Issues Mol. Biol. 2021, 43, 665–675. [Google Scholar] [CrossRef]
- Gallego, L.; Junquera, L.; Meana, A.; García, E.; García, V. Three-dimensional culture of mandibular human osteoblasts on a novel albumin scaffold: Growth, proliferation, and differentiation potential in vitro. Int. J. Oral Maxillofac. Implant. 2010, 25, 699–705. [Google Scholar]
- Gallego, L.; Junquera, L.; García, E.; García, V.; Álvarez-Viejo, M.; Costilla, S.; Fresno, M.F.; Meana, A. Repair of Rat Mandibular Bone Defects by Alveolar Osteoblasts in a Novel Plasma-Derived Albumin Scaffold. Tissue Eng. Part A 2010, 16, 1179–1187. [Google Scholar] [CrossRef]
- Gallego, L.; Pérez-Basterrechea, M.; García-Consuegra, L.; Álvarez-Viejo, M.; Megías, J.; Novoa, A.; Costilla, S.; Meana, A.; Junquera, L. Repair of segmental mandibular bone defects in sheep using bone marrow stromal cells and autologous serum scaffold: A pilot study. J. Clin. Periodontol. 2015, 42, 1143–1151. [Google Scholar] [CrossRef]
- Gallego, L.; Junquera, L.; Meana, Á.; Álvarez-Viejo, M.; Fresno, M. Ectopic bone formation from mandibular osteoblasts cultured in a novel human serum-derived albumin scaffold. J Biomater Appl. 2010, 25, 367–381. [Google Scholar] [CrossRef]
- Bar-Or, D.; Thomas, G.W.; Rael, L.T.; Gersch, E.D.; Rubinstein, P.; Brody, E. Low Molecular Weight Fraction of Commercial Human Serum Albumin Induces Morphologic and Transcriptional Changes of Bone Marrow-Derived Mesenchymal Stem Cells. Stem. Cells Transl. Med. 2015, 4, 945–955. [Google Scholar] [CrossRef]
- Frederick, E.D.; Hausburg, M.A.; Thomas, G.W.; Rael, L.T.; Brody, E.; Bar-Or, D. The low molecular weight fraction of human serum albumin upregulates COX2, prostaglandin E2, and prostaglandin D2 under inflammatory conditions in osteoarthritic knee synovial fibroblasts. Biochem. Biophys. Rep. 2016, 8, 68–74. [Google Scholar] [CrossRef]
- Salottolo, K.; Cole, B.; Bar-Or, D. Intra-articular injection of the anti-inflammatory compound LMWF-5A in adults with severe osteoarthritis: A double-blind prospective randomized controlled multi-center safety and efficacy trial. Patient Saf. Surg. 2018, 12, 11. [Google Scholar] [CrossRef]
- Bar-Or, D.; Thomas, G.; Rael, L.T.; Frederick, E.; Hausburg, M.; Bar-Or, R.; Brody, E. On the Mechanisms of Action of the Low Molecular Weight Fraction of Commercial Human Serum Albumin in Osteoarthritis. Curr. Rheumatol. Rev. 2019, 15, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Salottolo, K.M.; Loose, H.; Phillips, M.J.; McGrath, B.; Wei, N.; Borders, J.L.; Ervin, J.E.; Kivitz, A.; Hermann, M.; et al. A Randomized Clinical Trial to Evaluate Two Doses of an Intra-Articular Injection of LMWF-5A in Adults with Pain Due to Osteoarthritis of the Knee. PLoS ONE 2014, 9, e87910. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.; McGrath, B.; Salottolo, K.; Bar-Or, D. LMWF-5A for the Treatment of Severe Osteoarthritis of the Knee: Integrated Analysis of Safety and Efficacy. Orthopedics 2018, 41, e77–e83. [Google Scholar] [CrossRef]
- Schwappach, J.; Schultz, J.; Salottolo, K.; Bar-Or, D. Incidence of total knee replacement subsequent to intra-articular injection of the anti-inflammatory compound LMWF-5A versus saline: A long-term follow-up study to a randomized controlled trial. Patient Saf. Surg. 2018, 12, 14. [Google Scholar] [CrossRef]
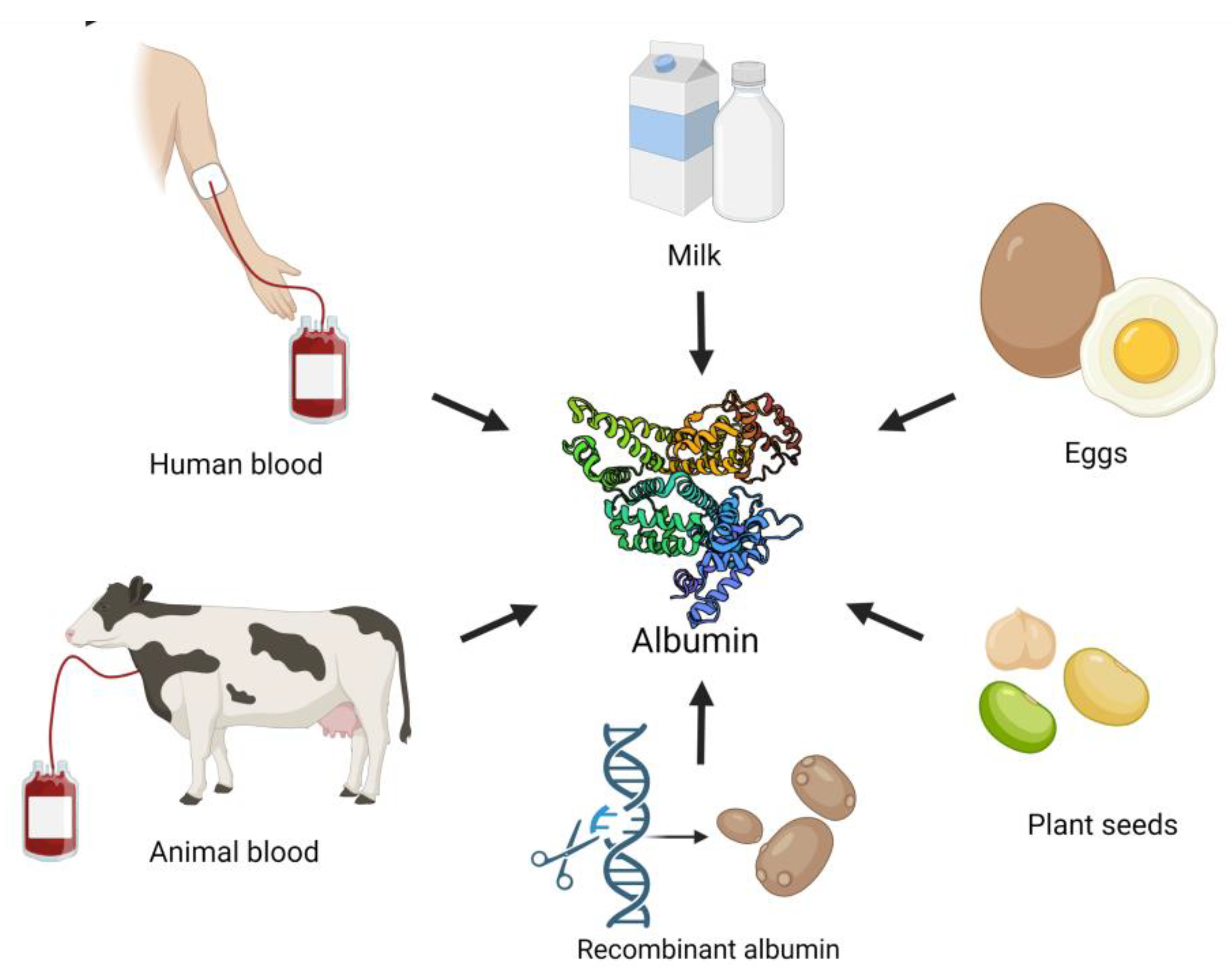

| Albumin Type | Molecular Weight | Structure | Main Characteristic | Production Method | Advantages | Limitations |
|---|---|---|---|---|---|---|
| Human serum albumin (HSA) | 66, 5–69 kDa 585 amino acids [4] |
|
|
|
| |
| Bovine serum albumin (BSA) | 67 kDa 583 amino acids [4] |
|
|
|
|
|
| Ovalbumin (OVA) | 47 kDa 385 amino acids [5] |
|
|
|
| Allergen [14] |
| Lactalbumin | 14 kDa 122–123 amino acids [15] |
|
|
|
| Allergen [16] |
| Plant albumin | 8–16 kDa [19,20] depending on the plant source |
|
|
| Allergen [23] | |
| Recombinant albumin | Structurally equivalent to HSA [24] | Structurally equivalent to HSA [24] |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuten Pella, O.; Hornyák, I.; Horváthy, D.; Fodor, E.; Nehrer, S.; Lacza, Z. Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 10557. https://doi.org/10.3390/ijms231810557
Kuten Pella O, Hornyák I, Horváthy D, Fodor E, Nehrer S, Lacza Z. Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine. International Journal of Molecular Sciences. 2022; 23(18):10557. https://doi.org/10.3390/ijms231810557
Chicago/Turabian StyleKuten Pella, Olga, István Hornyák, Dénes Horváthy, Eszter Fodor, Stefan Nehrer, and Zsombor Lacza. 2022. "Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine" International Journal of Molecular Sciences 23, no. 18: 10557. https://doi.org/10.3390/ijms231810557
APA StyleKuten Pella, O., Hornyák, I., Horváthy, D., Fodor, E., Nehrer, S., & Lacza, Z. (2022). Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine. International Journal of Molecular Sciences, 23(18), 10557. https://doi.org/10.3390/ijms231810557







