Reactive Nitrogen Species and Male Reproduction: Physiological and Pathological Aspects
Abstract
:1. Introduction
2. Mechanism of Formation of RNS
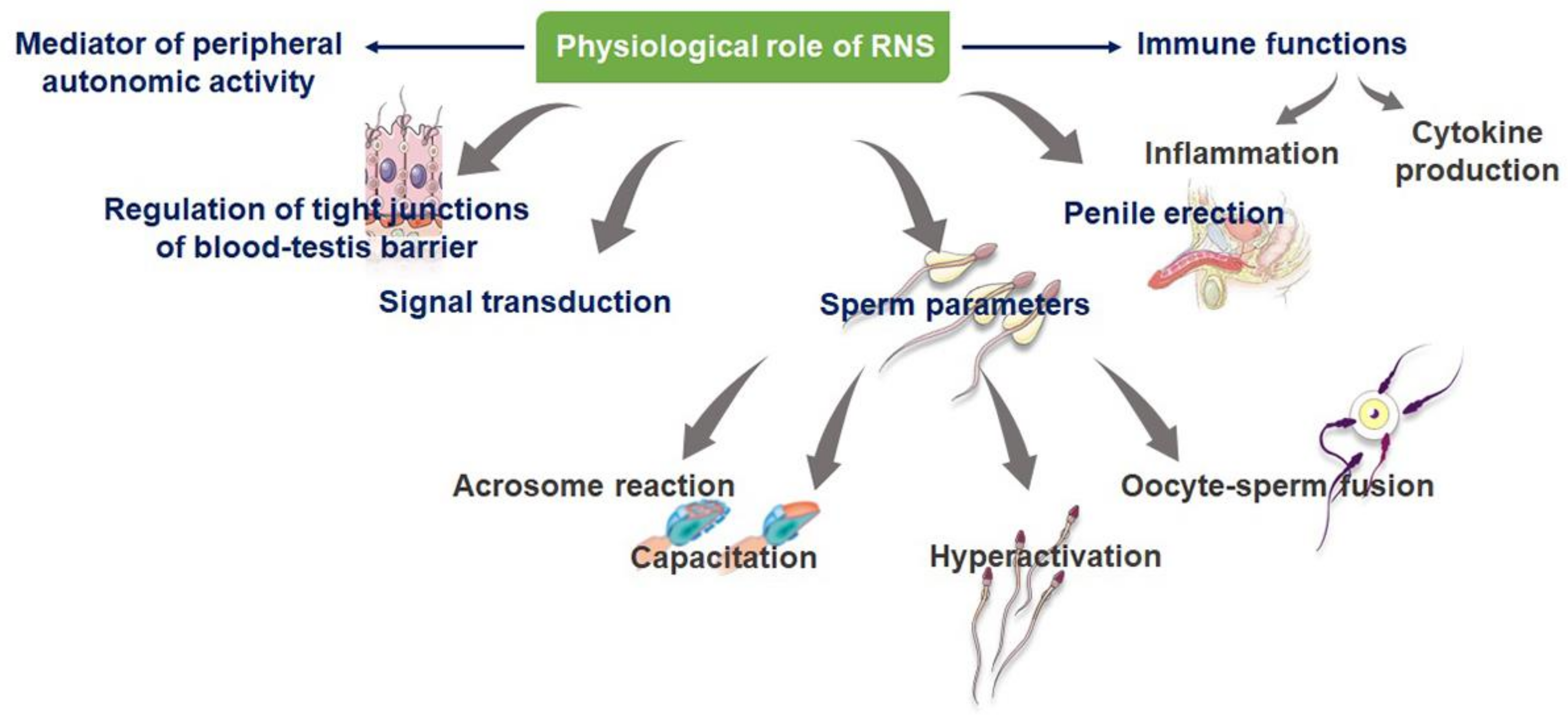
3. Physiological Significance of RNS
3.1. RNS as Cell Signal Transducers
3.2. RNS in the Formation of Blood–Testes Barrier (BTB)
3.3. RNS as Male Reproductive Immune Modulators
3.4. NO and Sperm Parameters
3.5. NO and Sperm Genetics
4. Pathological Effects of RNS
4.1. NO and Induction of Apoptosis in Testicular Cells
4.2. NO and Steroidogenesis
4.3. NO-Mediated Sperm Lipid Peroxidation
4.4. NO and Impaired Sperm Function
4.5. RNS and Leukocytospermia
4.6. Varicocele
4.7. Erectile Dysfunction (ED)
4.8. Diabetes Mellitus (DM)
4.9. Strategies to Measure RNS
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pryor, W.A.; Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. Lung Cell Mol. Physiol. 1995, 268, 699–722. [Google Scholar] [CrossRef]
- Ramya, T.; Misro, M.M.; Sinha, D.; Nandan, D.; Mithal, S. Altered levels of seminal nitric oxide, nitric oxide synthase, and enzymatic antioxidants and their association with sperm function in infertile subjects. Int. J. Fertil. Steril. 2011, 95, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Jourd’heuil, D.; Jourd’heuil, F.L.; Kutchukian, P.S.; Musah, R.A.; Wink, D.A.; Grisham, M.B. Reaction of Superoxide and Nitric Oxide with Peroxynitrite. Implications for Peroxynitrite-mediated oxidation reactions in vivo. J. Biol. Chem. 2001, 276, 28799–28805. [Google Scholar] [CrossRef] [PubMed]
- Eshraghian, A.; Taghavi, S.A. Systematic review: Endocrine abnormalities in patients with liver cirrhosis. Arch. Iran 2014, 17, 713–721. [Google Scholar]
- Baker, H.G. Reproductive effects of nontesticular illness. Endocrinol. Metab. Clin. N. Am. 1999, 27, 831–850. [Google Scholar] [CrossRef]
- Sabanegh, E.S., Jr.; Ragheb, A.M. Male fertility after cancer. Urol 2009, 73, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sengupta, P. Oxidative stress and its association with male infertility. In Male Infertility; Parekattil, S.J., Esteves, S.C., Agarwal, A., Eds.; Springer: Cham, Switzerland, 2020; pp. 57–68. [Google Scholar]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Perez-Lebena, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Baker, M.A.; Aitken, R.J. Reactive oxygen species in spermatozoa: Methods for monitoring and significance for the origins of genetic disease and infertility. Reprod. Biol. Endocrinol. 2005, 3, 67. [Google Scholar] [CrossRef]
- Cornwell, T.L.; Arnold, E.L.; Boerth, N.J.; Lincoln, T.M. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am. J. Physiol. Cell Physiol. 1994, 267, 1405–1413. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, R.L. Possible role of nitric oxide on fertile and asthenozoospermic infertile human sperm functions. Free Radic. Res. 1996, 25, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.P.; Cheng, C.Y. Nitric oxide/nitric oxide synthase, spermatogenesis, and tight junction dynamics. Biol. Reprod. 2004, 70, 267–276. [Google Scholar] [CrossRef]
- Rosselli, M.; Keller, R.J.; Dubey, R.K. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum. Reprod. Update 1998, 4, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Homa, S.T.; Vessey, W.; Perez-Miranda, A.; Riyait, T.; Agarwal, A. Reactive oxygen species (ROS) in human semen: Determination of a reference range. J. Assist. Reprod. Genet. 2015, 32, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Sezer, C.; Koksal, I.T.; Usta, M.F.; Gulkesen, K.H.; Erdogru, T.; Ciftcioglu, A.; Baykara, M. Relationship between mast cell and iNOS expression in testicular tissue associated with infertility. Arch. Androl. 2005, 51, 149–158. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic. Biol. Med. 1995, 18, 487–495. [Google Scholar] [CrossRef]
- Lee, N.P.; Cheng, C.Y. Nitric oxide and cyclic nucleotides: Their roles in junction dynamics and spermatogenesis. Adv. Exp. Med. Biol. 2008, 636, 172–185. [Google Scholar]
- Turker, K.I.; Erdogru, T.; Gülkesen, H.; Sezer, C.; Usta, M.; Ciftçioglu, A. The potential role of inducible nitric oxide synthase (iNOS) activity in the testicular dysfunction associated with varicocele: An experimental study. Int. Urol. Nephrol. 2004, 36, 67–72. [Google Scholar]
- O’Bryan, M.K.; Zini, A.; Cheng, C.Y.; Schlegel, P.N. Human sperm endothelial nitric oxide synthase expression: Correlation with sperm motility. Fertil Steril. 1998, 70, 1146–1147. [Google Scholar] [CrossRef]
- Bolanos, J.P.; Delgado-Esteban, M.; Herrero-Mendez, A.; Fernandez-Fernandez, S.; Almeida, A. Regulation of glycolysis and pentose–phosphate pathway by nitric oxide: Impact on neuronal survival. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Nobunaga, T.; Tokugawa, Y.; Hashimoto, K.; Kubota, Y.; Sawai, K.; Kimura, T.; Shimoya, K.; Takemura, M.; Matsuzaki, N.; Azuma, C.; et al. Elevated nitric oxide concentration in the seminal plasma of infertile males: Nitric oxide inhibits sperm motility. Am. J. Reprod. Immunol. 1996, 36, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.J.; East, S.J.; Barratt, C.L.; Bolton, A.E.; Cooke, I.D. Preliminary communication: Possible role of reactive nitrogen intermediates in leucocyte-mediated sperm dysfunction. Am. J. Reprod. Immunol. 1992, 27, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Archer, S. Measurement of nitric oxide in biological models. FASEB J. 1993, 7, 349–360. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, S.S.; Cabler, S.; McAlister, D.A.; Sabanegh, E.; Agarwal, A. The effect of obesity on sperm disorders and male infertility. Nat. Rev. Urol. 2010, 7, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress and male infertility. Indian J. Med. Res. 2009, 129, 357–368. [Google Scholar]
- Koppenol, W.H.; Moreno, J.J.; Pryor, W.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol. 1992, 5, 834–842. [Google Scholar] [CrossRef]
- Hall, C.N.; Garthwaite, J. What is the real physiological NO concentration in vivo? Nitric Oxide 2009, 21, 92–103. [Google Scholar] [CrossRef]
- Herrero, M.B.; de Lamirande, E.; Gagnon, C. Nitric oxide regulates human sperm capacitation and protein-tyrosine phosphorylation in vitro. Biol. Reprod. 1999, 61, 575–581. [Google Scholar] [CrossRef]
- Otasevic, V.; Korac, A.; Vucetic, M.; Macanovic, B.; vGaralejic, E.; Ivanovic-Burmazovic, I.; Filipovic, M.R.; Buzadzic, B.; Stancic, A.; Jankovic, A.; et al. Is manganese (II) pentaazamacrocyclic superoxide dismutase mimic beneficial for human sperm mitochondria function and motility? Antioxid. Redox. Signal 2013, 18, 170–178. [Google Scholar] [CrossRef]
- de Lamirande, E.; Lamothe, G.; Villemure, M. Control of superoxide and nitric oxide formation during human sperm capacitation. Free Radic. Biol. Med. 2009, 46, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Lamirande, E.D.; Gagnon, C. Nitric oxide is a signaling molecule in spermatozoa. Curr. Pharm. Des. 2003, 9, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Kothari, S.; Thompson, A.; Agarwal, A.; du Plessis, S.S. Free radicals: Their beneficial and detrimental effects on sperm function. Indian J. Exp. Biol. 2010, 48, 425–435. [Google Scholar]
- Vignini, A.; Nanetti, L.; Buldreghini, E.; Moroni, C.; Ricciardo-Lamonica, G.; Mantero, F.; Boscaro, M.; Mazzanti, L.; Balercia, G. The production of peroxynitrite by human spermatozoa may affect sperm motility through the formation of protein nitrotyrosine. Fertil. Steril. 2006, 85, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Cassina, A.; Silveira, P.; Cantu, L.; Montes, J.M.; Radi, R.; Sapiro, R. Defective human sperm cells are associated with mitochondrial dysfunction and oxidant production. Biol. Reprod. 2015, 93, 119. [Google Scholar] [CrossRef]
- Herrero, M.B.; de Lamirande, E.; Gagnon, C. Tyrosine nitration in human spermatozoa: A physiological function of peroxynitrite, the reaction product of nitric oxide and superoxide. Mol. Hum. Reprod. 2001, 7, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Beconi, M.T. Peroxynitrite participates in mechanisms involved in capacitation of cryopreserved cattle. Anim. Reprod. Sci. 2009, 110, 96–107. [Google Scholar] [CrossRef]
- Rosselli, M.; Dubey, R.K.; Imthurn, B.; Macas, E.; Keller, P.J. Andrology: Effects of nitric oxide on human spermatozoa: Evidence that nitric oxide decreases sperm motility and induces sperm toxicity. Hum. Reprod. 1995, 10, 1786–1790. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int.J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Meineke, V.; Frungieri, M.B.; Jessberger, B.; Vogt, H.J.; Mayerhofer, A. Human testicular mast cells contain tryptase: Increased mast cell number and altered distribution in the testes of infertile men. Fertil. Steril. 2000, 74, 239–246. [Google Scholar] [CrossRef]
- Tarpey, M.M.; Beckman, J.S.; Ischiropoulos, H.; Gore, J.Z.; Brock, T.A. Peroxynitrite stimulates vascular smooth muscle cell cyclic GMP synthesis. FEBS Lett. 1995, 364, 314–318. [Google Scholar] [CrossRef]
- Schuppe, H.C.; Meinhardt, A.; Allam, J.P.; Bergmann, M.; Weidner, W.; Haidl, G. Chronic orchitis: a neglected cause of male infertility? Andrologia 2008, 40, 84–91. [Google Scholar]
- Eduardo, R.S.; Eustache, F. Male reproductive physiology. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Elsevier: Waltham, MA, USA, 2018; Volume 1, pp. 408–416. [Google Scholar]
- Dutta, S.; Sengupta, P.; Chhikara, B.S. Reproductive inflammatory mediators and male infertility. Chem. Biol. Lett. 2020, 7, 73–74. [Google Scholar]
- Irez, T.; Bicer, S.; Sahin, E.; Dutta, S.; Sengupta, P. Cytokines and adipokines in the regulation of spermatogenesis and semen quality. Chem. Biol. Lett. 2020, 7, 131–139. [Google Scholar]
- Semenzato, G. Tumour necrosis factor: A cytokine with multiple biological activities. Br. J. Cancer 1990, 61, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Villalobo, A. Nitric oxide and cell proliferation. FEBS J. 2006, 273, 2329–2346. [Google Scholar] [CrossRef]
- Ferreiro, M.E.; Amarilla, M.S.; Glienke, L.; Méndez, C.S.; González, C.; Jacobo, P.V.; Sobarzo, C.M.; De Laurentiis, A.; Ferraris, M.J.; Theas, M.S. The inflammatory mediators TNFα and nitric oxide arrest spermatogonia GC-1 cell cycle. Reprod. Biol. 2019, 19, 329–339. [Google Scholar] [CrossRef]
- Lue, Y.; Sinha Hikim, A.P.; Wang, C.; Leung, A.; Swerdloff, R.S. Functional role of inducible nitric oxide synthase in the induction of male germ cell apoptosis, regulation of sperm number, and determination of testes size: Evidence from null mutant mice. Endocrinology 2003, 146, 3092–3400. [Google Scholar] [CrossRef] [Green Version]
- Auharek, S.A.; Lara, N.L.; Avelar, G.F.; Sharpe, R.M.; França, L.R. Effects of inducible nitric oxide synthase (iNOS) deficiency in mice on Sertoli cell proliferation and perinatal testis development. Int. J. Androl. 2012, 35, 741–751. [Google Scholar] [CrossRef]
- Sikka, S.C. Relative impact of oxidative stress on male reproductive function. Curr. Med. Chem. 2001, 8, 851–862. [Google Scholar] [CrossRef]
- Mack, SR.; Han, HL.; de Jonge, CJ.; Anderson, RA.; Zaneveld, LJ. The human sperm acrosome reaction does not depend on arachidonic acid metabolism via the cyclooxygenase and lipoxygenase pathways. J. Androl. 1992, 13, 551–559. [Google Scholar] [PubMed]
- Yeoman, RR.; Jones, WD.; Rizk, BM. Evidence for nitric oxide regulation of hamster sperm hyperactivation. J.Androl. 1998, 19, 19,58–64. [Google Scholar]
- Miraglia, E.; Rullo, M.L.; Bosia, A.; Massobrio, M.; Revelli, A.; Ghigo, D. Stimulation of the nitric oxide/cyclic guanosine monophosphate signaling pathway elicits human sperm chemotaxis in vitro. Fertil. Steril. 2007, 87, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Morielli, T.; O’Flaherty, C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015, 149, 113–123. [Google Scholar] [CrossRef]
- Donnelly, E.T.; Lewis, S.E.; Thompson, W.; Chakravarthy, U. Sperm nitric oxide and motility: The effects of nitric oxide synthase stimulation and inhibition. Mol. Hum. Reprod. 1997, 3, 755–762. [Google Scholar] [CrossRef]
- Lamirande, E.D.; Gagnon, C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int. J.Androl. 1993, 6, 21–25. [Google Scholar] [CrossRef]
- O’flaherty, C.; Beconi, M.; Beorlegui, N. Effect of natural antioxidants, superoxide dismutase and hydrogen peroxide on capacitation of frozen-thawed bull spermatozoa. Andrologia 1997, 29, 269–275. [Google Scholar] [CrossRef]
- Balercia, G.; Moretti, S.; Vignini, A.; Magagnini, M.; Mantero, F.; Boscaro, M.; Ricciardo-Lamonica, G.; Mazzanti, L. Role of nitric oxide concentrations on human sperm motility. J. Androl. 2004, 25, 246–249. [Google Scholar] [CrossRef]
- Goud, P.T.; Goud, A.P.; Joshi, N.; Puscheck, E.; Diamond, M.P.; Abu-Soud, H.M. Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis. Fertil. Steril. 2014, 102, 151–159. [Google Scholar] [CrossRef]
- Bisht, S.; Dada, R.J. Oxidative stress: Major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front. Biosci. (Schol Ed.) 2017, 9, 420–467. [Google Scholar]
- Blesbois, E.; Lessire, M.; Grasseau, I.; Hallouis, J.M.; Hermier, D. Effect of dietary fat on the fatty acid composition and fertilizing ability of fowl semen. Biol. Reprod. 1997, 56, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.B.; Dun, M.D.; Smith, N.D.; Curry, B.J.; Connaughton, H.S.; Aitken, R.J. The presence of a truncated base excision repair pathway in human spermatozoa that is mediated by OGG1. J. Cell Sci. 2013, 126, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; De Iuliis, G.N.; McLachlan, R.I. Biological and clinical significance of DNA damage in the male germ line. Int. J. Androl. 2009, 32, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Hajimohammad, M.; Stalf, T.; Hoogendijk, C.; Mehnert, C.; Menkveld, R.; Gips, H.; Schill, W.B.; Kruger, T.F. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil. Steril. 2004, 81, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Hud, N.V.; Allen, M.J.; Downing, K.H.; Lee, J.; Balhorn, R. Identification of the elemental packing unit of DNA in mammalian sperm cells by atomic force microscopy. Biochem. Biophys. Res. Commun. 1993, 193, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Otasevic, V.; Stancic, A.; Korac, A.; Jankovic, A.; Korac, B. Reactive oxygen, nitrogen, and sulfur species in human male fertility. A crossroad of cellular signaling and pathology. Biofactors 2020, 46, 206–219. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A.; O’Bryan, M. Shedding light on chemiluminescence: The application of chemiluminescence in diagnostic andrology. J. Androl. 2004, 25, 465. [Google Scholar] [CrossRef]
- Ito, N.; Ruegg, U.T.; Kudo, A.; Miyagoe-Suzuki, Y.; Takeda, S.I. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat. Med. 2013, 19, 101–106. [Google Scholar] [CrossRef]
- Lenzi, A.; Picardo, M.; Gandini, L.; Dondero, F. Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update 1996, 2, 246–256. [Google Scholar] [CrossRef]
- Lenzi, A.; Gandini, L.; Picardo, M.; Tramer, F.; Sandri, G.; Panfili, E. Lipoperoxidation damage of spermatozoa polyunsaturated fatty acids (PUFA): Scavenger mechanisms and possible scavenger therapies. Front. Biosci. 2000, 5, E1–E5. [Google Scholar]
- Bain, J. Testosterone and the aging male: To treat or not to treat? Maturitas 2010, 66, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Semenova, A.V.; Tomilova, I.K.; Panikratov, K.D.; Kadykova, E.L.; Basharin, A.V. The role of nitric oxide in fertility disorders in men. Urologiia 2005, 6, 31–36. [Google Scholar]
- Kruger, T.F.; Menkveld, R.; Stander, F.S.; Lombard, C.J.; Van der Merwe, J.P.; van Zyl, J.A.; Smith, K. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil. Steril. 1986, 46, 1118–1123. [Google Scholar] [CrossRef]
- Burnett, A.L.; Ricker, D.D.; Chamness, S.L.; Maguire, M.P.; Crone, J.K.; Bredt, D.S.; Snyder, S.H.; Chang, T.S. Localization of nitric oxide synthase in the reproductive organs of the male rat. Biol. Reprod. 1995, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.; Jones, J.; Khorram, O. Human seminal plasma nitric oxide: Correlation with sperm morphology and testosterone. Med. Sci. Monit. 2006, 12, CR103–CR106. [Google Scholar]
- Theam, O.C.; Dutta, S.; Sengupta, P. Role of leucocytes in reproductive tract infections and male infertility. Chem. Biol. Lett. 2020, 7, 124–130. [Google Scholar]
- Santoro, G.; Romeo, C.; Impellizzeri, P.; Ientile, R.; Cutroneo, G.; Trimarchi, F.; Pedale, S.; Turiaco, N.; Gentile, C. Nitric oxide synthase patterns in normal and varicocele testis in adolescents. BJU Int. 2001, 88, 967–973. [Google Scholar] [CrossRef]
- Shiraishi, K.; Naito, K. Nitric oxide produced in the testis is involved in dilatation of the internal spermatic vein that compromises spermatogenesis in infertile men with varicocele. BJU Int. 2007, 99, 1086–1090. [Google Scholar] [CrossRef]
- Mitropoulos, D.; Deliconstantinos, G.; Zervas, A.; Villiotou, V.; Dimopoulos, C.; Stavrides, J. Nitric oxide synthase and xanthine oxidase activities in the spermatic vein of patients with varicocele: A potential role for nitric oxide and peroxynitrite in sperm dysfunction. J. Urol. 1996, 156, 1952–1958. [Google Scholar] [CrossRef]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef]
- Agbaje, I.; Rogers, D.A.; McVicar, C.M.; McClure, N.; Atkinson, A.B.; Mallidis, C.; Lewis, S.E. Insulin dependant diabetes mellitus: Implications for male reproductive function. Hum. Reprod. 2007, 22, 1871–1877. [Google Scholar] [CrossRef]
- Dinulovic, D.; Radonjic, G. Diabetes mellitus/male infertility. Arch. Androl. 1990, 25, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prabakaran, S.A. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian J. Exp. Biol. 2005, 46, 963–974. [Google Scholar]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in male infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M. Oxidative stress, DNA damage and apoptosis in male infertility: A clinical approach. BJU Int. 2005, 95, 503–507. [Google Scholar] [CrossRef]
- Buettner, G.R. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance. Oxid. Med. Cell. Longev. 2012, 2012, 973927. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. A quantitative method to monitor reactive oxygen species production by electron paramagnetic resonance in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2014, 2014, 306179. [Google Scholar] [CrossRef]
- Shekarriz, M.; Thomas, A.J., Jr.; Agarwal, A. Incidence and level of seminal reactive oxygen species in normal men. Urology 1995, 46, 103–107. [Google Scholar] [CrossRef]
- Sharma, R.K.; Pasqualotto, A.E.; Nelson, D.R.; Thomas, A.J., Jr.; Agarwal, A. Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J. Androl. 2001, 22, 575–583. [Google Scholar]
- Gil-Guzman, E.; Ollero, M.; Lopez, M.C.; Sharma, R.K.; Alvarez, J.G.; Thomas, A.J., Jr.; Agarwal, A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum. Reprod. 2001, 16, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods Enzymol. 2002, 348, 93–112. [Google Scholar]
- Chirico, S.; Smith, C.; Marchant, C.; Mitchinson, M.J.; Halliwell, B. Lipid peroxidation in hyperlipidaemic patients. A study of plasma using an HPLC-based thiobarbituric acid test. Free Radic. Res. Commun. 1993, 19, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Grotto, D.; Santa Maria, L.D.; Boeira, S.; Valentini, J.; Charao, M.F.; Moro, A.M.; Nascimento, P.C.; Pomblum, V.J.; Garcia, S.C. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J. Pharm. Biomed. Anal. 2007, 46, 619–624. [Google Scholar] [CrossRef]
- Shapiro, H.M. Redox balance in the body: An approach to quantitation. J. Surg. Res. 1972, 13, 138–152. [Google Scholar] [CrossRef]
- Rael, L.T.; Bar-Or, R.; Aumann, R.M.; Slone, D.S.; Mains, C.W.; Bar-Or, D. Oxidation-reduction potential and paraoxonase-arylesterase activity in trauma patients. Biochem. Biophys. Res. Commun. 2007, 361, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Roychoudhury, S.; Sharma, R.; Gupta, S.; Majzoub, A.; Sabanegh, E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: Clinical utility in male factor infertility. Reprod. Biomed. Online 2017, 34, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Sharma, R.; Roychoudhury, S.; du Plessis, S.; Sabanegh, E. MiOXSYS: A novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil. Steril. 2016, 106, 566–573. [Google Scholar] [CrossRef]
- Agarwal, A.; Panner Selvam, M.K.; Arafa, M.; Okada, H.; Homa, S.; Killeen, A.; Balaban, B.; Saleh, R.; Armagan, A.; Roychoudhury, S.; et al. Multi-center evaluation of oxidation-reduction potential by the MiOXSYS in males with abnormal semen. Asian J. Androl. 2019, 21, 565–569. [Google Scholar] [CrossRef]
- Deepinder, F.; Agarwal, A. Determination of seminal oxidants (reactive oxygen species). In Infertility in the Male, 4th ed.; Lipshultz, L.I., Howards, S., Neiderberger, C.S., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 618–632. [Google Scholar]
- Agarwal, A.; Cocuzza, M.; Abdelrazik, H.; Sharma, R.K. Oxidative stress measurement in patients with male or female factor infertility. In Handbook of Chemiluminescent Methods in Oxidative Stress Assessment; Popov, I., Lewin, G., Eds.; Transworld Research Network: Trivandrum, India, 2008; pp. 195–218. [Google Scholar]
- Kobayashi, H.; Gil-Guzman, E.; Mahran, A.M.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J., Jr.; Agarwal, A. Quality control of reactive oxygen species measurement by luminol-dependent chemiluminescence assay. J. Androl. 2001, 22, 568–574. [Google Scholar]
- Agarwal, A.; Allamaneni, S.S.R.; Said, T.M. Chemiluminescence technique for measuring reactive oxygen species. Reprod. Biomed. Online 2004, 9, 466–468. [Google Scholar] [CrossRef]
- Benjamin, D.; Sharma, R.K.; Moazzam, A.; Agarwal, A. Methods for the detection of ROS in human sperm samples. In Studies on Men’s Health and Fertility. Oxidative Stress in Applied Basic Research and Clinical Practice; Agarwal, A., Aitken, R., Alvarez, J., Eds.; Humana Press: New York, NY, USA, 2012; pp. 257–274. [Google Scholar]
- Agarwal, A.; Ahmad, G.; Sharma, R. Reference values of reactive oxygen species in seminal ejaculates using chemiluminescence assay. J. Assist. Reprod. Genet. 2015, 32, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Buckingham, D.W.; West, K.M. Reactive oxygen species and human spermatozoa: Analysis of the cellular mechanisms involved in luminol- and lucigenin-dependent chemiluminescence. J. Cell. Physiol. 1992, 151, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Kashou, A.H.; Sharma, R.; Agarwal, A. Assessment of oxidative stress in sperm and semen. Methods Mol. Biol. 2013, 927, 351–361. [Google Scholar] [PubMed]
- Guthrie, H.D.; Welch, G.R. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J. Anim. Sci. 2006, 84, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, G.N.; Wingate, J.K.; Koppers, A.J.; McLaughlin, E.A.; Aitken, R.J. Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J. Clin. Endocrinol. Metab. 2006, 91, 1968–1975. [Google Scholar] [CrossRef]
- Mahfouz, R.; Sharma, R.; Lackner, J.; Aziz, N.; Agarwal, A. Evaluation of chemiluminescence and flow cytometry as tools in assessing production of hydrogen peroxide and superoxide anion in human spermatozoa. Fertil. Steril. 2009, 92, 819–827. [Google Scholar] [CrossRef]
- Halliwell, B.; Chirico, S. Lipid peroxidation: Its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993, 57, 715S–724S. [Google Scholar] [CrossRef] [Green Version]
- Roychoudhury, S.; Sharma, R.; Sikka, S.C.; Agarwal, A. Diagnostic application of total antioxidant capacity in seminal plasma to assess oxidative stress in male factor infertility. J. Assist. Reprod. Genet 2016, 33, 627–635. [Google Scholar] [CrossRef]
- Zarkovic, N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Aspects Med. 2003, 24, 281–291. [Google Scholar] [CrossRef]
- Spickett, C.M. The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox Biol. 2013, 1, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Milkovic, L.; Bennett, S.J.; Griffiths, H.R.; Zarkovic, N.; Grune, T. Measurement of HNE-protein adducts in human plasma and serum by ELISA-Comparison of two primary antibodies. Redox Biol. 2013, 1, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Khosrowbeygi, A.; Zarghami, N. Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clin. Pathol. 2007, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Amiri, I.; Sheikh, N.; Najafi, R. Nitric oxide level in seminal plasma and its relations with sperm DNA damages. Iran Biomed. J. 2007, 11, 259–264. [Google Scholar] [PubMed]


| Type(s) of RNS | Concentration | Experimental Model | Physiological and Pathological Effects | Reference(s) |
|---|---|---|---|---|
| TnNOS | - | Human | Aids in the process of steroidogenesis | [19,20] |
| eNOS | 50–100 nM | Human | Aberrant patterns of sperm eNOS expression associated with decreased sperm motility (r = −0.46; p < 0.05) | [21,22] |
| iNOS | >1 mM | Human | Structural association with various tight junction-proteins, including actin, occludin, vimentin, and α-tubulin, vital in modulating the Sertoli cells tight junctions maintaining the BTB | [14] |
| SNP | (i). 0.25–2.5 mM (ii). 10−6 to 10−4 M | Human | NO induced decreased in sperm motility (p < 0.01) and viability (p < 0.05). Reduction of sperm motility in a dose- and time-dependent manner by SNP. Sperm progressive motility, and concentration of motile cells also reduced by all SNP doses (p < 0.005) | [23,24,25] |
| SNAP | 0–1.2 nmol/106 spermatozoa | Human | A positive correlation was seen between the concentrations of NO and the percentage of immotile spermatozoa (p < 0.01). | [25] |
| Method(s) | Type | Principle | Advantage(s) | Reference(s) |
|---|---|---|---|---|
| Cytochrome C reduction test | Direct | Reduction of ferricytochrome C to ferrocytochrome C is used to detect superoxide formation. | Gold standard for measuring extracellular superoxide anions. | [87] |
| Electron spin resonance (ESR), electron paramagnetic resonance (EPR) | Direct | (i) Magnetic properties of unpaired electrons in free radicals enable them to absorb electromagnetic radiation on application of external magnetic field; this then generates absorption spectra utilizing the energy of the electron spin state, which is measured using ESR spectrophotometers. (ii) Provide direct detection of the “instantaneous” presence of free radical species in a sample. (iii) Play a major role in the assessment of most of the oxidants characterized by very short half-life (nanoseconds to microseconds) usually by using stabilizing molecules called spin-traps/probes. | (i) Used to measure oxidative stress on proteins and lipids. Simple, and have high sensitivity and specificity. (ii) Detects free radicals and paramagnetic molecules. The magnetic field-based EPR detection enables nondestructive (in vitro) and noninvasive (in vivo) measurements of biological samples. EPR spectroscopy, coupled with the use of paramagnetic probes, is a potential technique for accurate and precise determination of ROS concentrations in a variety of biological samples. | [86,90] |
| Xylenol orange-based assay | Direct | (i) Uses automated analyzer. (ii) ROS in semen oxidizes ferrous to ferric ion and this forms a colored complex with xylenol orange in an acidic medium, the color intensity of which can be measured spectrophotometrically. (iii) Results are expressed in μmol H O2 2 equiv./L. | It is rapid, easy, stable, inexpensive, reliable, and sensitive. | [104] |
| ROS measurement via chemiluminescence | Direct | (i) Measures real-time production of ROS. (ii) Uses two probes—luminol and lucigenin. (iii) Luminol measures global ROS levels, both extracellular and intracellular (superoxide anion, hydrogen peroxide, and hydroxyl radical). (iv) Lucigenin is specific for superoxide anion and hydroxyl radical. | Chemiluminescence is a robust, sensitive, and specific method. | [105,106,107,108,109,110,111] |
| Flow cytometry | Direct | (i) ROS measurement of hydrogen peroxide and superoxide anion via flow cytometry. (ii) Dihydroethidium measures intracellular superoxide anion and dichlorofluoroscein diacetate for intracellular hydrogen peroxide. | Requires very low amounts of spermatozoa, and high-specificity intracellular ROS in spermatozoa. | [102,112,113,114] |
| Endtz test | Indirect | (i) ROS is mainly generated by leukocytes. (ii) Myeloperoxidase is used to stain polymorphonuclear granulocytes, but does not provide any information regarding ROS generation by spermatozoa. | Indirect indicator of excessive ROS generation by leukocytes in semen. | [91,102,104] |
| Redox potential GSH/GSSG | Indirect | (i) Balance of reduced glutathione and its oxidized form (GSSG) gives an indication of ROS levels in vivo. (ii) GSH/GSSG levels are measured biochemically or using high-performance liquid chromatography. | Can be used to measure oxidative stress in vitro and in vivo. | [92,93] |
| Thiobarbituric acid assay (TBARS) | Indirect | (i) Measures lipid peroxidation. (ii) Detects malondialdehyde (MDA-TBA) adduct by colorimetry or fluoroscopy. | Simple but non-specific. | [103,104] |
| Oxidation-reduction potential | Indirect | (i) Measures the redox balance in a given biological system. (ii) It measures all known and unknown oxidants and antioxidants in a given sample. | Can be measured both in seminal ejaculates and in seminal plasma (both fresh and frozen). | [98,99,100] |
| Technique | Principle | Advantage(s) | Disadvantage(s) | Reference(s) |
|---|---|---|---|---|
| Thiobarbituric acid assay (TBARS) | MDA-TBA adduct detection using colorimetry or fluoroscopy | Simple but non-specific | Rigorous controls are required | [94,95] |
| Isoprostane | EIA/liquid chromatography–tandem mass spectrometry | Specificity, stable compound | Labor-intensive and expensive equipment required | [115] |
| HNE-His Adduct ELISA | ELISA | Rapid, helps in quantification | Chances of cross-reactivity | [117,118,119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, S.; Sengupta, P.; Das, S.; Slama, P.; Roychoudhury, S. Reactive Nitrogen Species and Male Reproduction: Physiological and Pathological Aspects. Int. J. Mol. Sci. 2022, 23, 10574. https://doi.org/10.3390/ijms231810574
Dutta S, Sengupta P, Das S, Slama P, Roychoudhury S. Reactive Nitrogen Species and Male Reproduction: Physiological and Pathological Aspects. International Journal of Molecular Sciences. 2022; 23(18):10574. https://doi.org/10.3390/ijms231810574
Chicago/Turabian StyleDutta, Sulagna, Pallav Sengupta, Sanghamitra Das, Petr Slama, and Shubhadeep Roychoudhury. 2022. "Reactive Nitrogen Species and Male Reproduction: Physiological and Pathological Aspects" International Journal of Molecular Sciences 23, no. 18: 10574. https://doi.org/10.3390/ijms231810574
APA StyleDutta, S., Sengupta, P., Das, S., Slama, P., & Roychoudhury, S. (2022). Reactive Nitrogen Species and Male Reproduction: Physiological and Pathological Aspects. International Journal of Molecular Sciences, 23(18), 10574. https://doi.org/10.3390/ijms231810574









