Activation of Neutrophils by Mucin–Vaterite Microparticles
Abstract
1. Introduction
2. Results
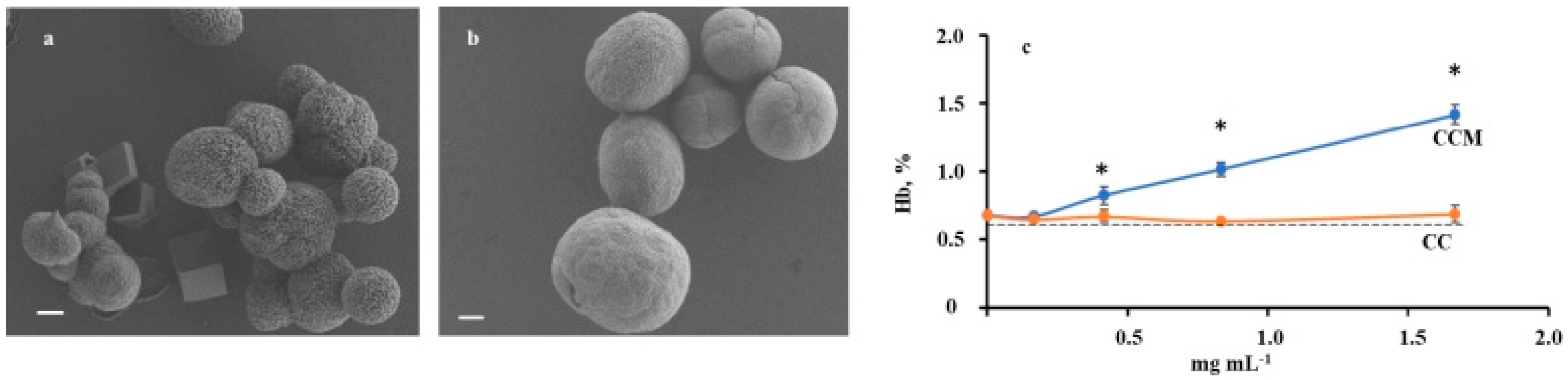
2.1. Characteristics of CC and CCM Microparticles
2.2. Hemolytic Activity of CC and CCM Microparticles
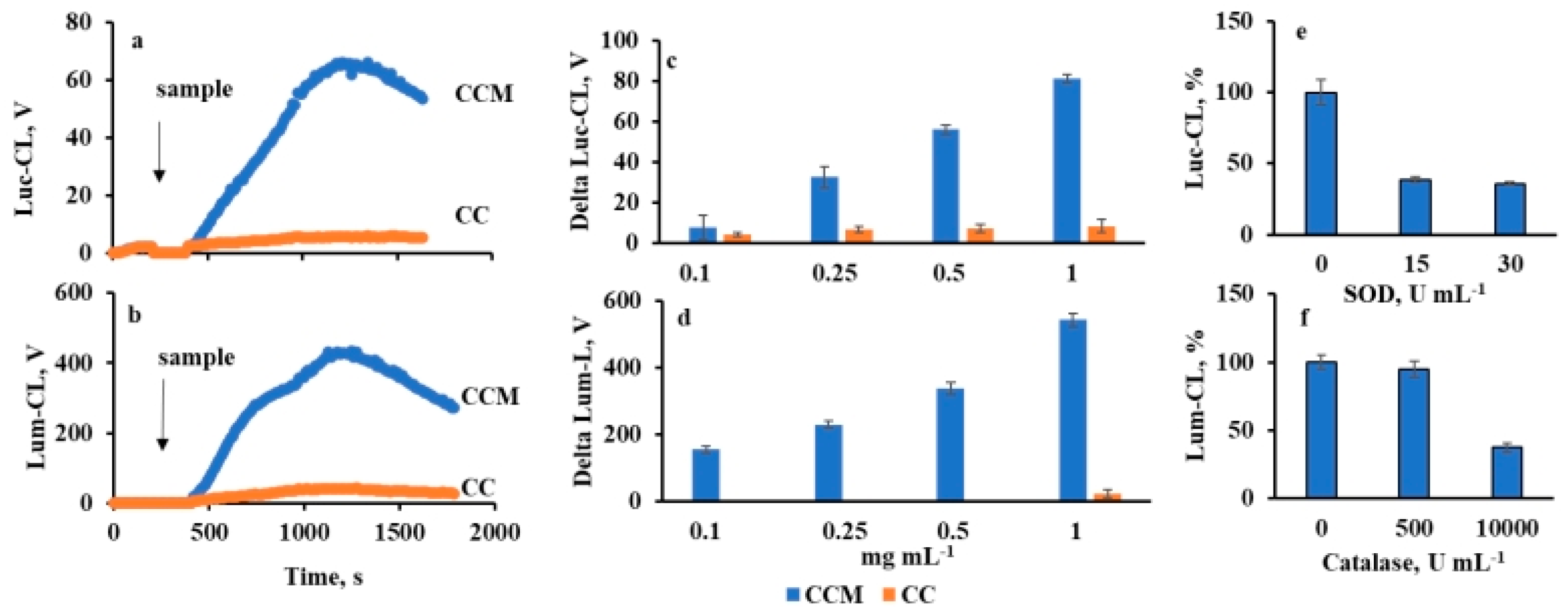
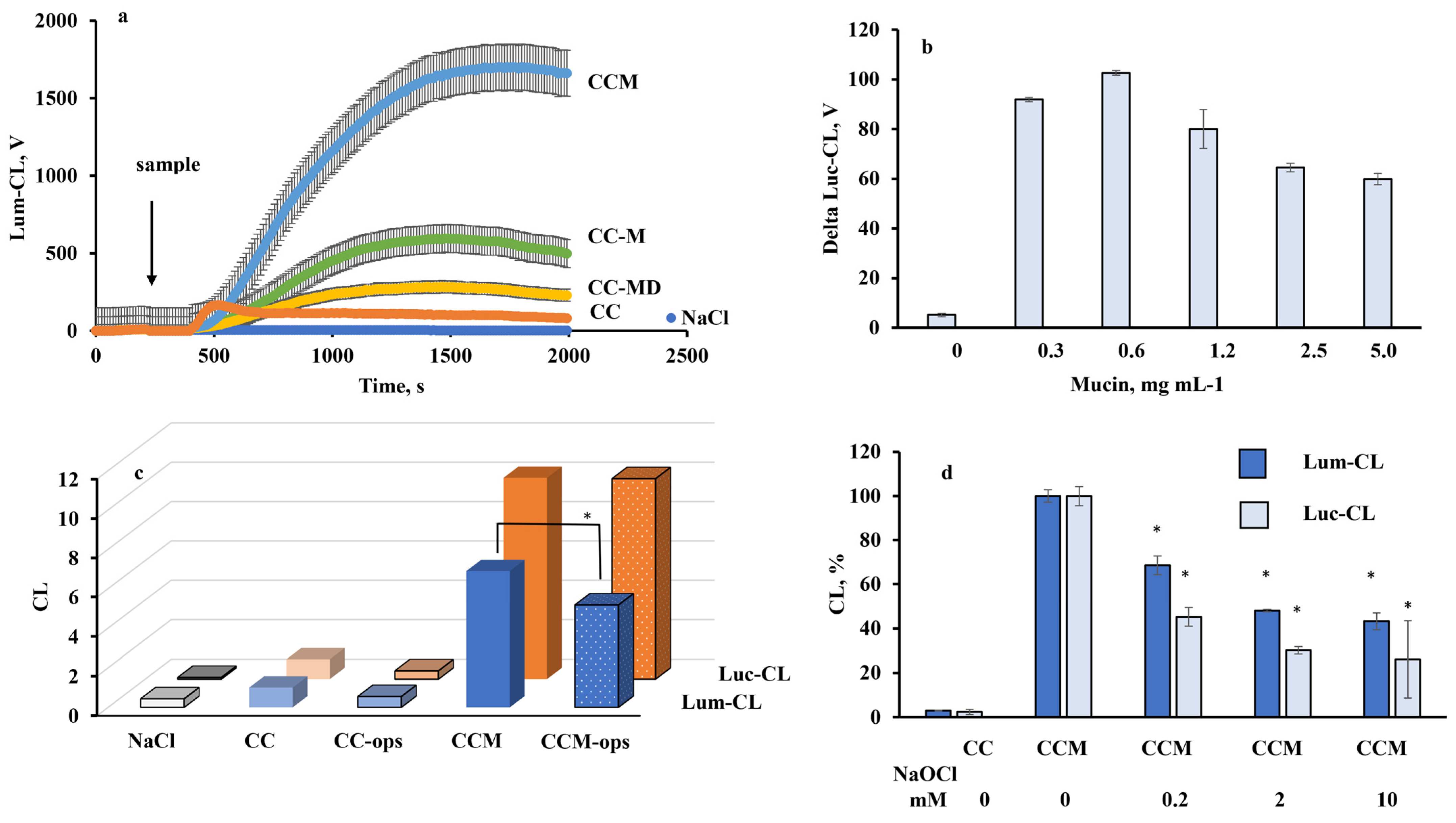
2.3. Neutrophil Chemiluminescence
2.4. Cytokine and Myeloperoxidase Release by Neutrophils
2.5. Light Microscopy
2.6. Analysis of Human Kidney Stones
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Analytical Determination of Mucin
4.3. Desialylation of Mucin
4.4. Fabrication of Vaterite Microparticles
4.5. Mucin Adsorption onto CC and Zymosan Particles
4.6. Opsonisation
4.7. Treatment of Microparticles with NaOCl
4.8. Scanning Electron Microscopy (SEM)
4.9. ζ- Potential Measurement
4.10. Nitrogen Adsorption–Desorption by the Brunauer–Emmett–Teller (BET) Method
4.11. Blood Cell Isolation
4.12. Hemolytic Activity Assay
4.13. Chemiluminescent Assay (CL)
4.14. Cytokine RNA Expression
4.15. Cytokine and Myeloperoxidase ELISA Assay
4.16. Light Microscopy
4.17. Kidney Stone Collection and Processing
4.18. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| SOD | superoxide dismutase |
| SEM | scanning electron microscopy |
| CC | vaterite microparticles |
| CCM | vaterite microparticles co-precipitated with mucin |
| CC-M | vaterite microparticles coated with mucin by adsorption |
| CL | chemiluminescent assay |
| ROS | reactive oxygen species |
| RHS | reactive halogen species |
| Zym | zymosan |
| MPO | myeloperoxidase |
| NET | neutrophil extracellular trap |
| St | kidney stone |
| Lum | luminol |
| Luc | lucigenin |
| PMB | polymyxin sulphate B |
References
- Volodkin, D. CaCO3 templated microbeads and-capsules for bioapplications. Adv. Coll. Interface Sci. 2014, 207, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.K.; Pan, H.; Huang, S.M.; Huang, N.L.; Yao, C.C.; Hsiao, K.M.; Wu, C.W. Calcium content of different compositions of gallstones and pathogenesis of calcium carbonate gallstones. Asian J. Surg. 2013, 36, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sayers, C.; Wyatt, J.; Soloway, R.D.; Taylor, D.R.; Stringer, M.D. Gallbladder mucin production and calcium carbonate gallstones in children. Pediatr. Surg. Int. 2007, 23, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Mashina, E.V.; Shanina, S.N. Amino acid composition of gallstones and its connection with the mineral component. Proc. Russ. Mineral. Soc. 2019, 148, 95–109. [Google Scholar]
- Konopacka-Łyskawa, D. Synthesis methods and favorable conditions for spherical vaterite precipitation: A review. Crystals 2019, 9, 223. [Google Scholar] [CrossRef]
- Miura, Y.; Iwazu, Y.; Shiizaki, K.; Akimoto, T.; Kotani, K.; Kurabayashi, M.; Kurosu, H.; Kuro-O, M. Identification and quantification of plasma calciprotein particles with distinct physical properties in patients with chronic kidney disease. Sci. Rep. 2018, 8, 1256. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hood, M.A.; Mauri, S.; Baio, J.E.; Bonn, M.; Muñoz-Espí, R.; Weidner, T. Biomimetic vaterite formation at surfaces structurally templated by oligo(glutamic acid) peptides. Chem. Commun. (Camb). 2015, 51, 15902–15905. [Google Scholar] [CrossRef]
- Yamasaki, T.; Chijiiwa, K.; Endo, M. Isolation of mucin from human hepatic bile and its induced effects on precipitation of cholesterol and calcium carbonate in vitro. Dig. Dis. Sci. 1993, 38, 909–915. [Google Scholar] [CrossRef]
- Van den Berg, A.A.; Van Buul, J.D.; Tytgat, G.N.J.; Groen, A.K.; Ostrow, J.D. Mucins and calcium phosphate precipitates additively stimulate cholesterol crystallization. J. Lipid Res. 1998, 39, 744–1751. [Google Scholar] [CrossRef]
- Gelli, R.; Martini, F.; Geppi, M.; Borsacchi, S.; Ridi, F.; Baglioni, P. Exploring the interplay of mucin with biologically-relevant amorphous magnesium-calcium phosphate nanoparticles. J. Coll. Interface Sci. 2021, 594, 802–811. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Kovalenko, E.A.; Mikhalchik, E.V.; Filatova, L.Y.; Volodkin, D.; Vikulina, A.S. Mucin adsorption on vaterite CaCO3 microcrystals for the prediction of mucoadhesive properties. J. Coll. Interface Sci. 2019, 545, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Balabushevich, N.G.; Sholina, E.A.; Mikhalchik, E.V.; Filatova, L.Y.; Vikulina, A.S.; Volodkin, D. Self-assembled mucin-containing microcarriers via hard templating on CaCO3 crystals. Micromachines 2018, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164. [Google Scholar] [CrossRef]
- Peng, H.H.; Liu, Y.J.; Ojcius, D.M.; Lee, C.M.; Chen, R.H.; Huang, P.R.; Martel, J.; Young, J.D. Mineral particles stimulate innate immunity through neutrophil extracellular traps containing HMGB1. Sci. Rep. 2017, 7, 16628. [Google Scholar] [CrossRef]
- Burt, H.M.; Jackson, J.K.; Taylor, D.R.; Crowther, R.S. Activation of human neutrophils by calcium carbonate polymorphs. Dig. Dis. Sci. 1997, 42, 1283–1289. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Kovalenko, E.A.; Le-Deygen, I.M.; Filatova, L.Y.; Volodkin, D.; Vikulina, A.S. Hybrid CaCO3-mucin crystals: Effective approach for loading and controlled release of cationic drugs. Mater. Des. 2019, 12, 108020. [Google Scholar] [CrossRef]
- Memar, M.Y.; Adibkia, K.; Farajnia, S.; Kafil, H.S.; Maleki-Diza, S.; Ghotaslou, R. Biocompatibility, cytotoxicity and antimicrobial effects of gentamicin-loaded CaCO3 as a drug delivery to osteomyelitis. J. Drug Del. Sci. Technol. 2019, 54, 101307. [Google Scholar] [CrossRef]
- Posiedelik, M.; Markowicz-Piasecka, M.; Sikora, J. Erythrocytes as model cells for biocompatibility assessment, cytotoxicity screening of xenobiotics and drug delivery. Chem. Biol. Inter. 2020, 332, 109305. [Google Scholar] [CrossRef]
- Gyllenhammar, H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J. Immunol. Methods 1987, 97, 209–213. [Google Scholar] [CrossRef]
- Brestel, E.P. Co-oxidation of luminol by hypochlorite and hydrogen peroxide implications for neutrophil chemiluminescence. Biochem. Biophys. Res. Commun. 1985, 126, 482–488. [Google Scholar] [CrossRef]
- Cantin, A.M.; Ouellet, C.; Cloutier, A.A.; McDonald, P.P. Airway mucins inhibit oxidative and non-oxidative bacterial killing by human neutrophils. Front. Pharmacol. 2020, 11, 554353. [Google Scholar] [CrossRef]
- Sandberg, T.; Carlsson, J.; Ott, M.K. Interactions between human neutrophils and mucin-coated surfaces. J. Mater. Sci. Mater. Med. 2008, 20, 621–631. [Google Scholar] [CrossRef][Green Version]
- Song, X.; Ju, H.; Lasanajak, Y.; Kudelka, M.R.; Smith, D.F.; Cummings, R.D. Oxidative release of natural glycans for functional glycomics. Nat. Methods 2016, 13, 528–534. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Kovalenko, E.A.; Filatova, L.Y.; Kirzhanova, E.A.; Mikhalchik, E.V.; Volodkin, D.; Vikulina, A.S. Hybrid mucin-vaterite microspheres for delivery of proteolytic enzyme chymotrypsin. Macromol. Biosci. 2022, 22, 2200005. [Google Scholar] [CrossRef]
- Schoonen, M.A.A.; Cohn, C.A.; Roemer, E.; Laffers, R.; Simon, S.R.; O’Riordan, T. Mineral-induced formation of reactive oxygen species. Rev. Mineral. Geochem. 2006, 64, 179–221. [Google Scholar] [CrossRef]
- Leppkes, M.; Maueröder, C.; Hirth, S.; Nowecki, S.; Günther, C.; Billmeier, U.; Paulus, S.; Biermann, M.; Muñoz, L.E.; Hoffmann, M.; et al. Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat. Commun. 2016, 7, 10973. [Google Scholar] [CrossRef]
- Parker, H.; Winterbourn, C.C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 2013, 3, 424. [Google Scholar] [CrossRef]
- Peng, H.H.; Wu, C.Y.; Young, D.; Martel, J.; Young, A.; Ojcius, D.M.; Lee, Y.H.; Young, J.D. Physicochemical and biological properties of biomimetic mineralo-protein nanoparticles formed spontaneously in biological fluids. Small 2013, 9, 2297–2307. [Google Scholar] [CrossRef]
- Muñoz, L.E.; Boeltz, S.; Bilyy, R.; Schauer, C.; Mahajan, A.; Widulin, N.; Grüneboom, A.; Herrmann, I.; Boada, E.; Rauh, M.; et al. Neutrophil extracellular traps initiate gallstone formation. Immunity 2019, 51, 443–450. [Google Scholar] [CrossRef]
- Schapher, M.; Koch, M.; Weidner, D.; Scholz, M.; Wirtz, S.; Mahajan, A.; Herrmann, I.; Singh, J.; Knopf, J.; Leppkes, M.; et al. Neutrophil extracellular traps promote the development and growth of human salivary stones. Cells 2020, 9, 2139. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Batool, F.; Özçelik, H.; Stutz, C.; Gegout, P.Y.; Benkirane-Jessel, N.; Petit, C.; Huck, O. Modulation of immune-inflammatory responses through surface modifications of biomaterials to promote bone healing and regeneration. J. Tissue Eng. 2021, 12, 20417314211041428. [Google Scholar] [CrossRef]
- Parakhonskiy, B.; Zyuzin, M.V.; Yashchenok, A.; Carregal-Romero, S.; Rejman, J.; Möhwald, H.; Parak, W.J.; Skirtach, A.G. The influence of the size and aspect ratio of anisotropic, porous CaCO3 particles on their uptake by cells. J. Nanobiotechnology 2015, 13, 53. [Google Scholar] [CrossRef]
- Tabei, Y.; Sugino, S.; Eguchi, K.; Tajika, M.; Abe, H.; Nakajima, Y.; Horie, M. Effect of calcium carbonate particle shape on phagocytosis and pro-inflammatory response in differentiated THP-1 macrophages. Biochem. Biophys. Res. Commun. 2017, 490, 499–505. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Senchukova, M.A. Brief review about the role of nanomaterials, mineral-organic nanoparticles, and extra-bone calcification in promoting carcinogenesis and tumor progression. Biomedicines 2019, 7, 65. [Google Scholar] [CrossRef]
- Underhill, D.M.; Goodridge, H.S. Information processing during phagocytosis. Nat. Rev. Immunol. 2012, 12, 492–502. [Google Scholar] [CrossRef]
- Nadra, I.; Boccaccini, A.R.; Philippidis, P.; Whelan, L.C.; McCarthy, G.; Haskard, D.O.; Landis, R.C. Effect of particle size on hydroxyapatite crystal-induced tumor necrosis factor alpha secretion by macrophages. Atherosclerosis 2008, 196, 98–105. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, M.K.; Kim, H.M.; Lee, J.K.; Jeong, J.; Kim, Y.R.; Oh, J.M.; Choi, S.J. The fate of calcium carbonate nanoparticles administered by oral route: Absorption and their interaction with biological matrices. Int. J. Nanomed. 2015, 10, 2273–2293. [Google Scholar]
- Peng, H.H.; Martel, J.; Lee, Y.H.; Ojcius, D.M.; Young, J.D. Serum-derived nanoparticles: De novo generation and growth in vitro, and internalization by mammalian cells in culture. Nanomedicine 2011, 6, 643–658. [Google Scholar] [CrossRef]
- Vakhrusheva, T.V.; Baikova, Y.P.; Balabushevich, N.G.; Gusev, S.A.; Lomakina, G.Y.; Sholina, E.A.; Moshkovskaya, M.A.; Shcherbakov, P.L.; Pobeguts, O.V.; Mikhalchik, E.V. Binding of mucin by E. coli from human gut. Bull. Exp. Biol. Med. 2017, 165, 235–238. [Google Scholar] [CrossRef]
- Mikhalchik, E.; Balabushevich, N.; Vakhrusheva, T.; Sokolov, A.; Baykova, J.; Rakitina, D.; Scherbakov, P.; Gusev, S.; Gusev, A.; Kharaeva, Z.; et al. Mucin adsorbed by E. coli can affect neutrophil activation in vitro. FEBS Open Bio. 2020, 10, 180–196. [Google Scholar] [CrossRef]
- Mikhalchik, E.V.; Boychenko, O.P.; Moskalets, A.P.; Morozova, O.V.; Klinov, D.V.; Basyreva, L.Y.; Gusev, S.A.; Panasenko, O.M.; Filatova, L.Y.; Balabushevich, N.G. Stimulation of neutrophil oxidative burst by calcium phosphate particles with sorbed mucin. Rus. Open Med. J. 2021, 10, CID e0428. [Google Scholar] [CrossRef]
- Turner, B.S.; Bhaskar, K.R.; Hadzopoulou-Cladaras, M.; LaMont, J.T. Cysteine-rich regions of pig gastric mucin contain von Willebrand factor and cystine knot domains at the carboxyl terminal. Biochim. Biophys. 1999, 1447, 77–92. [Google Scholar] [CrossRef]
- Marangella, M.; Vitale, C.; Bagnis, C.; Bruno, M.; Ramello, A. Idiopathic calcium nephrolithiasis. Nephron 1999, 81, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Aknin, M.L.; Berry, M.; Dick, A.D.; Khan-Lim, D. Normal but not altered mucins activate neutrophils. Cell Tissue Res. 2004, 318, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Bornhöfft, K.F.; Rebl, A.; Gallagher, M.E.; Viergutz, T.; Zlatina, K.; Reid, C.; Galuska, S.P. Sialylated cervical mucins inhibit the activation of neutrophils to form neutrophil extracellular traps in bovine in vitro model. Front. Immunol. 2019, 10, 2478. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Luis, M.J.; Armas-González, E.; Herrera-García, A.; Arce-Franco, M.; Feria, M.; Vicente-Manzanares, M.; Martínez-Ruiz, A.; Sánchez-Madrid, F.; Díaz-González, F. L-selectin expression is regulated by CXCL8-induced reactive oxygen species produced during human neutrophil rolling. Eur. J. Immunol. 2019, 49, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Yeh, Y.C.; Yang, K.D. Functions and therapeutic targets of Siglec-mediated infections, inflammations and cancers. J. Formos Med. Assoc. 2021, 120, 5–24. [Google Scholar] [CrossRef]
- Lizcano, A.; Secundino, I.; Döhrmann, S.; Corriden, S.; Rohena, C.; Diaz, S.; Ghosh, P.; Deng, L.; Nizet, V.; Varki, A. Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation. Blood 2017, 129, 3100–3110. [Google Scholar] [CrossRef]
- Green, P.J.; Yuen, C.-T.; Childs, R.A.; Chai, W.; Miyasaka, M.; Lemoine, R.; Lubineau, A.; Smith, B.; Ueno, H.; Nicolaou, K.C.; et al. Further studies of the binding specificity of the leukocyte adhesion molecule, L-selectin, towards sulphated oligosaccharides-suggestion of a link between the selectin- and the integrin-mediated lymphocyte adhesion systems. Glycobiology 1995, 5, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Crottet, P.; Kim, Y.J.; Varki, A. Subsets of sialated, sulfated mucins of diverse origins are recognized by L-selectin. Lack of evidence for unique oligosaccharide sequences mediating binding. Glycobiology 1996, 6, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [PubMed]
- Morris, J.C. The acid ionization constant of HOCl from 5 to 35°. J. Phys. Chem. 1966, 70, 3798–3805. [Google Scholar] [CrossRef]
- Melo-Gonzalez, F.; Fenton, T.M.; Forss, C.; Smedley, C.; Goenka, A.; MacDonald, A.S.; Thornton, D.J.; Travis, M.A. Intestinal mucin activates human dendritic cells and IL-8 production in a glycan-specific manner. J. Biol. Chem. 2018, 293, 8543–8553. [Google Scholar] [CrossRef]
- Desai, J.; Foresto-Neto, O.; Honarpisheh, M.; Steiger, S.; Nakazawa, D.; Popper, B.; Buhl, E.M.; Boor, P.; Mulay, S.R.; Anders, H.-J. Particles of different sizes and shapes induce neutrophil necroptosis followed by the release of neutrophil extracellular trap-like chromatin. Sci. Rep. 2017, 7, 15003. [Google Scholar] [CrossRef]
- Kirchner, T.; Möller, S.; Klinger, M.; Solbach, W.; Laskay, T.; Behnen, M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediat. Inflamm. 2012, 2012, 849136. [Google Scholar] [CrossRef]
- Wang, D.Q.-H. Aging per se is an independent risk factor for cholesterol gallstone formation in gallstone susceptible mice. J. Lipid Res. 2002, 43, 1950–1959. [Google Scholar] [CrossRef]
- Georgescu, D.; Ionita, I.; Lascu, A.; Hut, E.-F.; Dragan, S.; Ancusa, O.-E.; Ionita, M.; Calamar-Popovici, D.; Georgescu, L.-A.; Lighezan, D.-F. Gallstone disease and bacterial metabolic performance of gut microbiota in middle-aged and older patients. Int. J. Gen. Med. 2021, 15, 5513–5531. [Google Scholar] [CrossRef]
- Verdier, J.; Luedde, T.; Sellge, G. Biliary mucosal barrier and microbiome. Viszeralmedizin 2015, 31, 158–161. [Google Scholar] [CrossRef]
- Hu, F.-L.; Chen, H.-T.; Guo, F.-F.; Yang, M.; Jiang, X.; Yu, J.-H.; Zhang, F.-M.; Xu, G.-O. Biliary microbiota and mucin 4 impact the calcification of cholesterol gallstones. Hepatobiliary Pancreat Dis Int. 2021, 20, 61–66. [Google Scholar] [CrossRef]
- Hess, E.L.; Coburn, A.F.; Bates, R.C.; Murphy, P.A. New method for measuring sialic acid levels in serum and its application to rheumatic fever. J. Clin. Investig. 1957, 36, 449–455. [Google Scholar] [CrossRef]
- Morozova, O.V.; Sokolova, A.I.; Pavlova, E.R.; Isaeva, E.I.; Obraztsova, E.A.; Ivleva, E.A.; Klinov, D.V. Protein nanoparticles: Cellular uptake, intracellular distribution, biodegradation and induction of cytokine gene expression. Nanomedicine 2020, 30, 102293. [Google Scholar] [CrossRef]

| Samples | Lum-CL | Luc-CL | ||
|---|---|---|---|---|
| Suspension | Supernatant | Suspension | Supernatant | |
| CC | 1.0 ± 0.03 | 0.9 ± 0.01 | 1.0 ± 0.7 | 0.6 ± 0.3 |
| CCM | 44.7 ± 1.3 * | 3.9 ± 0.12 * | 75.5 ± 0.5 * | 4.1 ± 0.4 * |
| CC-M | 15.4 ± 0.4 *# | 2.2 ± 0.07 * | 36.7 ± 11.5 *# | 3.0 ± 0.5 * |
| Zym | 120 ± 0.9 | n.d. | 48.0 ± 5.4 | n.d. |
| Zym-M | 81.4 ± 2.6 ** | n.d. | 36.2 ± 4.0 ** | n.d. |
| Mucin 1 mg mL−1 | 2.2 ± 0.05 | 0.2 ± 0.01 | 6.2 ± 0.6 | 7.8 ± 0.4 |
| NaCl | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.4 ± 0.3 | 0.4 ± 0.3 |
| Samples | Control (0.15 M NaCl) | CC | CCM | E. coli |
|---|---|---|---|---|
| IL-1β, pg mL−1 | 0.7 ± 0.2 | 0.3 ± 0.2 | 0.9 ± 0.5 | 1.7 ± 0.5 * |
| IL-6, pg mL−1 | 11.1 ± 3.0 | 6.9 ± 3.2 | 5.4 ± 0.7 * | 12.9 ± 5.5 |
| IL-8, pg mL−1 | 45.3 ± 5.5 | 51 ± 2.4 | 58 ± 5 * | 51 ± 5 |
| IL-10, pg mL−1 | 2.7 ± 0.7 | 2.1 ± 0.2 | 3.3 ± 1.5 | 5.9 ± 3.2 * |
| TNF-α, pg mL−1 | 1.4 ± 0.5 | 0.6 ± 0.3 | 2.4 ± 1.1 | 6.1 ± 1.1 * |
| MPO, ng mL−1 | 307 ± 78 | 313 ± 7 | 175 ± 41 * | n.d. |
| Samples | 0.15 M NaCl | 20 µM PMB |
|---|---|---|
| St | 0.8 ± 0.4 | 1.0 ± 0.3 |
| St-M | 31.5 ± 0.8 * | 37.3 ± 7.3 * |
| O111:B4 | 5.6 ± 1.3 | 0.5 ± 0.2 # |
| CC | 1.2 ± 0.1 | 0.9 ± 0.2 |
| CCM | 46.7 ± 3.7 ** | 60.4 ± 13.5 |
| NaCl | 0.7 ± 0.4 | 0.6 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhalchik, E.; Basyreva, L.Y.; Gusev, S.A.; Panasenko, O.M.; Klinov, D.V.; Barinov, N.A.; Morozova, O.V.; Moscalets, A.P.; Maltseva, L.N.; Filatova, L.Y.; et al. Activation of Neutrophils by Mucin–Vaterite Microparticles. Int. J. Mol. Sci. 2022, 23, 10579. https://doi.org/10.3390/ijms231810579
Mikhalchik E, Basyreva LY, Gusev SA, Panasenko OM, Klinov DV, Barinov NA, Morozova OV, Moscalets AP, Maltseva LN, Filatova LY, et al. Activation of Neutrophils by Mucin–Vaterite Microparticles. International Journal of Molecular Sciences. 2022; 23(18):10579. https://doi.org/10.3390/ijms231810579
Chicago/Turabian StyleMikhalchik, Elena, Liliya Yu. Basyreva, Sergey A. Gusev, Oleg M. Panasenko, Dmitry V. Klinov, Nikolay A. Barinov, Olga V. Morozova, Alexander P. Moscalets, Liliya N. Maltseva, Lyubov Yu. Filatova, and et al. 2022. "Activation of Neutrophils by Mucin–Vaterite Microparticles" International Journal of Molecular Sciences 23, no. 18: 10579. https://doi.org/10.3390/ijms231810579
APA StyleMikhalchik, E., Basyreva, L. Y., Gusev, S. A., Panasenko, O. M., Klinov, D. V., Barinov, N. A., Morozova, O. V., Moscalets, A. P., Maltseva, L. N., Filatova, L. Y., Pronkin, E. A., Bespyatykh, J. A., & Balabushevich, N. G. (2022). Activation of Neutrophils by Mucin–Vaterite Microparticles. International Journal of Molecular Sciences, 23(18), 10579. https://doi.org/10.3390/ijms231810579







