cAMP and the Fibrous Sheath Protein CABYR (Ca2+-Binding Tyrosine-Phosphorylation-Regulated Protein) Is Required for 4D Sperm Movement
Abstract
:1. Introduction
2. Results
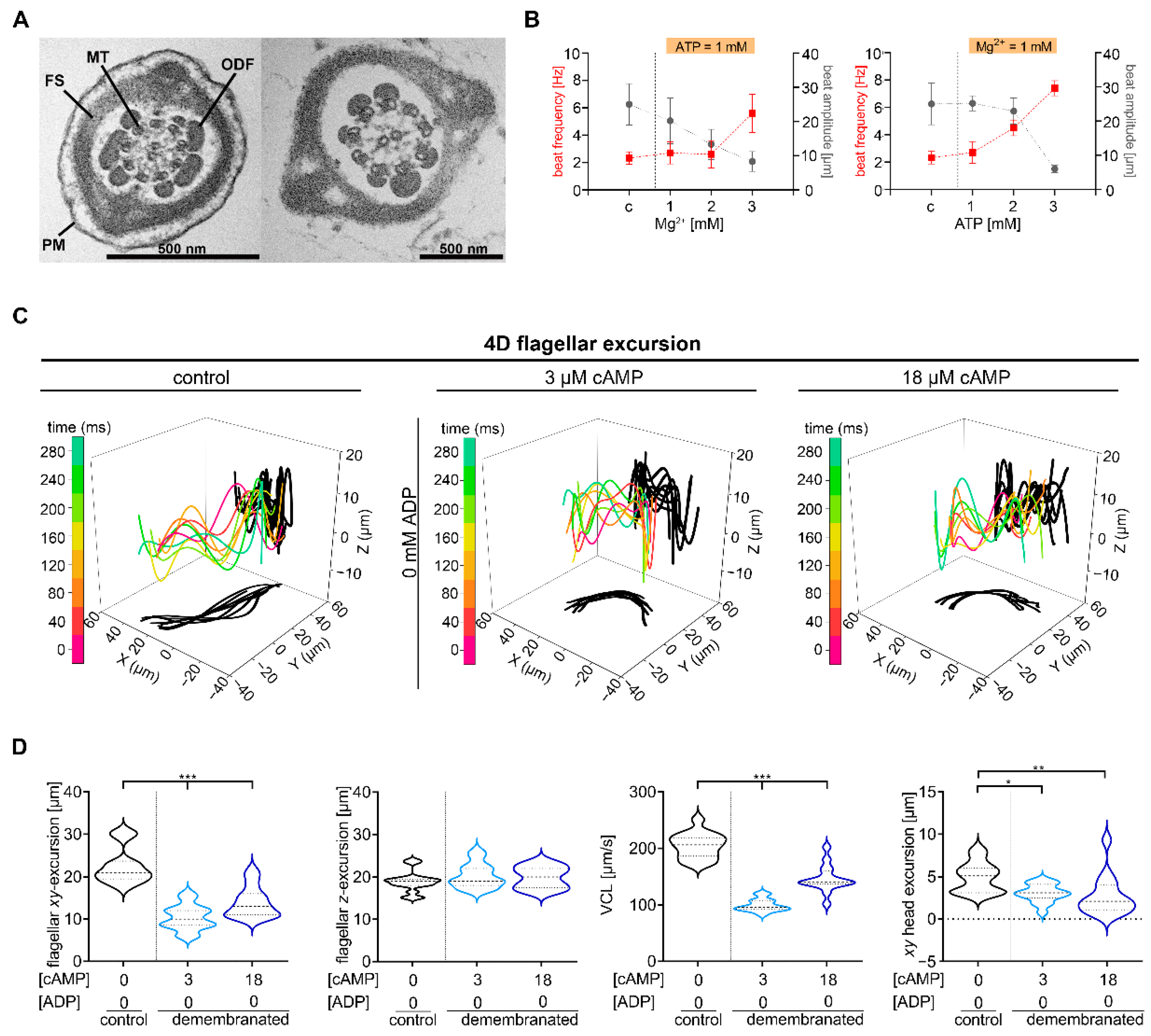
2.1. Defined cAMP and ADP Concentrations Are Necessary for Murine Chiral Movement
2.2. Rolling along the Long Axis and Conserved CW Path Chirality of Sperm Depends on ADP and cAMP
2.3. Invertebrate Sperm Show No Rolling along Their Long Axis
2.4. Cabyr Is Required for Conserved Clockwise Path Chirality of Mouse Sperm
3. Discussion
3.1. cAMP Plays a Central Role in Regulating Flagellar Movement
3.2. Linear Swimming Depends on Alternating Chirality of Rolling
3.3. CABYR Maintains Conserved Path Chirality
4. Materials and Methods
4.1. Chemical
4.2. Animals
4.3. Sperm Preparation and Media
4.4. Demembranation and Reactivation of Sperm and Perfusion
4.5. Computer-Assisted-Sperm-Analysis (CASA)
4.6. Digital Holographic Microscopy (DHM)
4.7. Path Chirality Analysis Using Procrustes Alignments
4.8. Sperm Beat Frequency and Waveform Analysis
4.9. Flagellar Movement and Trajectory Visualization in 3D
4.10. Simulation Methods
4.11. Transmission Electron Microscopy (TEM)
4.12. Image Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharlip, I.D.; Jarow, J.P.; Belker, A.M.; Lipshultz, L.I.; Sigman, M.; Thomas, A.J.; Schlegel, P.N.; Howards, S.S.; Nehra, A.; Damewood, M.D.; et al. Best practice policies for male infertility. Fertil. Steril. 2002, 77, 873–882. [Google Scholar] [CrossRef]
- Brokaw, C.J. Calcium-induced asymmetrical beating of triton-demembranated sea urchin sperm flagella. J. Cell Biol. 1979, 82, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.E.; Hille, B.; Babcock, D.F. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev. Biol. 2007, 312, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, S.; Baba, S.A.; Mohri, H.; Suarez, S.S. Quantitative analysis of flagellar movement in hyperactivated and acrosome-reacted golden hamster spermatozoa. Mol. Reprod. Dev. 2002, 61, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Control of hyperactivation in sperm. Hum. Reprod Update 2008, 14, 647–657. [Google Scholar] [CrossRef]
- Carlson, A.E.; Westenbroek, R.E.; Quill, T.; Ren, D.J.; Clapham, D.E.; Hille, B.; Garbers, D.L.; Babcock, D.F. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. USA 2003, 100, 14864–14868. [Google Scholar] [CrossRef]
- Stauss, C.R.; Votta, T.J.; Suarez, S.S. Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biol. Reprod. 1995, 53, 1280–1285. [Google Scholar] [CrossRef]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signaling mechanisms in mammalian sperm motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [CrossRef]
- Xie, F.; Garcia, M.A.; Carlson, A.E.; Schuh, S.M.; Babcock, D.F.; Jaiswal, B.S.; Gossen, J.A.; Esposito, G.; van Duin, M.; Conti, M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev. Biol. 2006, 296, 353–362. [Google Scholar] [CrossRef]
- Wennemuth, G.; Westenbroek, R.E.; Xu, T.; Hille, B.; Babcock, D.F. CaV2.2 and CaV2.3 (N- and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. J. Biol. Chem. 2000, 275, 21210–21217. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.A.; Babcock, D.F.; Wennemuth, G.; Brown, W.; Burton, K.A.; McKnight, G.S. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc. Natl. Acad. Sci. USA 2004, 101, 13483–13488. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.; Alvarez, L.; Balbach, M.; Strunker, T.; Hegemann, P.; Kaupp, U.B.; Wachten, D. Controlling fertilization and cAMP signaling in sperm by optogenetics. eLife 2015, 4, e05161. [Google Scholar] [CrossRef] [PubMed]
- Wennemuth, G.; Carlson, A.E.; Harper, A.J.; Babcock, D.F. Bicarbonate actions on flagellar and Ca2+ -channel responses: Initial events in sperm activation. Development 2003, 130, 1317–1326. [Google Scholar] [CrossRef]
- Kirichok, Y.; Navarro, B.; Clapham, D.E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006, 439, 737–740. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef]
- Qi, H.Y.; Moran, M.M.; Navarro, B.; Chong, J.A.; Krapivinsky, G.; Krapivinsky, L.; Kirichok, Y.; Ramsey, I.S.; Quill, T.A.; Clapham, D.E. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA 2007, 104, 1219–1223. [Google Scholar] [CrossRef]
- Ren, D.J.; Navarro, B.; Perez, G.; Jackson, A.C.; Hsu, S.F.; Shi, Q.; Tilly, J.L.; Clapham, D.E. A sperm ion channel required for sperm motility and male fertility. Nature 2001, 413, 603–609. [Google Scholar] [CrossRef]
- Seifert, R.; Flick, M.; Bonigk, W.; Alvarez, L.; Trotschel, C.; Poetsch, A.; Muller, A.; Goodwin, N.; Pelzer, P.; Kashikar, N.D.; et al. The CatSper channel controls chemosensation in sea urchin sperm. EMBO J. 2015, 34, 379–392. [Google Scholar] [CrossRef]
- Strunker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471, 382–386. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Wiesehoefer, C.; Shah, N.B.; Reetz, E.; Hwang, J.Y.; Huang, X.; Wang, T.E.; Lishko, P.V.; Davies, K.M.; et al. 3D structure and in situ arrangements of CatSper channel in the sperm flagellum. Nat. Commun. 2022, 13, 3439. [Google Scholar] [CrossRef]
- Wang, H.; McGoldrick, L.L.; Chung, J.J. Sperm ion channels and transporters in male fertility and infertility. Nat. Rev. Urol. 2021, 18, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Young, S.A.; Miyata, H.; Satouh, Y.; Aitken, R.J.; Baker, M.A.; Ikawa, M. CABYR is essential for fibrous sheath integrity and progressive motility in mouse spermatozoa. J. Cell Sci. 2016, 129, 4379–4387. [Google Scholar] [CrossRef]
- Lindemann, C.B.; de Pinho, T.G.; Lesich, K.A. The physiological role of ADP and Mg2+ in maintaining a stable beat cycle in bull sperm. Cytoskeleton 2014, 71, 638–648. [Google Scholar] [CrossRef]
- Lesich, K.A.; de Pinho, T.G.; Dang, L.; Lindemann, C.B. Ultrastructural evidence that motility changes caused by variations in ATP, Mg2+, and ADP correlate to conformational changes in reactivated bull sperm axonemes. Cytoskeleton 2014, 71, 649–661. [Google Scholar] [CrossRef]
- Lesich, K.A.; Pelle, D.W.; Lindemann, C.B. Insights into the mechanism of ADP action on flagellar motility derived from studies on bull sperm. Biophys. J. 2008, 95, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, C.B. A cAMP-induced increase in the motility of demembranated bull sperm models. Cell 1978, 13, 9–18. [Google Scholar] [CrossRef]
- Lindemann, C.B.; Gibbons, I.R. Adenosine triphosphate-induced motility and sliding of filaments in mammalian sperm extracted with Triton X-100. J. Cell Biol. 1975, 65, 147–162. [Google Scholar] [CrossRef]
- Lindemann, C.B.; Goltz, J.S.; Kanous, K.S. Regulation of activation state and flagellar wave form in epididymal rat sperm: Evidence for the involvement of both Ca2+ and cAMP. Cell Motil. Cytoskelet. 1987, 8, 324–332. [Google Scholar] [CrossRef]
- Wennemuth, G.; Babcock, D.F.; Hille, B. Calcium clearance mechanisms of mouse sperm. J. Gen. Physiol. 2003, 122, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Lindemann, C.B.; Lesich, K.A. Detergent-extracted models for the study of cilia or flagella. Methods Mol. Biol. 2009, 586, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Usui, T.; Yamashita, M.; Kanemori, Y.; Baba, T. Surfing and Swimming of Ejaculated Sperm in the Mouse Oviduct. Biol Reprod 2016, 94, 89. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Clapham, D.E. Rheotaxis guides mammalian sperm. Curr. Biol. 2013, 23, 443–452. [Google Scholar] [CrossRef]
- Kantsler, V.; Dunkel, J.; Blayney, M.; Goldstein, R.E. Rheotaxis facilitates upstream navigation of mammalian sperm cells. eLife 2014, 3, e02403. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.; Dai, L.; Friedrich, B.M.; Kashikar, N.D.; Gregor, I.; Pascal, R.; Kaupp, U.B. The rate of change in Ca2+ concentration controls sperm chemotaxis. J Cell Biol. 2012, 196, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Gomez, H.V.; Tuval, I.; Guerrero, A.; Darszon, A. Analysis of sperm chemotaxis. Methods Cell Biol. 2019, 151, 473–486. [Google Scholar] [CrossRef]
- De Toni, L.; Dipresa, S.; Foresta, C.; Garolla, A. Molecular Bases of Sperm Thermotaxis: Old and New Knowledges. Protein Pept Lett 2018, 25, 446–450. [Google Scholar] [CrossRef]
- Babcock, D.F.; Wandernoth, P.M.; Wennemuth, G. Episodic rolling and transient attachments create diversity in sperm swimming behavior. BMC Biol. 2014, 12, 67. [Google Scholar] [CrossRef]
- Muschol, M.; Wenders, C.; Wennemuth, G. Four-dimensional analysis by high-speed holographic imaging reveals a chiral memory of sperm flagella. PLoS ONE 2018, 13, e0199678. [Google Scholar] [CrossRef]
- Zaferani, M.; Javi, F.; Mokhtare, A.; Li, P.; Abbaspourrad, A. Rolling controls sperm navigation in response to the dynamic rheological properties of the environment. eLife 2021, 10, e68693. [Google Scholar] [CrossRef]
- Gadadhar, S.; Alvarez Viar, G.; Hansen, J.N.; Gong, A.; Kostarev, A.; Ialy-Radio, C.; Leboucher, S.; Whitfield, M.; Ziyyat, A.; Toure, A.; et al. Tubulin glycylation controls axonemal dynein activity, flagellar beat, and male fertility. Science 2021, 371, eabd4914. [Google Scholar] [CrossRef]
- Schiffer, C.; Rieger, S.; Brenker, C.; Young, S.; Hamzeh, H.; Wachten, D.; Tuttelmann, F.; Ropke, A.; Kaupp, U.B.; Wang, T.; et al. Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca2+ signaling. EMBO J. 2020, 39, e102363. [Google Scholar] [CrossRef]
- Wiesehofer, C.; Wiesehofer, M.; Dankert, J.T.; Chung, J.J.; von Ostau, N.E.; Singer, B.B.; Wennemuth, G. CatSper and its CaM-like Ca2+ sensor EFCAB9 are necessary for the path chirality of sperm. Faseb. J. 2022, 36, e22288. [Google Scholar] [CrossRef]
- Inaba, K. Sperm flagella: Comparative and phylogenetic perspectives of protein components. Mol. Hum. Reprod. 2011, 17, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Naaby-Hansen, S.; Mandal, A.; Wolkowicz, M.J.; Sen, B.; Westbrook, V.A.; Shetty, J.; Coonrod, S.A.; Klotz, K.L.; Kim, Y.H.; Bush, L.A.; et al. CABYR, a novel calcium-binding tyrosine phosphorylation-regulated fibrous sheath protein involved in capacitation. Dev. Biol. 2002, 242, 236–254. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Liang, J.; Zhu, C. Low-expressed testis-specific calcium-binding protein CBP86-IV (CABYR) is observed in idiopathic asthenozoospermia. World J. Urol. 2015, 33, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, B.H.; Gibbons, I. Flagellar movement and adenosine triphosphatase activity in sea urchin sperm extracted with Triton X-100. J. Cell Biol. 1972, 54, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, U.B.; Alvarez, L. Sperm as microswimmers—Navigation and sensing at the physical limit. Eur. Phys. J. Spec. Top. 2016, 225, 2119–2139. [Google Scholar] [CrossRef]
- Chang, H.; Kim, B.J.; Kim, Y.S.; Suarez, S.S.; Wu, M. Different migration patterns of sea urchin and mouse sperm revealed by a microfluidic chemotaxis device. PLoS ONE 2013, 8, e60587. [Google Scholar] [CrossRef]
- Chen, Y.; Cann, M.J.; Litvin, T.N.; Iourgenko, V.; Sinclair, M.L.; Levin, L.R.; Buck, J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 2000, 289, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Litvin, T.N.; Kamenetsky, M.; Zarifyan, A.; Buck, J.; Levin, L.R. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J. Biol. Chem. 2003, 278, 15922–15926. [Google Scholar] [CrossRef] [PubMed]
- Zippin, J.H.; Chen, Y.; Straub, S.G.; Hess, K.C.; Diaz, A.; Lee, D.; Tso, P.; Holz, G.G.; Sharp, G.W.; Levin, L.R.; et al. CO2/HCO3− and calcium-regulated soluble adenylyl cyclase as a physiological ATP sensor. J. Biol. Chem. 2013, 288, 33283–33291. [Google Scholar] [CrossRef] [PubMed]
- Wennemuth, G. Bicarbonate action on early events in sperm activation. Ann. Anat. 2004, 186, 293–294. [Google Scholar] [CrossRef]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Leclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995, 121, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Krahling, A.M.; Alvarez, L.; Debowski, K.; Van, Q.; Gunkel, M.; Irsen, S.; Al-Amoudi, A.; Strunker, T.; Kremmer, E.; Krause, E.; et al. CRIS-a novel cAMP-binding protein controlling spermiogenesis and the development of flagellar bending. PLoS Genet. 2013, 9, e1003960. [Google Scholar] [CrossRef]
- Yoshimura, A.; Nakano, I.; Shingyoji, C. Inhibition by ATP and activation by ADP in the regulation of flagellar movement in sea urchin sperm. Cell Motil. Cytoskelet. 2007, 64, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Demarco, I.A.; Espinosa, F.; Edwards, J.; Sosnik, J.; De La Vega-Beltran, J.L.; Hockensmith, J.W.; Kopf, G.S.; Darszon, A.; Visconti, P.E. Involvement of a Na+/HCO3− cotransporter in mouse sperm capacitation. J. Biol. Chem. 2003, 278, 7001–7009. [Google Scholar] [CrossRef]
- Wang, D.; King, S.M.; Quill, T.A.; Doolittle, L.K.; Garbers, D.L. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat. Cell Biol. 2003, 5, 1117–1122. [Google Scholar] [CrossRef]
- Zeng, Y.; Oberdorf, J.A.; Florman, H.M. pH regulation in mouse sperm: Identification of Na+-, Cl−-, and HCO3−-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev. Biol. 1996, 173, 510–520. [Google Scholar] [CrossRef]
- Brokaw, C.J. Regulation of Sperm Flagellar Motility by Calcium and Camp-Dependent Phosphorylation. J. Cell Biochem. 1987, 35, 175–184. [Google Scholar] [CrossRef]
- De Jonge, C.J.; Han, H.L.; Lawrie, H.; Mack, S.R.; Zaneveld, L.J. Modulation of the human sperm acrosome reaction by effectors of the adenylate cyclase/cyclic AMP second-messenger pathway. J. Exp. Zool. 1991, 258, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Lefievre, L.; Jha, K.N.; de Lamirande, E.; Visconti, P.E.; Gagnon, C. Activation of protein kinase A during human sperm capacitation and acrosome reaction. J. Androl. 2002, 23, 709–716. [Google Scholar]
- Gong, A.; Rode, S.; Gompper, G.; Kaupp, U.B.; Elgeti, J.; Friedrich, B.M.; Alvarez, L. Reconstruction of the three-dimensional beat pattern underlying swimming behaviors of sperm. Eur. Phys. J. E Soft Matter 2021, 44, 87. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, C.J. Bending moments in free-swimming flagella. J. Exp. Biol. 1970, 53, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Jikeli, J.F.; Alvarez, L.; Friedrich, B.M.; Wilson, L.G.; Pascal, R.; Colin, R.; Pichlo, M.; Rennhack, A.; Brenker, C.; Kaupp, U.B. Sperm navigation along helical paths in 3D chemoattractant landscapes. Nat. Commun. 2015, 6, 7985. [Google Scholar] [CrossRef]
- Eddy, E.M.; Toshimori, K.; O’Brien, D.A. Fibrous sheath of mammalian spermatozoa. Microsc. Res. Tech. 2003, 61, 103–115. [Google Scholar] [CrossRef]
- Ficarro, S.; Chertihin, O.; Westbrook, V.A.; White, F.; Jayes, F.; Kalab, P.; Marto, J.A.; Shabanowitz, J.; Herr, J.C.; Hunt, D.F.; et al. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J. Biol. Chem. 2003, 278, 11579–11589. [Google Scholar] [CrossRef]
- Mannowetz, N.; Wandernoth, P.; Hornung, J.; Ruffing, U.; Raubuch, M.; Wennemuth, G. Early activation of sperm by HCO3− is regulated hormonally in the murine uterus. Int. J. Androl. 2011, 34, 153–164. [Google Scholar] [CrossRef]
- Saggiorato, G.; Alvarez, L.; Jikeli, J.F.; Kaupp, U.B.; Gompper, G.; Elgeti, J. Human sperm steer with second harmonics of the flagellar beat. Nat. Commun. 2017, 8, 1415. [Google Scholar] [CrossRef]
- Hu, J.; Yang, M.; Gompper, G.; Winkler, R.G. Modelling the mechanics and hydrodynamics of swimming E. coli. Soft Matter 2015, 11, 7867–7876. [Google Scholar] [CrossRef]
- Lauga, E.; Eloy, C. Shape of optimal active flagella. J. Fluid Mech. 2013, 730, R1. [Google Scholar] [CrossRef] [Green Version]
- Söderlind. Numerical Algorithms; Springer: Berlin/Heidelberg, Germany, 2002; Volume 31. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frintrop, L.; Wiesehöfer, C.; Stoskus, A.; Hilken, G.; Dubicanac, M.; von Ostau, N.E.; Rode, S.; Elgeti, J.; Dankert, J.T.; Wennemuth, G. cAMP and the Fibrous Sheath Protein CABYR (Ca2+-Binding Tyrosine-Phosphorylation-Regulated Protein) Is Required for 4D Sperm Movement. Int. J. Mol. Sci. 2022, 23, 10607. https://doi.org/10.3390/ijms231810607
Frintrop L, Wiesehöfer C, Stoskus A, Hilken G, Dubicanac M, von Ostau NE, Rode S, Elgeti J, Dankert JT, Wennemuth G. cAMP and the Fibrous Sheath Protein CABYR (Ca2+-Binding Tyrosine-Phosphorylation-Regulated Protein) Is Required for 4D Sperm Movement. International Journal of Molecular Sciences. 2022; 23(18):10607. https://doi.org/10.3390/ijms231810607
Chicago/Turabian StyleFrintrop, Linda, Caroline Wiesehöfer, Aura Stoskus, Gero Hilken, Marko Dubicanac, Nicola Edith von Ostau, Sebastian Rode, Jens Elgeti, Jaroslaw Thomas Dankert, and Gunther Wennemuth. 2022. "cAMP and the Fibrous Sheath Protein CABYR (Ca2+-Binding Tyrosine-Phosphorylation-Regulated Protein) Is Required for 4D Sperm Movement" International Journal of Molecular Sciences 23, no. 18: 10607. https://doi.org/10.3390/ijms231810607
APA StyleFrintrop, L., Wiesehöfer, C., Stoskus, A., Hilken, G., Dubicanac, M., von Ostau, N. E., Rode, S., Elgeti, J., Dankert, J. T., & Wennemuth, G. (2022). cAMP and the Fibrous Sheath Protein CABYR (Ca2+-Binding Tyrosine-Phosphorylation-Regulated Protein) Is Required for 4D Sperm Movement. International Journal of Molecular Sciences, 23(18), 10607. https://doi.org/10.3390/ijms231810607






