Physiological and Biochemical Regulation Mechanism of Exogenous Hydrogen Peroxide in Alleviating NaCl Stress Toxicity in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn)
Abstract
:1. Introduction
2. Results
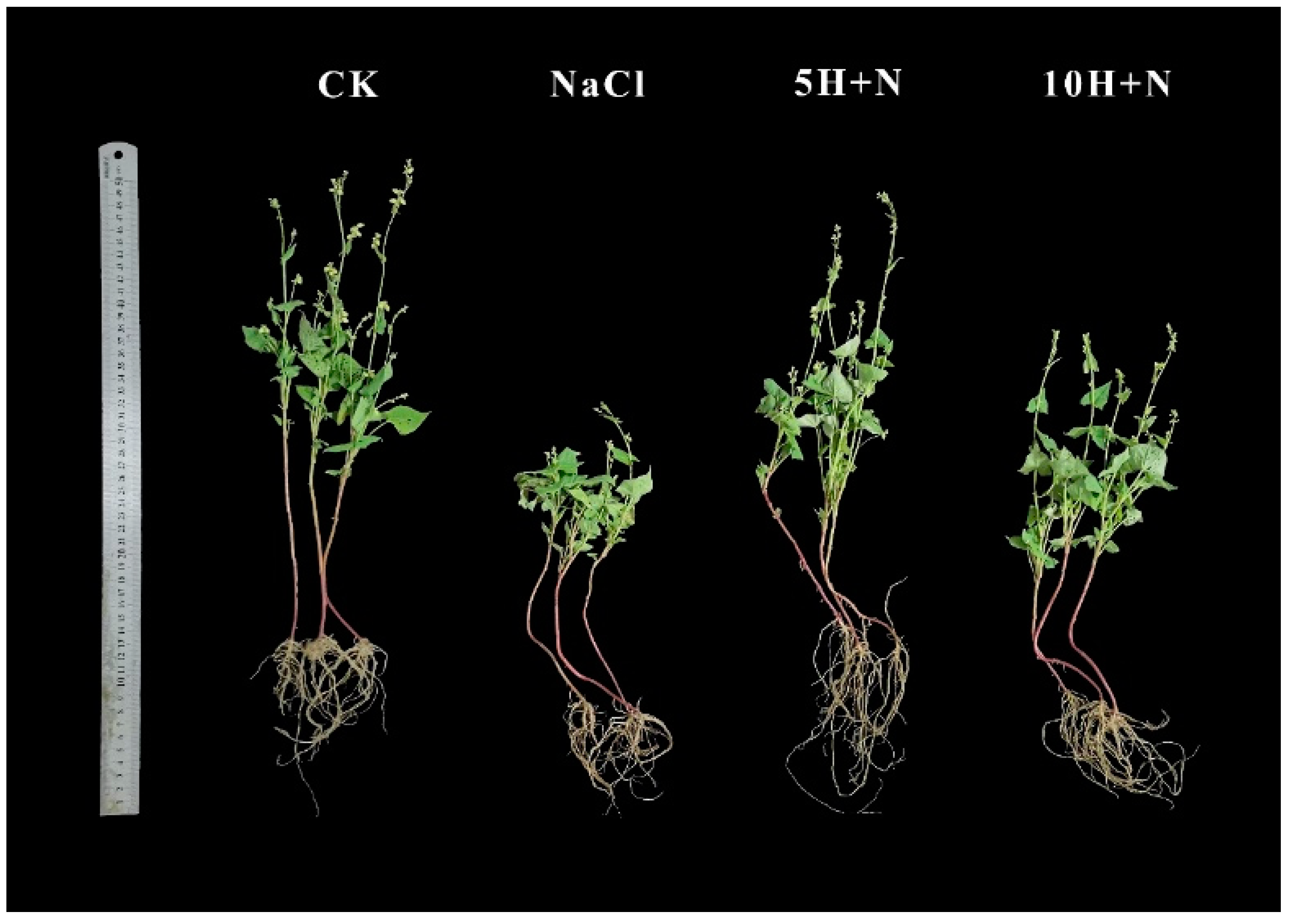
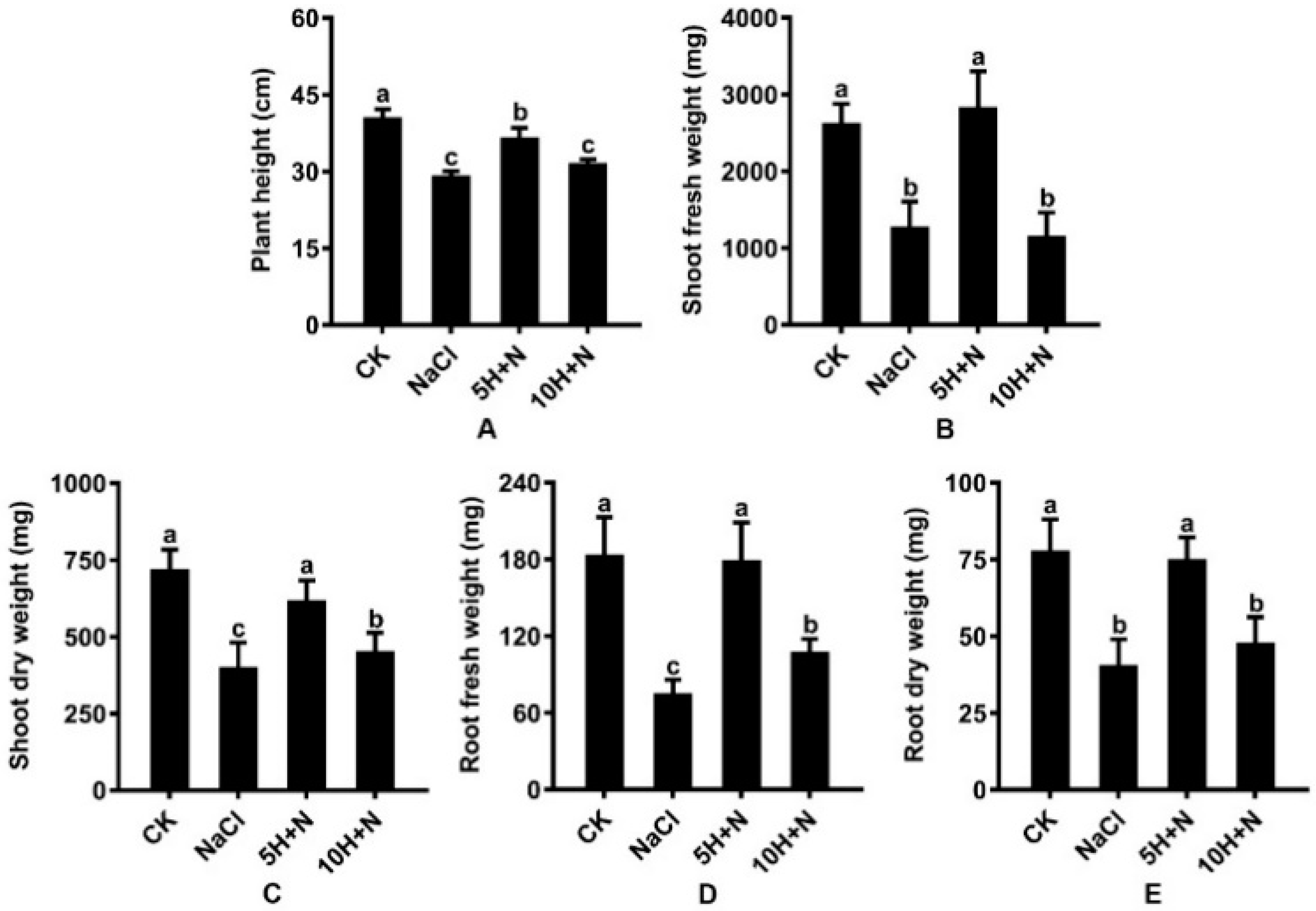
2.1. Effect of Exogenous H2O2 on Plant Height and Biomass under NaCl Stress
2.2. Effect of Exogenous H2O2 and NaCl Stress on Leaf Growth and Relative Water Content
2.3. Effect of Exogenous H2O2 and NaCl Stress on Leaf Photosynthetic Pigments
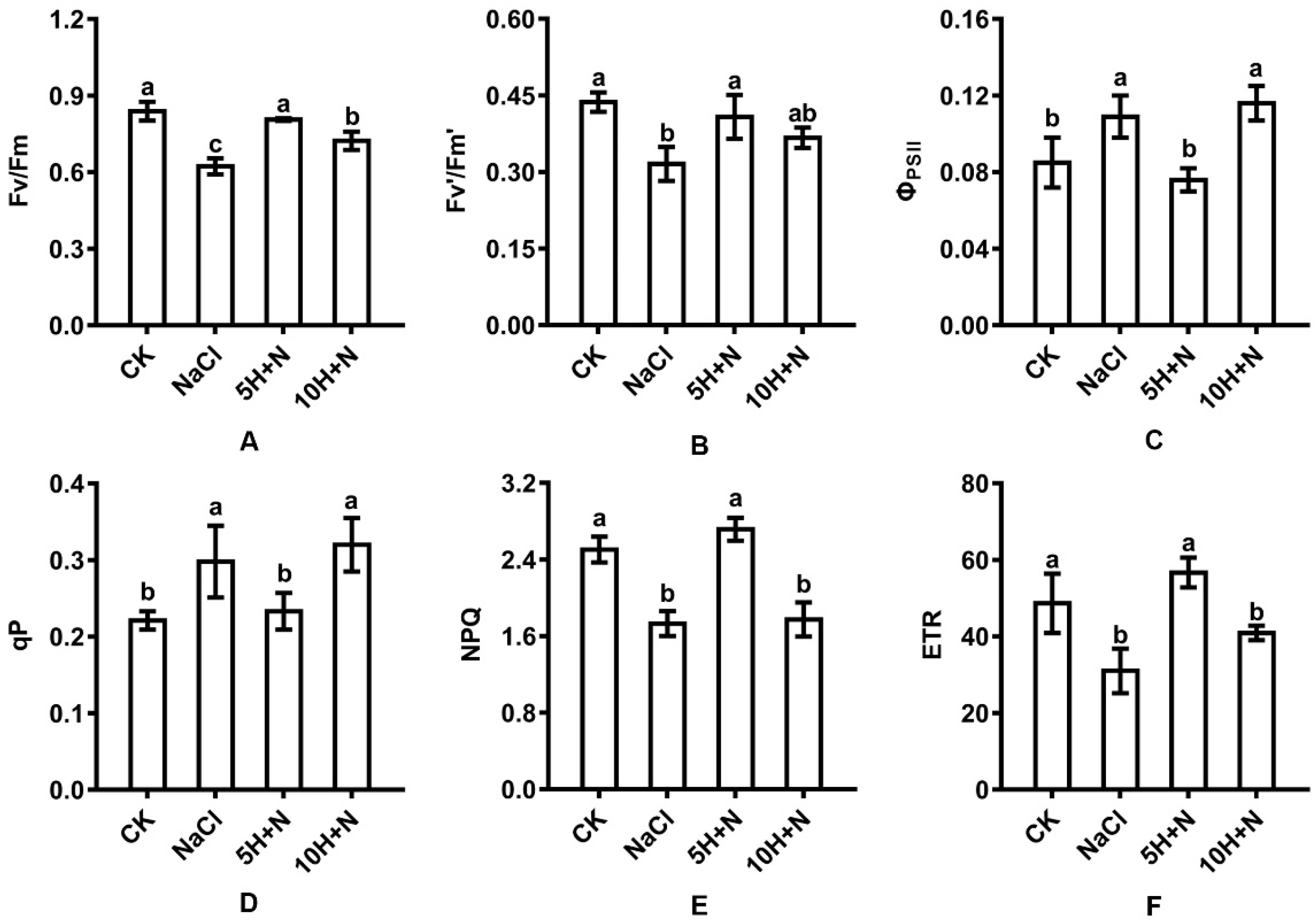
2.4. Effect of Exogenous H2O2 and NaCl Stress on Leaf Fluorescence Parameters
2.5. Effect of Exogenous H2O2 and NaCl Stress on Antioxidant Enzymes
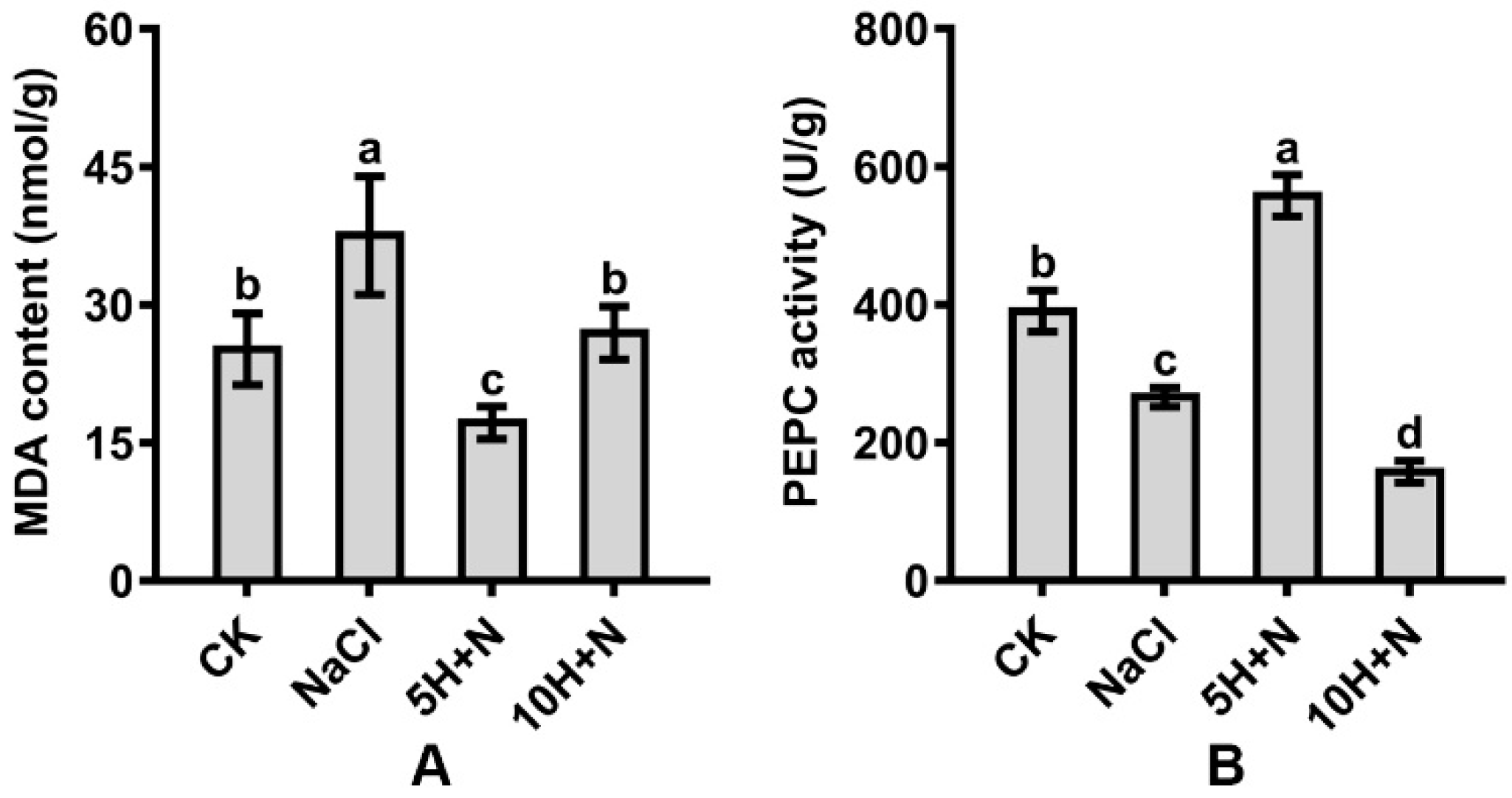
2.6. Effect of Exogenous H2O2 and NaCl Stress on MDA and Phosphoenolpyruvate Carboxylase (PEPC)
2.7. Effect of Exogenous H2O2 and NaCl Stress on Relative Expression of Stress Response Genes
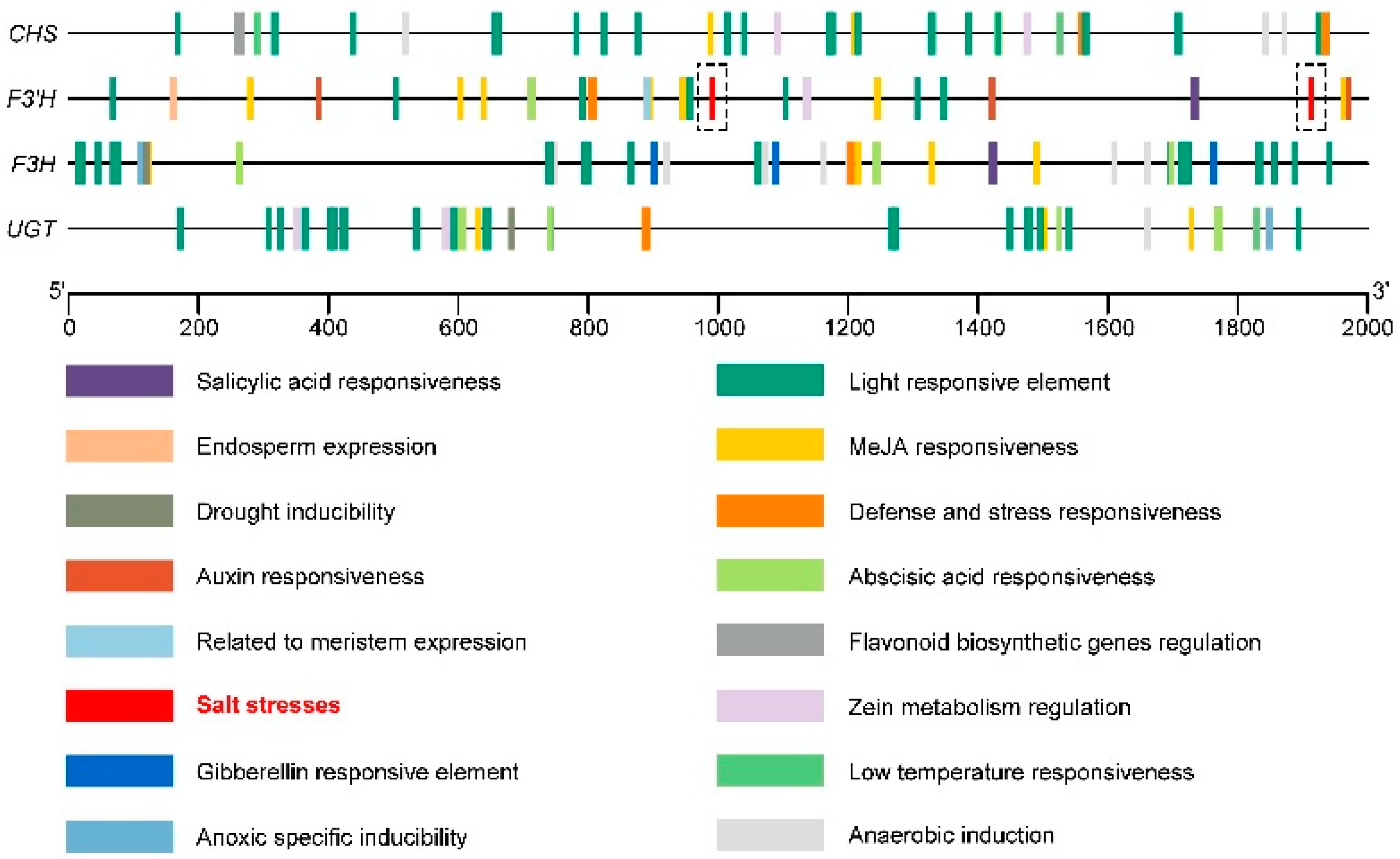
2.8. Effect of Exogenous H2O2 and NaCl Stress on Relative Expression of Key Genes of the Flavonoid Synthesis Pathway
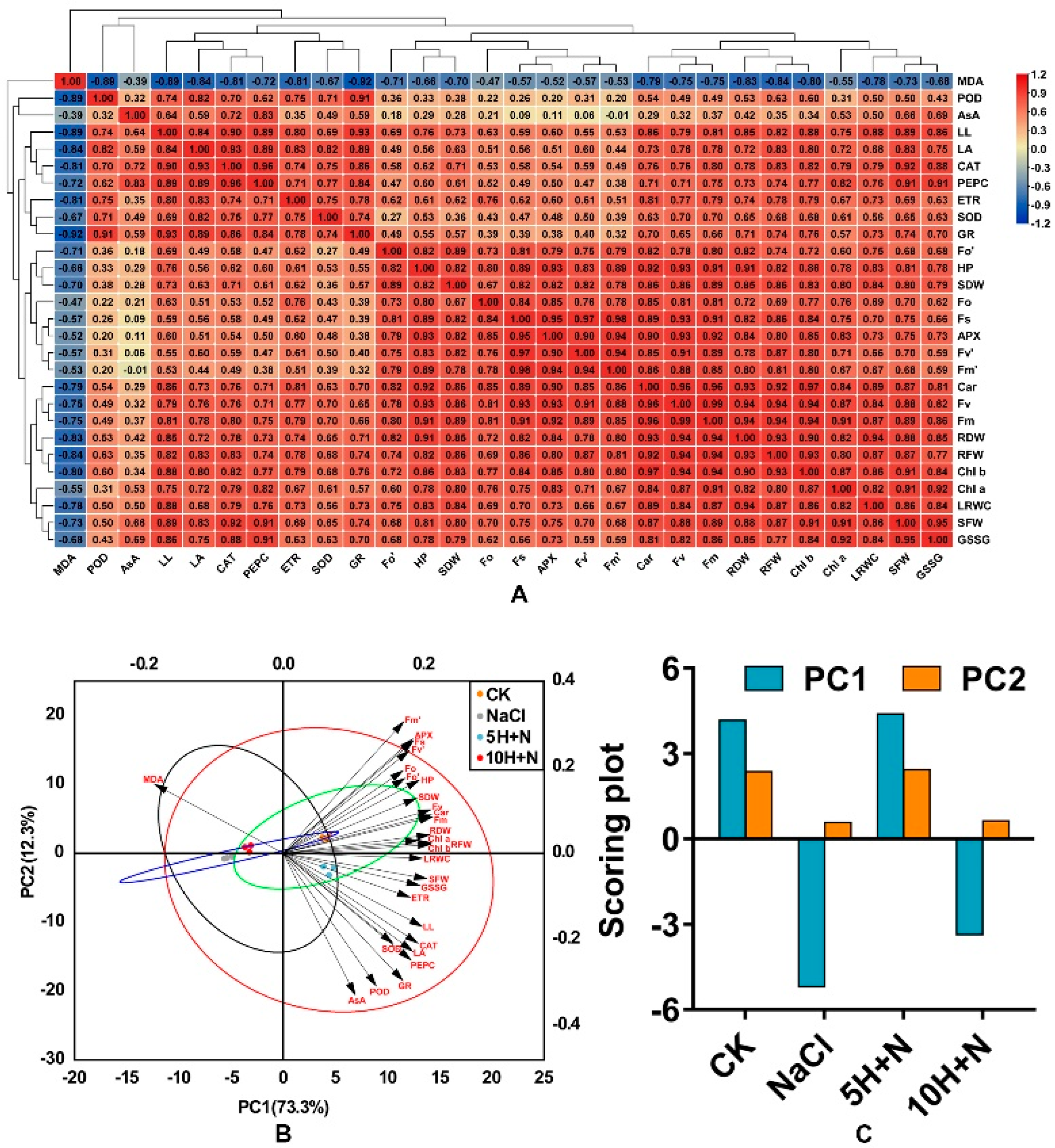
2.9. Correlation Analysis, Hierarchical Cluster Analysis, and Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Experiment Materials
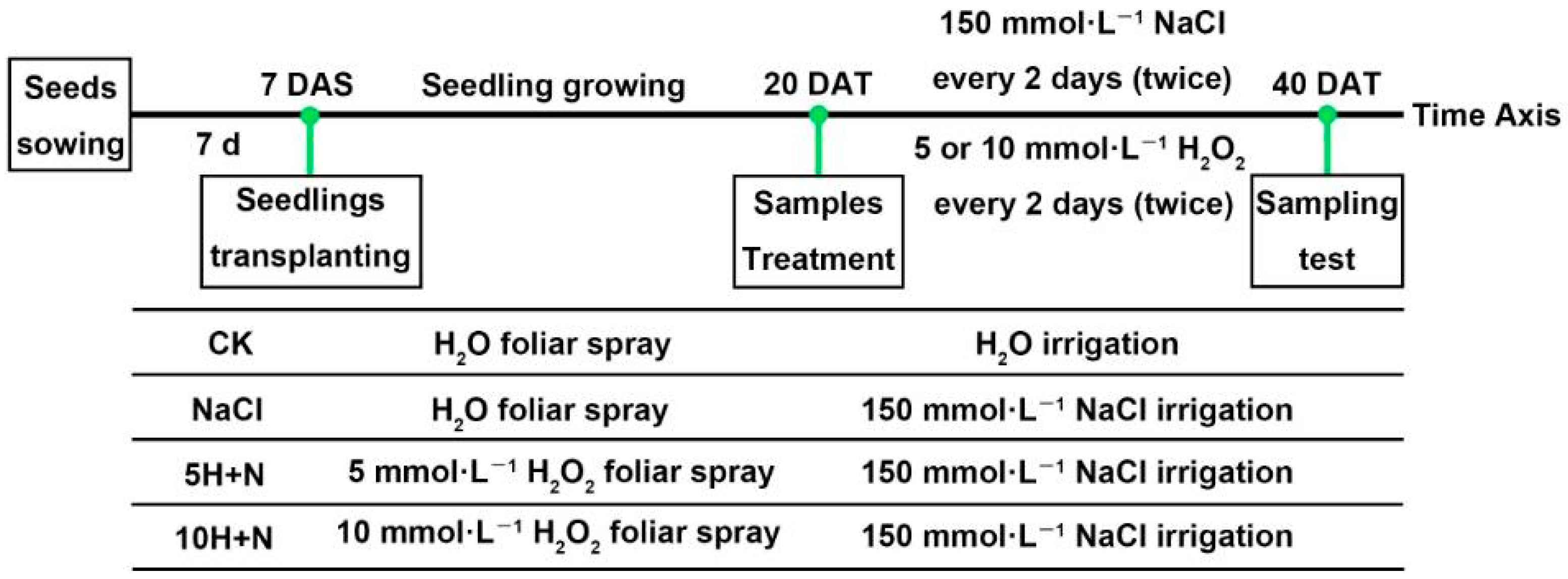
4.2. Experiment Design and Treatments
4.3. Measurement of Plant Height and Biomass
4.4. Determination of Leaf Phenotype and Relative Leaf Water Content
4.5. Determination of Photosynthetic Pigment Content
4.6. Measurement of Photosynthetic Fluorescence Parameters
4.7. Estimation of Biochemical Indicators
4.7.1. Antioxidant Enzyme Activity
4.7.2. Antioxidant Enzyme Content
4.7.3. Malondialdehyde Content
4.7.4. Phosphoenolpyruvate Carboxylase (PEPC) Activity
4.8. Total RNA Extraction and Reverse Transcription PCR (RT-PCR)
4.9. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.10. Prediction of Cis-Acting Element
4.11. Statistics and Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, X.; Zhou, M.; Ruan, J.; Peng, Y.; Yang, H.; Tang, Y.; Gao, A.; Cheng, J. Pretreatment with H2O2 Alleviates the Negative Impacts of NaCl Stress on Seed Germination of Tartary Buckwheat (Fagopyrum tataricum). Plants 2021, 10, 1784. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Tang, Y.; Fan, Y.; Li, W.; Lai, D.L.; Yan, M.L.; Zhang, K.X.; Zhou, M.L. Collection and taxonomic identification of rare germplasm resources of the genus Buckwheat in six provinces (regions) in western China. J. Plant Genet. Resour. 2021, 22, 963–970, (In Chinese with English abstract). [Google Scholar]
- Tan, Y.R.; Tao, B.B.; Guan, Y.F.; Ming, J.; Zhao, G.H. Research status and prospect of flavonoids in tartary buckwheat. Food Ind. Sci. Technol. 2012, 33, 377–381, (In Chinese with English abstract). [Google Scholar]
- Sun, Z.; Wang, X.; Liu, R.; Du, W.; Ma, M.; Han, Y.; Li, H.; Liu, L.; Hou, S. Comparative transcriptomic analysis reveals the regulatory mechanism of the gibberellic acid pathway of Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.) dwarf mutants. BMC Plant Biol. 2021, 21, 240. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ding, M.Q.; Zhang, K.X.; Yang, K.L.; Tang, Y.; Zhang, Z.W.; Fang, W.; Yan, J.; Zhou, M.L. Overview of Buckwheat Germplasm Resources. J. Plant Genet. Resour. 2019, 20, 813–828, (In Chinese with English abstract). [Google Scholar]
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat—the source of antioxidant activity in functional foods. Food Res. Int. 2022, 35, 207–211. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, M.; Sun, W.; Huang, L.; Wu, Q.; Bu, T.; Li, C.; Chen, H. Genome-wide identification and expression analysis of the trihelix transcription factor family in tartary buckwheat (Fagopyrum tataricum). BMC Plant Biol. 2019, 19, 344. [Google Scholar] [CrossRef]
- Martin, N.G.; Eaves, L.J.; Heath, A.C. Prospects for detecting genotype X environment interactions in twins with breast cancer. Acta Genet. Med. Gemellol. 1987, 36, 5–20. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Kravcov, N.; Schaffasz, A.; Snowdon, R.J.; Wittkop, B.; Windpassinger, S. Genetic Architecture of Novel Sources for Reproductive Cold Tolerance in Sorghum. Front. Plant Sci. 2021, 12, 772177. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2015, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Karle, S.B.; Guru, A.; Dwivedi, P.; Kumar, K. Insights into the Role of Gasotransmitters Mediating Salt Stress Responses in Plants. J. Plant Growth Regul. 2021, 40, 2259–2275. [Google Scholar] [CrossRef]
- Kumar, P.; Choudhary, M.; Halder, T.; Prakash, N.R.; Singh, V.; Vineeth, T.V.; Sheoran, S.; Ravikiran, K.T.; Longmei, N.; Rakshit, S.; et al. Salinity stress tolerance and omics approaches: Revisiting the progress and achievements in major cereal crops. Heredity 2022, 128, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.Q.; Wang, D.; Wang, J.R.; Lan, M.; Zhao, J.; Dong, S.Q.; Song, X.E.; Alam, S.; Yuan, X.Y.; Wang, Y.G.; et al. Effects of exogenous nitric oxide on sorghum seed germination and starch transformation under salt stress. Chin. Agric. Sci. 2019, 52, 4119–4128, (In Chinese with English abstract). [Google Scholar]
- Song, C.; Zhang, Y.; Chen, R.; Zhu, F.; Wei, P.; Pan, H.; Chen, C.; Dai, J. Label-Free Quantitative Proteomics Unravel the Impacts of Salt Stress on Dendrobium huoshanense. Front. Plant Sci. 2022, 13, 874579. [Google Scholar] [CrossRef]
- Noreen, S.; Sultan, M.; Akhter, M.S.; Shah, K.H.; Ummara, U.; Manzoor, H.; Ulfat, M.; Alyemeni, M.N.; Ahmad, P. Foliar fertigation of ascorbic acid and zinc improves growth, antioxidant enzyme activity and harvest index in barley (Hordeum vulgare L.) grown under salt stress. Plant Physiol. Biochem. 2021, 158, 244–254. [Google Scholar] [CrossRef]
- Li, Z.; Geng, W.; Tan, M.; Ling, Y.; Zhang, Y.; Zhang, L.; Peng, Y. Differential Responses to Salt Stress in Four White Clover Genotypes Associated with Root Growth, Endogenous Polyamines Metabolism, and Sodium/Potassium Accumulation and Transport. Front. Plant Sci. 2022, 13, 896436. [Google Scholar] [CrossRef]
- Sheikh-Mohamadi, M.H.; Etemadi, N.; Aalifar, M.; Pessarakli, M. Salt stress triggers augmented levels of Na+, K+ and ROS alters salt-related gene expression in leaves and roots of tall wheatgrass (Agropyron elongatum). Plant Physiol. Biochem. 2022, 183, 9–22. [Google Scholar] [CrossRef]
- Cembrowska-Lech, D. Tissue Printing and Dual Excitation Flow Cytometry for Oxidative Stress-New Tools for Reactive Oxygen Species Research in Seed Biology. Int. J. Mol. Sci. 2022, 21, 8656. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shu, S.; Li, C.; Sun, J.; Guo, S. Spermidine-mediated hydrogen peroxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol. Biochem. 2018, 128, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Shan, W.; Zhao, G.P.; Lyu, L.D. H2O2 concentration-dependent kinetics of gene expression: Linking the intensity of oxidative stress and mycobacterial physiological adaptation. Emerg. Microbes Infect. 2022, 11, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Kaviraj, M.; Panneerselvam, P.; Priya, H.; Chakraborty, K.; Swain, P.; Chatterjee, S.N.; Sharma, S.G.; Nayak, P.K.; Nayak, A.K. Ascorbic acid formulation for survivability and diazotrophic efficacy of Azotobacter chroococcum Avi2 (MCC 3432) under hydrogen peroxide stress and its role in plant-growth promotion in rice (Oryza sativa L.). Plant Physiol. Biochem. 2019, 139, 419–427. [Google Scholar] [CrossRef]
- Ren, Y.F.; He, J.Y.; Yang, J.; Wei, Y.J. Effects of exogenous H2O2 on seed germination and seedling physiological characteristics of Chinese cabbage under salt stress. Acta Ecol. Sin. 2019, 39, 7745–7756, (In Chinese with English abstract). [Google Scholar]
- Lomovatskaya, L.A.; Kuzakova, O.V.; Goncharova, A.M.; Romanenko, A.S. Implication of cAMP in Regulation of Hydrogen Peroxide Level in Pea Seedling Roots under Biotic Stress. Russ. J. Plant Physiol. 2020, 67, 435–442. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Gao, Y.; Wang, C.; Guo, G.; Luo, Y.; Huang, Y.; Hu, W.; Sheteiwy, M.S.; Guan, Y.; et al. The Synergistic Priming Effect of Exogenous Salicylic Acid and H2O2 on Chilling Tolerance Enhancement during Maize (Zea mays L.) Seed Germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef]
- Khan, M.I.; Khan, N.A.; Masood, A.; Per, T.S.; Asgher, M. Hydrogen Peroxide Alleviates Nickel-Inhibited Photosynthetic Responses through Increase in Use-Efficiency of Nitrogen and Sulfur, and Glutathione Production in Mustard. Front. Plant Sci. 2016, 7, 44. [Google Scholar] [CrossRef]
- Yao, X.; Liu, T.T.; Ruan, J.J.; Peng, Y.; Tang, Y.; Yang, H.; Gao, A.J.; Cheng, J.P. Effects of seed soaking with H2O2 on root growth and antioxidant enzyme activity of tartary buckwheat seedlings. Mol. Plant Breed. 2021, 9, 1–17. [Google Scholar]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Kempen, B.; de Sousa, L. Global mapping of soil salinity change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- Xu, Y.; Magwanga, R.O.; Cai, X.; Zhou, Z.; Wang, X.; Wang, Y.; Zhang, Z.; Jin, D.; Guo, X.; Wei, Y.; et al. Deep Transcriptome Analysis Reveals Reactive Oxygen Species (ROS) Network Evolution, Response to Abiotic Stress, and Regulation of Fiber Development in Cotton. Int. J. Mol. Sci. 2019, 20, 1863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, Q.; Zheng, J.; Li, Z.; Li, Y.; Feng, Y.; Han, Y.; Li, Y. GmLecRlk, a Lectin Receptor-like Protein Kinase, Contributes to Salt Stress Tolerance by Regulating Salt-Responsive Genes in Soybean. Int. J. Mol. Sci. 2022, 23, 1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Xu, S. Is salinity the main ecological factor that influences foliar nutrient resorption of desert plants in a hyper-arid environment? BMC Plant Biol. 2020, 20, 461. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elyousr, K.; Mousa, M.; Ibrahim, O.; Alshareef, N.O.; Eissa, M.A. Calcium-Rich Biochar Stimulates Salt Resistance in Pearl Millet (Pennisetum glaucum L.) Plants by Improving Soil Quality and Enhancing the Antioxidant Defense. Plants 2022, 11, 1301. [Google Scholar] [CrossRef] [PubMed]
- Cocozza, C.; Bartolini, P.; Brunetti, C.; Miozzi, L.; Pignattelli, S.; Podda, A.; Scippa, G.S.; Trupiano, D.; Rotunno, S.; Brilli, F.; et al. Modulation of class III peroxidase pathways and phenylpropanoids in Arundo donax under salt and phosphorus stress. Plant Physiol. Biochem. 2022, 183, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Borrajo, C.I.; Sánchez-Moreiras, A.M.; Reigosa, M.J. Ecophysiological Responses of Tall Wheatgrass Germplasm to Drought and Salinity. Plants 2022, 11, 1548. [Google Scholar] [CrossRef]
- Bhardwaj, R.D.; Singh, N.; Sharma, A.; Joshi, R.; Srivastava, P. Hydrogen peroxide regulates antioxidant responses and redox related proteins in drought stressed wheat seedlings. Physiol. Mol. Biol. Plants 2021, 27, 151–163. [Google Scholar] [CrossRef]
- Ellouzi, H.; Oueslati, S.; Hessini, K.; Rabhi, M.; Abdelly, C. Seed-priming with H2O2 alleviates subsequent salt stress by preventing ROS production and amplifying antioxidant defense in cauliflower seeds and seedlings. Sci. Hortic. 2021, 288, 110360. [Google Scholar] [CrossRef]
- Park, H.S.; Kazerooni, E.A.; Kang, S.M.; Al-Sadi, A.M.; Lee, I.J. Melatonin Enhances the Tolerance and Recovery Mechanisms in Brassica juncea (L.) Czern. Under Saline Conditions. Front. Plant Sci. 2021, 12, 593717. [Google Scholar] [CrossRef]
- Verslues, P.E.; Kim, Y.S.; Zhu, J.K. Altered ABA, proline and hydrogen peroxide in an Arabidopsis glutamate: Glyoxylate aminotransferase mutant. Plant Mol. Biol. 2007, 64, 205–217. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Jawad Hassan, M.; Ali Raza, M.; Khan, I.; Ahmad Meraj, T.; Ahmed, M.; Abbas Shah, G.; Ansar, M.; Awan, S.A.; Khan, N.; Iqbal, N.; et al. Selenium and Salt Interactions in Black Gram (Vigna mungo L.): Ion Uptake, Antioxidant Defense System, and Photochemistry Efficiency. Plants 2020, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Ou, X.B.; Wang, J.C. Effects of exogenous hydrogen peroxide on chlorophyll fluorescence parameters and photosynthetic carbon assimilation enzymes activities in naked oat seedlings under lanthanum stress. Acta Ecol. Sin. 2019, 39, 2833–2841, (In Chinese with English abstract). [Google Scholar]
- He, X.; Richmond, M.; Williams, D.V.; Zheng, W.; Wu, F. Exogenous Glycinebetaine Reduces Cadmium Uptake and Mitigates Cadmium Toxicity in Two Tobacco Genotypes Differing in Cadmium Tolerance. Int. J. Mol. Sci. 2019, 20, 1612. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, Y.F.; Li, F.X.; Xiao, M.J.; Tao, Y.; Xu, W.J.; Huang, R.D. Effects of salt stress on photosynthesis and fluorescence characteristics of sorghum seedlings. Chin. Agric. Sci. 2012, 45, 3265–3272, (In Chinese with English abstract). [Google Scholar]
- Rapacz, M. Chlorophyll a fluorescence transient during freezing and recovery in winter wheat. Photosynthetica 2007, 45, 409–418. [Google Scholar] [CrossRef]
- Sixto, H.; Aranda, I.; Grau, J.M. Assessment of salt tolerance in Populus alba clones using chlorophyll fluorescence. Photosynthetica 2006, 44, 169–173. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Sun, Y.; Zheng, S.; Wang, J.; Zhang, T. Hydrogen peroxide is involved in strigolactone induced low temperature stress tolerance in rape seedlings (Brassica rapa L.). Plant Physiol. Biochem. 2020, 157, 402–415. [Google Scholar] [CrossRef]
- Guo, J.; Shan, C.; Zhang, Y.; Wang, X.; Tian, H.; Han, G.; Zhang, Y.; Wang, B. Mechanisms of Salt Tolerance and Molecular Breeding of Salt-Tolerant Ornamental Plants. Front. Plant Sci. 2022, 13, 854116. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Papolu, P.K.; Satish, L.; Vinod, K.K.; Wei, Q.; Sharma, A.; Emamverdian, A.; Zou, L.H.; Zhou, M. Redox status of the plant cell determines epigenetic modifications under abiotic stress conditions and during developmental processes. J. Adv. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H. Selenium Promotes the Growth and Photosynthesis of Tomato Seedlings Under Salt Stress by Enhancing Chloroplast Antioxidant Defense System. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Correia, P.; da Silva, A.B.; Vaz, M.; Carmo-Silva, E.; Marques da Silva, J. Efficient Regulation of CO2 Assimilation Enables Greater Resilience to High Temperature and Drought in Maize. Front. Plant Sci. 2021, 12, 675546. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhong, X.; Cong, Y.; Wang, T.; Yang, S.; Li, Y.; Gai, J. Genome-wide Analysis of Phosphoenolpyruvate Carboxylase Gene Family and Their Response to Abiotic Stresses in Soybean. Sci. Rep. 2016, 6, 38448. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, C.; Hou, L.; Wu, X.; Wang, D.; Zhang, L.; Liu, P. Exogenous SA Affects Rice Seed Germination under Salt Stress by Regulating Na+/K+ Balance and Endogenous GAs and ABA Homeostasis. Int. J. Mol. Sci. 2022, 23, 3293. [Google Scholar] [CrossRef]
- Nutan, K.K.; Kumar, G.; Singla-Pareek, S.L.; Pareek, A. A Salt Overly Sensitive Pathway Member from Brassica juncea BjSOS3 Can Functionally Complement ΔAtsos3 in Arabidopsis. Curr. Genomics 2018, 19, 60–69. [Google Scholar] [CrossRef]
- Chung, J.S.; Zhu, J.K.; Bressan, R.A.; Hasegawa, P.M.; Shi, H. Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J. 2008, 53, 554–565. [Google Scholar] [CrossRef]
- Fan, W.; Deng, G.; Wang, H.; Zhang, H.; Zhang, P. Elevated compartmentalization of Na+ into vacuoles improves salt and cold stress tolerance in sweet potato (Ipomoea batatas). Physiol. Plant. 2015, 154, 560–571. [Google Scholar] [CrossRef]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen peroxide modulate photosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef]
- Asgher, M.; Ahmed, S.; Sehar, Z.; Gautam, H.; Gandhi, S.G.; Khan, N.A. Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa L.). J. Hazard. Mater. 2021, 401, 123365. [Google Scholar] [CrossRef]
- Su, X.; Fan, X.; Shao, R.; Guo, J.; Wang, Y.; Yang, J.; Yang, Q.; Guo, L. Physiological and iTRAQ-based proteomic analyses reveal that melatonin alleviates oxidative damage in maize leaves exposed to drought stress. Plant Physiol. Biochem. 2019, 142, 263–274. [Google Scholar] [CrossRef]
- Hossain, M.S.; Li, J.; Sikdar, A.; Hasanuzzaman, M.; Uzizerimana, F.; Muhammad, I.; Yuan, Y.; Zhang, C.; Wang, C.; Feng, B. Exogenous Melatonin Modulates the Physiological and Biochemical Mechanisms of Drought Tolerance in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). Molecules 2020, 25, 2828. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Q.F.; Li, J.; Xiong, J.; Zhou, L.N.; He, S.L.; Zhang, J.Q.; Chen, Z.A.; He, S.G.; Liu, H. Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Sci. Rep. 2019, 9, 7397. [Google Scholar] [CrossRef]
- Kong, L.; Huo, H.; Mao, P. Antioxidant response and related gene expression in aged oat seed. Front. Plant Sci. 2015, 6, 158. [Google Scholar] [CrossRef]
- Devi, S.R.; Prasad, M.N. Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: Response of antioxidant enzymes and antioxidants. Plant Sci. 1998, 138, 157–165. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Aoyagi, K.; Bassham, J.A. Pyruvate orthophosphate dikinase in wheat leaves. Plant Physiol. 1983, 73, 853–854. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]




| Primer Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Functions |
|---|---|---|---|
| FtH3 | AATTCGCAAGTACCAGAAG | CCAACAAGGTATGCCTCAGC | Actin |
| FtNHX1 | CGTTGCTAGGACGCAATGTTCCA | ACAGTCCACGTCGGATGCCTTAT | Stress-related genes |
| FtSOS1 | CCTTACACCGTACCCGCTC | CCGGAAGAAACACAGCCAACA | |
| FtNAC6 | GATTCAATTCCCCGGCTCCA | AACGGGGACAACTCATTCCC | |
| FtNAC9 | CTGAGGGTGTAATTCCGGGT | TCAACGGTAGGGGTAGAAGC | |
| FtWRKY46 | TGTTCCGCCTTCTGATGGTT | CAGCACTGTGGGGTCATCAT | |
| FtbZIP83 | ACCGAGTATTCCGCAAGCTC | AACTCTCCCCAAAACCCACC | |
| CAT | GAGTTTGGTTCCCTTGCTT | TTCATACACTTCACTGGCGT | Enzyme-related genes |
| POD | GTTCTGGTTGGGCTTGG | TTGTCCTCGTCTGTTGGTC | |
| CuSOD | ATGGTGCTCCTGACGATG | CCACTGCCCTTCCAATAAT | |
| MnSOD | GTCTACGGTCCTCCTTCTACAT | TAACAACAGCACACTTCTTTCT | |
| APX | TACTCCGAGGTTGTGTGCC | CAATCAAGGTGTTCCAGTCA | |
| GR | TTGAGTGGAGAGAAGGAAGG | CATAGTCGGCAAAGAAAGC | |
| PEPC | AAGTCTCCACATTCGGTCTC | ATCTCCAAGTGCCTGGTTAT | |
| CHS | GAGGAGATCAGAAAGGCACAAAGGG | GTCGGCTTGGTAGATACAGTTAGGC | Flavonoid-synthesis-related genes |
| F3′H | TCAAGGAGAATGGCGGAGTT | TGGGTGGTTCAGGAGGAGTG | |
| F3H | GCCTGTTGAGGGTGCCTTTGTC | TGGCGATTGAAAGACGGCTGAAG | |
| UGT | CAGCTTCTTCACCACCGAATCCTC | TCTCGCCCGCTAACCCATCTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Zhou, M.; Ruan, J.; Peng, Y.; Ma, C.; Wu, W.; Gao, A.; Weng, W.; Cheng, J. Physiological and Biochemical Regulation Mechanism of Exogenous Hydrogen Peroxide in Alleviating NaCl Stress Toxicity in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). Int. J. Mol. Sci. 2022, 23, 10698. https://doi.org/10.3390/ijms231810698
Yao X, Zhou M, Ruan J, Peng Y, Ma C, Wu W, Gao A, Weng W, Cheng J. Physiological and Biochemical Regulation Mechanism of Exogenous Hydrogen Peroxide in Alleviating NaCl Stress Toxicity in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). International Journal of Molecular Sciences. 2022; 23(18):10698. https://doi.org/10.3390/ijms231810698
Chicago/Turabian StyleYao, Xin, Meiliang Zhou, Jingjun Ruan, Yan Peng, Chao Ma, Weijiao Wu, Anjing Gao, Wenfeng Weng, and Jianping Cheng. 2022. "Physiological and Biochemical Regulation Mechanism of Exogenous Hydrogen Peroxide in Alleviating NaCl Stress Toxicity in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn)" International Journal of Molecular Sciences 23, no. 18: 10698. https://doi.org/10.3390/ijms231810698
APA StyleYao, X., Zhou, M., Ruan, J., Peng, Y., Ma, C., Wu, W., Gao, A., Weng, W., & Cheng, J. (2022). Physiological and Biochemical Regulation Mechanism of Exogenous Hydrogen Peroxide in Alleviating NaCl Stress Toxicity in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). International Journal of Molecular Sciences, 23(18), 10698. https://doi.org/10.3390/ijms231810698







