Morphofunctional State and Circadian Rhythms of the Liver of Female Rats under the Influence of Chronic Alcohol Intoxication and Constant Lighting
Abstract
:1. Introduction
2. Results
2.1. Influence of Constant Lighting and CAI on the Morphofunctional Condition of the Liver
2.2. Influence of Constant Lighting and CAI on the Micromorphometric Parameters of Hepatocytes of Rats
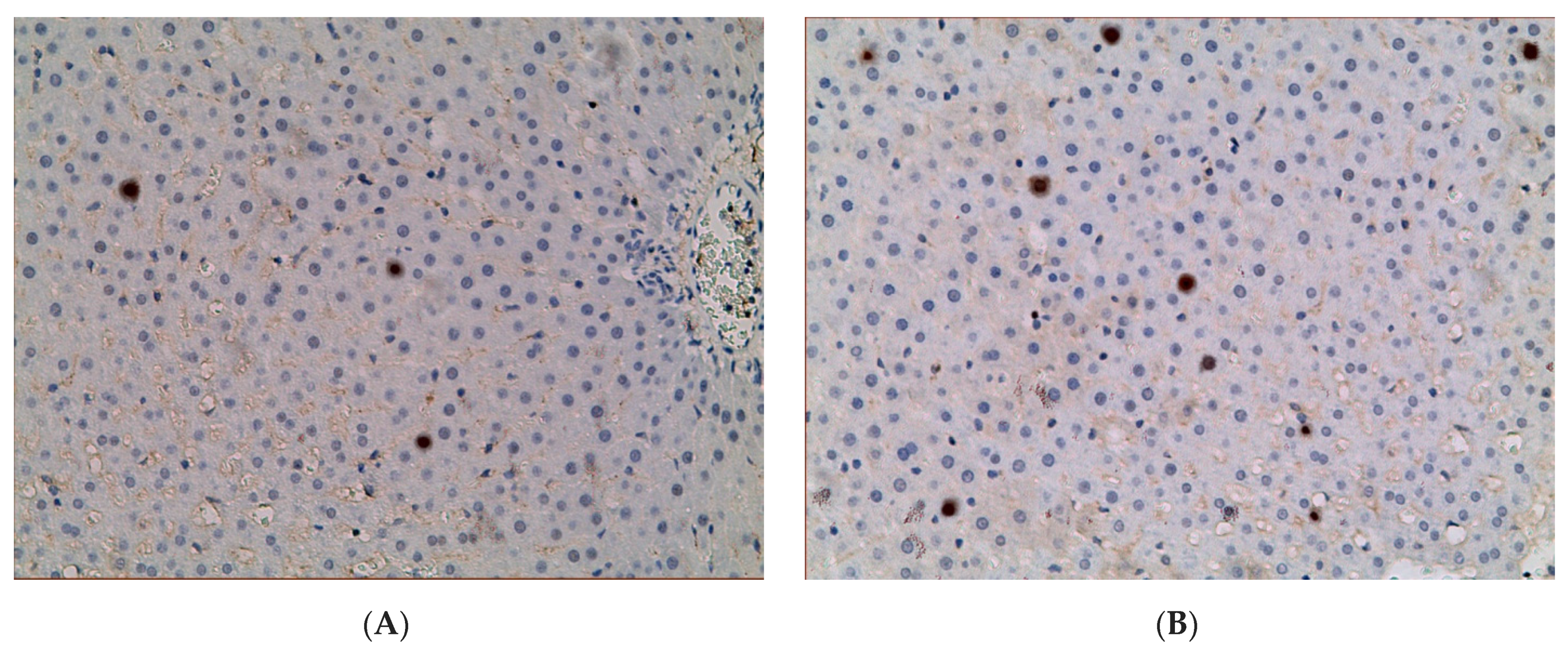
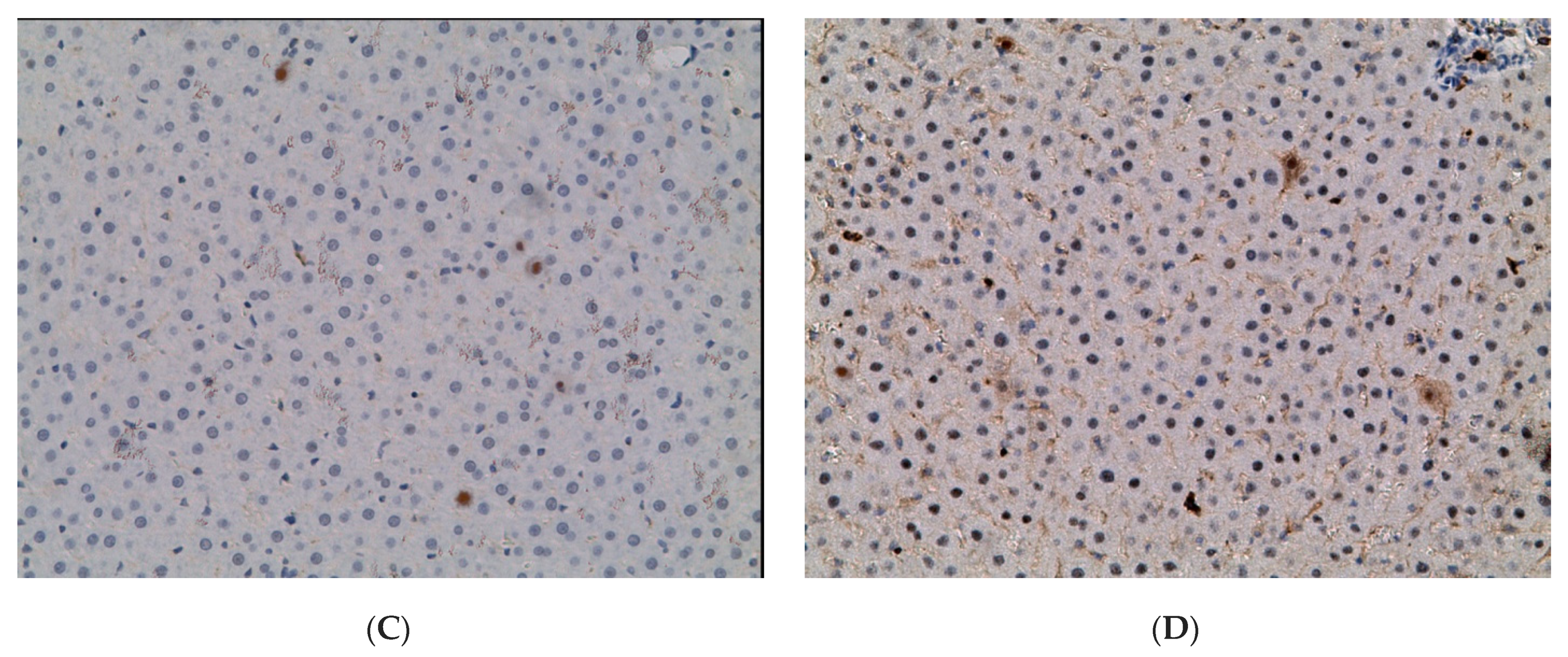
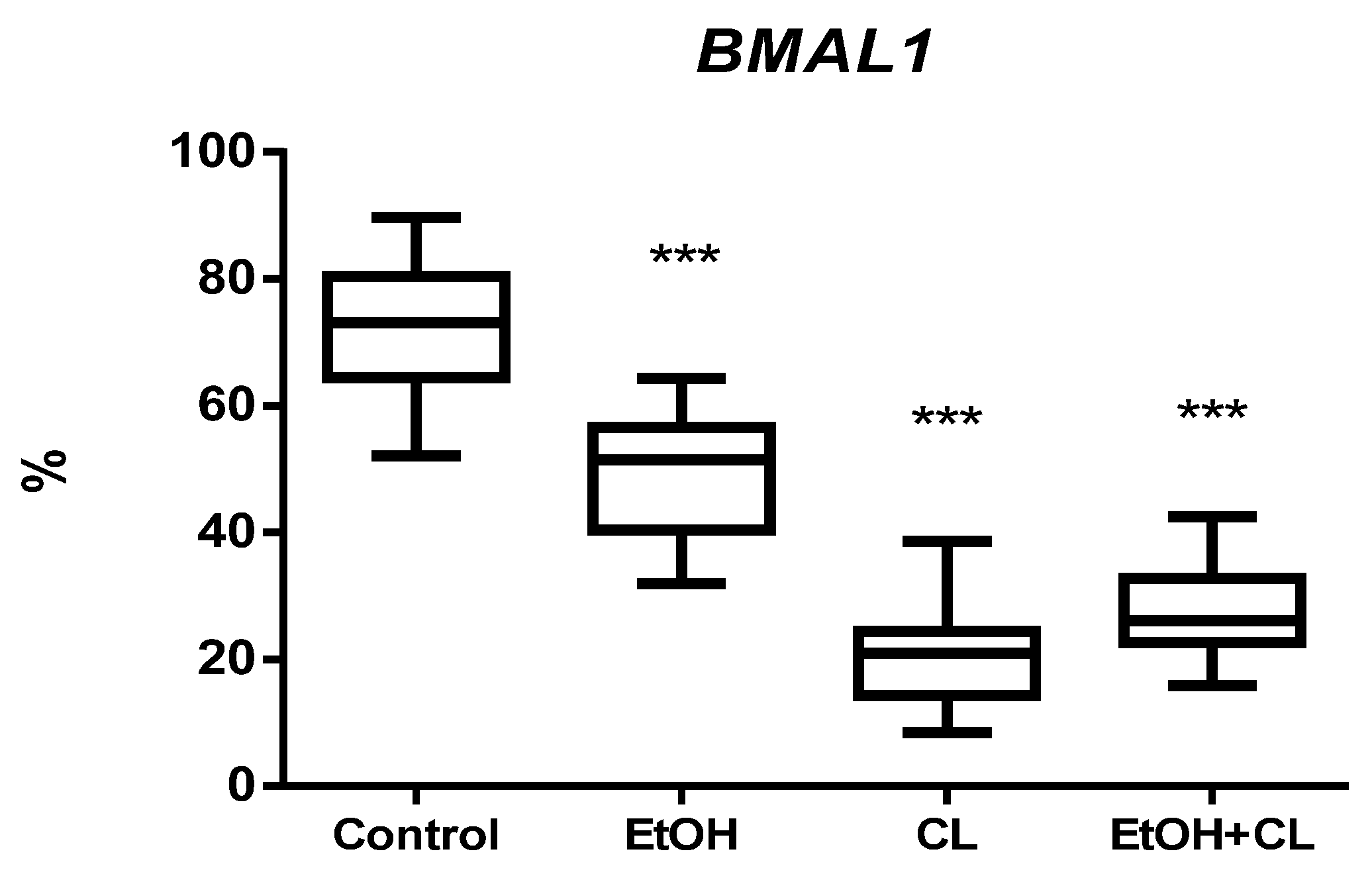
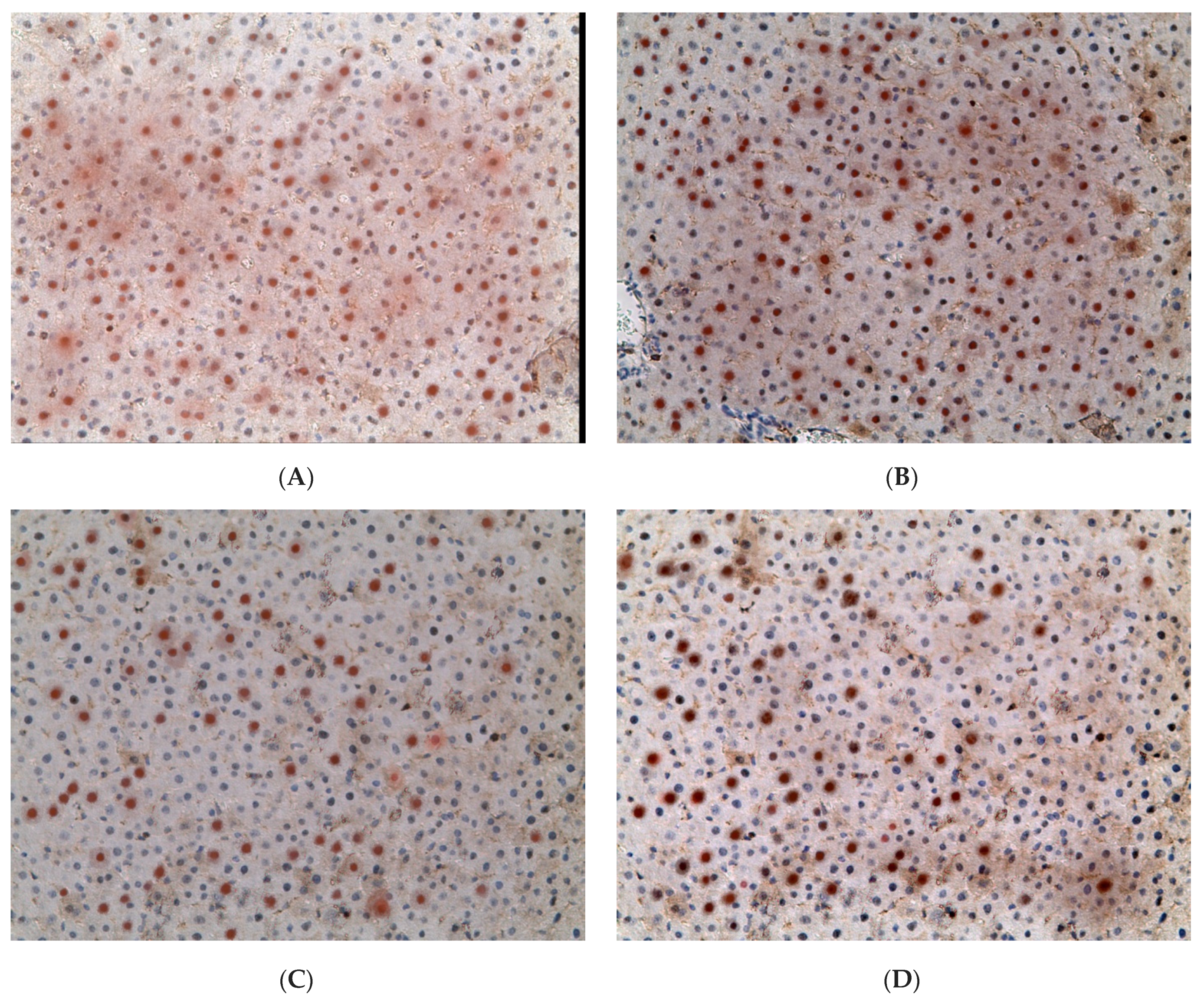
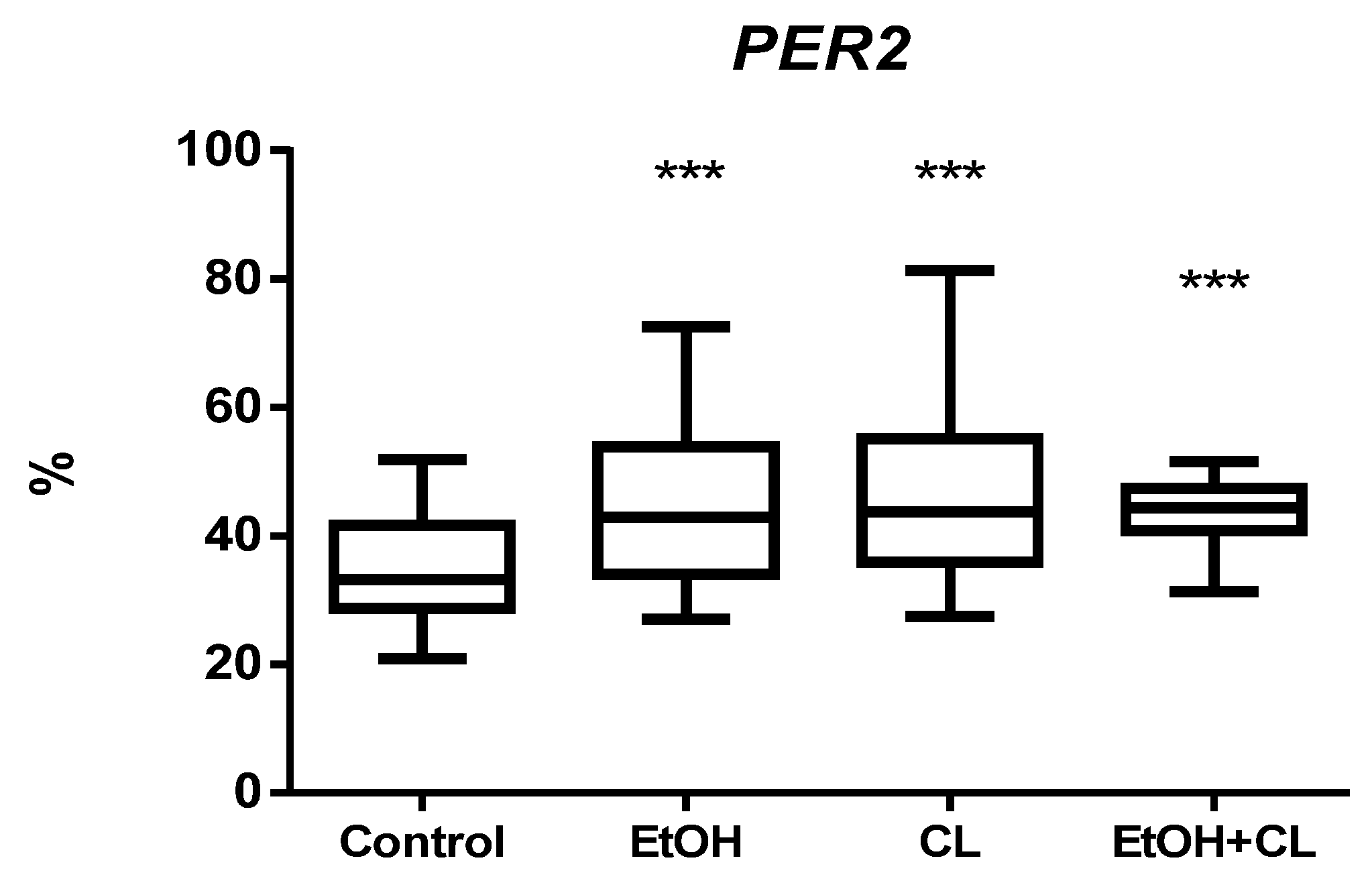
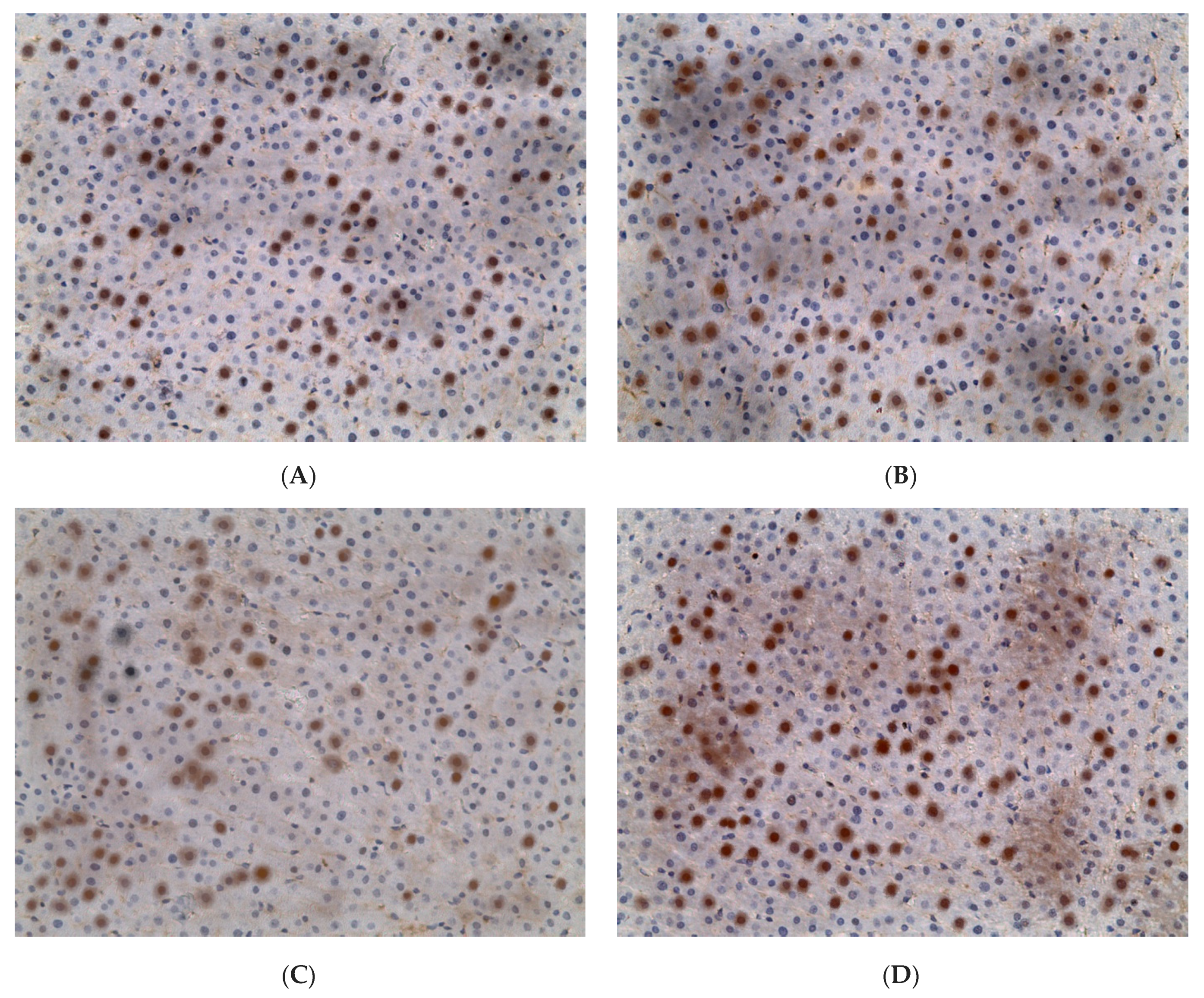
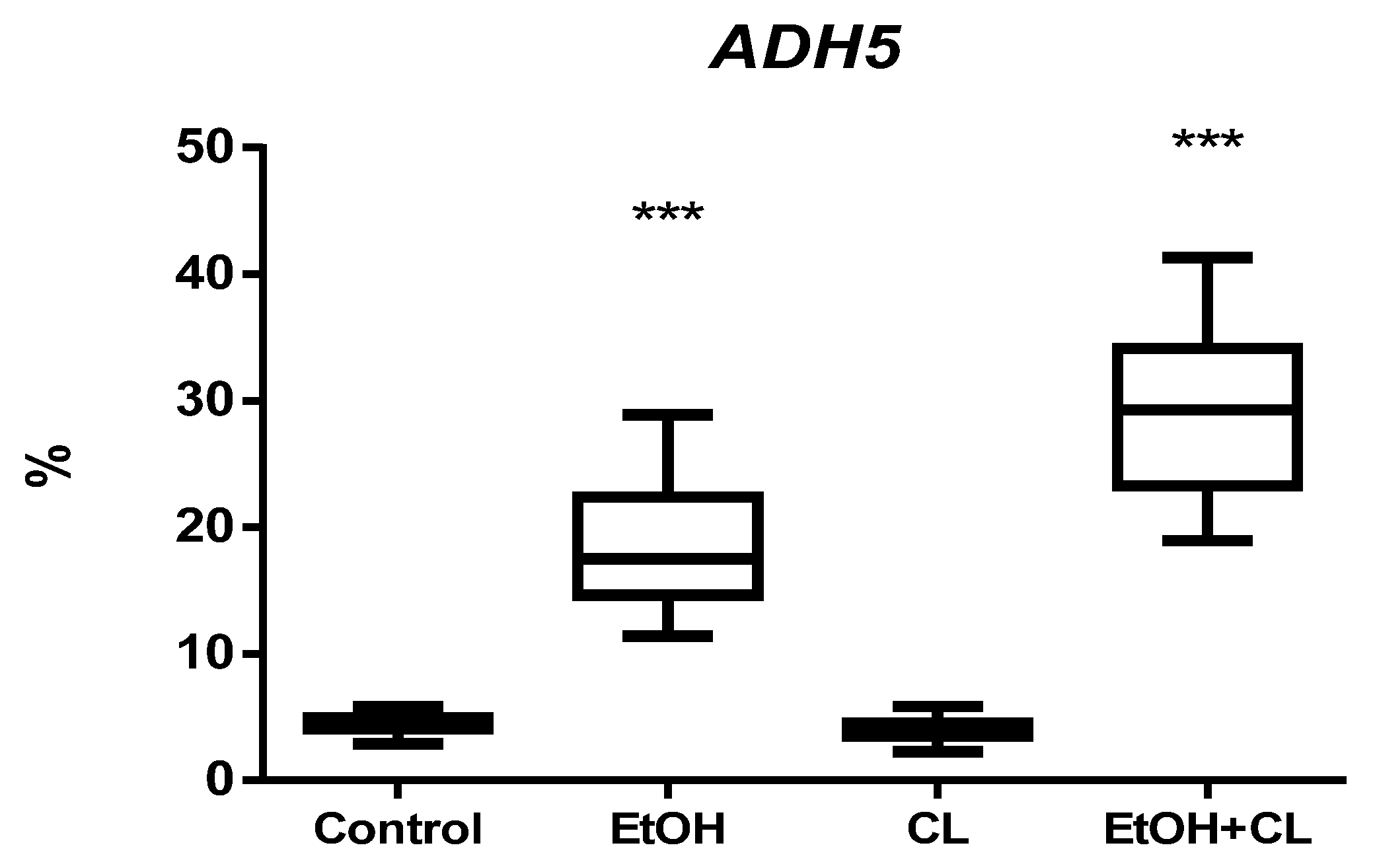
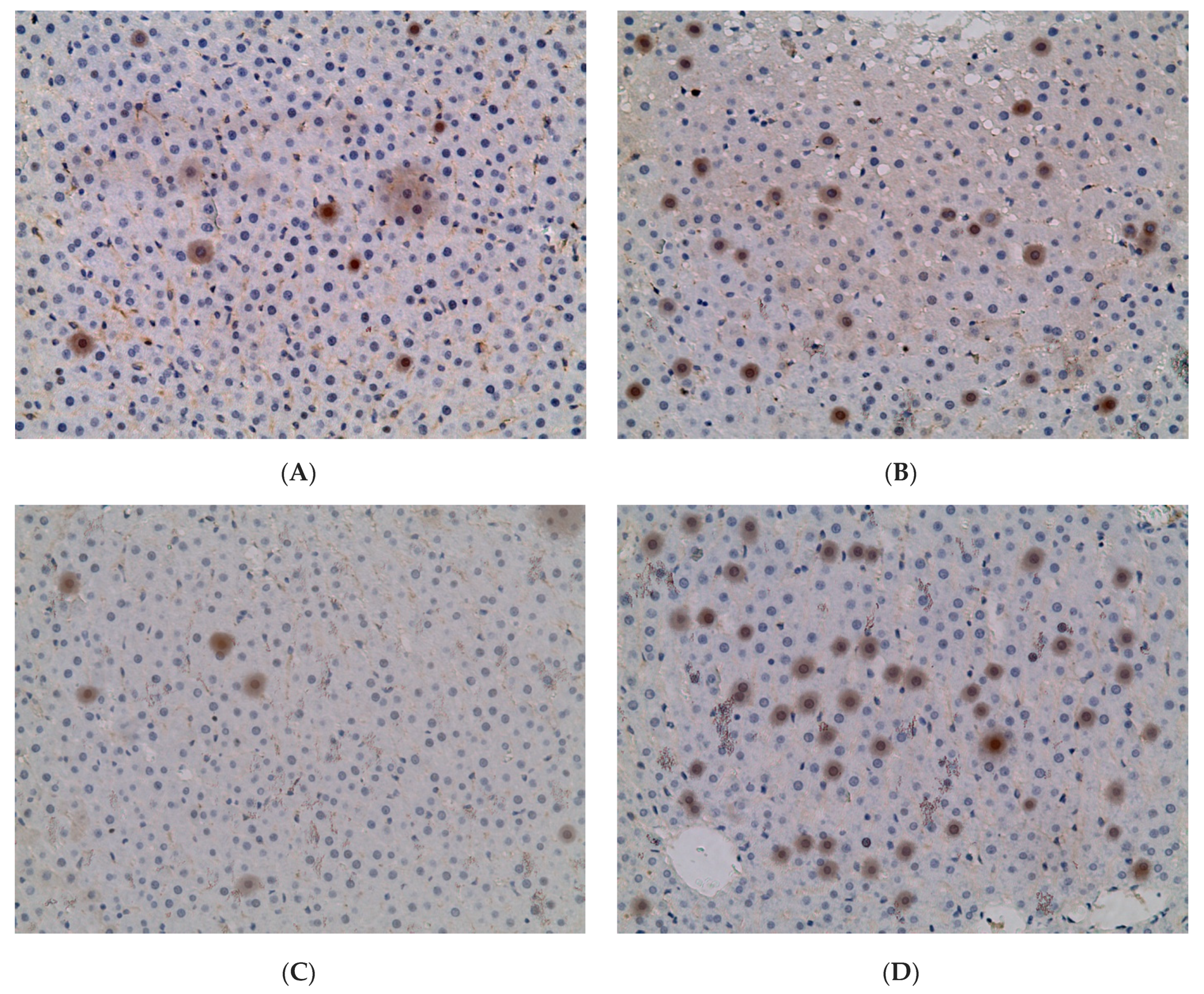
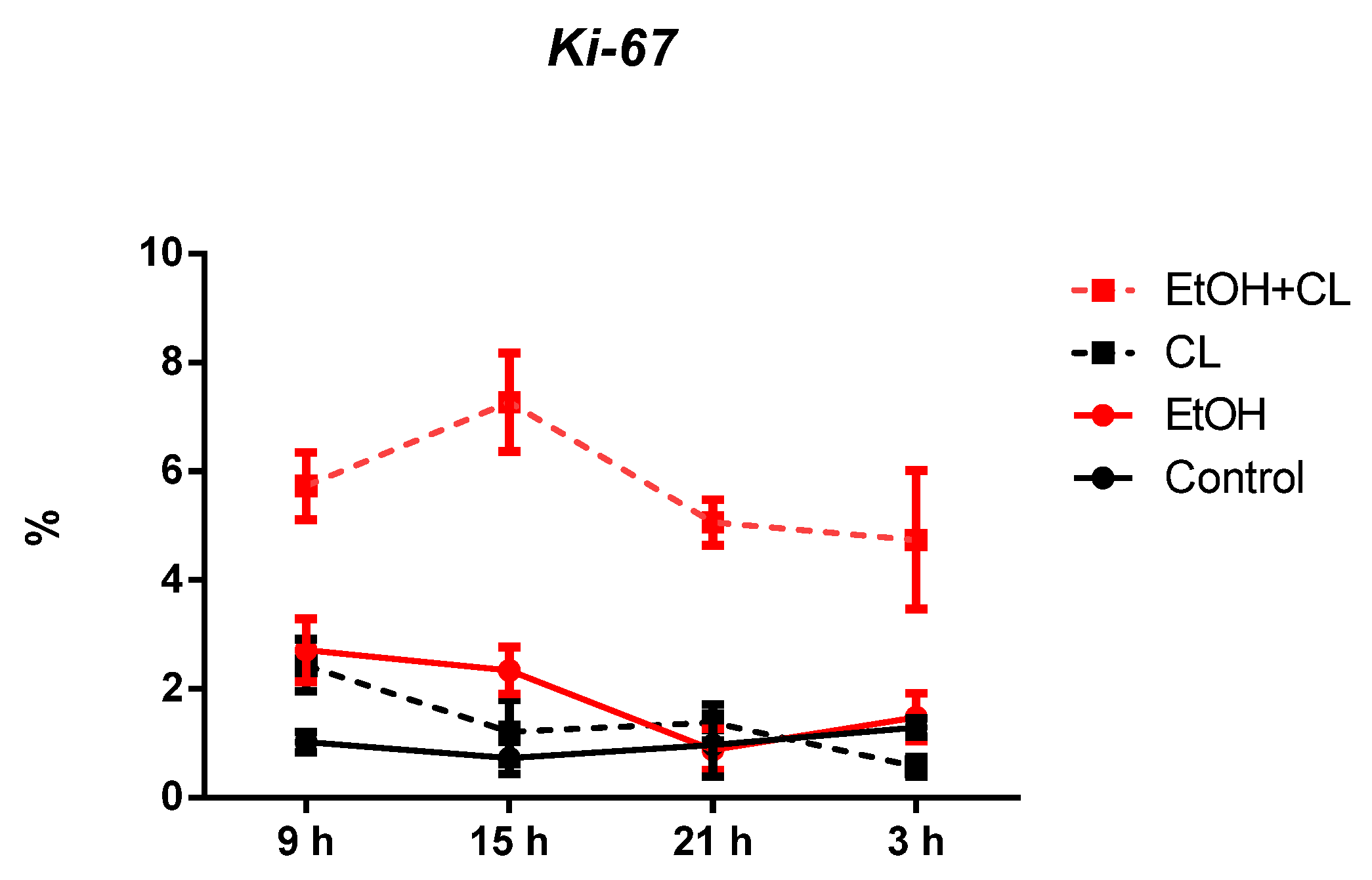
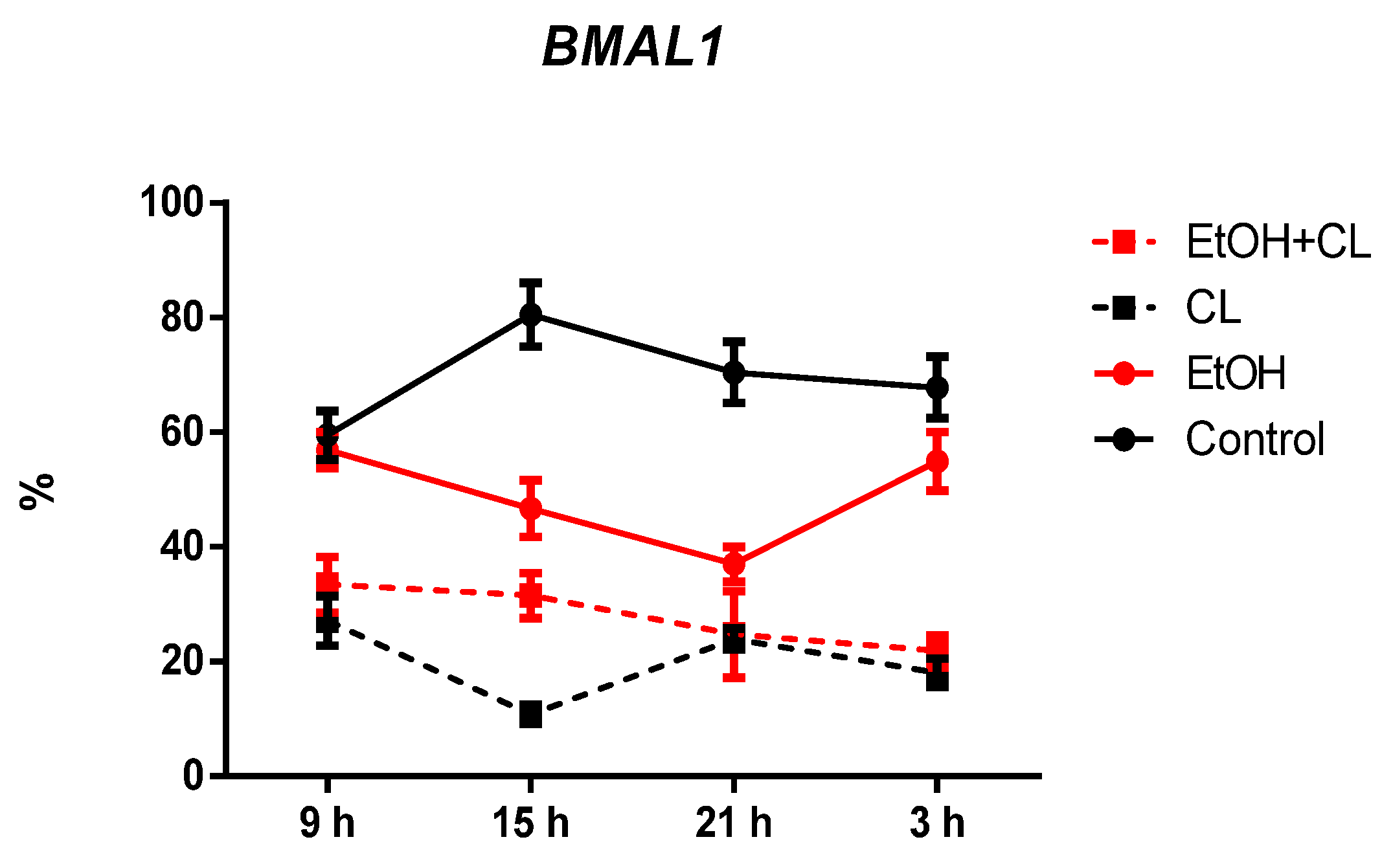
2.3. Influence of Constant Lighting and CAI on Gene Expression
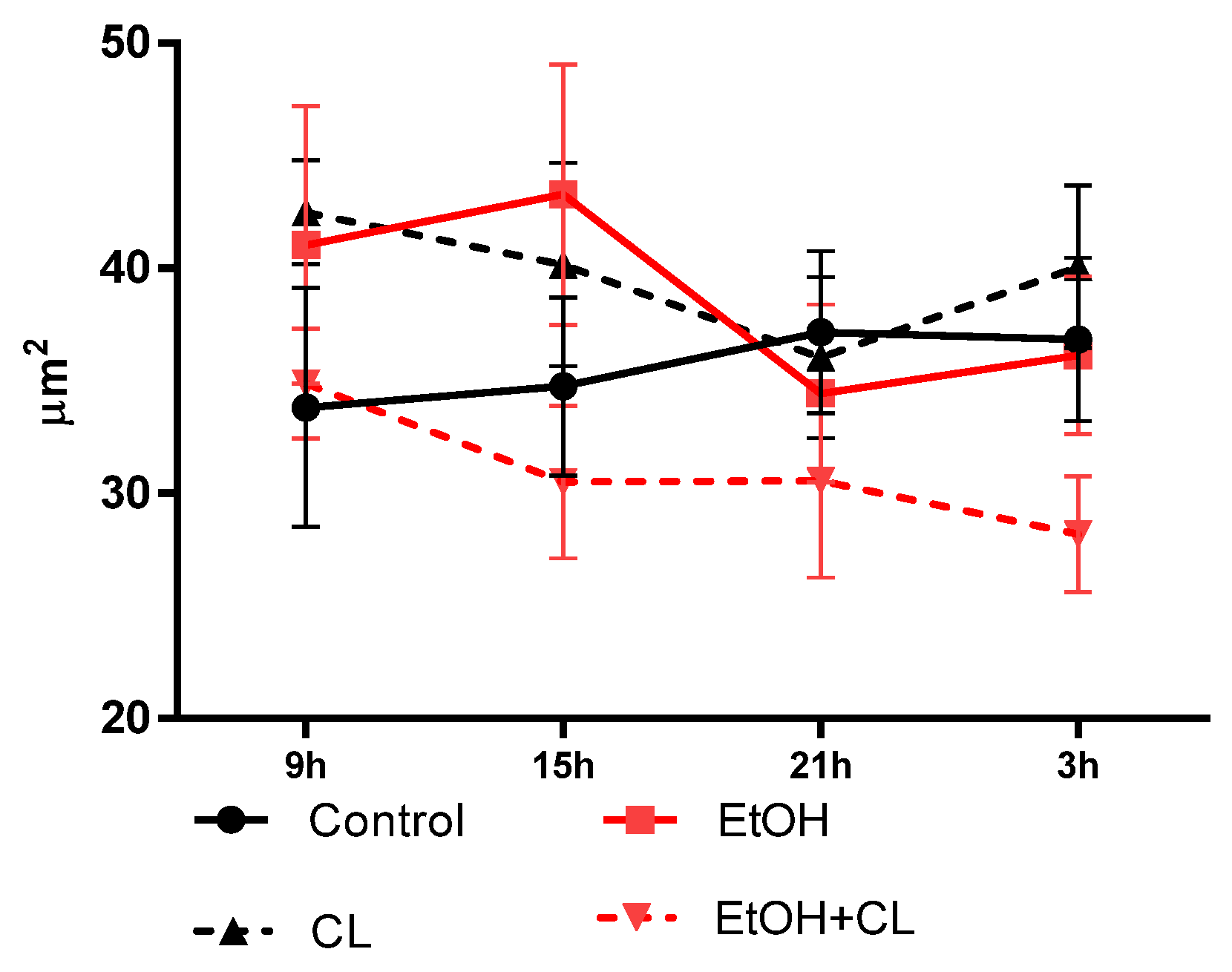
2.4. Influence of CAI and Constant Lighting on Circadian Dynamics of Morphometric Parameters of Hepatocytes
2.5. Influence of Constant Illumination and CAI on the Organization of Circadian Rhythms of the Expression of the Studied Genes
3. Discussion
4. Materials and Methods
4.1. Object of Study
4.2. Design of Study
4.3. Morphological, Morphometric, and Histochemical Methods
4.4. Immunohistochemical Methods
- Ki-67–Rabbit polyclonal (Cloud-Clone Corp., Houston, TX, USA), 1:300;
- PER2–Rabbit polyclonal (Cloud-Clone Corp., Houston, TX, USA), 1:200;
- BMAL1–Rabbit polyclonal (Cloud-Clone Corp., Houston, TX, USA), 1:200;
- p53–Rabbit polyclonal (Cloud-Clone Corp., Houston, TX, USA), 1:200;
- ADH5–Rabbit polyclonal (Cloud-Clone Corp., Houston, TX, USA), 1:300.
4.5. Methods of Statistical Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKenna, H.; Van Der Horst, G.T.J.; Reiss, I.; Martin, D. Clinical chronobiology: A timely consideration in critical care medicine. Crit. Care 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Serin, Y.; Tek, N.A. Effect of Circadian Rhythm on Metabolic Processes and the Regulation of Energy Balance. Ann. Nutr. Metab. 2019, 74, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, J.C. Molecular Bases for Circadian Clocks. Cell 1999, 96, 271–290. [Google Scholar] [CrossRef]
- Foster, R.G.; Roenneberg, T. Human Responses to the Geophysical Daily, Annual and Lunar Cycles. Curr. Biol. 2008, 18, R784–R794. [Google Scholar] [CrossRef]
- Michel, S.; Meijer, J.H. From clock to functional pacemaker. Eur. J. Neurosci. 2019, 51, 482–493. [Google Scholar] [CrossRef]
- Harmer, S.L.; Panda, S.; Kay, S.A. Molecular bases of circadian rhythms. Annu. Rev. Cell Dev. Biol. 2001, 17, 215–253. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Verlande, A.; Masri, S. Circadian Clocks and Cancer: Timekeeping Governs Cellular Metabolism. Trends Endocrinol. Metab. 2019, 30, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N. Light desynchronosis and health. Light Eng. 2019, 27, 14–25. [Google Scholar] [CrossRef]
- Ueda, H.R.; Hayashi, S.; Chen, W.; Sano, M.; Machida, M.; Shigeyoshi, Y.; Iino, M.; Hashimoto, S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 2005, 37, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Shibata, S. Circadian rhythms of liver physiology and disease: Experimental and clinical evidence. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, Y.; Aviram, R.; Asher, G. The emerging roles of lipids in circadian control. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 1017–1025. [Google Scholar] [CrossRef]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef]
- Kornmann, B.; Schaad, O.; Reinke, H.; Saini, C.; Schibler, U. Regulation of Circadian Gene Expression in Liver by Systemic Signals and Hepatocyte Oscillators. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 319–330. [Google Scholar] [CrossRef]
- Vollmers, C.; Gill, S.; DiTacchio, L.; Pulivarthy, S.R.; Le, H.D.; Panda, S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 21453–21458. [Google Scholar] [CrossRef]
- Wang, J.; Mauvoisin, D.; Martin, E.; Atger, F.; Galindo, A.N.; Dayon, L.; Sizzano, F.; Palini, A.; Kussmann, M.; Waridel, P.; et al. Nuclear Proteomics Uncovers Diurnal Regulatory Landscapes in Mouse Liver. Cell Metab. 2016, 25, 102–117. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Kinouchi, K.; Welz, P.-S.; Smith, J.G.; Zinna, V.M.; Shi, J.; Samad, M.; Chen, S.; Magnan, C.N.; Kinchen, J.M.; et al. Defining the Independence of the Liver Circadian Clock. Cell 2019, 177, 1448–1462.e14. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Zhang, W.; Chen, S.; Rabearivony, A.; Shi, Y.; Liu, J.; Corton, C.J.; Liu, C. Endogenous circadian time genes expressions in the liver of mice under constant darkness. BMC Genom. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Mukherji, A.; Bailey, S.M.; Staels, B.; Baumert, T.F. The circadian clock and liver function in health and disease. J. Hepatol. 2019, 71, 200–211. [Google Scholar] [CrossRef]
- Saran, A.R.; Dave, S.; Zarrinpar, A. Circadian Rhythms in the Pathogenesis and Treatment of Fatty Liver Disease. Gastroenterology 2020, 158, 1948–1966.e1. [Google Scholar] [CrossRef]
- Chen, P.; Han, Z.; Yang, P.; Zhu, L.; Hua, Z.; Zhang, J. Loss of clock gene mPer2 promotes liver fibrosis induced by carbon tetrachloride. Hepatol. Res. 2010, 40, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Xiao, R.; Tseng, H.-T.; Shan, L.; Fu, L.; Moore, D.D. Circadian Dysregulation Disrupts Bile Acid Homeostasis. PLoS ONE 2009, 4, e6843. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.B.; Coiffard, B.; Leone, M.; Mezouar, S.; Mege, J.-L. For Whom the Clock Ticks: Clinical Chronobiology for Infectious Diseases. Front. Immunol. 2020, 11, 1457. [Google Scholar] [CrossRef]
- Feillet, C.; van der Horst, G.T.J.; Levi, F.; Rand, D.A.; Delaunay, F. Coupling between the Circadian Clock and Cell Cycle Oscillators: Implication for Healthy Cells and Malignant Growth. Front. Neurol. 2015, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zheng, M.; Sun, B.; Wang, Y.; Ye, L.; Zhang, X. A Long Noncoding RNA Perturbs the Circadian Rhythm of Hepatoma Cells to Facilitate Hepatocarcinogenesis. Neoplasia 2015, 17, 79–88. [Google Scholar] [CrossRef]
- Kettner, N.M.; Voicu, H.; Finegold, M.J.; Coarfa, C.; Sreekumar, A.; Putluri, N.; Katchy, C.A.; Lee, C.; Moore, D.D.; Fu, L. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell 2016, 30, 909–924. [Google Scholar] [CrossRef]
- Ozturk, N.; Ozturk, D.; Kavakli, I.H.; Okyar, A. Molecular Aspects of Circadian Pharmacology and Relevance for Cancer Chronotherapy. Int. J. Mol. Sci. 2017, 18, 2168. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, Z.; Varghese, E.; Büsselberg, D. Cisplatin’s dual-effect on the circadian clock triggers proliferation and apoptosis. Neurobiol. Sleep Circadian Rhythm. 2020, 9, 100054. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.; Guo, Y.; Liu, R.; Qi, F.; Li, X.; Yu, H.; Cheng, S.; Wang, Z. Circadian gene Clock participates in mitochondrial apoptosis pathways by regulating mitochondrial membrane potential, mitochondria out membrane permeablization and apoptosis factors in AML12 hepatocytes. Mol. Cell. Biochem. 2020, 467, 65–75. [Google Scholar] [CrossRef]
- Fárková, E.; Schneider, J.; Šmotek, M.; Bakštein, E.; Herlesová, J.; Kopřivová, J.; Šrámková, P.; Pichlerová, D.; Fried, M. Weight loss in conservative treatment of obesity in women is associated with physical activity and circadian phenotype: A longitudinal observational study. Biopsychosoc. Med. 2019, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.A.; Garde, A.H.; Kristiansen, J.; Nabe-Nielsen, K.; Hansen, M. The effect of the number of consecutive night shifts on diurnal rhythms in cortisol, melatonin and heart rate variability (HRV): A systematic review of field studies. Int. Arch. Occup. Environ. Health 2015, 89, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Yamazaki, S.; McMahon, D.G. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 2005, 8, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D. Melatonin attenuates light-at-night effects on systolic blood pressure and body temperature but does not affect di-astolic blood pressure and heart rate circadian rhythms. Biol. Rhythm. Res. 2020, 51, 780–793. [Google Scholar] [CrossRef]
- Armstrong, S.M. Melatonin and circadian control in mammals. Experientia 1989, 45, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.C.; Silva, C.M.; Balieiro, L.C.T.; Fahmy, W.M.; Crispim, C.A. Social jetlag and metabolic control in non-communicable chronic diseases: A study addressing different obesity statuses. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795–1803. [Google Scholar] [CrossRef]
- Yalçin, M.; El-Athman, R.; Ouk, K.; Priller, J.; Relógio, A. Analysis of the Circadian Regulation of Cancer Hallmarks by a Cross-Platform Study of Colorectal Cancer Time-Series Data Reveals an Association with Genes Involved in Huntington’s Disease. Cancers 2020, 12, 963. [Google Scholar] [CrossRef]
- Rosenwasser, A.M. Chronobiology of ethanol: Animal models. Alcohol 2015, 49, 311–319. [Google Scholar] [CrossRef]
- Davis, B.T.; Voigt, R.M.; Shaikh, M.; Forsyth, C.B.; Keshavarzian, A. Circadian Mechanisms in Alcohol Use Disorder and Tissue Injury. Alcohol. Clin. Exp. Res. 2018, 42, 668–677. [Google Scholar] [CrossRef]
- Gaucher, J.; Kinouchi, K.; Ceglia, N.; Montellier, E.; Peleg, S.; Greco, C.M.; Schmidt, A.; Forne, I.; Masri, S.; Baldi, P.; et al. Distinct metabolic adaptation of liver circadian pathways to acute and chronic patterns of alcohol intake. Proc. Natl. Acad. Sci. USA 2019, 116, 25250–25259. [Google Scholar] [CrossRef]
- Prosser, R.; Mangrum, C.; Glass, J. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience 2008, 152, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Schluter, P.J.; Turner, C.; Benefer, C. Long working hours and alcohol risk among Australian and New Zealand nurses and midwives: A cross-sectional study. Int. J. Nurs. Stud. 2012, 49, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.B.; Ruby, C.L.; Brager, A.J.; Prosser, R.A.; Glass, J.D. Environmental Modulation of Alcohol Intake in Hamsters: Effects of Wheel Running and Constant Light Exposure. Alcohol. Clin. Exp. Res. 2010, 34, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I.; Wu, D.; Mari, M.; Bai, J. CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Free Radic. Biol. Med. 2001, 31, 1539–1543. [Google Scholar] [CrossRef]
- Pronko, P.; Bardina, L.; Satanovskaya, V.; Kuzmich, A.; Zimatkin, S. Effect of chronic alcohol consumption on the ethanol- and acetaldehyde-metabolizing systems in the rat gastrointestinal tract. Alcohol. Alcohol. 2002, 37, 229–235. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Holmes, E.; Patel, M.; Iber, F.; Fields, J.Z.; Pethkar, S. Leaky Gut in Alcoholic Cirrhosis: A Possible Mechanism for Alcohol-Induced Liver Damage. Am. J. Gastroenterol. 1999, 94, 200–207. [Google Scholar] [CrossRef]
- Bailey, S.M. Emerging role of circadian clock disruption in alcohol-induced liver disease. Am. J. Physiol. Liver Physiol. 2018, 315, G364–G373. [Google Scholar] [CrossRef]
- Swanson, G.R.; Gorenz, A.; Shaikh, M.; Desai, V.; Kaminsky, T.; Berg, J.V.D.; Murphy, T.; Raeisi, S.; Fogg, L.F.; Vitaterna, M.H.; et al. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am. J. Physiol. Liver Physiol. 2016, 311, G192–G201. [Google Scholar] [CrossRef]
- Wetterberg, L.; Aperia, B.; Gorelick, D.A.; Gwirtzman, H.E.; McGuire, M.T.; Serafetinides, E.A.; Yuwiler, A. Age, alcoholism and de-pression are associated with low levels of urinary melatonin. J. Psychiatry Neurosci. 1992, 17, 215–224. [Google Scholar]
- Fonzi, S.; Solinas, G.P.; Costelli, P.; Parodi, C.; Murialdo, G.; Bo, P.; Albergati, A.; Montalbetti, L.; Savoldi, F.; Polleri, A. Melatonin and cortisol circadian secretion during ethanol withdrawal in chronic alcoholics. Chronobiologia 1994, 21, 109–112. [Google Scholar]
- Zucker, I.; Beery, A. Males still dominate animal studies. Nature 2010, 465, 690. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuljis, D.A.; Loh, D.; Truong, D.; Vosko, A.M.; Ong, M.L.; McClusky, R.; Arnold, A.P.; Colwell, C. Gonadal- and Sex-Chromosome-Dependent Sex Differences in the Circadian System. Endocrinology 2013, 154, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.K.; Chatterjee, B. Sexual Dimorphism in the Liver. Annu. Rev. Physiol. 1983, 45, 37–50. [Google Scholar] [CrossRef]
- Justo, R.; Boada, J.; Frontera, M.; Oliver, J.O.; Bermudez, J.; Gianotti, M. Gender dimorphism in rat liver mitochondrial oxidative metabolism and biogenesis. Am. J. Physiol. Physiol. 2005, 289, C372–C378. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, M.A.; Kirillov, Y.A.; Makartseva, L.A.; Chernov, I.; Areshidze, D.A. Morphofunctional State and Circadian Rhythms of the Liver under the Influence of Chronic Alcohol Intoxication and Constant Lighting. Int. J. Mol. Sci. 2021, 22, 13007. [Google Scholar] [CrossRef]
- Kirillov, Y.A.; Kozlova, M.A.; Makartseva, L.A.; Areshidze, D.A.; Chernov, I.A.; Shtemplevskaya, E.V. Character of the Daily Dynamics of a Number of Morphometric Parameters of Hepatocytes of Wistar Rats at Chronic Alcohol Intoxica-tion. World Heart J. 2021, 13, 215–217. [Google Scholar]
- Areshidze, D.A.; Kozlova, M.A.; Makartseva, L.A.; Chernov, I.A.; Sinelnikov, M.Y.; Kirillov, Y.A. Influence of constant lightning on liver health: An experimental study. Environ. Sci. Pollut. Res. 2022, 1–12. [Google Scholar] [CrossRef]
- Jansson, J.-O.; Edén, S.; Isaksson, O. Sexual Dimorphism in the Control of Growth Hormone Secretion*. Endocr. Rev. 1985, 6, 128–150. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, X.; Antonson, P.; Gustafsson, J.; Li, Z. Genomics of sex hormone receptor signaling in hepatic sexual dimorphism. Mol. Cell. Endocrinol. 2018, 471, 33–41. [Google Scholar] [CrossRef]
- Bur, I.M.; Cohen-Solal, A.M.; Carmignac, D.; Abecassis, P.-Y.; Chauvet, N.; Martin, A.O.; van der Horst, G.T.; Robinson, I.C.; Maurel, P.; Mollard, P.; et al. The Circadian Clock Components CRY1 and CRY2 Are Necessary to Sustain Sex Dimorphism in Mouse Liver Metabolism. J. Biol. Chem. 2009, 284, 9066–9073. [Google Scholar] [CrossRef] [PubMed]
- Guillaumond, F.; Gréchez-Cassiau, A.; Subramaniam, M.; Brangolo, S.; Peteri-Brünback, B.; Staels, B.; Fiévet, C.; Spelsberg, T.C.; Delaunay, F.; Teboul, M. Kruppel-Like Factor KLF10 Is a Link between the Circadian Clock and Metabolism in Liver. Mol. Cell. Biol. 2010, 30, 3059–3070. [Google Scholar] [CrossRef] [PubMed]
- Berthier, A.; Johanns, M.; Zummo, F.P.; Lefebvre, P.; Staels, B. PPARs in liver physiology. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166097. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mendoza, M.; Rivera-Zavala, J.B.; Rodríguez-Guadarrama, A.H.; Montoya-Gomez, L.M.; Carmona-Castro, A.; Díaz-Muñoz, M.; Miranda-Anaya, M. Daily cycle in hepatic lipid metabolism in obese mice, Neotomodon alstoni: Sex differences. Chronobiol. Int. 2018, 35, 643–657. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Zhang, D.; Jin, T.; Cai, D.-J.; Wu, Q.; Lu, Y.; Liu, J.; Klaassen, C.D. Diurnal Variation of Hepatic Antioxidant Gene Expression in Mice. PLoS ONE 2012, 7, e44237. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Hui, L. Cell Plasticity in Liver Regeneration. Trends Cell Biol. 2020, 30, 329–338. [Google Scholar] [CrossRef]
- Junatas, K.L.; Tonar, Z.; Kubíková, T.; Liška, V.; Pálek, R.; Mik, P.; Králíčková, M.; Witter, K. Stereological analysis of size and density of hepatocytes in the porcine liver. J. Anat. 2017, 230, 575–588. [Google Scholar] [CrossRef]
- Rowell, R.J.; Quentin, M.A. An overview of the genetics, mechanisms and management of NAFLD and ALD. Clin. Med. 2015, 15, s77–s82. [Google Scholar] [CrossRef]
- Shetty, A.; Hsu, J.W.; Manka, P.P. Role of the Circadian Clock in the Metabolic Syndrome and Nonalcoholic Fatty Liver Dis-ease. Dig. Dis. Sci. 2018, 63, 3187–3206. [Google Scholar] [CrossRef]
- Kobayashi, T.; Saito, Y.; Ohtake, Y.; Maruko, A.; Yamamoto, Y.; Yamamoto, F.; Kuwahara, Y.; Fukumoto, M.; Fukumoto, M.; Ohkubo, Y. Effect of aging on norepinephrine-related proliferative response in primary cultured periportal and perivenous hepatocytes. Am. J. Physiol. Liver Physiol. 2012, 303, G861–G869. [Google Scholar] [CrossRef]
- Lang, F. Mechanisms and Significance of Cell Volume Regulation. J. Am. Coll. Nutr. 2007, 26, 613S–623S. [Google Scholar] [CrossRef] [PubMed]
- Corona-Pérez, A.; Díaz-Muñoz, M.; Rodríguez, I.S.; Cuevas, E.; Martínez-Gómez, M.; Castelán, F.; Rodríguez-Antolín, J.; Nicolás-Toledo, L. High Sucrose Intake Ameliorates the Accumulation of Hepatic Triacylglycerol Promoted by Restraint Stress in Young Rats. Lipids 2015, 50, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Ma, S.; Li, X.; An, S.; Li, T.; Guo, K.; Lin, M.; Qu, W.; Dong, X.; Han, X.; et al. Long-term stress with hyperglucocorti-coidemiainduced hepatic steatosis with VLDL overproduction is dependent on both 5-HT2 receptor and 5-HT synthesis in liver. Int. J. Biol. Sci. 2016, 12, 219–234. [Google Scholar] [CrossRef] [Green Version]
- Schott, M.B.; Rasineni, K.; Weller, S.G.; Schulze, R.J.; Sletten, A.C.; Casey, C.A.; McNiven, M.A. β-Adrenergic induction of lipolysis in hepatocytes is inhibited by ethanol exposure. J. Biol. Chem. 2017, 292, 11815–11828. [Google Scholar] [CrossRef] [PubMed]
- Derdak, Z.; Lang, C.H.; Villegas, K.A.; Tong, M.; Mark, N.M.; de la Monte, S.M.; Wands, J.R. Activation of p53 enhances apoptosis and insulin resistance in a rat model of alcoholic liver disease. J. Hepatol. 2011, 54, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Yatsuji, S.; Hashimoto, E.; Tobari, M.; Tokushige, K.; Shiratori, K. Influence of age and gender in Japanese patients with non-alcoholic steatohepatitis. Hepatol. Res. 2007, 37, 1034–1043. [Google Scholar] [CrossRef]
- Tobari, M.; Hashimoto, E.; Kabutake, A.; Yatsuji, S.; Taniai, M.; Tokushige, K.; Shiratori, K. Genderspecific clinicopathological features in nonalcoholic steatohepatitis. Gastroenterology 2007, 132, 815. [Google Scholar]
- Panasiuk, A.; Dzięcioł, J.; Panasiuk, B.; Prokopowicz, D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J. Gastroenterol. 2006, 12, 6198–6202. [Google Scholar] [CrossRef]
- Stöppeler, S.; Palmes, D.; Fehr, M.; Hölzen, J.P.; Zibert, A.; Siaj, R.; Schmidt, H.H.-J.; Spiegel, H.-U.; Bahde, R. Gender and strain-specific differences in the development of steatosis in rats. Lab. Anim. 2013, 47, 43–52. [Google Scholar] [CrossRef]
- Veteläinen, R.; Bennink, R.J.; van Vliet, A.K.; van Gulik, T.M. Mild steatosis impairs functional recovery after liver resection in an experimental model. Br. J. Surg. 2007, 94, 1002–1008. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, M.; Xu, L.; Cheng, J.; Shen, J.; Yang, T.; Zhang, L. Bmal1 Regulates Macrophage Polarize Through Glycolytic Pathway in Alcoholic Liver Disease. Front. Pharmacol. 2021, 12, 640521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tong, X.; Nelson, B.B.; Jin, E.; Sit, J.; Charney, N.; Yang, M.; Omary, M.B.; Yin, L. The hepatic BMAL1/AKT/lipogenesis axis protects against alcoholic liver disease in mice via promoting PPARα pathway. Hepatology 2018, 68, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.R.; Gorenz, A.; Shaikh, M.; Desai, V.; Forsyth, C.; Fogg, L.; Burgess, H.J.; Keshavarzian, A. Decreased melatonin secretion is associated with increased intestinal permeability and marker of endotoxemia in alcoholics. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G1004-11. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Meng, F.; Francis, H.; Wu, N.; Chen, L.; Kennedy, L.; Zhou, T.; Franchitto, A.; Onori, P.; Gaudio, E.; et al. Melatonin and circadian rhythms in liver diseases: Functional roles and potential therapies. J. Pineal Res. 2020, 68, e12639. [Google Scholar] [CrossRef]
- González-Fernández, B.; Sánchez, D.I.; Crespo, I.; San-Miguel, B.; De Urbina, J.O.; González-Gallego, J.; Tuñón, M.J. Melatonin Attenuates Dysregulation of the Circadian Clock Pathway in Mice With CCl4-Induced Fibrosis and Human Hepatic Stellate Cells. Front. Pharmacol. 2018, 9, 556. [Google Scholar] [CrossRef]
- Fu, L.; Pelicano, H.; Liu, J.; Huang, P.; Lee, C.C. The circadian gene period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 2002, 111, 41–50. [Google Scholar] [CrossRef]
- Zhanfeng, N.; Chengquan, W.; Hechun, X.; Jun, W.; Lijian, Z.; Dede, M.; Wenbin, L.; Lei, Y. Period2 downregulation inhibits glioma cell apoptosis by activating the MDM2-TP53 pathway. Oncotarget 2016, 7, 27350–27362. [Google Scholar] [CrossRef]
- Miki, T.; Matsumoto, T.; Zhao, Z.; Lee, C.C. p53 regulates Period2 expression and the circadian clock. Nat. Commun. 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Gallego, M.; Virshup, D.M. Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol. 2007, 8, 139–148. [Google Scholar] [CrossRef]
- Altman, B.J. Cancer Clocks Out for Lunch: Disruption of Circadian Rhythm and Metabolic Oscillation in Cancer. Front. Cell Dev. Biol. 2016, 4, 62. [Google Scholar] [CrossRef]
- Uchiyama, Y. Rhythms in morphology and function of hepatocytes. J. Gastroenterol. Hepatol. 1990, 5, 321–333. [Google Scholar] [CrossRef]
- Morin, L.P. Neuroanatomy of the extended circadian rhythm system. Exp. Neurol. 2012, 243, 4–20. [Google Scholar] [CrossRef]
- Bailey, M.; Silver, R. Sex differences in circadian timing systems: Implications for disease. Front. Neuroendocr. 2013, 35, 111–139. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; Wang, A.; Sasanian, J.; Silver, R. A Role for Androgens in Regulating Circadian Behavior and the Suprachiasmatic Nucleus. Endocrinology 2007, 148, 5487–5495. [Google Scholar] [CrossRef] [Green Version]
- Karatsoreos, I.N.; Butler, M.P.; LeSauter, J.; Silver, R. Androgens Modulate Structure and Function of the Suprachiasmatic Nucleus Brain Clock. Endocrinology 2011, 152, 1970–1978. [Google Scholar] [CrossRef]
- Kriegsfeld, L.J.; Silver, R. The regulation of neuroendocrine function: Timing is everything. Horm. Behav. 2006, 49, 557–574. [Google Scholar] [CrossRef]
- Silver, R.; Kriegsfeld, L.J. Circadian rhythms have broad implications for understanding brain and behavior. Eur. J. Neurosci. 2014, 39, 1866–1880. [Google Scholar] [CrossRef]
- Güldner, F.-H. Suprachiasmatic nucleus: Numbers of synaptic appositions and various types of synapses. Cell Tissue Res. 1984, 235, 449–452. [Google Scholar] [CrossRef]
- Collado, P.; Beyer, C.; Hutchison, J.; Holman, S. Hypothalamic distribution of astrocytes is gender-related in Mongolian gerbils. Neurosci. Lett. 1995, 184, 86–89. [Google Scholar] [CrossRef]
- A Hofman, M.; Fliers, E.; Goudsmit, E.; Swaab, D.F. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: Sex differences and age-dependent changes. J. Anat. 1988, 160, 127–143. [Google Scholar]
- McCarthy, M.M.; Arnold, A.P.; Ball, G.; Blaustein, J.D.; de Vries, G. Sex Differences in the Brain: The Not So Inconvenient Truth. J. Neurosci. 2012, 32, 2241–2247. [Google Scholar] [CrossRef]
- Oishi, K.; Amagai, N.; Shirai, H.; Kadota, K.; Ohkura, N.; Ishida, N. Genome-wide Expression Analysis Reveals 100 Adrenal Gland-dependent Circadian Genes in the Mouse Liver. DNA Res. 2005, 12, 191–202. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Kino, T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress 2007, 10, 213–219. [Google Scholar] [CrossRef]
- Kloehn, I.; Pillai, S.B.; Officer, L.; Klement, C.; Gasser, P.J.; Evans, J.A. Sexual Differentiation of Circadian Clock Function in the Adrenal Gland. Endocrinology 2016, 157, 1895–1904. [Google Scholar] [CrossRef]
- Moisan, M.-P. Sexual Dimorphism in Glucocorticoid Stress Response. Int. J. Mol. Sci. 2021, 22, 3139. [Google Scholar] [CrossRef]
- Balsalobre, A.; Brown, S.A.; Marcacci, L.; Tronche, F.; Kellendonk, C.; Reichardt, H.M.; Schütz, G.; Schibler, U. Resetting of Circadian Time in Peripheral Tissues by Glucocorticoid Signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef]
- Oishi, K.; Miyazaki, K.; Kadota, K.; Kikuno, R.; Nagase, T.; Atsumi, G.; Ohkura, N.; Azama, T.; Mesaki, M.; Yukimasa, S.; et al. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J. Biol. Chem. 2003, 278, 41519-27, Epub 5 July 2003. [Google Scholar] [CrossRef]
- Rodd-Henricks, Z.A.; Bell, R.L.; Kuc, K.A.; Murphy, J.M.; McBride, W.J.; Lumeng, L.; Li, T.K. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alco-hol-preferring (P) rats: I. Periadolescent exposure. Alcohol. Clin. Exp. Res. 2002, 26, 1632–1641. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harb. Protoc. 2008, 2008, pdb-prot4986. [Google Scholar] [CrossRef]
- Christman, J.F.; Werner, H.J. Chromatography and Biological Stains IV. Preparation of a Suitable Fat Stain from Commercial Sudan III. Stain Technol. 1953, 28, 259–264. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef]
- Broeke, J.; Pérez, J.M.M.; Pascau, J. Image Processing with ImageJ; Packt Publishing: Birmingham, UK, 2015; p. 346. [Google Scholar]
- Smitha, T.; Sharada, P.; Girish, H. Morphometry of the basal cell layer of oral leukoplakia and oral squamous cell carcinoma using computer-aided image analysis. J. Oral Maxillofac. Pathol. 2011, 15, 26–33. [Google Scholar] [CrossRef]
- Kim, S.-W.; Roh, J.; Park, C.-S. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef]
- Rubin Grandis, J.; Melhem, M.F.; Barnes, E.L.; Tweardy, D.J. Quantitative immunohistochemical analysis of transforming growth factor-alpha and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer 1996, 78, 1284–1292. [Google Scholar] [CrossRef]
- Cornelissen, G. Cosinor-based rhythmometry. Theor. Biol. Med Model. 2014, 11, 16. [Google Scholar] [CrossRef] [Green Version]


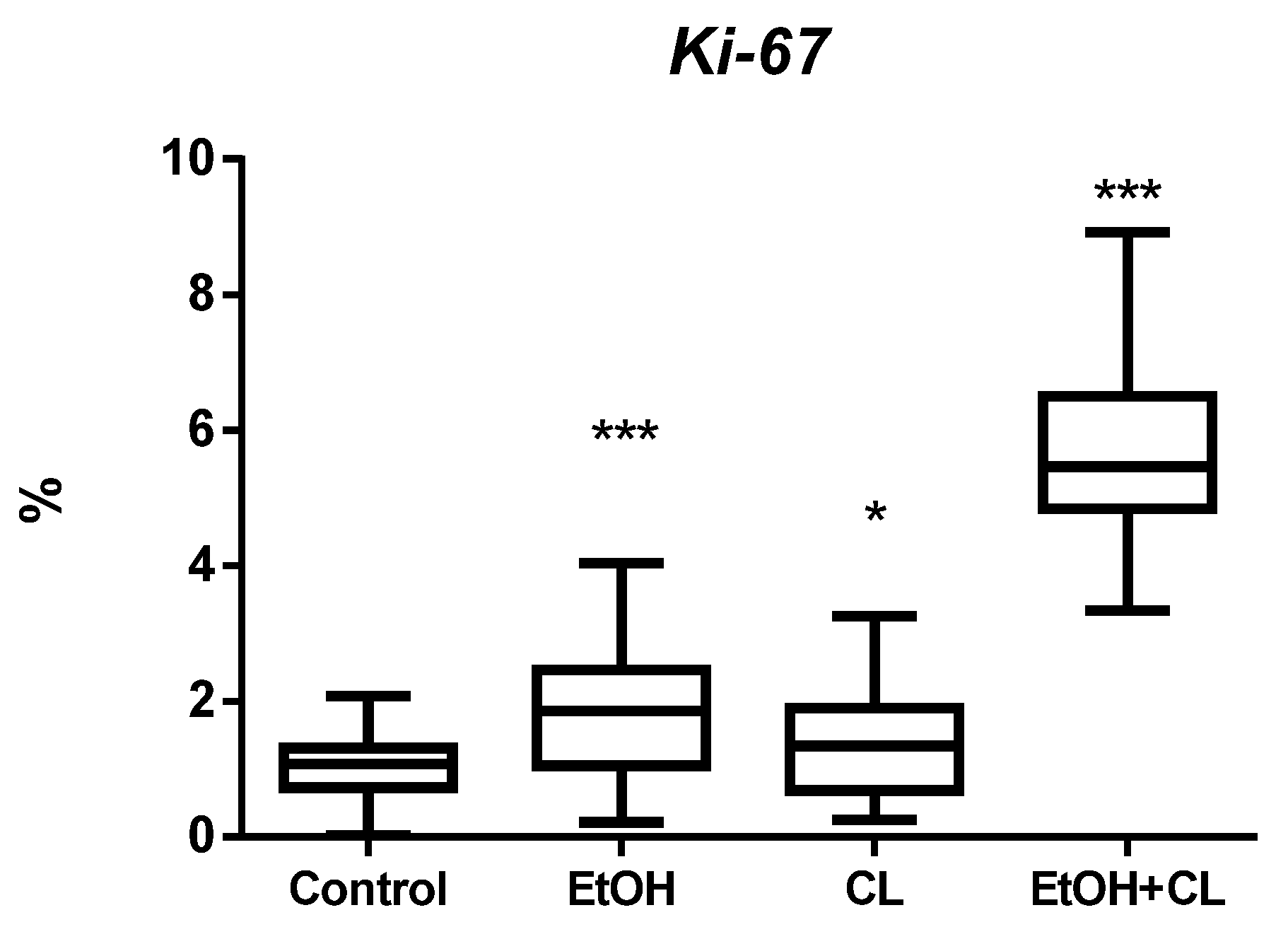
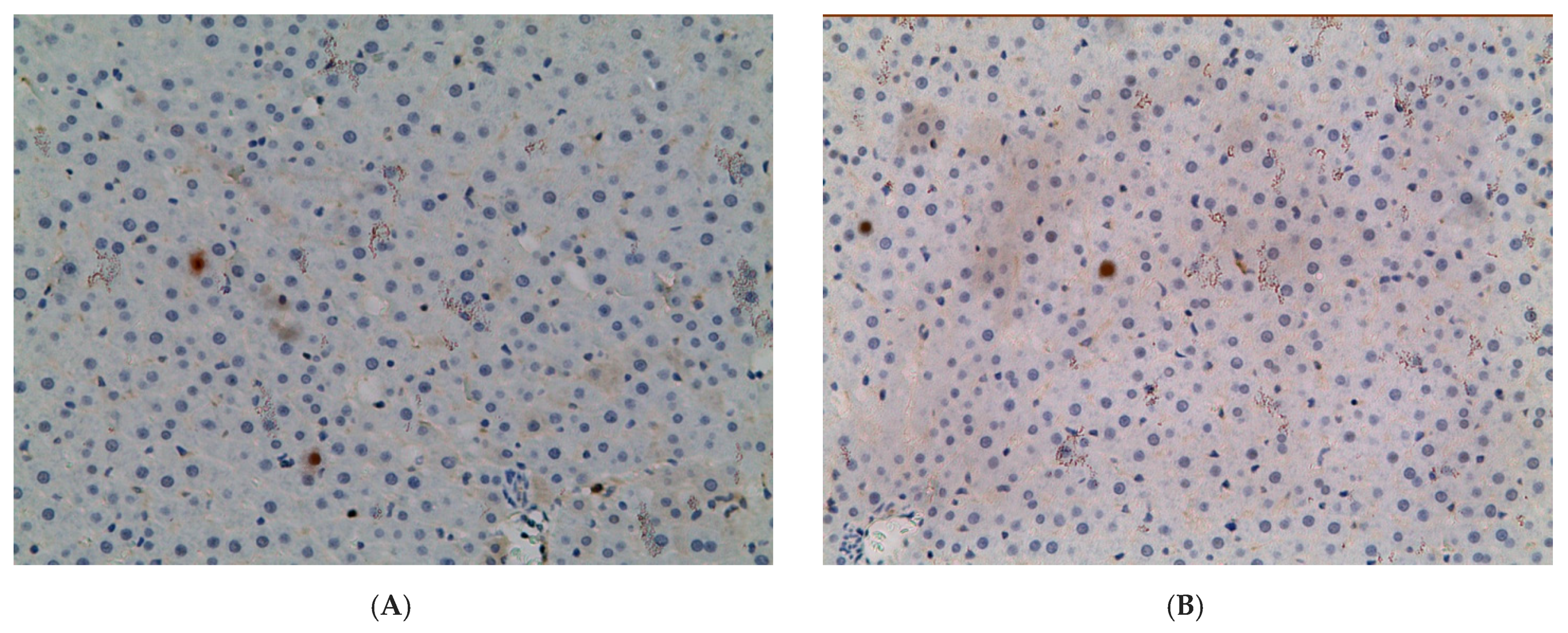
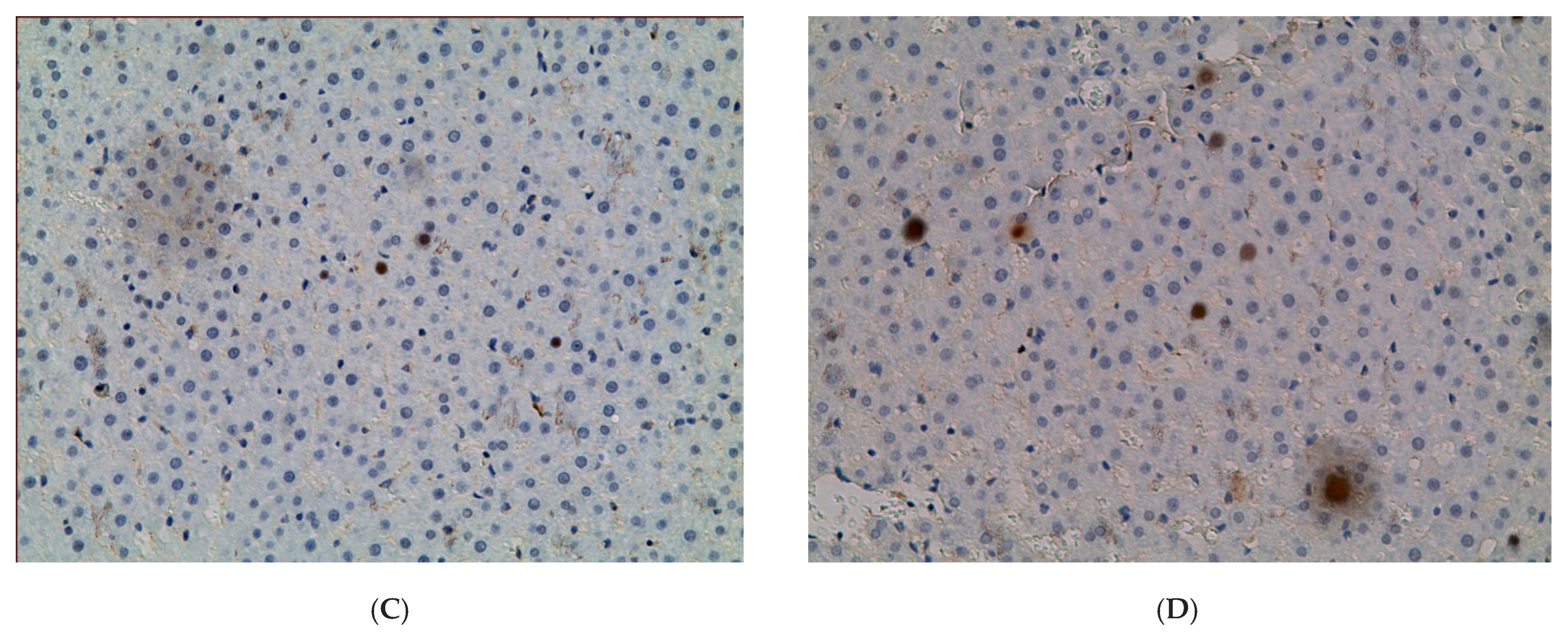
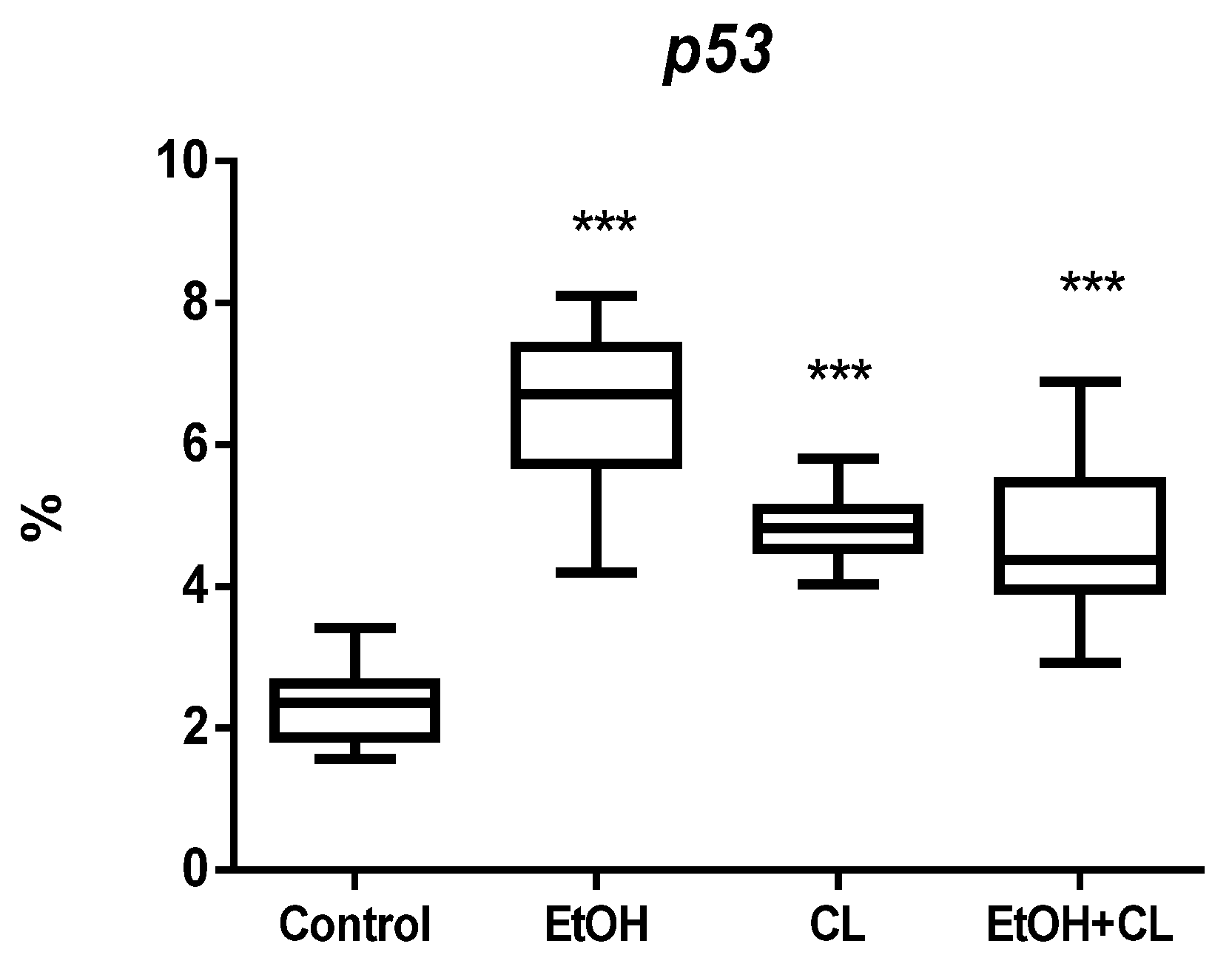





| Control | EtOH | CL | EtOH + CL | |
|---|---|---|---|---|
| Cross-sectional area of nuclei, µm2 | 36.54 ± 4.26 | 38.72 ± 6.0 | 39.69 ± 4.17 * | 31.03 ± 3.98 ** |
| Volume of nuclei, µm3 | 130.80 ± 28.73 | 149.40 ± 39.79 | 144.20 ± 50.23 | 108.30 ± 20.36 |
| Perimeter of nuclei, µm | 24.83 ± 2.46 | 23.59 ± 1.98 | 26.28 ± 2.05 | 21.38 ± 1.28 *** |
| Nucleus volume to nucleus area ratio (V/A coefficient) | 3.70 ± 0.85 | 3.82 ± 0.48 | 3.61 ± 1.11 | 3.51 ± 0.56 |
| Long diameter of nuclei, µm | 6.78 ± 0.40 | 6.90 ± 0.54 | 7.23 ± 0.63 ** | 6.44 ± 0.39 |
| Small diameter of nuclei, µm | 5.76 ± 0.69 | 6.36 ± 0.90 ** | 5.81 ± 1.53 | 5.29 ± 0.44 |
| Mean diameter of nuclei, µm | 6.26 ± 0.45 | 6.63 ± 0.55 * | 6.52 ± 0.71 | 5.85 ± 0.37 * |
| Elongation index of nucleus | 1.19 ± 0.13 | 1.10 ± 0.15 | 1.39 ± 0.64 ** | 1.22 ± 0.09 |
| Contour index of nucleus | 4.19 ± 0.56 | 3.80 ± 0.13 * | 4.19 ± 0.39 | 3.85 ± 0.06 |
| Coefficient of form of nucleus | 0.75 ± 0.16 | 0.87 ± 0.07 | 0.73 ± 0.12 | 0.85 ± 0.02 |
| Cell area, µm2 | 128.60 ± 20.51 | 199.30 ± 31.57 *** | 171.8 ± 21.36 *** | 139.5 ± 16.62 |
| Cell volume, µm3 | 1118.0 ± 268.80 | 2050.0 ± 494.1 *** | 1571.4 ± 302.7 *** | 1196.0 ± 214.1 |
| NCR | 0.29 ± 0.07 | 0.20 ± 0.02 *** | 0.24 ± 0.04 *** | 0.22 ± 0.026 *** |
| Group | Acrophase | Amplitude |
|---|---|---|
| Area of Nuclei of Hepatocytes, μm2 | ||
| Control | 2339 | 1.96 |
| EtOH | 1208 | 4.84 |
| CL | 904 | 4.63 |
| EtOH + CL | 1051 | 2.31 |
| Area of Hepatocytes, μm2 | ||
| Control | 454 | 8.99 |
| EtOH | 1226 | 22.34 |
| CL | Not significant CR | |
| EtOH + CL | Not significant CR | |
| NCR | ||
| Control | 2052 | 0.02 |
| EtOH | Not significant CR | |
| CL | 1103 | 0.033 |
| EtOH + CL | 1457 | 0.017 |
| Group | Acrophase | Amplitude |
|---|---|---|
| Ki-67, % | ||
| Control | 347 | 0.29 |
| EtOH | 1040 | 1.04 |
| CL | 1006 | 0.62 |
| EtOH + CL | Not significant CR | |
| p53, % | ||
| Control | 1706 | 0.23 |
| EtOH | 1854 | 0.24 |
| CL | 1119 | 1.65 |
| EtOH + CL | Not significant CR | |
| BMAL1, % | ||
| Control | 419 | 11.14 |
| EtOH | 730 | 10.78 |
| CL | 456 | 3.95 |
| EtOH + CL | Not significant CR | |
| PER2, % | ||
| Control | 316 | 9.22 |
| EtOH | 1339 | 14.41 |
| CL | 1316 | 15.03 |
| EtOH + CL | Not significant CR | |
| ADH5, % | ||
| Control | 1501 | 0.46 |
| EtOH | 156 | 6.34 |
| CL | 2205 | 0.62 |
| EtOH + CL | 1219 | 7.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Areshidze, D.A.; Kozlova, M.A. Morphofunctional State and Circadian Rhythms of the Liver of Female Rats under the Influence of Chronic Alcohol Intoxication and Constant Lighting. Int. J. Mol. Sci. 2022, 23, 10744. https://doi.org/10.3390/ijms231810744
Areshidze DA, Kozlova MA. Morphofunctional State and Circadian Rhythms of the Liver of Female Rats under the Influence of Chronic Alcohol Intoxication and Constant Lighting. International Journal of Molecular Sciences. 2022; 23(18):10744. https://doi.org/10.3390/ijms231810744
Chicago/Turabian StyleAreshidze, David A., and Maria A. Kozlova. 2022. "Morphofunctional State and Circadian Rhythms of the Liver of Female Rats under the Influence of Chronic Alcohol Intoxication and Constant Lighting" International Journal of Molecular Sciences 23, no. 18: 10744. https://doi.org/10.3390/ijms231810744





