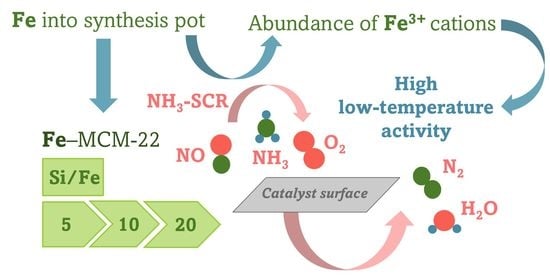
Catalytic Performance and Sulfur Dioxide Resistance of One-Pot Synthesized Fe-MCM-22 in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia (NH3-SCR)—The Effect of Iron Content
Abstract
1. Introduction
2. Results
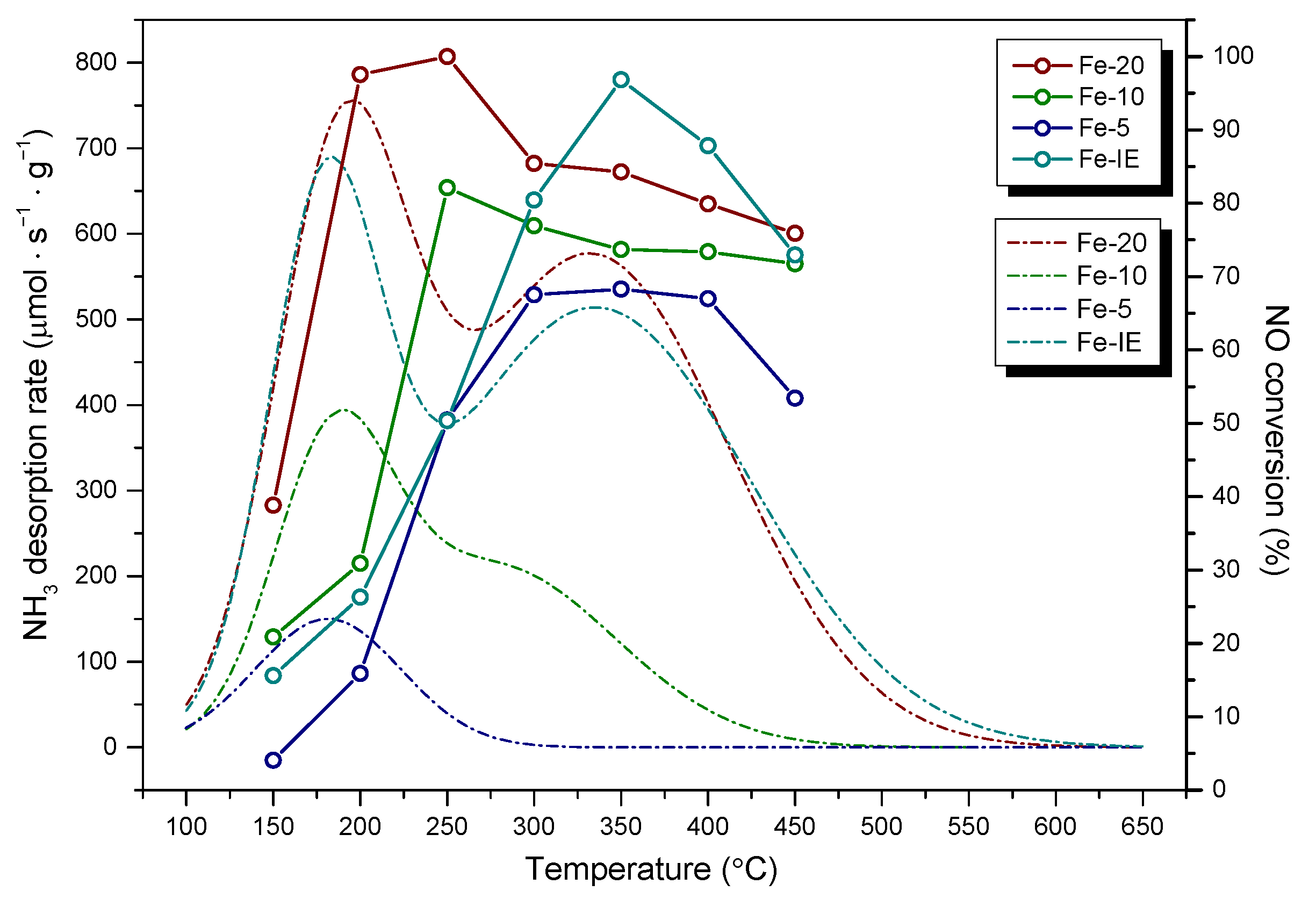
2.1. Catalytic Performance of the Fresh Catalysts
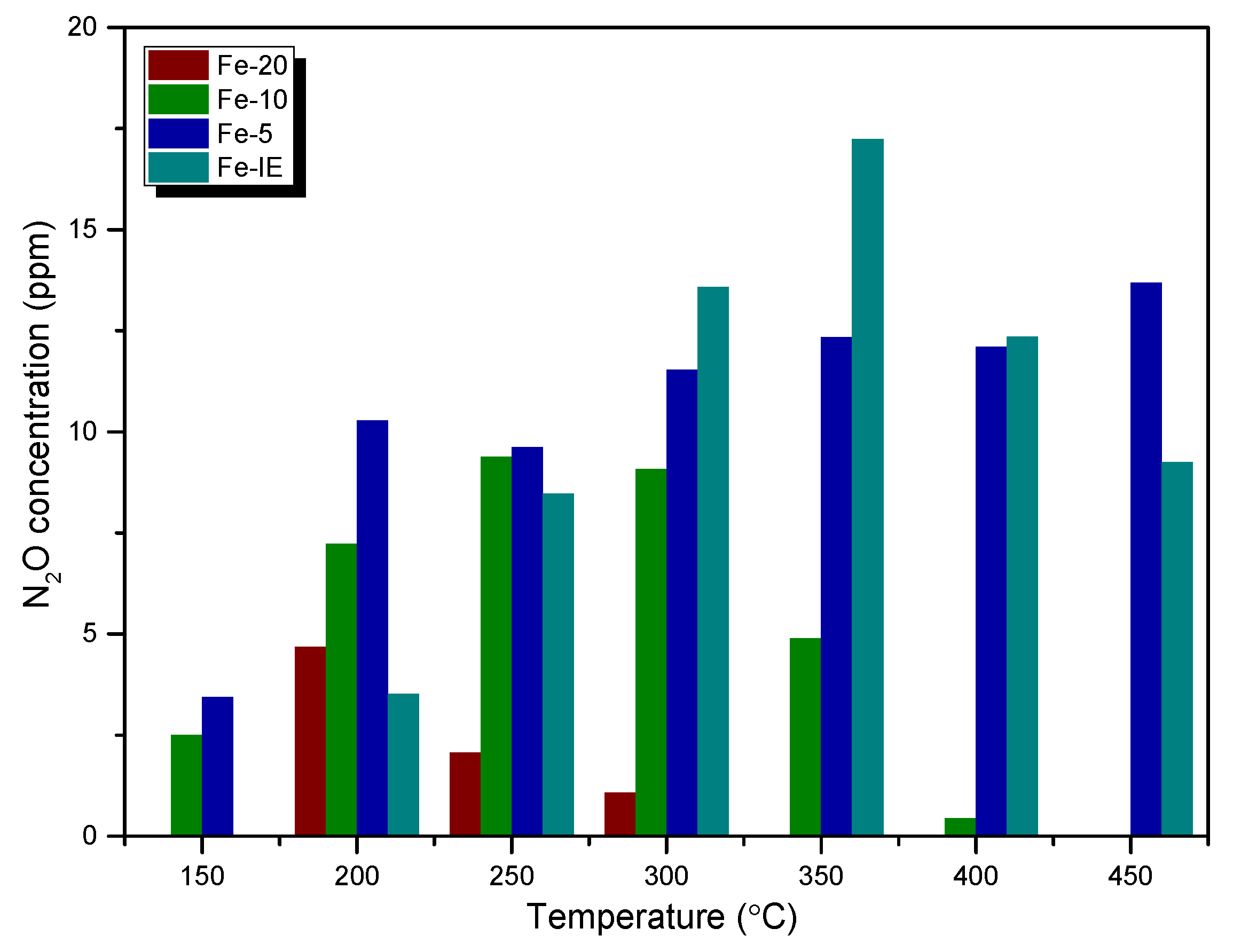
2.2. Catalytic Performance of the SO2-Poisoned Catalysts
2.3. Characterization of the Catalysts
2.3.1. Chemical Composition and Crystal Structure
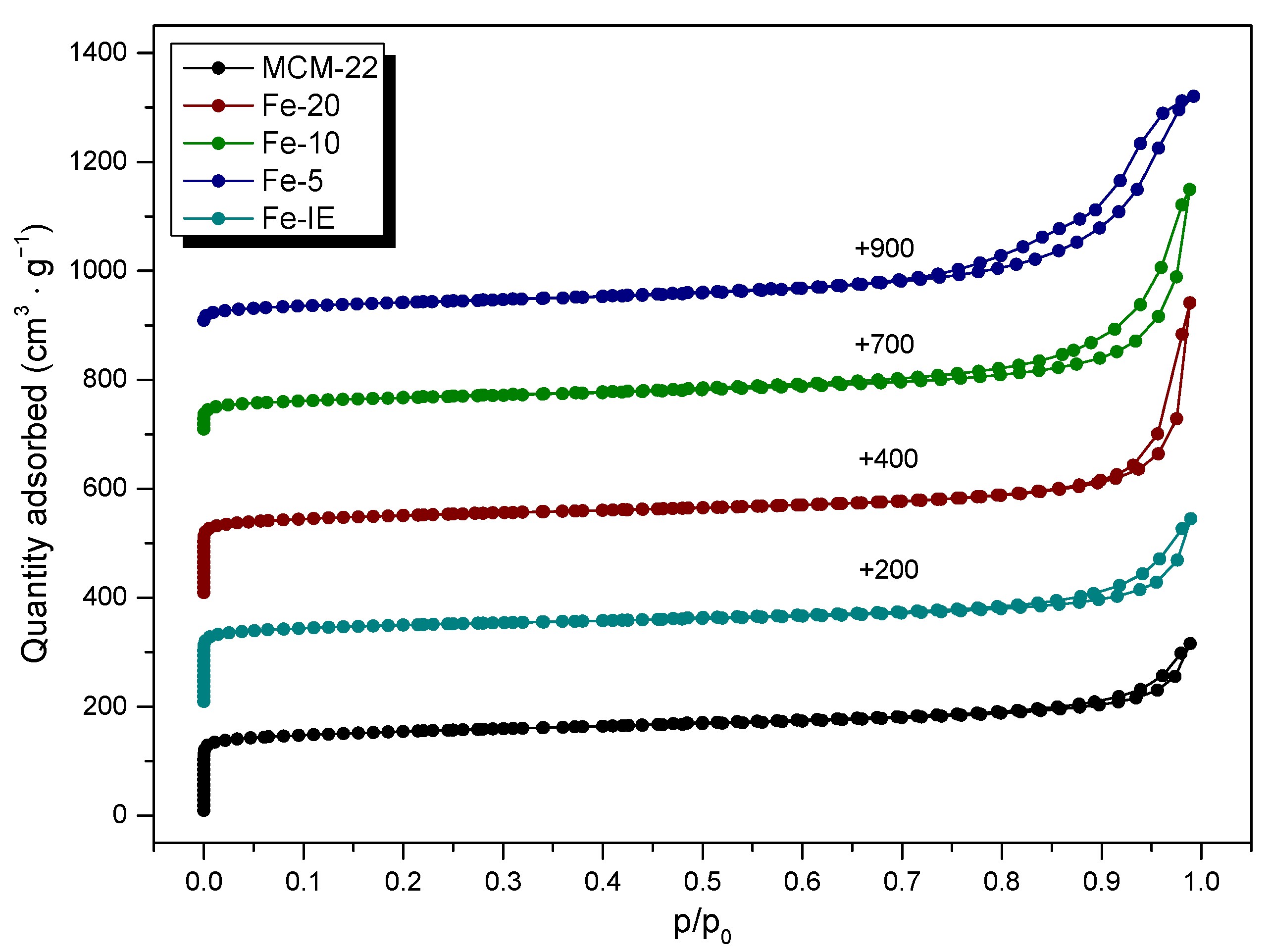
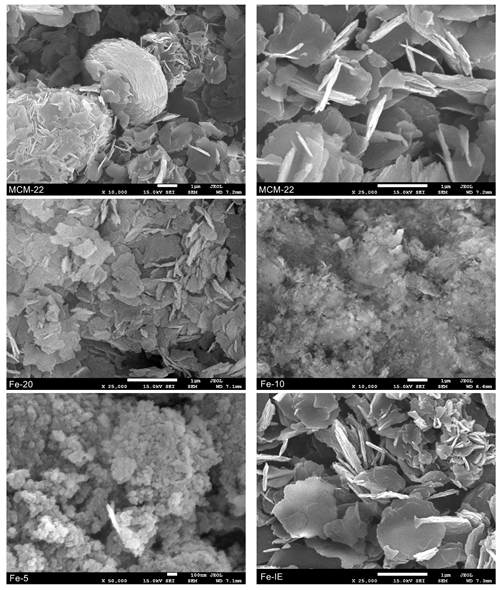
2.3.2. Structural and Surface Properties
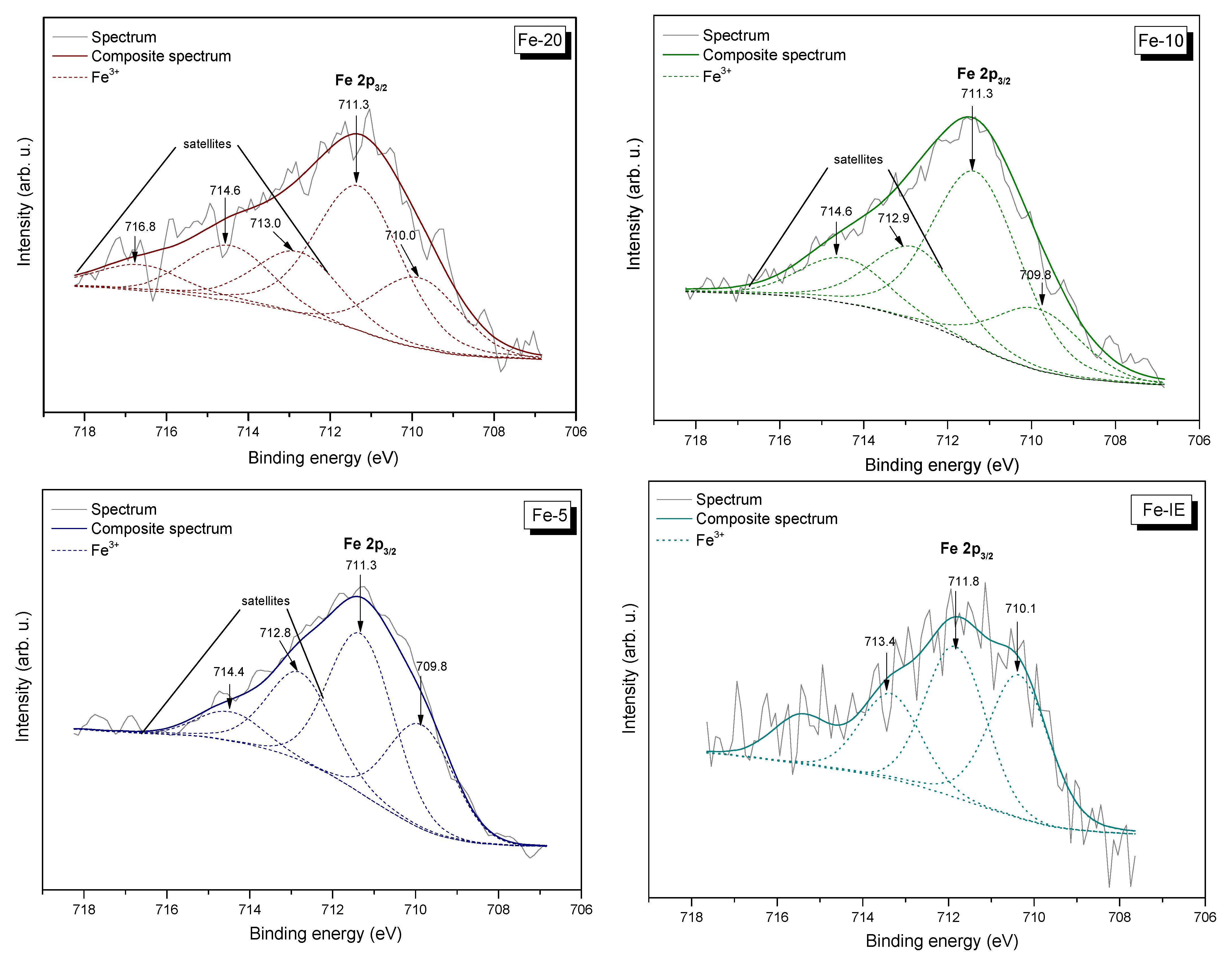
2.3.3. Speciation and Distribution of Iron
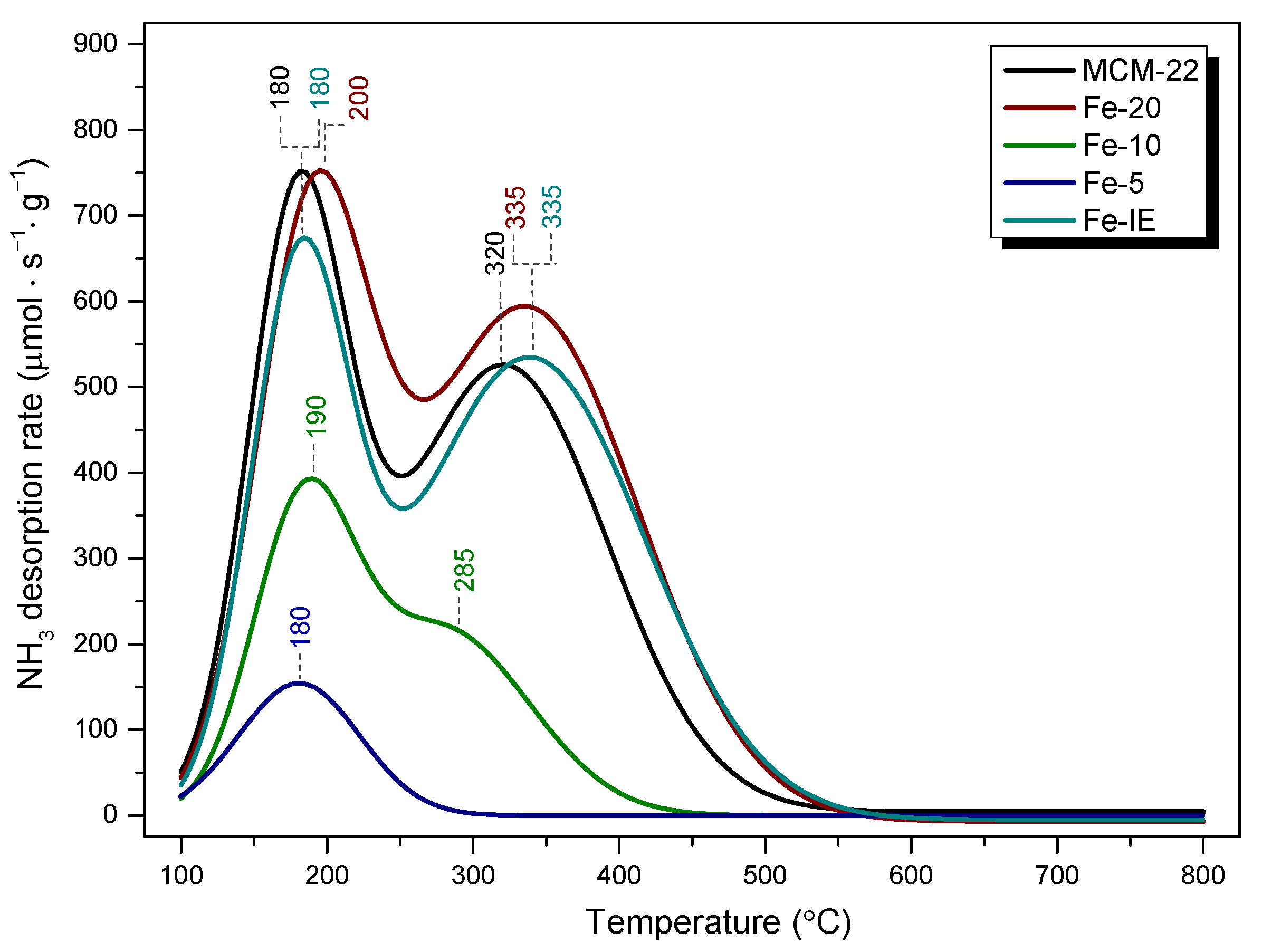
2.3.4. Surface Acidity
3. Discussion
4. Materials and Methods
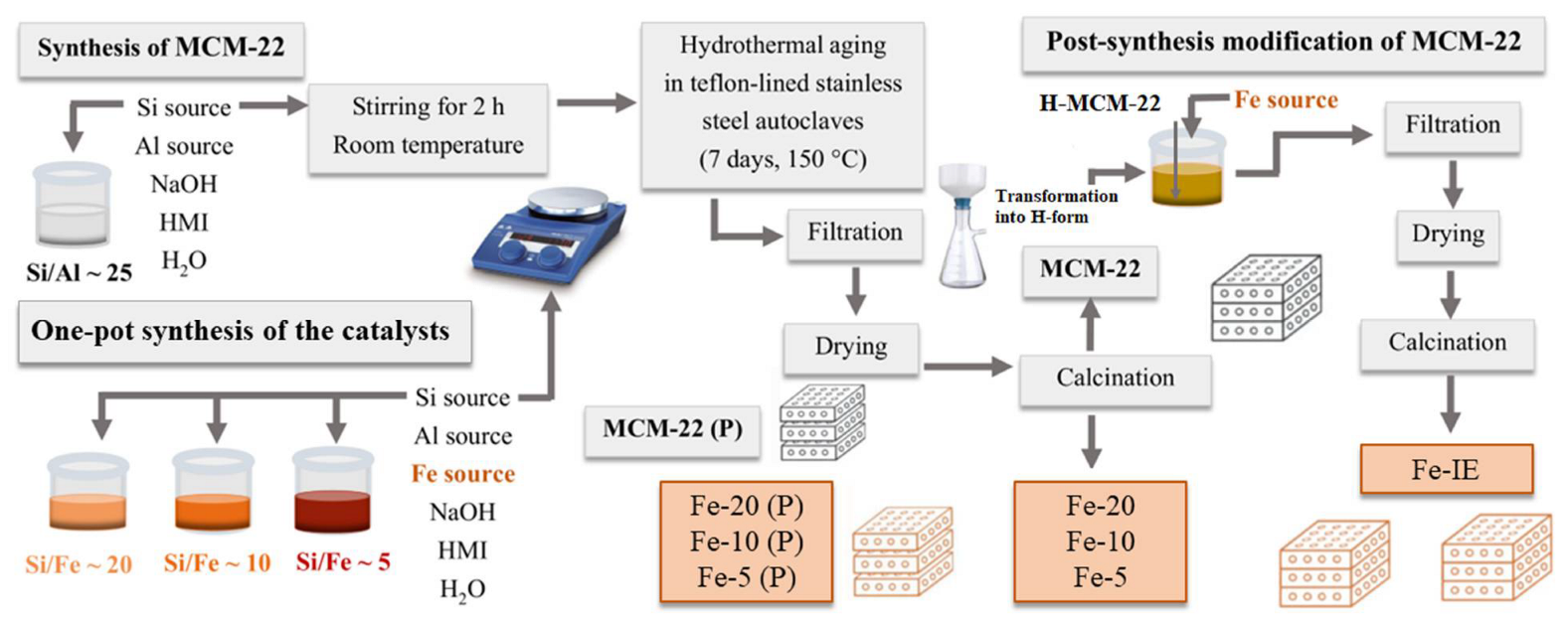
4.1. Catalyst Preparation
4.1.1. Synthesis of MCM-22 Zeolite
4.1.2. Synthesis of the Catalysts
4.2. Catalytic Activity Measurement
4.3. Characterization of the Catalysts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BET | Brunauer: Emmet, and Teller specific surface area |
| BJH | Barret-Joyner-Halenda pore size and volume |
| FT-IR | Fourier transform infrared spectroscopy |
| ICP-OES | Inductively couples plasma optical emission spectroscopy |
| MWW | Mobile Twenty Two type of framework |
| NH3-SCR | Selective catalytic reduction of nitrogen oxides with ammonia |
| NH3-TPD | Temperature-programmed desorption of ammonia |
| SEM/EDS | Scanning electron microscopy and electron-dispersive X-ray spectroscopy |
| Si/Al | Silicon to aluminum molar ratio |
| Si/Fe | Silicon to iron molar ratio |
| TEM BF | Bright-field transmission electron microscopy |
| TEM HAADF | High-angle annular dark-field scanning transmission electron microscopy |
| TEM HR | High resolution transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| UV-Vis-DRS | Ultraviolet-Visible diffuse reflectance spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Dmitrienko, M.A.; Nyashina, G.S.; Strizhak, P.A. Major gas emissions from combustion of slurry fuels based on coal, coal waste, and coal derivatives. J. Clean. Prod. 2018, 177, 284–301. [Google Scholar] [CrossRef]
- Shan, W.; Yu, Y.; Zhang, Y.; He, G.; Peng, Y.; Li, J.; He, H. Theory and practice of metal oxide catalyst design for the selective catalytic reduction of NOx with NH3. Catal. Today 2020, 376, 292–301. [Google Scholar] [CrossRef]
- Gonçalves, A.A.S.; Ciesielczyk, F.; Samojeden, B.; Jaroniec, M. Toward development of single-atom ceramic catalysts for selective catalytic reduction of NO with NH3. J. Hazard. Mater. 2021, 401, 123413. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NO x with NH 3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Toops, T.J. Selective Catalytic Reduction: From Basic Science to deNOx Applications. Catalysts 2021, 11, 250. [Google Scholar] [CrossRef]
- Lian, Z.; Li, Y.; Shan, W.; He, H. Recent Progress on Improving Low-Temperature Activity of Vanadia-Based Catalysts for the Selective Catalytic Reduction of NO x with Ammonia. Catalysts 2020, 10, 1421. [Google Scholar] [CrossRef]
- Kuma, R.; Kitano, T.; Tsujiguchi, T.; Tanaka, T. In Situ XANES Characterization of V2O5/TiO2-SiO2-MoO3Catalyst for Selective Catalytic Reduction of NO by NH3. Ind. Eng. Chem. Res. 2020, 59, 13467–13476. [Google Scholar] [CrossRef]
- Wu, R.; Li, L.; Zhang, N.; He, J.; Song, L.; Zhang, G.; Zhang, Z.; He, H. Enhancement of low-temperature NH3-SCR catalytic activity and H2O & SO2 resistance over commercial V2O5-MoO3/TiO2 catalyst by high shear-induced doping of expanded graphite. Catal. Today 2021, 376, 302–310. [Google Scholar] [CrossRef]
- Kang, T.H.; Youn, S.; Kim, D.H. Improved catalytic performance and resistance to SO2 over V2O5-WO3/TiO2 catalyst physically mixed with Fe2O3 for low-temperature NH3-SCR. Catal. Today 2020, 376, 95–103. [Google Scholar] [CrossRef]
- Zyrkowski, M.; Motak, M.; Samojeden, B.; Szczepanek, K. Deactivation of V2O5-WO3/TiO2 DeNOx Catalyst under Commercial Conditions in Power Production Plant. Energies 2020, 13, 6200. [Google Scholar] [CrossRef]
- Szymaszek, A.; Samojeden, B.; Motak, M. The Deactivation of Industrial SCR Catalysts—A Short Review. Energies 2020, 13, 3870. [Google Scholar] [CrossRef]
- Chang, H.; Shi, C.; Li, M.; Zhang, T.; Wang, C.; Jiang, L.; Wang, X. The effect of cations (NH4+, Na+, K+, and Ca2+) on chemical deactivation of commercial SCR catalyst by bromides. Cuihua Xuebao/Chinese J. Catal. 2018, 39, 710–717. [Google Scholar] [CrossRef]
- Li, S.; Huang, W.; Xu, H.; Chen, T.; Ke, Y.; Qu, Z.; Yan, N. Alkali-induced deactivation mechanism of V2O5-WO3/TiO2 catalyst during selective catalytic reduction of NO by NH3 in aluminum hydrate calcining flue gas. Appl. Catal. B Environ. 2020, 270, 118872. [Google Scholar] [CrossRef]
- Du, X.; Xue, J.; Wang, X.; Chen, Y.; Ran, J.; Zhang, L. Oxidation of Sulfur Dioxide over V2O5/TiO2 Catalyst with Low Vanadium Loading: A Theoretical Study. J. Phys. Chem. C 2018, 122, 4517–4523. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, J.; Lin, Y.; Guo, J.; Zhu, T. Distribution of SO2 Oxidation Products in the SCR of NO over V2O5/TiO2 Catalysts at Different Temperatures. Ind. Eng. Chem. Res. 2020, 59, 5177–5185. [Google Scholar] [CrossRef]
- Samojeden, B.; Grzybek, T. The influence of nitrogen groups introduced onto activated carbons by high- or low-temperature NH3treatment on SO2sorption capacity. Adsorpt. Sci. Technol. 2017, 35, 572–581. [Google Scholar] [CrossRef]
- Chai, Y.; Zhang, G.; He, H.; Sun, S. Theoretical study of the catalytic activity and anti-SO2 poisoning of a MoO3/V2O5 selective catalytic reduction catalyst. ACS Omega 2020, 5, 26978–26985. [Google Scholar] [CrossRef]
- Youn, S.; Song, I.; Lee, H.; Cho, S.J.; Kim, D.H. Effect of pore structure of TiO2 on the SO2 poisoning over V2O5/TiO2 catalysts for selective catalytic reduction of NOx with NH3. Catal. Today 2018, 303, 19–24. [Google Scholar] [CrossRef]
- Guo, M.; Liu, Q.; Zhao, P.; Han, J.; Li, X.; Ha, Y.; Fu, Z.; Song, C.; Ji, N.; Liu, C.; et al. Promotional effect of SO2 on Cr2O3 catalysts for the marine NH3-SCR reaction. Chem. Eng. J. 2019, 361, 830–838. [Google Scholar] [CrossRef]
- Xu, L.; Wang, C.; Chang, H.; Wu, Q.; Zhang, T.; Li, J. New Insight into SO2 Poisoning and Regeneration of CeO2-WO3/TiO2 and V2O5-WO3/TiO2 Catalysts for Low-Temperature NH3-SCR. Environ. Sci. Technol. 2018, 52, 7064–7071. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Chen, Y. Promotion of transition metal oxides on the NH3-SCR performance of ZrO2-CeO2 catalyst. Front. Environ. Sci. Eng. 2017, 11, 6. [Google Scholar] [CrossRef]
- Szymaszek, A.; Samojeden, B.; Motak, M. Selective catalytic reduction of NOx with ammonia (NH3-SCR) over transition metal-based catalysts—influence of the catalysts support. Physicochem. Probl. Miner. Process. 2019, 55, 1429–1441. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, F.; Zhu, M.; Dan, J.; Wang, X.; Zhang, J.; Dai, B. Enhanced low temperature NO reduction performance via MnOx-Fe2O3/vermiculite monolithic honeycomb catalysts. Catalysts 2018, 8, 100. [Google Scholar] [CrossRef]
- Sun, B.; Sheng, M.; Gao, W.; Zhang, L.; Arowo, M.; Liang, Y.; Shao, L.; Chu, G.W.; Zou, H.; Chen, J.F. Absorption of Nitrogen Oxides into Sodium Hydroxide Solution in a Rotating Packed Bed with Preoxidation by Ozone. Energy Fuels 2017, 31, 11019–11025. [Google Scholar] [CrossRef]
- Saad, M.; Szymaszek, A.; Białas, A.; Samojeden, B.; Motak, M. So2 poisoning and recovery of copper-based activated carbon catalysts for selective catalytic reduction of no with nh3 at low temperature. Catalysts 2020, 10, 1426. [Google Scholar] [CrossRef]
- Samojeden, B.; Drużkowska, J.; Duraczyńska, D.; Poddębniak, M.; Motak, M. Use of iron and copper-promoted cenospheres as catalysts in the selective catalytic reduction of nitrogen(II) oxide with ammonia. Przem. Chem. 2019, 1, 55–59. [Google Scholar] [CrossRef]
- Wang, B.; Ma, J.; Wang, D.; Gong, Z.; Shi, Q.; Gao, C.; Lu, C.; Crittenden, J. Acid-pretreated red mud for selective catalytic reduction of NOx with NH3: Insights into inhibition mechanism of binders. Catal. Today 2021, 376, 247–254. [Google Scholar] [CrossRef]
- Bendrich, M.; Scheuer, A.; Hayes, R.E.; Votsmeier, M. Unified mechanistic model for Standard SCR, Fast SCR, and NO2 SCR over a copper chabazite catalyst. Appl. Catal. B Environ. 2018, 222, 76–87. [Google Scholar] [CrossRef]
- Lei, H.; Rizzotto, V.; Guo, A.; Ye, D.; Simon, U.; Chen, P. Recent understanding of low-temperature copper dynamics in Cu-chabazite NH3-SCR catalysts. Catalysts 2021, 11, 52. [Google Scholar] [CrossRef]
- Rutkowska, M.; Pacia, I.; Basa, S.; Kowalczyk, A.; Piwowarska, Z.; Duda, M. Catalytic performance of commercial Cu-ZSM-5 zeolite modi fi ed by desilication in NH3-SCR and NH3-SCO processes. 2017, 246, 193–206. Micropor. Mesopor. Mater. 2017, 246, 193–206. [Google Scholar] [CrossRef]
- Zhang, T.; Chang, H.; You, Y.; Shi, C.; Li, J. Excellent Activity and Selectivity of One-Pot Synthesized Cu—SSZ-13 Catalyst in the Selective Catalytic Oxidation of Ammonia to Nitrogen. Environ. Sci. Technol. 2018, 52, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Díaz, U.; Corma, A. Layered zeolitic materials: An approach to designing versatile functional solids. Dalt. Trans. 2014, 43, 10292–10316. [Google Scholar] [CrossRef] [PubMed]
- Marosz, M.; Samojeden, B.; Kowalczyk, A.; Rutkowska, M.; Motak, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. MCM-22, MCM-36, and ITQ-2 zeolites with different Si/Al molar ratios as effective catalysts of methanol and ethanol dehydration. Materials 2020, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, B.; Andrade, H.M.C.; Mascarenhas, A.J.S. Oxidative dehydration of glycerol over alternative H, Fe-MCM-22 catalysts: Sustainable production of acrylic acid. Microporous Mesoporous Mater. 2019, 278, 366–377. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, D.; Chu, X.; Li, D.; Wang, J.; Song, W. Highly selective isomerization of biomass b -pinene over hierarchically acidic MCM-22 catalyst. Microporous Mesoporous Mater. 2017, 237, 180–188. [Google Scholar] [CrossRef]
- Aleixo, R.; Elvas-leitão, R.; Martins, F.; Carvalho, A.P.; Brigas, A.; Martins, A.; Nunes, N. Kinetic study of Friedel-Crafts acylation reactions over hierarchical MCM-22 zeolites. Mol. Catal. 2017, 434, 175–183. [Google Scholar] [CrossRef]
- Xiao, P.; Wang, Y.; Osuga, R.; Kondo, J.N.; Yokoi, T. One-pot synthesis of highly active Fe-containing MWW zeolite catalyst: Elucidation of Fe species and its impact on catalytic performance. Adv. Powder Technol. 2021, 32, 1070–1080. [Google Scholar] [CrossRef]
- Chen, J.; Peng, G.; Liang, T.; Zhang, W.; Zheng, W.; Zhao, H.; Guo, L.; Wu, X. Catalytic performances of cu/mcm-22 zeolites with different cu loadings in nh3-scr. Nanomaterials 2020, 10, 2170. [Google Scholar] [CrossRef]
- Díaz, U. Layered Materials with Catalytic Applications Pillared and Delaminated Zeolites. Int. Sch. Res. Netw. 2012, 2012, 537164. [Google Scholar]
- Palomares, A.E.; Franch, C.; Corma, A. Determining the characteristics of a Co-zeolite to be active for the selective catalytic reduction of NO x with hydrocarbons. Catal. Today 2011, 176, 239–241. [Google Scholar] [CrossRef]
- Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Cu and Fe modified derivatives of 2D MWW-type zeolites (MCM-22, ITQ-2 and MCM-36) as new catalysts for DeNOx process. Appl. Catal. B Environ. 2015, 168–169, 531–539. [Google Scholar] [CrossRef]
- Chen, J.; Peng, G.; Zheng, W.; Zhang, W.; Guo, L.; Wu, X. Excellent performance of one-pot synthesized Fe-containing MCM-22 zeolites for the selective catalytic reduction of NO: Xwith NH3. Catal. Sci. Technol. 2020, 10, 6583–6598. [Google Scholar] [CrossRef]
- Auvray, X.; Arvanitidou, M.; Högström, Å.; Jansson, J.; Fouladvand, S.; Olsson, L. Comparative Study of SO2 and SO2/SO3 Poisoning and Regeneration of Cu/BEA and Cu/SSZ-13 for NH3 SCR. Emiss. Control Sci. Technol. 2021, 7, 232–246. [Google Scholar] [CrossRef]
- Hammershøi, P.S.; Jensen, A.D.; Janssens, T.V.W. Impact of SO2-poisoning over the lifetime of a Cu-CHA catalyst for NH3-SCR. Appl. Catal. B Environ. 2018, 238, 104–110. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Elsener, M. Selective catalytic reduction of NO and N2O at low temperatures. Catal. Today 2002, 73, 239–247. [Google Scholar] [CrossRef]
- Rutkowska, M.; Jankowska, A.; Różycka-Dudek, E.; Dubiel, W.; Kowalczyk, A.; Piwowarska, Z.; Llopis, S.; Díaz, U.; Chmielarz, L. Modification of mcm-22 zeolite and its derivatives with iron for the application in n2o decomposition. Catalysts 2020, 10, 1139. [Google Scholar] [CrossRef]
- Delahay, G.; Coq, B.; Kieger, S.; Neveu, B. The origin of N2O formation in the selective catalytic reduction of NOx by NH3 in O2 rich atmosphere on Cu-faujasite catalysts. Catal. Today 1999, 54, 431–438. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, H.; Su, W.; Li, K.; Zhang, J.; Shi, J.; Tian, J.; Wang, J. Mineral-derived catalysts optimized for selective catalytic reduction of NOx with NH3. J. Clean. Prod. 2021, 289, 125756. [Google Scholar] [CrossRef]
- Roth, W.J.; Dorset, D.L. Expanded view of zeolite structures and their variability based on layered nature of 3-D frameworks. Microporous Mesoporous Mater. 2011, 142, 32–36. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Makowski, W.; Sławek, A.; Korzeniowska, A.; Grzybek, J.; Siwek, M.; Michorczyk, P. Framework-substituted cerium MCM-22 zeolite and its interlayer expanded derivative MWW-IEZ. Catal. Sci. Technol. 2016, 6, 2742–2753. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Nawab, M.; Barot, S.; Bandyopadhyay, R. Solvent-free selective oxidation of toluene over metal-doped MCM-22. New J. Chem. 2019, 43, 4406–4412. [Google Scholar] [CrossRef]
- Ahmad, A.; Naqvi, S.R.; Rafique, M.; Nasir, H.; Sarosh, A. Synthesis, characterization and catalytic testing of MCM-22 derived catalysts for n-hexane cracking. Sci. Rep. 2020, 10, 21786. [Google Scholar] [CrossRef]
- Thakkar, R.; Bandyopadhyay, R. Preparation, characterization, and post-synthetic modification of layered MCM-22 zeolite precursor. J. Chem. Sci. 2017, 129, 1671–1676. [Google Scholar] [CrossRef]
- Sahu, P.; Eniyarppu, S.; Ahmed, M.; Sharma, D.; Sakthivel, A. Cerium ion-exchanged layered MCM-22: Preparation, characterization and its application for esterification of fatty acids. J. Porous Mater. 2018, 25, 999–1005. [Google Scholar] [CrossRef]
- Zaitan, H.; Bianchi, D.; Achak, O.; Chafik, T. A comparative study of the adsorption and desorption of o-xylene onto bentonite clay and alumina. J. Hazard. Mater. 2008, 153, 852–859. [Google Scholar] [CrossRef]
- Kumar, A.; Lingfa, P. Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD. Mater. Today Proc. 2020, 22, 737–742. [Google Scholar] [CrossRef]
- Onida, B.; Geobaldo, F.; Testa, F.; Aiello, R.; Garrone, E. H-bond formation and proton transfer in H-MCM-22 zeolite as compared to H-ZSM-5 and H-MOR: An FTIR study. J. Phys. Chem. B 2002, 106, 1684–1690. [Google Scholar] [CrossRef]
- Onida, B.; Geobaldo, F.; Testa, F.; Crea, F.; Garrone, E. FTIR investigation of the interaction at 77 K of diatomic molecular probes on MCM-22 zeolite. Microporous Mesoporous Mater. 1999, 30, 119–127. [Google Scholar] [CrossRef]
- Onida, B.; Borello, L.; Bonelli, B.; Geobaldo, F.; Garrone, E. IR study of the acidity of ITQ-2, an “all-surface” zeolitic system. J. Catal. 2003, 214, 191–199. [Google Scholar] [CrossRef]
- Favvas, E.P.; Tsanaktsidis, C.G.; Sapalidis, A.A.; Tzilantonis, G.T.; Papageorgiou, S.K.; Mitropoulos, A.C. Clinoptilolite, a natural zeolite material: Structural characterization and performance evaluation on its dehydration properties of hydrocarbon-based fuels. Microporous Mesoporous Mater. 2016, 225, 385–391. [Google Scholar] [CrossRef]
- Hawn, D.D.; DeKoven, B.M. Deconvolution as a correction for photoelectron inelastic energy losses in the core level XPS spectra of iron oxides. Surf. Interface Anal. 1987, 10, 63–74. [Google Scholar] [CrossRef]
- Gurgul, J.; Łątka, K.; Hnat, I.; Rynkowski, J.; Dzwigaj, S. Identification of iron species in FeSiBEA by DR UV-vis, XPS and Mössbauer spectroscopy: Influence of Fe content. Microporous Mesoporous Mater. 2013, 168, 1–6. [Google Scholar] [CrossRef]
- Milanesio, M.; Croce, G.; Viterbo, D.; Pastore, H.O.; Dos Santos Mascarenhas, A.J.; De Oliveira Munsignatti, E.C.; Meda, L. A Combined High-Resolution X-ray Powder Diffraction, Computational, and XPS Study of the Local Structure of Extra- Framework Copper Ions in Over-Exchanged Cu-MCM22 Zeolite. J. Phys. Chem. A 2008, 112, 13745. [Google Scholar] [CrossRef][Green Version]
- Grzybek, T. Węgle Aktywne Promowane Fe3+ Jako Katalizatory Redukcji Tlenku Azotu Amoniakiem; Wydawnictwo Naukowe AGH: Kraków, Poland, 1994. [Google Scholar]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database. J. Chem. Educ. 1996, 70, A25. [Google Scholar] [CrossRef]
- Bordiga, S.; Buzzoni, R.; Geobaldo, F.; Lamberti, C.; Giamello, E.; Zecchina, A.; Leofanti, G.; Petrini, G.; Tozzola, G.; Vlaic, G. Structure and reactivity of framework and extraframework iron in Fe-silicalite as investigated by spectroscopic and physicochemical methods. J. Catal. 1996, 158, 486–501. [Google Scholar] [CrossRef]
- Kumar, M.S.; Schwidder, M.; Grünert, W.; Brückner, A. On the nature of different iron sites and their catalytic role in Fe-ZSM-5 DeNOx catalysts: New insights by a combined EPR and UV/VIS spectroscopic approach. J. Catal. 2004, 227, 384–397. [Google Scholar] [CrossRef]
- Boroń, P.; Chmielarz, L.; Gurgul, J.; Łątka, K.; Gil, B.; Marszałek, B.; Dzwigaj, S. Influence of iron state and acidity of zeolites on the catalytic activity of FeHBEA, FeHZSM-5 and FeHMOR in SCR of NO with NH3 and N2O decomposition. Microporous Mesoporous Mater. 2015, 203, 73–85. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The determination of the activities of different iron species in Fe-ZSM-5 for SCR of NO by NH3. Appl. Catal. B Environ. 2010, 95, 348–357. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Elsener, M.; Kröcher, O. The Significance of Lewis Acid Sites for the Selective Catalytic Reduction of Nitric Oxide on Vanadium-Based Catalysts. Angew. Chemie—Int. Ed. 2016, 55, 11989–11994. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Romero, M.; Camposeco, R.; Castillo, S.; Marín, J.; Rodríguez-González, V.; García-Serrano, L.A.; Mejía-Centeno, I. Acidity, surface species, and catalytic activity study on V2O5-WO3/TiO2 nanotube catalysts for selective NO reduction by NH3. Fuel 2017, 198, 123–133. [Google Scholar] [CrossRef]
- Kapustin, G.I.; Brueva, T.R.; Klyachko, A.L.; Beran, S.; Wichterlova, B. Determination of the number and acid strength of acid sites in zeolites by ammonia adsorption. Comparison of calorimetry and temperature-programmed desorption of ammonia. Appl. Catal. 1988, 42, 239–246. [Google Scholar] [CrossRef]
- Ates, A. Characteristics of Fe-exchanged natural zeolites for the decomposition of N2O and its selective catalytic reduction with NH3. Appl. Catal. B Environ. 2007, 76, 282–290. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Q.; Meng, X.; Müller, U.; Feyen, M.; Dai, D.; Maurer, S.; McGuire, R.; Moini, A.; Parvulescu, A.N.; et al. Recent advances in the preparation of zeolites for the selective catalytic reduction of NOx in diesel engines. React. Chem. Eng. 2019, 4, 975–985. [Google Scholar] [CrossRef]
- Song, S.; Wu, G.; Dai, W.; Guan, N.; Li, L. Al-free Fe-beta as a robust catalyst for selective reduction of nitric oxide by ammonia. Catal. Sci. Technol. 2016, 6, 8325–8335. [Google Scholar] [CrossRef]
- Ryu, T.; Kang, Y.; Nam, I.S.; Hong, S.B. Iron-exchanged high-silica LTA zeolites as hydrothermally stable NH3-SCR catalysts. React. Chem. Eng. 2019, 4, 1050–1058. [Google Scholar] [CrossRef]
- Andonova, S.; Tamm, S.; Montreuil, C.; Lambert, C.; Olsson, L. The effect of iron loading and hydrothermal aging on one-pot synthesized Fe/SAPO-34 for ammonia SCR. Appl. Catal. B Environ. 2016, 180, 775–787. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Wokaun, A.; Tissler, A.; Althoff, R. The role of Brønsted acidity in the selective catalytic reduction of NO with ammonia over Fe-ZSM-5. J. Catal. 2009, 268, 297–306. [Google Scholar] [CrossRef]
- Luo, J.Y.; Hou, X.; Wijayakoon, P.; Schmieg, S.J.; Li, W.; Epling, W.S. Spatially resolving SCR reactions over a Fe/zeolite catalyst. Appl. Catal. B Environ. 2011, 102, 110–119. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, R.T. N2O Formation Pathways over Zeolite-Supported Cu and Fe Catalysts in NH3-SCR. Energy and Fuels 2018, 32, 2170–2182. [Google Scholar] [CrossRef]
- Delahay, G.; Mauvezin, M.; Coq, B.; Kieger, S. Selective catalytic reduction of nitrous oxide by ammonia on iron zeolite beta catalysts in an oxygen rich atmosphere: Effect of iron contents. J. Catal. 2001, 202, 156–162. [Google Scholar] [CrossRef]
- Shi, Y.J.; Shu, H.; Zhang, Y.H.; Fan, H.M.; Zhang, Y.P.; Yang, L.J. Formation and decomposition of NH4HSO4 during selective catalytic reduction of NO with NH3 over V2O5-WO3/TiO2 catalysts. Fuel Process. Technol. 2016, 150, 141–147. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Zhang, Z.; Liu, Q. The latest research progress of nh3-scr in the so2 resistance of the catalyst in low temperatures for selective catalytic reduction of nox. Catalysts 2020, 10, 1034. [Google Scholar] [CrossRef]
- Nagaishi, T.; Ishiyama, S.; Matsumoto, M.; Yoshinaga, S. Reactions between ammonium sulphate and metal oxides (metal=Cr, Mn and Fe) and thermal decomposition of the products. J. Therm. Anal. 1984, 29, 121–129. [Google Scholar] [CrossRef]
- Song, X.; Zhao, J.; Li, Y.; Sun, Z.; Yu, J. Thermal decomposition mechanism of ammonium sulfate catalyzed by ferric oxide. Front. Chem. Sci. Eng. 2013, 7, 210–217. [Google Scholar] [CrossRef]
- Alemany, L.J.; Berti, F.; Busca, G.; Ramis, G.; Robba, D.; Toledo, G.P.; Trombetta, M. Characterization and composition of commercial V2O5-WO3- TiO2 SCR catalysts. Appl. Catal. B Environ. 1996, 10, 299–311. [Google Scholar] [CrossRef]
- Husnain, N.; Wang, E.; Li, K.; Anwar, M.T.; Mehmood, A.; Gul, M.; Li, D.; Mao, J. Iron oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3. Rev. Chem. Eng. 2019, 35, 239–264. [Google Scholar] [CrossRef]
- Lee Ashley, T.; Bai, H. Byproduct analysis of so2 poisoning on nh3-scr over mnfe/tio2 catalysts at medium to low temperatures. Catalysts 2019, 9, 265. [Google Scholar] [CrossRef]
- Ming, S.; Pang, L.; Chen, Z.; Guo, Y.; Guo, L.; Liu, Q.; Liu, P.; Dong, Y.; Zhang, S.; Li, T. Insight into SO2 poisoning over Cu-SAPO-18 used for NH3-SCR. Microporous Mesoporous Mater. 2020, 303, 110294. [Google Scholar] [CrossRef]
- Wijayanti, K.; Leistner, K.; Chand, S.; Kumar, A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Deactivation of Cu-SSZ-13 by SO2 exposure under SCR conditions. Catal. Sci. Technol. 2016, 6, 2565–2579. [Google Scholar] [CrossRef]
- Wang, S.; Fan, C.; Zhao, Z.; Liu, Q.; Xu, G.; Wu, M.; Chen, J.; Li, J. A facile and controllable in situ sulfation strategy for CuCeZr catalyst for NH3-SCR. Appl. Catal. A Gen. 2020, 597, 117554. [Google Scholar] [CrossRef]
- Corma, A.; Corell, C. Synthesis and characterization of the MCM-22 zeolite. Zeolites 1995, 15, 2–8. [Google Scholar] [CrossRef]
- Xing, E.; Shi, Y.; Zheng, A.; Zhang, J.; Gao, X.; Liu, D.; Xin, M.; Xie, W.; Zhang, F.; Mu, X.; et al. Transformation from NaA to MCM-49 Zeolite and Its Catalytic Alkylation Performance. Ind. Eng. Chem. Res. 2015, 54, 3123–3135. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET equation applicable to microporous adsorbents ? Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar]



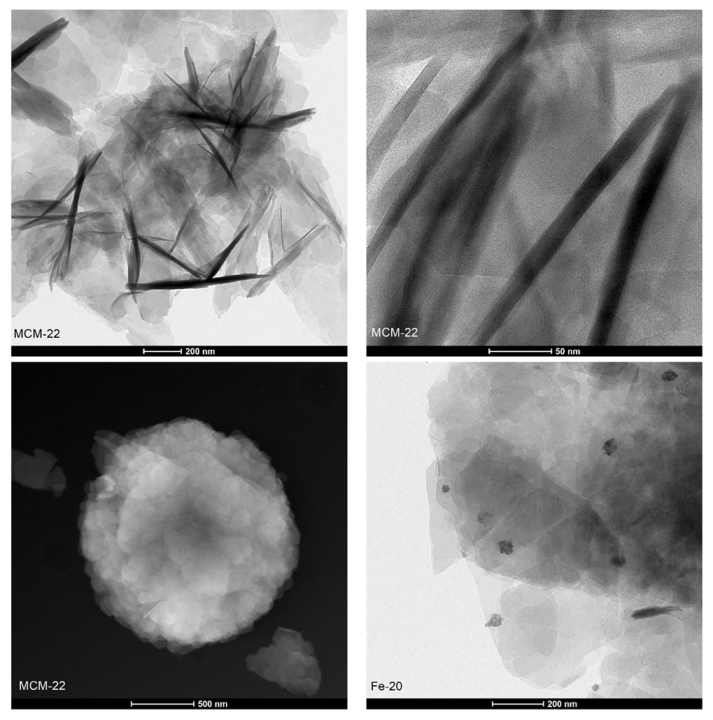

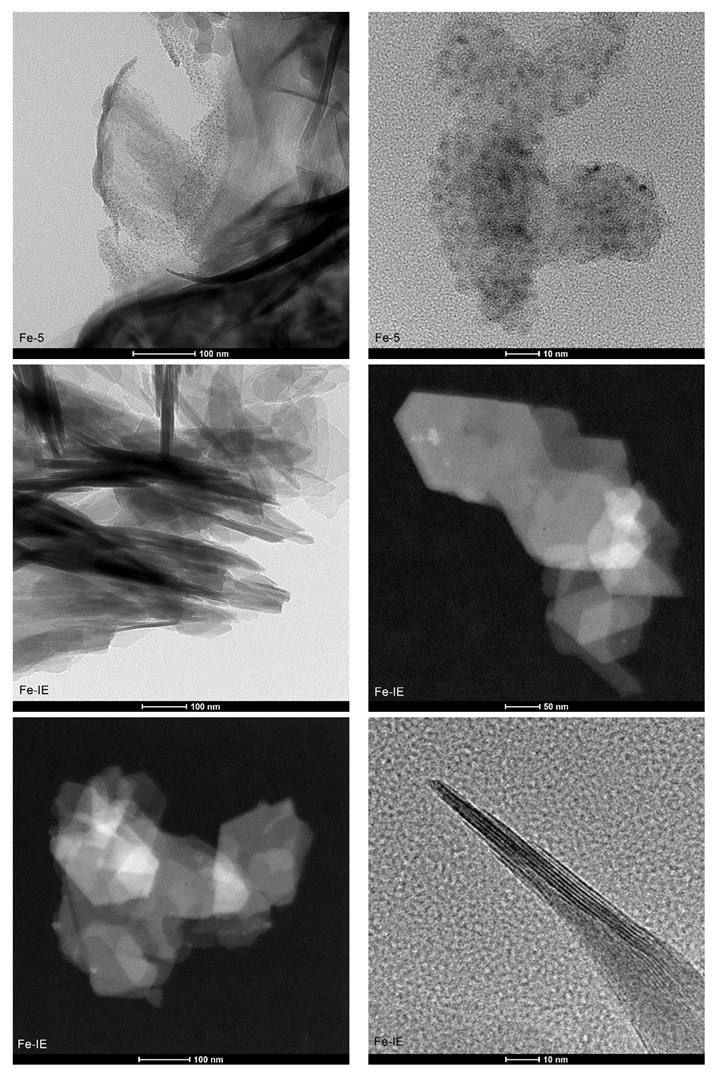
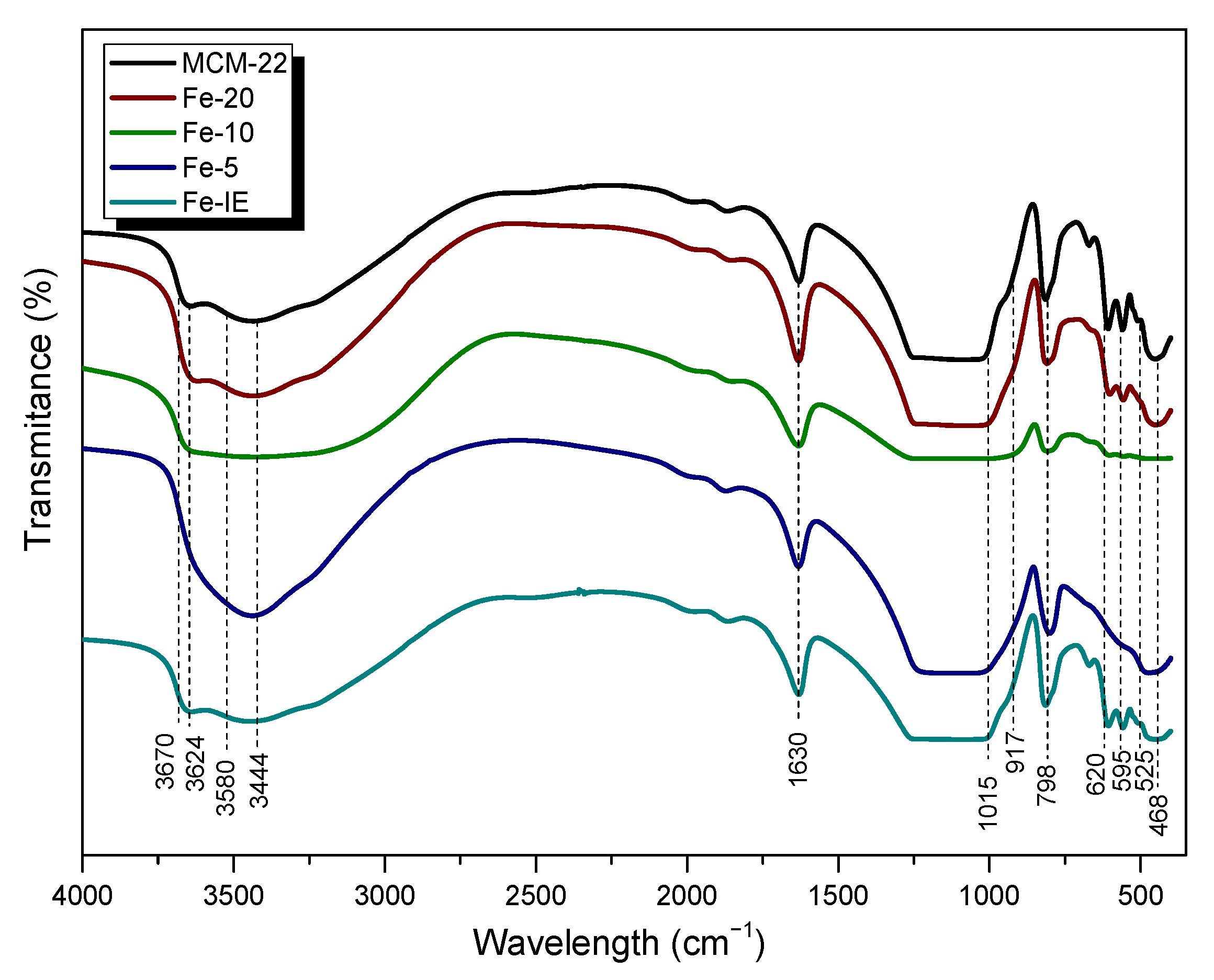


| Temperature (°C) | Fe-20 | Fe-10 | Fe-5 | Fe-IE | ||||
|---|---|---|---|---|---|---|---|---|
| Fresh | SO2 | Fresh | SO2 | Fresh | SO2 | Fresh | SO2 | |
| 150 | 0 | 0 | 3 | 1 | 1 | 38 | 0 | 0 |
| 200 | 5 | 12 | 7 | 8 | 2 | 33 | 4 | 4 |
| 250 | 2 | 22 | 9 | 15 | 10 | 31 | 8 | 10 |
| 300 | 1 | 22 | 9 | 17 | 12 | 32 | 14 | 19 |
| 350 | 5 | 19 | 5 | 16 | 12 | 32 | 17 | 21 |
| 400 | 0 | 15 | 0 | 15 | 12 | 32 | 12 | 18 |
| 450 | 0 | 15 | 0 | 14 | 14 | 24 | 9 | 17 |
| Sample Code | Si (wt.%) | Al (wt.%) | Fe (wt.%) | Si/Al | RC (%) |
|---|---|---|---|---|---|
| MCM-22 | 33.23 | 1.41 | 0 | 23 | 100.0 |
| Fe-20 | 36.38 | 1.17 | 4.78 | 29 | 65.5 |
| Fe-10 | 36.58 | 1.60 | 8.43 | 21 | 15.8 |
| Fe-5 | 33.32 | 1.40 | 14.90 | 23 | 0.0 |
| Fe-IE | 30.48 | 1.19 | 5.74 | 25 | 90.3 |
| Sample Code | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | ||||
|---|---|---|---|---|---|---|
| SBET a | Smicro b | SExt b | Vtotal | Vmicro b | Vmeso c | |
| MCM-22 | 479 | 367 | 112 | 0.518 | 0.192 | 0.116 |
| Fe-20 | 419 | 335 | 84 | 0.479 | 0.160 | 0.196 |
| Fe-10 | 216 | 116 | 101 | 0.475 | 0.055 | 0.246 |
| Fe-5 | 144 | 15 | 130 | 0.379 | 0.007 | 0.170 |
| Fe-IE | 372 | 263 | 109 | 0.418 | 0.129 | 0.119 |
| Sample Code | Weight Loss in the Temperature Range (%) | ||
|---|---|---|---|
| 30–150 °C | 150–400 °C | 400–800 °C | |
| MCM-22 | 4.3 | 0.6 | 0.6 |
| Fe-20 | 7.5 | 1.4 | 0.7 |
| Fe-10 | 3.3 | 0.9 | 0.6 |
| Fe-5 | 2.1 | 0.9 | 0.5 |
| Fe-IE | 5.1 | 1.0 | 0.6 |
| Core Excitation | Binding Energy (eV) | Relative Area of the Component (at.%) in the Sample | |||
|---|---|---|---|---|---|
| Fe-20 | Fe-10 | Fe-5 | Fe-IE | ||
| Si 2p | 103.0 | 26.3 | 26.0 | 24.3 | 25.8 |
| Al 2p | 74.4 | 1.1 | 1.2 | 1.1 | 1.4 |
| O 1s * | 534.2–530.5 | 62.4 | 60.8 | 64.4 | 56.9 |
| Fe 2p3/2 ** | 709.9 | 0.9 | 1.2 | 2.4 | 0.8 |
| Sample Code | Concentration of Acid Sites (μmol g−1) | ||
|---|---|---|---|
| Weak Sites | Medium and Strong Sites | Total Amount of Sites | |
| MCM-22 | 747 | 524 | 1271 |
| Fe-20 | 745 | 597 | 1342 |
| Fe-10 | 398 | 219 | 617 |
| Fe-5 | 153 | 0 | 153 |
| Fe-IE | 670 | 533 | 1203 |
| Sample Code | Si/Fe |
|---|---|
| MCM-22 | non-modified support |
| Fe-20 | 20 * |
| Fe-10 | 10 * |
| Fe-5 | 5 * |
| Fe-IE | 11 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymaszek-Wawryca, A.; Díaz, U.; Duraczyńska, D.; Świerczek, K.; Samojeden, B.; Motak, M. Catalytic Performance and Sulfur Dioxide Resistance of One-Pot Synthesized Fe-MCM-22 in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia (NH3-SCR)—The Effect of Iron Content. Int. J. Mol. Sci. 2022, 23, 10754. https://doi.org/10.3390/ijms231810754
Szymaszek-Wawryca A, Díaz U, Duraczyńska D, Świerczek K, Samojeden B, Motak M. Catalytic Performance and Sulfur Dioxide Resistance of One-Pot Synthesized Fe-MCM-22 in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia (NH3-SCR)—The Effect of Iron Content. International Journal of Molecular Sciences. 2022; 23(18):10754. https://doi.org/10.3390/ijms231810754
Chicago/Turabian StyleSzymaszek-Wawryca, Agnieszka, Urbano Díaz, Dorota Duraczyńska, Konrad Świerczek, Bogdan Samojeden, and Monika Motak. 2022. "Catalytic Performance and Sulfur Dioxide Resistance of One-Pot Synthesized Fe-MCM-22 in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia (NH3-SCR)—The Effect of Iron Content" International Journal of Molecular Sciences 23, no. 18: 10754. https://doi.org/10.3390/ijms231810754
APA StyleSzymaszek-Wawryca, A., Díaz, U., Duraczyńska, D., Świerczek, K., Samojeden, B., & Motak, M. (2022). Catalytic Performance and Sulfur Dioxide Resistance of One-Pot Synthesized Fe-MCM-22 in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia (NH3-SCR)—The Effect of Iron Content. International Journal of Molecular Sciences, 23(18), 10754. https://doi.org/10.3390/ijms231810754