The Underlying Relationship between Keratoconus and Down Syndrome
Abstract
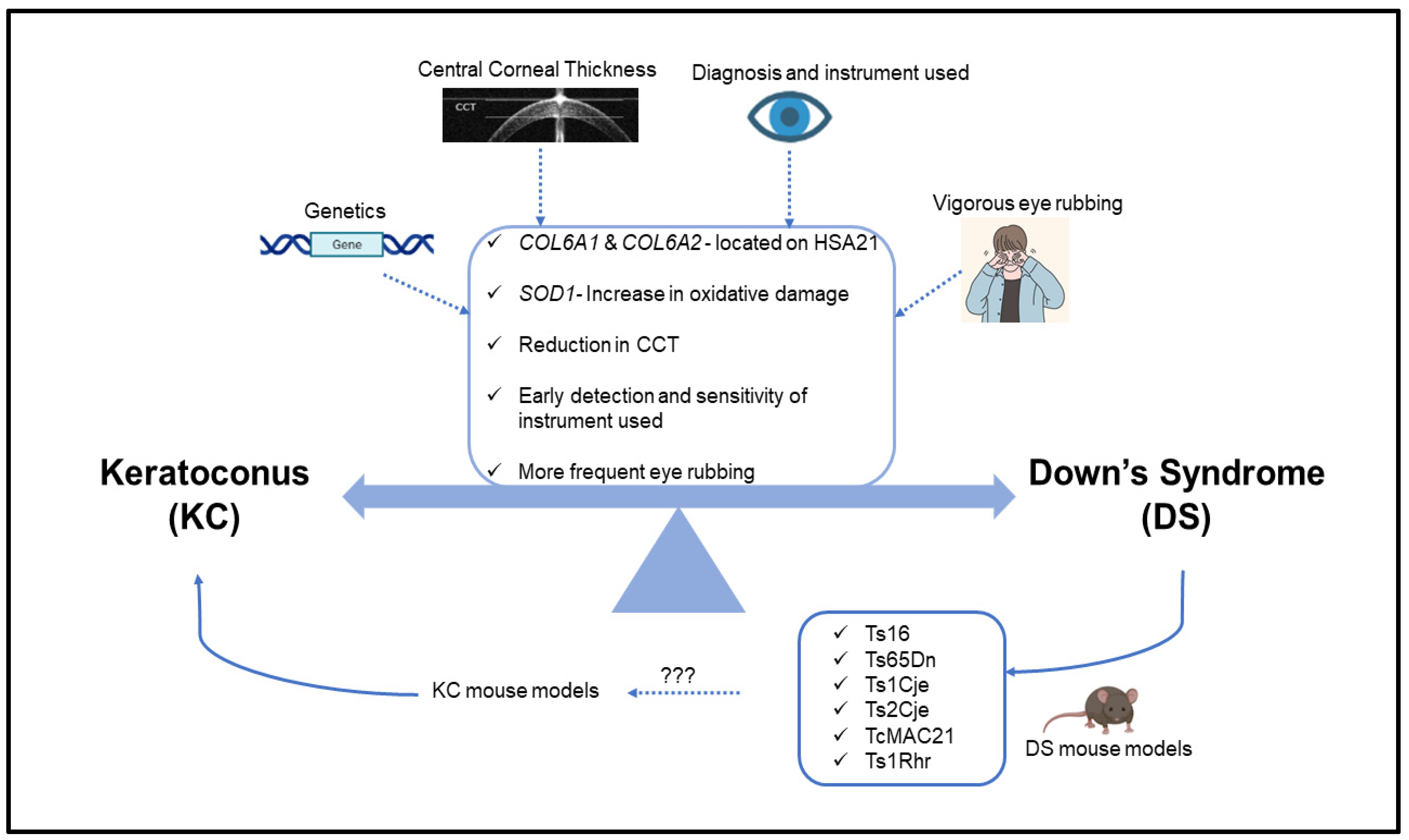
1. Introduction
2. Ophthalmic Manifestations of Down Syndrome
3. Genetics of Keratoconus and Down Syndrome
4. Central Corneal Thickness (CCT) of Keratoconus and Down Syndrome Patients
5. Eye Rubbing in Keratoconus and Down Syndrome Patients
6. Diagnosis/Treatment of Keratoconus in Down Syndrome Patients
7. Mouse Models of DS for KC
8. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnal, E.; Peris-Martínez, C.; Menezo, J.L.; Johnsen-Soriano, S.; Romero, F.J. Oxidative Stress in Keratoconus? Investig. Opthalmol. Vis. Sci. 2011, 52, 8592–8597. [Google Scholar] [CrossRef] [PubMed]
- Loukovitis, E.; Sfakianakis, K.; Syrmakesi, P.; Tsotridou, E.; Orfanidou, M.; Bakaloudi, D.R.; Stoila, M.; Kozei, A.; Koronis, S.; Zachariadis, Z.; et al. Genetic Aspects of Keratoconus: A Literature Review Exploring Potential Genetic Contributions and Possible Genetic Relationships with Comorbidities. Ophthalmol. Ther. 2018, 7, 263–292. [Google Scholar] [CrossRef]
- Sugar, J.; Macsai, M.S. What Causes Keratoconus? Cornea 2012, 31, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Coco, G.; Kheirkhah, A.; Foulsham, W.; Dana, R.; Ciolino, J.B. Keratoconus progression associated with hormone replacement therapy. Am. J. Ophthalmol. Case Rep. 2019, 15, 100519. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Ambati, B.K. Pediatric keratoconus: A review of the literature. Int. Ophthalmol. 2018, 38, 2257–2266. [Google Scholar] [CrossRef]
- Kennedy, R.H.; Bourne, W.M.; Dyer, J.A. A 48-Year Clinical and Epidemiologic Study of Keratoconus. Am. J. Ophthalmol. 1986, 101, 267–273. [Google Scholar] [CrossRef]
- Godefrooij, D.A.; de Wit, G.A.; Uiterwaal, C.S.; Imhof, S.M.; Wisse, R.P. Age-specific Incidence and Prevalence of Keratoconus: A Nationwide Registration Study. Am. J. Ophthalmol. 2017, 175, 169–172. [Google Scholar] [CrossRef]
- Karamichos, D.; Escandon, P.; Vasini, B.; Nicholas, S.E.; Van, L.; Dang, D.H.; Cunningham, R.L.; Riaz, K.M. Anterior pituitary, sex hormones, and keratoconus: Beyond traditional targets. Prog. Retin. Eye Res. 2021, 88, 101016. [Google Scholar] [CrossRef]
- Georgiou, T.; Funnell, C.L.; Cassels-Brown, A.; O’Conor, R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye 2004, 18, 379–383. [Google Scholar] [CrossRef]
- Alzahrani, K.; Al-Rashah, A.; Al-Salem, S.; Al-Murdif, Y.; Al-Rashah, A.; Alrashah, A.; Al-Faify, N.; Ibrahim, M. Keratoconus Epidemiology Presentations at Najran Province, Saudi Arabia. Clin. Optom. 2021, ume 13, 175–179. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Rodrigues, P.F.; Lamazales, L.L. Keratoconus epidemiology: A review. Saudi J. Ophthalmol. 2022, 36, 3–6. [Google Scholar] [CrossRef]
- Akowuah, P.K.; Kobia-Acquah, E.; Donkor, R.; Adjei-Anang, J.; Ankamah-Lomotey, S. Keratoconus in Africa: A systematic review and meta-analysis. Ophthalmic Physiol. Opt. 2021, 41, 736–747. [Google Scholar] [CrossRef]
- Kristianslund, O.; Drolsum, L. Prevalence of Keratoconus in Persons with Down Syndrome in a National Registry in Norway. JAMA Netw. Open 2021, 4, e210814. [Google Scholar] [CrossRef]
- Papali’I-Curtin, A.T.; Cox, R.; Ma, T.; Woods, L.; Covello, A.; Hall, R.C. Keratoconus Prevalence Among High School Students in New Zealand. Cornea 2019, 38, 1382–1389. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Chen, Y. Application of a scheimpflug-based biomechanical analyser and tomography in the early detection of subclinical keratoconus in chinese patients. BMC Ophthalmol. 2021, 21, 339. [Google Scholar] [CrossRef]
- Ferrari, G.; Rama, P. The keratoconus enigma: A review with emphasis on pathogenesis. Ocul. Surf. 2020, 18, 363–373. [Google Scholar] [CrossRef]
- Gordon-Shaag, A.; Millodot, M.; Shneor, E. The Epidemiology and Etiology of Keratoconus. Int. J. Keratoconus Ectatic Corneal Dis. 2012, 1, 7–15. [Google Scholar] [CrossRef]
- Edwards, M.; McGhee, C.N.; Dean, S. The genetics of keratoconus. Clin. Exp. Ophthalmol. 2001, 29, 345–351. [Google Scholar] [CrossRef]
- Sharif, R.; Bak-Nielsen, S.; Hjortdal, J.; Karamichos, D. Pathogenesis of Keratoconus: The intriguing therapeutic potential of Prolactin-inducible protein. Prog. Retin. Eye Res. 2018, 67, 150–167. [Google Scholar] [CrossRef]
- Galvis, V.; Sherwin, T.; Tello, A.; Merayo, J.; Barrera, R.; Acera, A. Keratoconus: An inflammatory disorder? Eye 2015, 29, 843–859. [Google Scholar] [CrossRef]
- Naderan, M.; Jahanrad, A. Topographic, tomographic and biomechanical corneal changes during pregnancy in patients with keratoconus: A cohort study. Acta Ophthalmol. 2016, 95, e291–e296. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I. Keratoconus and the ehlers.danlos syndrome: A new aspect of Keratoconus. Med. J. Aust. 1975, 1, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Mediero, S.; Mardero, O.D.; Bueis, A.B.D.L.; Martín, S.N.; García-Miñaur, S. Keratoconus associated with Williams-Beuren syndrome: A new case report. Int. J. Ophthalmol. 2017, 10, 658–660. [Google Scholar] [CrossRef]
- Sharif, K.W.; Casey, T.A.; Coltart, J. Prevalence of Mitral Valve Prolapse in Keratoconus Patients. J. R. Soc. Med. 1992, 85, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Lichter, H.; Loya, N.; Sagie, A.; Cohen, N.; Muzmacher, L.; Yassur, Y.; Weinberger, D. Keratoconus and mitral valve prolapse. Am. J. Ophthalmol. 2000, 129, 667–668. [Google Scholar] [CrossRef]
- Akcay, E.K.; Akcay, M.; Uysal, B.S.; Kosekahya, P.; Aslan, A.N.; Caglayan, M.; Koseoglu, C.; Yulek, F.; Cagil, N. Impaired Corneal Biomechanical Properties and the Prevalence of Keratoconus in Mitral Valve Prolapse. J. Ophthalmol. 2014, 2014, 402193. [Google Scholar] [CrossRef]
- Fransen, E.; Valgaeren, H.; Janssens, K.; Sommen, M.; De Ridder, R.; Vandeweyer, G.; Bisceglia, L.; Soler, V.; Hoischen, A.; Mortier, G.; et al. Resequencing of candidate genes for Keratoconus reveals a role for Ehlers–Danlos Syndrome genes. Eur. J. Hum. Genet. 2021, 29, 1745–1755. [Google Scholar] [CrossRef]
- Kristianslund, O.; Drolsum, L. Prevalence of keratoconus in persons with Down syndrome: A review. BMJ Open Ophthalmol. 2021, 6, e000754. [Google Scholar] [CrossRef]
- Burdon, K.P.; Vincent, A.L. Insights into keratoconus from a genetic perspective. Clin. Exp. Optom. 2013, 96, 146–154. [Google Scholar] [CrossRef]
- Alio, J.L.; Vega-Estrada, A.; Sanz, P.; Osman, A.A.; Kamal, A.M.; Mamoon, A.; Soliman, H. Corneal Morphologic Characteristics in Patients with Down Syndrome. JAMA Ophthalmol. 2018, 136, 971–978. [Google Scholar] [CrossRef]
- Cullen, J.F.; Butler, H.G. Mongolism (down’s syndrome) and keratoconus. Br. J. Ophthalmol. 1963, 47, 321–330. [Google Scholar] [CrossRef]
- Roizen, N.J.; Mets, M.B.; Blondis, T.A. Ophthalmic disorders in children with down syndrome. Dev. Med. Child Neurol. 2008, 36, 594–600. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, J.-M.; Kim, H.J.; Yu, Y.S. Characteristic ocular findings in Asian children with Down syndrome. Eye 2002, 16, 710–714. [Google Scholar] [CrossRef]
- Kim, U.; Hwang, J.-M. Refractive errors and strabismus in Asian patients with Down syndrome. Eye 2008, 23, 1560–1564. [Google Scholar] [CrossRef]
- Fox, A.R.; Alward, W.L.; Fingert, J.H. Aqueous Misdirection After Trabeculectomy in a Down Syndrome Patient with Angle-closure Glaucoma. J. Glaucoma 2021, 30, e269–e270. [Google Scholar] [CrossRef]
- Vega-Estrada, A.; Fariselli, C.; Alio, J.L. Posterior corneal features in patients with Down syndrome and their relation with keratoconus. Br. J. Ophthalmol. 2020, 104, 1683–1689. [Google Scholar] [CrossRef]
- Crawford, A.Z.; Zhang, J.; Gokul, A.; McGhee, C.N.; Ormonde, S.E. The Enigma of Environmental Factors in Keratoconus. Asia-Pac. J. Ophthalmol. 2020, 9, 549–556. [Google Scholar] [CrossRef]
- Imbornoni, L.M.; Wise, R.E.; Taravella, M.J.; Hickey, F.; McCourt, E.A. Keratoconus and corneal morphology in patients with Down syndrome at a pediatric hospital. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2020, 24, 140.e1–140.e5. [Google Scholar] [CrossRef]
- Asgari, S.; Aghamirsalim, M.; Mehravaran, S.; Hashemi, H. Effect of Down syndrome and keratoconus on corneal density and volume: A triple comparative study. Sci. Rep. 2020, 10, 9098. [Google Scholar] [CrossRef] [PubMed]
- Haugen, O.H.; Høvding, G.; Eide, G.E. Biometric measurements of the eyes in teenagers and young adults with Down syndrome. Acta Ophthalmol. Scand. 2001, 79, 616–625. [Google Scholar] [CrossRef]
- Vincent, A.L.; Weiser, B.A.; Cupryn, M.; Stein, R.M.; Abdolell, M.; Levin, A.V. Clinical Science. Computerized corneal topography in a paediatric population with Down syndrome. Clin. Exp. Ophthalmol. 2005, 33, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Aslan, L.; Aslankurt, M.; Yüksel, E.; Özdemir, M.; Aksakal, E.; Gümüşalan, Y.; Özdemir, G. Corneal thickness measured by Scheimpflug imaging in children with Down syndrome. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2013, 17, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Fong, A.H.C.; Shum, J.; Ng, A.L.K.; Li, K.; McGhee, S.; Wong, D. Prevalence of ocular abnormalities in adults with Down syndrome in Hong Kong. Br. J. Ophthalmol. 2013, 97, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Najmi, H.; Mobarki, Y.; Mania, K.; AlTowairqi, B.; Basehi, M.; Mahfouz, M.S.; Elmahdy, M. The correlation between keratoconus and eye rubbing: A review. Int. J. Ophthalmol. 2019, 12, 1775–1781. [Google Scholar] [CrossRef]
- Haseeb, A.; Huynh, E.; ElSheikh, R.H.; ElHawary, A.S.; Scelfo, C.; Ledoux, D.M.; Maidana, D.E.; Elhusseiny, A.M. Down syndrome: A review of ocular manifestations. Ther. Adv. Ophthalmol. 2022, 14, 25158414221101718. [Google Scholar] [CrossRef]
- Mathan, J.J.; Simkin, S.K.; Gokul, A.; McGhee, C.N.J. Down syndrome and the eye: Ocular characteristics and ocular assessment. Surv. Ophthalmol. 2022, 14, 15. [Google Scholar] [CrossRef]
- Fimiani, F.; Iovine, A.; Carelli, R.; Pansini, M.; Sebastio, G.; Magli, A. Incidence of Ocular Pathologies in Italian Children with down Syndrome. Eur. J. Ophthalmol. 2007, 17, 817–822. [Google Scholar] [CrossRef]
- Da Cunha, R.P.; Moreira, J.B.D.C. Ocular Findings in Down’s Syndrome. Am. J. Ophthalmol. 1996, 122, 236–244. [Google Scholar] [CrossRef]
- Yurdakul, N.S.; Ugurlu, S.; Maden, A. Strabismus in Down syndrome. J. Pediatr. Ophthalmol. Strabismus 2006, 43, 27–30. [Google Scholar]
- Haargaard, B. Down’s syndrome and early cataract. Br. J. Ophthalmol. 2006, 90, 1024–1027. [Google Scholar] [CrossRef]
- Li, E.Y.; Chan, T.C.; Lam, N.M.; Jhanji, V. Cataract surgery outcomes in adult patients with Down’s syndrome: Table 1. Br. J. Ophthalmol. 2014, 98, 1273–1276. [Google Scholar] [CrossRef]
- Terai, T.; Eda, S.; Sugasawa, J.; Tonari, M.; Matsuo, J.; Oku, H.; Ikeda, T. Ocular findings in Japanese children with Down syndrome: The course of visual acuity and refraction, and systemic and ocular anomalies. Clin. Ophthalmol. 2018, ume 12, 1637–1643. [Google Scholar] [CrossRef]
- Berk, A.T.; Saatci, A.O.; Ercal, M.D.; Tunc, M.; Ergin, M. Ocular findings in 55 patients with Down’s syndrome. Ophthalmic Genet. 1996, 17, 15–19. [Google Scholar] [CrossRef]
- Ahmad, A.; Pruett, R.C. The fundus in mongolism. Arch. Ophthalmol. (Chic. Ill. 1960) 1976, 94, 772–776. [Google Scholar] [CrossRef]
- Van Splunder, J.; Stilma, J.S.; Bernsen, R.M.; Evenhuis, H.M. Prevalence of ocular diagnoses found on screening 1539 adults with intellectual disabilities. Ophthalmology 2004, 111, 1457–1463. [Google Scholar] [CrossRef]
- Lin, K.-K.; Lee, J.-S.; Hou, C.-H.; Chen, W.-M.; Hsiao, C.-H.; Chen, Y.-W.; Yeh, C.-T.; See, L.-C. The Sociodemographic and Risk Factors for Keratoconus: Nationwide Matched Case-Control Study in Taiwan, 1998-2015. Am. J. Ophthalmol. 2021, 223, 140–148. [Google Scholar] [CrossRef]
- Karlica, D.; Skelin, S.; Culic, V.; Galetović, D.; Znaor, L.; Karlica, H.; Pavelić, J. The ophthalmic anomalies in children with Down syndrome in Split-Dalmatian County. Coll. Antropol. 2011, 35, 1115–1118. [Google Scholar]
- Wong, V.; Ho, D. Ocular abnormalities in down syndrome: An analysis of 140 Chinese children. Pediatr. Neurol. 1997, 16, 311–314. [Google Scholar] [CrossRef]
- Liza-Sharmini, A.T.; Azlan, Z.N.; Zilfalil, B.A. Ocular findings in Malaysian children with Down syndrome. Singap. Med. J. 2006, 47, 14–19. [Google Scholar]
- Asgari, S.; Mehravaran, S.; Aghamirsalim, M.; Hashemi, H. Tomography-based definition of keratoconus for Down syndrome patients. Eye Vis. 2020, 7, 49. [Google Scholar] [CrossRef]
- Chatzis, N.; Hafezi, F. Progression of Keratoconus and Efficacy of Corneal Collagen Cross-linking in Children and Adolescents. J. Refract. Surg. 2012, 28, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Dimacali, V.; Balidis, M.; Adamopoulou, A.; Kozei, A.; Kozeis, N. A Case of Early Keratoconus Associated with Eye Rubbing in a Young Child. Ophthalmol. Ther. 2020, 9, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Olivo-Payne, A.; Abdala-Figuerola, A.; Hernandez-Bogantes, E.; Pedro-Aguilar, L.; Chan, E.; Godefrooij, D. Optimal management of pediatric keratoconus: Challenges and solutions. Clin. Ophthalmol. 2019, ume 13, 1183–1191. [Google Scholar] [CrossRef]
- Shehadeh, M.M.; Akkawi, M.T.; Aghbar, A.A. Keratoconus in a 4-year-old Girl with a Strong Family History of Keratoconus. US Ophthalmic Rev. 2018, 11, 56–58. [Google Scholar] [CrossRef]
- Bermudez, B.E.B.V.; Amaral, M.E.d.S.D.; Gomes, C.D.S.; Novadzki, I.M.; de Oliveira, C.M.; Serpe, C.C. Ophthalmological abnormalities in Down syndrome among Brazilian patients. Am. J. Med. Genet. Part A 2020, 182, 2641–2645. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.J.; Bullock, J.; Gray, C.; Spencer, A.; Cunningham, C. Emmetropisation, axial length, and corneal topography in teenagers with Down’s syndrome. Br. J. Ophthalmol. 1998, 82, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated review. Contact Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef] [PubMed]
- De Asua, D.R.; Quero, M.; Moldenhauer, F.; Suarez, C. Clinical profile and main comorbidities of Spanish adults with Down syndrome. Eur. J. Intern. Med. 2015, 26, 385–391. [Google Scholar] [CrossRef]
- Van Allen, M.I.; Fung, J.; Jurenka, S.B. Health care concerns and guidelines for adults with Down syndrome. Am. J. Med. Genet. 1999, 89, 100–110. [Google Scholar] [CrossRef]
- Hashemi, H.; Asgari, S.; Panahi, P.; Mehravaran, S.; Fotouhi, A.; Ambrósio, R. Corneal ectasia in mothers of Down syndrome children. Sci. Rep. 2021, 11, 22436. [Google Scholar] [CrossRef]
- Hashemi, H.; Miraftab, M.; Amanzadeh, K.; Seyedian, M.A.; Vinciguerra, R.; Ambrósio, R.; Roberts, C.; Makateb, A.; Vinciguerra, P.; Asgari, S. Keratoconus detection by novel indices in patients with Down syndrome: A cohort population-based study. Jpn. J. Ophthalmol. 2020, 64, 285–291. [Google Scholar] [CrossRef]
- Haugen, O.H.; Høvding, G.; Eide, G.E.; Bertelsen, T. Corneal grafting for keratoconus in mentally retarded patients. Acta Ophthalmol. Scand. 2001, 79, 609–615. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Kalantan, H.; Al-Muammar, A.M. Analysis of the VSX1 gene in keratoconus patients from Saudi Arabia. Mol. Vis. 2011, 17, 667–672. [Google Scholar]
- Gordon-Shaag, A.; Millodot, M.; Shneor, E.; Liu, Y. The Genetic and Environmental Factors for Keratoconus. BioMed Res. Int. 2015, 2015, 795738. [Google Scholar] [CrossRef]
- Hardcastle, A.J.; Liskova, P.; Bykhovskaya, Y.; McComish, B.J.; Davidson, A.E.; Inglehearn, C.F.; Li, X.; Choquet, H.; Habeeb, M.; Lucas, S.E.M.; et al. A multi-ethnic genome-wide association study implicates collagen matrix integrity and cell differentiation pathways in keratoconus. Commun. Biol. 2021, 4, 266. [Google Scholar] [CrossRef]
- Pritchard, M.A.; Kola, I. The “gene dosage effect” hypothesis versus the “amplified developmental instability” hypothesis in Down syndrome. J. Neural Transm. Suppl. 1999, 57, 293–303. [Google Scholar]
- Antonarakis, S.E.; Lyle, R.; Dermitzakis, E.T.; Reymond, A.; Deutsch, S. Chromosome 21 and Down syndrome: From genomics to pathophysiology. Nat. Rev. Genet. 2004, 5, 725–738. [Google Scholar] [CrossRef]
- Gab-Alla, A.A. Reference Values of the Central Corneal Thickness with Different Refractive Errors for the Adult Egyptian Population. Clin. Ophthalmol. (Auckl. N. Z.) 2020, 14, 3465–3474. [Google Scholar]
- Li, X.; Bykhovskaya, Y.; Canedo, A.L.C.; Haritunians, T.; Siscovick, D.; Aldave, A.J.; Szczotka-Flynn, L.; Iyengar, S.K.; Rotter, J.I.; Taylor, K.D.; et al. Genetic Association of COL5A1 Variants in Keratoconus Patients Suggests a Complex Connection between Corneal Thinning and Keratoconus. Investig. Opthalmol. Vis. Sci. 2013, 54, 2696–2704. [Google Scholar] [CrossRef]
- Karolak, J.A.; Kulinska, K.; Nowak, D.M.; Pitarque, J.A.; Molinari, A.; Rydzanicz, M.; Bejjani, B.A.; Gajecka, M. Sequence variants in COL4A1 and COL4A2 genes in Ecuadorian families with keratoconus. Mol. Vis. 2011, 17, 827–843. [Google Scholar]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Oxlund, H.; Simonsen, A.H. Biochemical studies of normal and keratoconus corneas. Acta Ophthalmol. 2009, 63, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Loukovitis, E.; Kozeis, N.; Gatzioufas, Z.; Kozei, A.; Tsotridou, E.; Stoila, M.; Koronis, S.; Sfakianakis, K.; Tranos, P.; Balidis, M.; et al. The Proteins of Keratoconus: A Literature Review Exploring Their Contribution to the Pathophysiology of the Disease. Adv. Ther. 2019, 36, 2205–2222. [Google Scholar] [CrossRef] [PubMed]
- Khaled, M.L.; Helwa, I.; Drewry, M.; Seremwe, M.; Estes, A.; Liu, Y. Molecular and Histopathological Changes Associated with Keratoconus. BioMed Res. Int. 2017, 2017, 7803029. [Google Scholar] [CrossRef]
- Alkanaan, A.; Barsotti, R.; Kirat, O.; Khan, A.; AlMubrad, T.; Akhtar, S. Collagen fibrils and proteoglycans of peripheral and central stroma of the keratoconus cornea—Ultrastructure and 3D transmission electron tomography. Sci. Rep. 2019, 9, 19963. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Tur, V.M.; MacGregor, C.; Jayaswal, R.; O’Brart, D.; Maycock, N. A review of keratoconus: Diagnosis, pathophysiology, and genetics. Surv. Ophthalmol. 2017, 62, 770–783. [Google Scholar] [CrossRef]
- McKay, T.B.; Priyadarsini, S.; Karamichos, D. Mechanisms of Collagen Crosslinking in Diabetes and Keratoconus. Cells 2019, 8, 1239. [Google Scholar] [CrossRef]
- Ihanamäki, T.; Pelliniemi, L.J.; Vuorio, E. Collagens and collagen-related matrix components in the human and mouse eye. Prog. Retin. Eye Res. 2004, 23, 403–434. [Google Scholar] [CrossRef]
- Meek, K.M. Corneal collagen—its role in maintaining corneal shape and transparency. Biophys. Rev. 2009, 1, 83–93. [Google Scholar] [CrossRef]
- Chwa, M.; Kenney, M.; Khin, H.; Brown, N.J. Altered Type VI Collagen Synthesis by Keratoconus Keratocytesin Vitro. Biochem. Biophys. Res. Commun. 1996, 224, 760–764. [Google Scholar] [CrossRef]
- Meek, K.; Tuft, S.J.; Huang, Y.; Gill, P.S.; Hayes, S.; Newton, R.H.; Bron, A.J. Changes in Collagen Orientation and Distribution in Keratoconus Corneas. Investig. Opthalmol. Vis. Sci. 2005, 46, 1948–1956. [Google Scholar] [CrossRef]
- Karousou, E.; Stachtea, X.; Moretto, P.; Viola, M.; Vigetti, D.; D’Angelo, M.L.; Raio, L.; Ghezzi, F.; Pallotti, F.; De Luca, G.; et al. New insights into the pathobiology of Down syndrome—hyaluronan synthase-2 overexpression is regulated by collagen VIα2 chain. FEBS J. 2013, 280, 2418–2430. [Google Scholar] [CrossRef]
- Cescon, M.; Gattazzo, F.; Chen, P.; Bonaldo, P. Collagen VI at a glance. J. Cell Sci. 2015, 128, 3525–3531. [Google Scholar] [CrossRef]
- Chen, S.; Mienaltowski, M.J.; Birk, D.E. Regulation of corneal stroma extracellular matrix assembly. Exp. Eye Res. 2015, 133, 69–80. [Google Scholar] [CrossRef]
- Kabza, M.; Karolak, J.; Rydzanicz, M.; Szcześniak, M.; Nowak-Malczewska, D.; Ginter-Matuszewska, B.; Polakowski, P.; Ploski, R.; Szaflik, J.P.; Gajecka, M. Collagen synthesis disruption and downregulation of core elements of TGF-β, Hippo, and Wnt pathways in keratoconus corneas. Eur. J. Hum. Genet. 2017, 25, 582–590. [Google Scholar] [CrossRef]
- Hao, X.-D.; Chen, X.-N.; Zhang, Y.-Y.; Chen, P.; Wei, C.; Shi, W.-Y.; Gao, H. Multi-level consistent changes of the ECM pathway identified in a typical keratoconus twin’s family by multi-omics analysis. Orphanet J. Rare Dis. 2020, 15, 227. [Google Scholar] [CrossRef]
- Groot, A.C.G.-D.; Bartram, U.; Oosthoek, P.W.; Bartelings, M.M.; Hogers, B.; Poelmann, R.E.; Jongewaard, I.N.; Klewer, S.E. Collagen type VI expression during cardiac development and in human fetuses with trisomy 21. Anat. Rec. 2003, 275A, 1109–1116. [Google Scholar] [CrossRef]
- Davies, G.E.; Howard, C.M.; Farrer, M.J.; Coleman, M.M.; Bennett, L.B.; Cullen, L.M.; Wyse, R.K.H.; Burn, J.; Williamson, R.; Kessling, A.M. Genetic variation in the COL6A1 region is associated with congenital heart defects in trisomy 21 (Down’s syndrome). Ann. Hum. Genet. 1995, 59, 253–269. [Google Scholar] [CrossRef]
- Grossman, T.R.; Gamliel, A.; Wessells, R.J.; Taghli-Lamallem, O.; Jepsen, K.; Ocorr, K.; Korenberg, J.R.; Peterson, K.L.; Rosenfeld, M.G.; Bodmer, R.; et al. Over-Expression of DSCAM and COL6A2 Cooperatively Generates Congenital Heart Defects. PLoS Genet. 2011, 7, e1002344. [Google Scholar] [CrossRef]
- Von Kaisenberg, C.S. Collagen type VI gene expression in the skin of trisomy 21 fetuses*1. Obstet. Gynecol. 1998, 91, 319–323. [Google Scholar] [CrossRef]
- Quarello, E.; Guimiot, F.; Moalic, J.-M.; Simoneau, M.; Ville, Y.; Delezoide, A.-L. Quantitative evaluation of collagen type VI and SOD gene expression in the nuchal skin of human fetuses with trisomy 21. Prenat. Diagn. 2007, 27, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Lampe, A.K. Collagen VI related muscle disorders. J. Med. Genet. 2005, 42, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Weil, D.; Mattei, M.G.; Passage, E.; N’Guyen, V.C.; Pribula-Conway, D.; Mann, K.; Deutzmann, R.; Timpl, R.; Chu, M.L. Cloning and chromosomal localization of human genes encoding the three chains of type VI collagen. Am. J. Hum. Genet. 1988, 42, 435–445. [Google Scholar]
- Klewer, S.E.; Krob, S.L.; Kolker, S.J.; Kitten, G.T. Expression of type VI collagen in the developing mouse heart. Dev. Dyn. 1998, 211, 248–255. [Google Scholar] [CrossRef]
- Hauser, J.W.M.A. The Genetics of Keratoconus: A Review. Reprod. Syst. Sex. Disord. 2012, 1 (Suppl. S6), 001. [Google Scholar]
- Moschos, M.M.; Kokolakis, N.; Gazouli, M.; Chatziralli, I.P.; Droutsas, D.; Anagnou, N.P.; Ladas, I.D. Polymorphism Analysis of VSX1 and SOD1 Genes in Greek Patients with Keratoconus. Ophthalmic Genet. 2013, 36, 213–217. [Google Scholar] [CrossRef]
- Gadelha, D.N.B.; Feitosa, A.F.B.; Da Silva, R.G.; Antunes, L.T.; Muniz, M.C.; De Oliveira, M.A.; Andrade, D.D.O.; Silva, N.M.D.P.; Cronemberger, S.; Schamber-Reis, B.L.F. Screening for Novel LOX and SOD1 Variants in Keratoconus Patients from Brazil. J. Ophthalmic Vis. Res. 2020, 15, 138–148. [Google Scholar] [CrossRef]
- Udar, N.; Atilano, S.R.; Brown, D.J.; Holguin, B.; Small, K.; Nesburn, A.B.; Kenney, M.C. SOD1: A Candidate Gene for Keratoconus. Investig. Opthalmol. Vis. Sci. 2006, 47, 3345–3351. [Google Scholar] [CrossRef]
- Nejabat, M.; Naghash, P.; Dastsooz, H.; Mohammadi, S.; Alipour, M.; Fardaei, M. VSX1 and SOD1 Mutation Screening in Patients with Keratoconus in the South of Iran. J. Ophthalmic Vis. Res. 2017, 12, 135–140. [Google Scholar] [CrossRef]
- Al-Muammar, A.M.; Kalantan, H.; Azad, T.A.; Sultan, T.; Abu-Amero, K.K. Analysis of the SOD1 Gene in Keratoconus Patients from Saudi Arabia. Ophthalmic Genet. 2014, 36, 373–375. [Google Scholar] [CrossRef]
- Lopes, A.G.; Júnior, G.C.D.A.; Teixeira, R.M.; De Mattos, L.C.; De Mattos, C.C.B.; Castiglioni, L. Absence of the c.169+50delTAAACAG mutation of SOD1 gene in a sample of keratoconus patients in Brazilian population. BMC Res. Notes 2020, 13, 328. [Google Scholar] [CrossRef]
- De Bonis, P.; Laborante, A.; Pizzicoli, C.; Stallone, R.; Barbano, R.; Longo, C.; Mazzilli, E.; Zelante, L.; Bisceglia, L. Mutational screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in keratoconus. Mol. Vis. 2011, 17, 2482–2494. [Google Scholar]
- Lucas, S.E.; Burdon, K.P. Genetic and Environmental Risk Factors for Keratoconus. Annu. Rev. Vis. Sci. 2020, 6, 25–46. [Google Scholar] [CrossRef]
- Perluigi, M.; Butterfield, D.A. Oxidative Stress and Down Syndrome: A Route toward Alzheimer-Like Dementia. Curr. Gerontol. Geriatr. Res. 2011, 2012, 724904. [Google Scholar] [CrossRef]
- Gulesserian, T.; Seidl, R.; Hardmeier, R.; Cairns, N.; Lubec, G. Superoxide Dismutase SOD1, Encoded on Chromosome 21, but Not SOD2 Is Overexpressed in Brains of Patients with Down Syndrome. J. Investig. Med. 2001, 49, 41–46. [Google Scholar] [CrossRef]
- Turrens, J. Increased superoxide dismutase and Down’s syndrome. Med. Hypotheses 2001, 56, 617–619. [Google Scholar] [CrossRef]
- Izzo, A.; Mollo, N.; Nitti, M.; Paladino, S.; Calì, G.; Genesio, R.; Bonfiglio, F.; Cicatiello, R.; Barbato, M.; Sarnataro, V.; et al. Mitochondrial dysfunction in down syndrome: Molecular mechanisms and therapeutic targets. Mol. Med. 2018, 24, 2. [Google Scholar] [CrossRef]
- Cowley, P.M.; Nair, D.R.; DeRuisseau, L.; Keslacy, S.; Atalay, M.; DeRuisseau, K.C. Oxidant production and SOD1 protein expression in single skeletal myofibers from Down syndrome mice. Redox Biol. 2017, 13, 421–425. [Google Scholar] [CrossRef]
- Bruijn, M.; Lutter, R.; Eldering, E.; Bos, A.P.; Van Woensel, J.B.M. Effect of oxidative stress on respiratory epithelium from children with Down syndrome. Eur. Respir. J. 2012, 42, 1037–1045. [Google Scholar] [CrossRef]
- Marucci, G.; Morandi, L.; Bartolomei, I.; Salvi, F.; Pession, A.; Righi, A.; Lauria, G.; Foschini, M.P. Amyotrophic lateral sclerosis with mutation of the Cu/Zn superoxide dismutase gene (SOD1) in a patient with Down syndrome. Neuromuscul. Disord. 2007, 17, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Jafarinasab, M.R.; Karimian, F.; Hasanpour, H.; Masudi, A. Central and Peripheral Corneal Thickness Measurement in Normal and Keratoconic Eyes Using Three Corneal Pachymeters. J. Ophthalmic Vis. Res. 2014, 9, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Brautaset, R.L.; Nilsson, M.; Miller, W.L.; Leach, N.E.; Tukler, J.H.; Bergmanson, J.P.G. Central and Peripheral Corneal Thinning in Keratoconus. Cornea 2013, 32, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Galgauskas, S.; Juodkaite, G.; Tutkuviene, J. Age-related changes in central corneal thickness in normal eyes among the adult Lithuanian population. Clin. Interv. Aging 2014, 9, 1145–1151. [Google Scholar] [CrossRef]
- Martín-Suárez, E.; Molleda, C.; Tardón, R.; Galán, A.; Gallardo, J.; Molleda, J. Diurnal variations of central corneal thickness and intraocular pressure in dogs from 8:00 a.m. to 8:00 p.m. Can. Veter.-J. 2014, 55, 361–365. [Google Scholar]
- Pan, C.-W.; Li, J.; Zhong, H.; Shen, W.; Niu, Z.; Yuan, Y.; Chen, Q. Ethnic Variations in Central Corneal Thickness in a Rural Population in China: The Yunnan Minority Eye Studies. PLoS ONE 2015, 10, e0135913. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Makateb, A.; Mehravaran, S.; Fotouhi, A.; Shariati, F.; Asgari, S. Mapping the corneal thickness and volume in patients with Down syndrome: A comparative population-based study. Arq. Bras. Oftalmol. 2020, 83, 196–201. [Google Scholar] [CrossRef]
- Evereklioglu, C.; Yilmaz, K.; Bekir, N.A. Decreased Central Corneal Thickness in Children with Down Syndrome. J. Pediatr. Ophthalmol. Strabismus 2002, 39, 274–277. [Google Scholar] [CrossRef]
- Akinci, A.; Oner, O.; Munir, K. Central Corneal Thickness in Children With Intellectual Disability: A Controlled Study. Cornea 2010, 29, 159–161. [Google Scholar] [CrossRef]
- Alió, J.L.; Shabayek, M.H. Corneal Higher Order Aberrations: A Method to Grade Keratoconus. J. Refract. Surg. 2006, 22, 539–545. [Google Scholar] [CrossRef]
- Hawkes, E.; Nanavaty, M. Eye Rubbing and Keratoconus: A Literature Review. Int. J. Keratoconus Ectatic Corneal Dis. 2014, 3, 118–121. [Google Scholar] [CrossRef]
- Ioannidis, A.S.; Speedwell, L.; Nischal, K.K. Unilateral keratoconus in a child with chronic and persistent eye rubbing. Am. J. Ophthalmol. 2005, 139, 356–357. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Mohan, S.; Pye, D.C.; Willcox, M.D.P. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012, 90, e303–e309. [Google Scholar] [CrossRef]
- Ollivier, F.J.; Gilger, B.; Barrie, K.P.; Kallberg, M.E.; Plummer, C.E.; O’Reilly, S.; Gelatt, K.N.; Brooks, D.E. Proteinases of the cornea and preocular tear film. Veter.-Ophthalmol. 2007, 10, 199–206. [Google Scholar] [CrossRef]
- Collier, S.A. Is the corneal degradation in keratoconus caused by matrix-metalloproteinases? Clin. Exp. Ophthalmol. 2001, 29, 340–344. [Google Scholar] [CrossRef]
- Mackiewicz, Z.; Määttä, M.; Stenman, M.; Konttinen, L.; Tervo, T.; Konttinen, Y.T. Collagenolytic Proteinases in Keratoconus. Cornea 2006, 25, 603–610. [Google Scholar] [CrossRef]
- Seppälä, H.P.S.; Määttä, M.; Rautia, M.; Mackiewicz, Z.; Tuisku, I.; Tervo, T.; Konttinen, Y.T. EMMPRIN and MMP-1 in Keratoconus. Cornea 2006, 25, 325–330. [Google Scholar] [CrossRef]
- Smith, V.A.; Hoh, H.B.; Littleton, M.; Easty, D.L. Over-expression of a gelatinase a activity in keratoconus. Eye 1995, 9, 429–433. [Google Scholar] [CrossRef]
- Smith, V.; Matthews, F.; Majid, M.; Cook, S. Keratoconus: Matrix metalloproteinase-2 activation and TIMP modulation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2006, 1762, 431–439. [Google Scholar] [CrossRef]
- Shetty, R.; Ghosh, A.; Lim, R.R.; Subramani, M.; Mihir, K.; Ranganath, A.; Nagaraj, S.; Nuijts, R.M.; Beuerman, R.; Shetty, R.; et al. Elevated Expression of Matrix Metalloproteinase-9 and Inflammatory Cytokines in Keratoconus Patients Is Inhibited by Cyclosporine A. Investig. Opthalmol. Vis. Sci. 2015, 56, 738–750. [Google Scholar] [CrossRef]
- Shoshani, Y.; Pe’Er, J.; Doviner, V.; Frucht-Pery, J.; Solomon, A. Increased Expression of Inflammatory Cytokines and Matrix Metalloproteinases in Pseudophakic Corneal Edema. Investig. Opthalmol. Vis. Sci. 2005, 46, 1940–1947. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Che, M.; Yuan, J.; Yu, Y.; Cao, C.; Qin, X.-Y.; Cheng, Y. Aberrations in circulating inflammatory cytokine levels in patients with Down syndrome: A meta-analysis. Oncotarget 2017, 8, 84489–84496. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.N.; Douraghi, M.; Mohammadi, A.M.; Nikmanesh, B. Altered serum pro-inflammatory cytokines in children with Down’s syndrome. Eur. Cytokine Netw. 2012, 23, 64–67. [Google Scholar] [CrossRef]
- Sullivan, K.D.; Evans, D.; Pandey, A.; Hraha, T.H.; Smith, K.P.; Markham, N.; Rachubinski, A.L.; Wolter-Warmerdam, K.; Hickey, F.; Espinosa, J.M.; et al. Trisomy 21 causes changes in the circulating proteome indicative of chronic autoinflammation. Sci. Rep. 2017, 7, 14818. [Google Scholar] [CrossRef]
- Shapiro, M.B.; France, T.D. The Ocular Features of Down’s Syndrome. Am. J. Ophthalmol. 1985, 99, 659–663. [Google Scholar] [CrossRef]
- Stoiber, J.; Muss, W.; Ruckhofer, J.; Grabner, G. Acute keratoconus with perforation in a patient with Down’s syndrome. Br. J. Ophthalmol. 2003, 87, 120. [Google Scholar] [CrossRef] [PubMed]
- Mathan, J.J.; Gokul, A.; Simkin, S.K.; Meyer, J.J.; Patel, D.V.; McGhee, C.N.J.; Boptom(Hons), J.J.M.; BOptom(Hons), A.G.P.; BOptom(Hons), S.K.S.P.; Mph, J.J.M.M.; et al. Topographic screening reveals keratoconus to be extremely common in Down syndrome. Clin. Exp. Ophthalmol. 2020, 48, 1160–1167. [Google Scholar] [CrossRef]
- Makino, S. Acute Corneal Hydrops in Down Syndrome. J. Clin. Case Rep. 2012, 2, 235. [Google Scholar] [CrossRef]
- Aslankurt, M.; Aslan, L.; Aksoy, A.; Kurt, M.M.; Özdemir, M. Evaluation of early corneal topographic changes in children with Down syndrome. Turk. J. Med. Sci. 2013, 43, 810–814. [Google Scholar] [CrossRef]
- Ozcan, A.A.; Ersoz, T.R. Severe acute corneal hydrops in a patient with Down syndrome and persistent eye rubbing. Ann. Ophthalmol. 2007, 39, 158–160. [Google Scholar] [CrossRef]
- Wylegala, E.; Tarnawska, D. Amniotic Membrane Transplantation with Cauterization for Keratoconus Complicated by Persistent Hydrops in Mentally Retarded Patients. Ophthalmology 2006, 113, 561–564. [Google Scholar] [CrossRef]
- Espandar, L.; Meyer, J. Keratoconus: Overview and Update on Treatment. Middle East Afr. J. Ophthalmol. 2010, 17, 15–20. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Shi, L.; Lewis, J.W.L.; Wang, M. Infrared Retinoscopy. Photonics 2014, 1, 303–322. [Google Scholar] [CrossRef]
- Mannion, L.S.; Tromans, C.; O’Donnell, C. Reduction in Corneal Volume with Severity of Keratoconus. Curr. Eye Res. 2011, 36, 522–527. [Google Scholar] [CrossRef]
- Gold, J.; Chauhan, V.; Rojanasthien, S.; Fitzgerald, J. Munson’s Sign: An Obvious Finding to Explain Acute Vision Loss. Clin. Pract. Cases Emerg. Med. 2019, 3, 312–313. [Google Scholar] [CrossRef]
- Kemp, A.P.; Mathew, J.H.; Goosey, J.D.; Bergmanson, J.P. Life Outside Fleischer’s Ring: Pathological Changes in Peripheral Keratconic Corneas. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1103. [Google Scholar]
- Andreanos, K.D.; Hashemi, K.; Petrelli, M.; Droutsas, K.; Georgalas, I.; Kymionis, G. Keratoconus Treatment Algorithm. Ophthalmol. Ther. 2017, 6, 245–262. [Google Scholar] [CrossRef]
- Parker, J.S.; van Dijk, K.; Melles, G.R. Treatment options for advanced keratoconus: A review. Surv. Ophthalmol. 2015, 60, 459–480. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Masoumi, A.; Mirghorbani, M.; Shahraki, K.; Hashemi, H. Updates on corneal collagen cross-linking: Indications, techniques and clinical outcomes. J. Curr. Ophthalmol. 2017, 29, 235–247. [Google Scholar] [CrossRef]
- Rabinowitz, Y.S. INTACS for Keratoconus. Int. Ophthalmol. Clin. 2006, 46, 91–103. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Heidari, Z.; Hashemi, H. Updates on Managements for Keratoconus. J. Curr. Ophthalmol. 2017, 30, 110–124. [Google Scholar] [CrossRef]
- Jhanji, V.; Sharma, N.; Vajpayee, R.B. Management of keratoconus: Current scenario. Br. J. Ophthalmol. 2010, 95, 1044–1050. [Google Scholar] [CrossRef]
- Marsack, J.D.; Benoit, J.S.; Kollbaum, P.S.; Anderson, H.A. Application of Topographical Keratoconus Detection Metrics to Eyes of Individuals with Down Syndrome. Optom. Vis. Sci. 2019, 96, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.E.; Klusek, J.; Estigarribia, B.; Roberts, J.E. Language Characteristics of Individuals with Down Syndrome. Top. Lang. Disord. 2009, 29, 112–132. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.A.J.; Power, B.; Malata, D.; Quill, B.; Murphy, C.C.; Power, W.J. Management of Keratoconus in Down Syndrome and Other Intellectual Disability. Cornea 2021, 41, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Perlman, R.L. Mouse Models of Human Disease: An Evolutionary Perspective. Evol. Med. Public Health 2016, 2016, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Justice, M.J.; Dhillon, P. Using the mouse to model human disease: Increasing validity and reproducibility. Dis. Model. Mech. 2016, 9, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Xu, T. The expanding role of mouse genetics for understanding human biology and disease. Dis. Model. Mech. 2008, 1, 56–66. [Google Scholar] [CrossRef]
- Peiffer, R.L., Jr.; Werblin, T.P.; Patel, A.S. Keratoconus in a Rhesus Monkey. J. Med. Primatol. 1987, 16, 403–406. [Google Scholar] [CrossRef]
- Thakar, J.H.; Percy, D.H.; Strickland, K.P. Ocular abnormalities in the myopathic hamster (UM-X7.1 strain). Investig. Ophthalmol. Vis. Sci. 1977, 16, 1047–1052. [Google Scholar]
- Tachibana, M.; Adachi, W.; Kinoshita, S.; Kobayashi, Y.; Honma, Y.; Hiai, H.; Matsushima, Y. Androgen-dependent hereditary mouse keratoconus: Linkage to an MHC region. Investig. Ophthalmol. Vis. Sci. 2002, 43, 51–57. [Google Scholar]
- Tachibana, M.; Okamoto, M.; Sakamoto, M.; Matsushima, Y. Hereditary keratoconus-like keratopathy in Japanese wild mice mapped to mouse Chromosome 13. Mamm. Genome 2002, 13, 692–695. [Google Scholar] [CrossRef]
- Khaled, M.L.; Bykhovskaya, Y.; Gu, C.; Liu, A.; Drewry, M.D.; Chen, Z.; Mysona, B.A.; Parker, E.; McNabb, R.P.; Yu, H.; et al. PPIP5K2 and PCSK1 are Candidate Genetic Contributors to Familial Keratoconus. Sci. Rep. 2019, 9, 19406. [Google Scholar] [CrossRef]
- Wallang, B.S.; Das, S. Keratoglobus. Eye 2013, 27, 1004–1012. [Google Scholar] [CrossRef]
- Puk, O.; Dalke, C.; Calzada-Wack, J.; Ahmad, N.; Klaften, M.; Wagner, S.; de Angelis, M.H.; Graw, J. Reduced Corneal Thickness and Enlarged Anterior Chamber in a Novel ColVIIIa2G257DMutant Mouse. Investig. Opthalmol. Vis. Sci. 2009, 50, 5653–5661. [Google Scholar] [CrossRef]
- Hopfer, U.; Fukai, N.; Hopfer, H.; Wolf, G.; Joyce, N.; Li, E.; Olsen, B.R. Targeted disruption of Col8a1 and Col8a2 genes in mice leads to anterior segment abnormalities in the eye. FASEB J. 2005, 19, 1232–1244. [Google Scholar] [CrossRef]
- Segev, F.; He’on, E.; Cole, W.G.; Wenstrup, R.J.; Young, F.; Slomovic, A.R.; Rootman, D.S.; Whitaker-Menezes, D.; Chervoneva, I.; Birk, D.E. Structural Abnormalities of the Cornea and Lid Resulting from Collagen V Mutations. Investig. Opthalmol. Vis. Sci. 2006, 47, 565–573. [Google Scholar] [CrossRef]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nat. Rev. Dis. Prim. 2020, 6, 9. [Google Scholar] [CrossRef]
- Davisson, M.T.; Bechtel, L.J.; Akeson, E.C.; Fortnab, A.; Slavovb, D.; Gardinerb, K. Evolutionary Breakpoints on Human Chromosome 21. Genomics 2001, 78, 99–106. [Google Scholar] [CrossRef]
- Tost, F.; Wolfinger, J.; Giebel, J.; Buselmaier, W. Corneal anomalies in murine trisomy 16. Ophthalmologe 2005, 102, 64–69. [Google Scholar] [CrossRef]
- Vacano, G.; Duval, N.; Patterson, D. The Use of Mouse Models for Understanding the Biology of Down Syndrome and Aging. Curr. Gerontol. Geriatr. Res. 2012, 2012, 717315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reeves, R.H.; Irving, N.G.; Moran, T.H.; Wohn, A.; Kitt, C.; Sisodia, S.S.; Schmidt, C.; Bronson, R.T.; Davisson, M.T. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat. Genet. 1995, 11, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Delabar, J.M.; Aflalo-Rattenbac, R.; Créau, N. Developmental Defects in Trisomy 21 and Mouse Models. Sci. World J. 2006, 6, 1945–1964. [Google Scholar] [CrossRef]
- Rueda, N.; Flórez, J.; Martínez-Cué, C. Mouse Models of Down Syndrome as a Tool to Unravel the Causes of Mental Disabilities. Neural Plast. 2012, 2012, 584071. [Google Scholar] [CrossRef] [PubMed]
- Hyde, L.A.; Frisone, D.F.; Crnic, L.S. Ts65Dn mice, a model for Down syndrome, have deficits in context discrimination learning suggesting impaired hippocampal function. Behav. Brain Res. 2001, 118, 53–60. [Google Scholar] [CrossRef]
- Scott-McKean, J.J.; Chang, B.; Hurd, R.E.; Nusinowitz, S.; Schmidt, C.; Davisson, M.T.; Costa, A.C.S. The Mouse Model of Down Syndrome Ts65Dn Presents Visual Deficits as Assessed by Pattern Visual Evoked Potentials. Investig. Opthalmol. Vis. Sci. 2010, 51, 3300–3308. [Google Scholar] [CrossRef] [PubMed]
- Villar, A.J.; Belichenko, P.V.; Gillespie, A.M.; Kozy, H.M.; Mobley, W.C.; Epstein, C.J. Identification and characterization of a new Down syndrome model, Ts[Rb(12.1716)]2Cje, resulting from a spontaneous Robertsonian fusion between T(1716)65Dn and mouseChromosome 12. Mamm. Genome 2005, 16, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Kazuki, Y.; Gao, F.J.; Li, Y.; Moyer, A.J.; Devenney, B.; Hiramatsu, K.; Miyagawa-Tomita, S.; Abe, S.; Kazuki, K.; Kajitani, N.; et al. A non-mosaic transchromosomic mouse model of Down syndrome carrying the long arm of human chromosome 21. eLife 2020, 9, e56223. [Google Scholar] [CrossRef]
- Dunlevy, L.; Bennett, M.; Slender, A.; Lana-Elola, E.; Tybulewicz, V.L.; Fisher, E.M.; Mohun, T. Down’s syndrome-like cardiac developmental defects in embryos of the transchromosomic Tc1 mouse. Cardiovasc. Res. 2010, 88, 287–295. [Google Scholar] [CrossRef]
- Das, I.; Reeves, R.H. The use of mouse models to understand and improve cognitive deficits in Down syndrome. Dis. Model. Mech. 2011, 4, 596–606. [Google Scholar] [CrossRef]
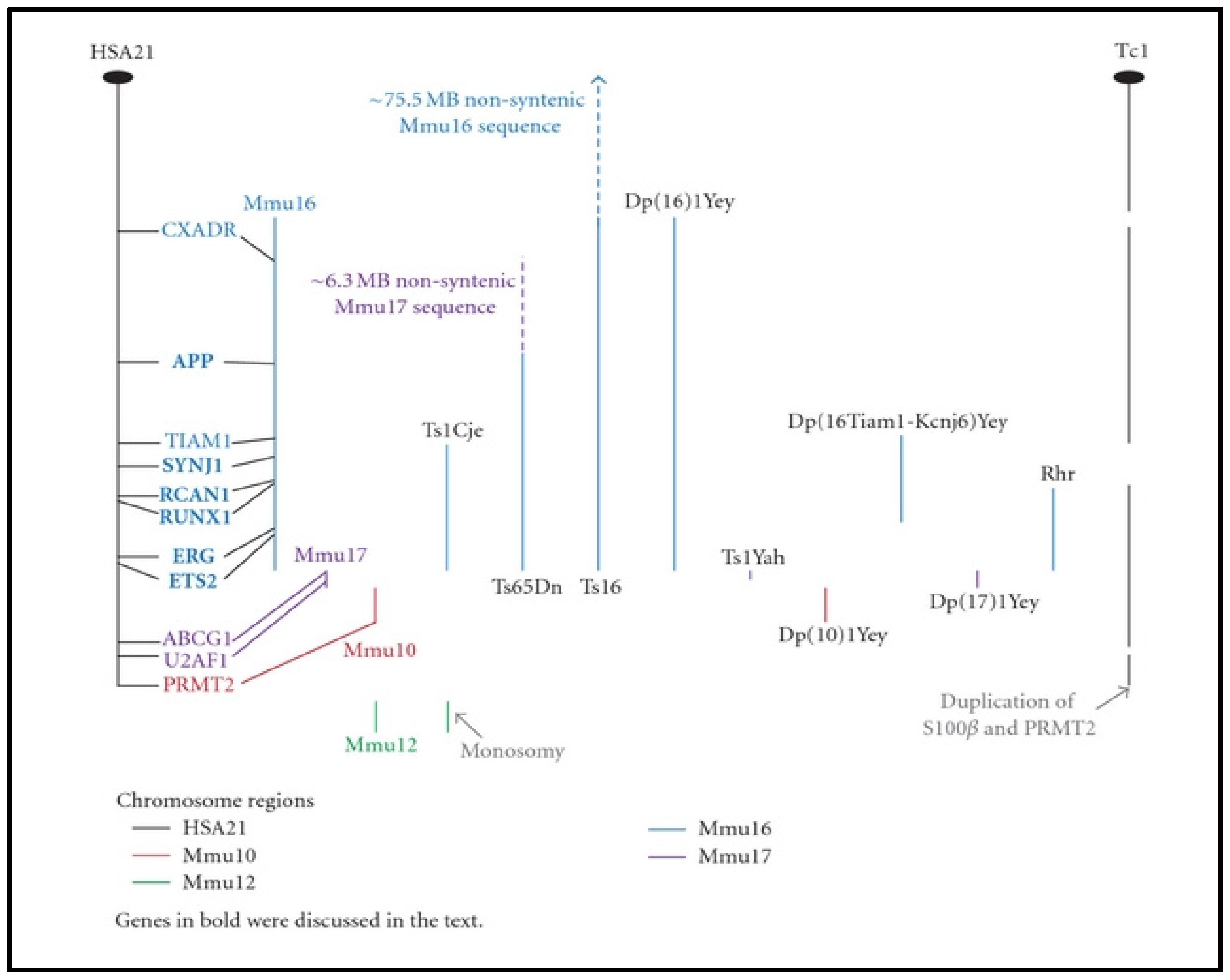
| Title of Article | Sample Size | Mean Age/Age Range of DS Patients | Ethnicity | KC Diagnosis and/or Instrument Used | Findings | Reference |
|---|---|---|---|---|---|---|
| Corneal Morphologic Characteristics in Patients with Down Syndrome | 112 DS patients | 14.88 ± 15.76 years Range: 3 months–60 years | DS group—59% Caucasians, and 41% Arabs | Visual, refractive, anterior, and posterior corneal characteristics were assessed and compared in both groups using Placido disc/Scheimpflug camera topographer (Sirius, CSO) | Patients with DS have steeper, thinner corneas and more corneal aberrations compared to those without genetic alterations. | [30] |
| Ophthalmological abnormalities in Down syndrome among Brazilian patients | 1207 DS patients | No mean age reported Range: 0–42 years | Brazilians | Slit-lamp evaluation, corneal pachymetry, and topography were used for KC detection | A prevalence of KC of 27.2 % was observed among 1207 persons with Down syndrome | [65] |
| Emmetropisation, axial length, and corneal topography in teenagers with Down’s syndrome | 50 DS patients | 17.4 years Range: 15–22 years | Mancunians | TMS-1 machine was used for corneal topographic mapping. Slit lamp examination was also conducted | Prevalence of KC in this cohort was 2%, but 6% had corneal topography with inferior steepening, which may represent a preclinical keratoconic process. | [66] |
| Clinical profile and main comorbidities of Spanish adults with Down syndrome | 144 DS patients | 35 ± 12 years Range: 17–65 years | Spanish | Not mentioned | KC is a prominent ocular feature associated with DS in individuals > 50 years. | [68] |
| Corneal thickness measured by Sheimpflug imaging in children with Down syndrome | 27 DS patients | 8.94 ± 2.35 years Range: 5–12 years | Turkish | CCT, TP, and CV were analyzed using Pentacam Scheimpflug imaging system | Corneal thickness was lower in DS children than in healthy control subjects. | [42] |
| Prevalence of ocular abnormalities in adults with Down syndrome in Hong Kong | 91 DS patients | 38 years ± 6.5 years Range: 30–56 years | Chinese | Corneal pachymetry was measured using a handheld ultrasound pachymeter | There is a high prevalence of corneal problems (including KC) in Chinese DS patients. | [43] |
| The ophthalmic anomalies in children with Down Syndrome in Split-Dalmatian County | 153 DS patients | 11.7 ± 3.2 years Range: 0–18 years | Croatians | Biomicroscopic examination of the anterior eye segment | Though less common than other refractive disorders, KC was observed in patients with DS. | [57] |
| Incidence of ocular pathologies in Italian children with Down Syndrome | 157 DS patients | 5.28 years Range: 1 month –18 years | Italians | Slit lamp biomicroscopy | No KC was observed in this cohort of DS patients. | [47] |
| Ocular findings in Malaysian children with Down syndrome | 60 DS patients | 6.72 ± 3.38 years Range: 1 month –17 years | Malaysians | Slit lamp biomicroscopy and Placido disc | No KC was observed in this cohort of DS patients. | [59] |
| Computerized corneal topography in a pediatric population with Down syndrome | 21 DS patients | 6.9 years Range: 10 months–18 years | Caucasians | Slit-lamp biomicroscopy, scissoring or oil-drop reflex on retinoscopy, corneal topography mapping using using the EyeSys Computer-Assisted Videokeratoscope | Corneal curvature in DS children was significantly steeper than in the control population. | [41] |
| Prevalence of ocular diagnoses found on screening 1539 adults with intellectual disabilities | 1539 DS patients | 45.7 years Range: 20.2–88.7 years | Dutch | Slit-lamp biomicroscopy | KC is independently related to DS (OR 7.65, 95% CI, 3.91 to 14.96). | [55] |
| Characteristic ocular findings in Asian children with Down syndrome | 123 DS patients | 6.5 years Range: 6 months–14 years | Koreans | Visual acuity assessment, slit-lamp biomicroscopy | KC was not observed in this cohort of Asian DS patients. | [33] |
| Biometric measurements of the eyes in teenagers and young adults with Down syndrome | 47 DS patients | 20.0 ± 3.9 years Range: 14–26 years | Norwegians | Retinoscopy reflexes, central corneal thickness and anterior chamber depth were measured with a A Nidek Model EAS-1000 anterior eye segment analysis system. Corneal topography was analyzed with a TechnoMed C-Scan V2.0.0. Corneal curvature was also measured with a hand-held Nidek autokeratometer KM-500 | DS patients have thinner corneas and higher keratometry values compared to control. | [40] |
| Ocular abnormalities in Down syndrome: an analysis of 140 Chinese children | 140 DS patients | 3.74 years Range: 3 months–13 years | Chinese | Visual acuity assessment, slit-lamp biomicroscopy | No KC was observed in this DS cohort. | [58] |
| Health care concerns and guidelines for adults with Down syndrome | 38 DS patients | 36.2 years for middle aged DS patients Range: 30–43 years old 59.7 years for elderly DS patients Range: 47–68 years old | Canadians | Not mentioned | KC was observed in 15.8% of all DS adults. | [69] |
| Corneal ectasia in mothers of Down syndrome children | 77 MDS and 63 MNC controls | Mean age at the time of examination was 48.81 ± 6.93 years in MDS and 48.46 ± 8.35 years in MNC | Iranians | Slit-lamp biomicroscopy, corneal tomography was assessed using Oculus Pentacam HR and corneal biomechanics was also measured using Corneal Visualization Scheimpflug Technology | Mild KC was identified in 30.9% (21 cases) in the MDS in comparison with 14.3% (9 cases) MNC. | [70] |
| Prevalence of Keratoconus in Persons with Down Syndrome in a National Registry in Norway | 4342 DS patients | 37.1 years Range: Not reported | Norwegians | Data analysis from International Classification of Diseases and Related Health Problems, Tenth Revision diagnosis codes Q90 for Down syndrome and H18.6 for KC from 1 January 2010, to 31 December 2019 | A prevalence of KC of 238 (5.5%) was observed among 4342 DS patients. | [13] |
| Keratoconus detection by novel indices in patients with Down syndrome: a cohort population-based study | 250 DS patients | 17.0 ± 4.7 years Range: 10–30 years | Iranians | Slit-lamp examinations, topographic indices were measured by Pentacam HR and corneal biomechanics were examined using the Corvis ST | A prevalence of KC of 28 (12.39%) was observed among the DS patients. | [71] |
| Corneal grafting for keratoconus in mentally retarded patients | 33 DS patients | 36.7 ± 10.8 years Range: 18–60 years | Norwegians | Not reported | KC was observed in 33 mentally retarded patients with DS. | [72] |
| Title of Paper | Sample Size | Ethnicity | Extract from Study | Reference |
|---|---|---|---|---|
| Corneal Morphologic Characteristics in Patients with Down Syndrome | 112 DS patients | DS group—59% Caucasians, and 41% Arabs | “Furthermore, it has been reported that patients with DS frequently rub their eyes which is a habit related to keratoconus development owing to the inflammation process and biomechanical alterations linked to eye rubbing habit” | [30] |
| Prevalence of ocular abnormalities in adults with Down syndrome in Hong Kong | 91 DS patients | Chinese | “This, together with frequent eye rubbing, may predispose DS patients to keratoconus” | [43] |
| The Ocular Features of Down’s Syndrome | 53 DS patients | Caucasians | “The cause of this increased incidence of keratoconus in Down’s syndrome is unknown. Several investigators have suggested eye rubbing as a probable cause” | [145] |
| Acute keratoconus with perforation in a patient with Down’s syndrome | 1 DS patient | Austrian | “Habitual eye rubbing, which is frequently observed in patients with Down’s syndrome and other forms of mental deficiency, has been postulated as an important factor not only for the development of keratoconus itself” | [146] |
| Posterior corneal features in patients with Down syndrome and their relation with keratoconus | 20 DS patients | Spanish | “Furthermore, patients with DS used to rub their eyes, and it is well known that the eye rubbing habit is an important risk factor for keratoconus development” | [36] |
| Computerized corneal topography in a pediatric population with Down syndrome | 21 DS patients | Canadians | “Some authors have attributed the high prevalence of keratoconus in patients with Down syndrome to chronic eye rubbing because of irritation caused by blepharitis, seen in 7–47% of patients with Down syndrome” | [41] |
| Keratoconus and corneal morphology in patients with Down syndrome at a pediatric hospital | 31 DS patients | 16 Caucasians, 10 Hispanics, 3 “other” patients, and 1 unreported patient | “Eye rubbing may also play a role in development of keratoconus and eye rubbing is commonly reported in patients with DS” | [38] |
| Biometric measurements of the eyes in teenagers and young adults with Down syndrome | 47 DS patients | Norwegians | “A thin cornea must be assumed to be particularly vulnerable to eye rubbing, and together these factors might lead to clinically manifest keratoconus in many Down syndrome patients” | [40] |
| Ocular findings in Malaysian children with Down syndrome | 60 DS patients | Malaysians | “However, there has been no established evidence to link genetic abnormality of Down syndrome to keratoconus. It is thought to be due to eye rubbing or underlying structural abnormalities of the cornea” | [59] |
| Topographic screening reveals keratoconus to be extremely common in Down syndrome | 48 DS patients | New Zealanders | “Eye rubbing behaviour is also common in DS and may further contribute to the predisposition to keratoconus development” | [147] |
| Acute Corneal Hydrops in Down Syndrome | 1 DS patient | Japanese | “Habitual eye rubbing, which is frequently observed in patients with Down syndrome and other forms of mental deficiency, has been postulated as an important factor, not only for the development of keratoconus” | [148] |
| Evaluation of early corneal topographic changes in children with Down syndrome | 27 DS patients | Caucasians | “Habitual eye rubbing, which is also frequently observed in patients with DS, has been postulated as a crucial factor either for the development of keratoconus or the progression of the disease” | [149] |
| Keratoconus detection by novel indices in patients with Down syndrome: a cohort population-based study | 250 DS patients | Iranians | “Eye rubbing was reported in 18.1% of the healthy individuals, in 13.6% of those with suspected KC, in 19.6% of those with KC, and in 21.1% of those with progressive KC” | [71] |
| Severe acute corneal hydrops in a patient with Down syndrome and persistent eye rubbing | 1 DS patient | Turkish | “Eye-rubbing may play a role in the pathogenesis of acute hydrops,” | [150] |
| Amniotic membrane transplantation with cauterization for keratoconus complicated by persistent hydrops in mentally retarded patients | 8 DS patients | Polish | “Vigorous eye rubbing is a cause of extensive hydrops in mentally retarded patients with keratoconus” | [151] |
| Mouse Model | Description | Clinical Phenotype in DS | Limitation(s) | Reference(s) |
|---|---|---|---|---|
| Ts16 (Trisomy 16) | First mouse model released in 1980, trisomic for all parts of mouse chromosome 16 in synteny with human chromosome 21 | Early developmental abnormalities | Barely survived to term by dying in utero, syntenic with other regions of human chromosome 3 and human chromosome 8 | [181,182,183,184] |
| Ts65Dn | Segmentally trisomic for about 55% of genes on mouse chromosome 16 that are homologous to segment MRP139-ZNF295 on human chromosome 21 | Impaired vision and hearing, congenital heart defects, learning and memory deficits | Difficulty in using model in vision research due to retinal degeneration, male mice are sterile | [181,183,185,186] |
| Ts1Cje | Produced from the reciprocal translocation between the distal portion of mouse chromosome 16 and the end of chromosome 12. Trisomic for a shorter region of mouse chromosome 16, which is smaller than that in Ts65Dn mouse spanning from Sod1 to Mx1 genes | Craniofacial abnormalities | Loss of a functional variant of superoxide dismutase1 | [181,183] |
| Ts2Cje (Derivative of Ts65Dn) | Developed from Ts65Dn mice by a Robertsonian translocation of the extra chromosome of Ts65Dn mice onto mouse chromosome 12 | Similar features as seen in Ts65Dn mice—congenital heart defects, learning and memory deficits | Although improved compared to the Ts65Dn, males still have moderate fertility | [181,187] |
| Tc1 | First transchromosomic (Tc) mouse DS model with a nearly complete fragment of human chromosome 21 including most of the gene orthologs located on mouse chromosome 10, 16, and 17 | Congenital heart defects, learning and memory deficits, motor coordination defects | Mosaic trisomy, where the human chromosome 21 is lost randomly in a large number of cells | [178,188,189] |
| TcMAC21 | Humanized DS mouse model containing a clone of the long arm of the human chromosome 21 as an artificial mouse chromosome | Learning and memory deficits, craniofacial abnormalities, heart anomalies | Made up of some deletions that affect about 8% of genes found on the long arm human chromosome 21 | [178,188] |
| Ts1Rhr | Segmental trisomic model containing segments of mouse chromosome 16 that are homologous to human chromosome 21. The model is also trisomic for the DS-critical region (DSCR) known to contain genes that may influence mental abnormalities in DS | Learning and memory impairment, craniofacial abnormalities | May not show accurate phenotypic feature in DS due to the inability of the trisomic region for the DSCR to impair hippocampal function in the brain | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akoto, T.; Li, J.J.; Estes, A.J.; Karamichos, D.; Liu, Y. The Underlying Relationship between Keratoconus and Down Syndrome. Int. J. Mol. Sci. 2022, 23, 10796. https://doi.org/10.3390/ijms231810796
Akoto T, Li JJ, Estes AJ, Karamichos D, Liu Y. The Underlying Relationship between Keratoconus and Down Syndrome. International Journal of Molecular Sciences. 2022; 23(18):10796. https://doi.org/10.3390/ijms231810796
Chicago/Turabian StyleAkoto, Theresa, Jiemin J. Li, Amy J. Estes, Dimitrios Karamichos, and Yutao Liu. 2022. "The Underlying Relationship between Keratoconus and Down Syndrome" International Journal of Molecular Sciences 23, no. 18: 10796. https://doi.org/10.3390/ijms231810796
APA StyleAkoto, T., Li, J. J., Estes, A. J., Karamichos, D., & Liu, Y. (2022). The Underlying Relationship between Keratoconus and Down Syndrome. International Journal of Molecular Sciences, 23(18), 10796. https://doi.org/10.3390/ijms231810796






