Hepatitis Viruses Control Host Immune Responses by Modifying the Exosomal Biogenesis Pathway and Cargo
Abstract
1. Introduction
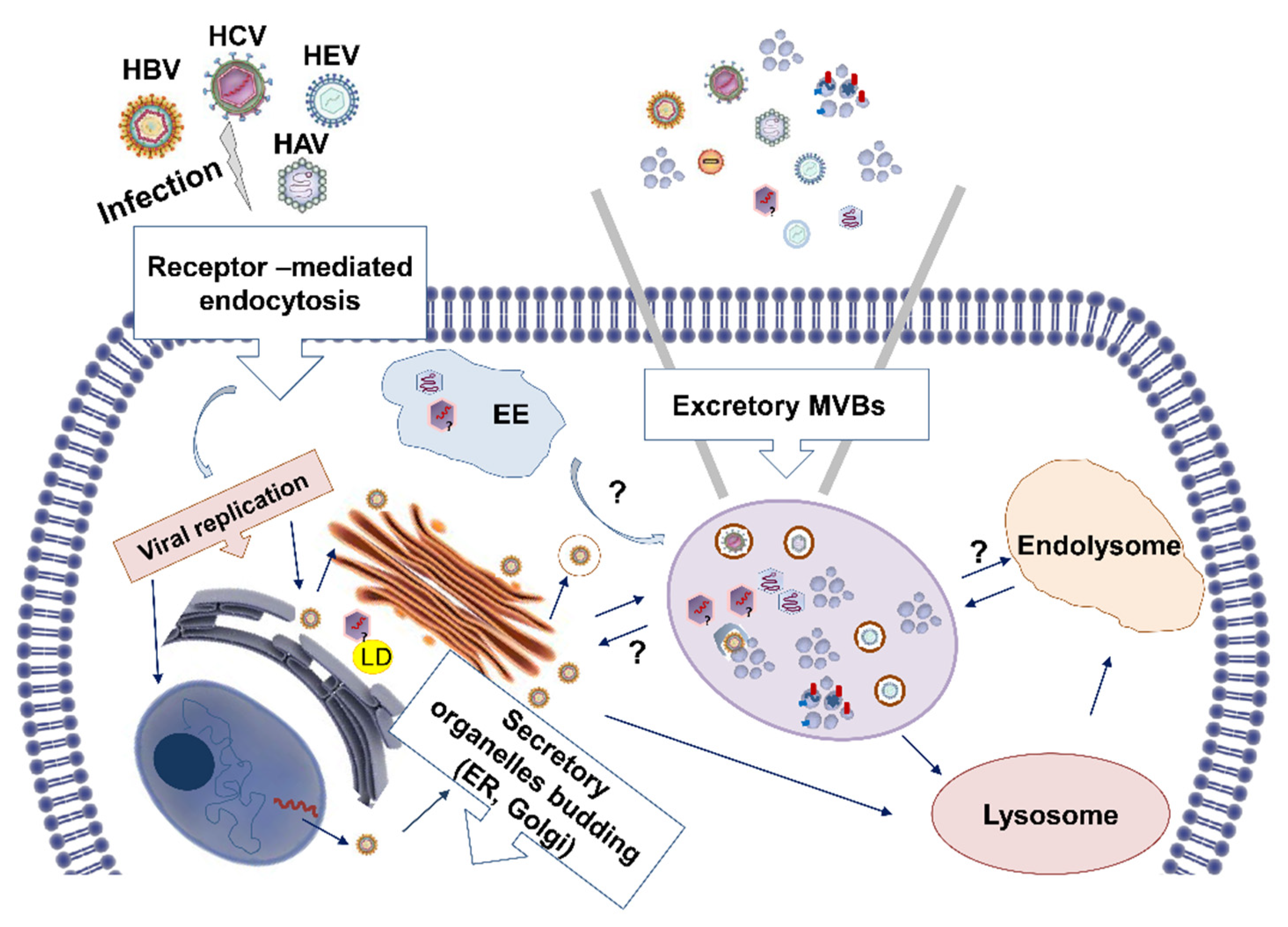
2. Exosomes: The Faithful Assistants of Hepatitis Viruses
2.1. The Making, the Taking, and the Loading
2.2. Hepatitis Viruses Exploit the Exosomal Biogenesis Pathway to Egress
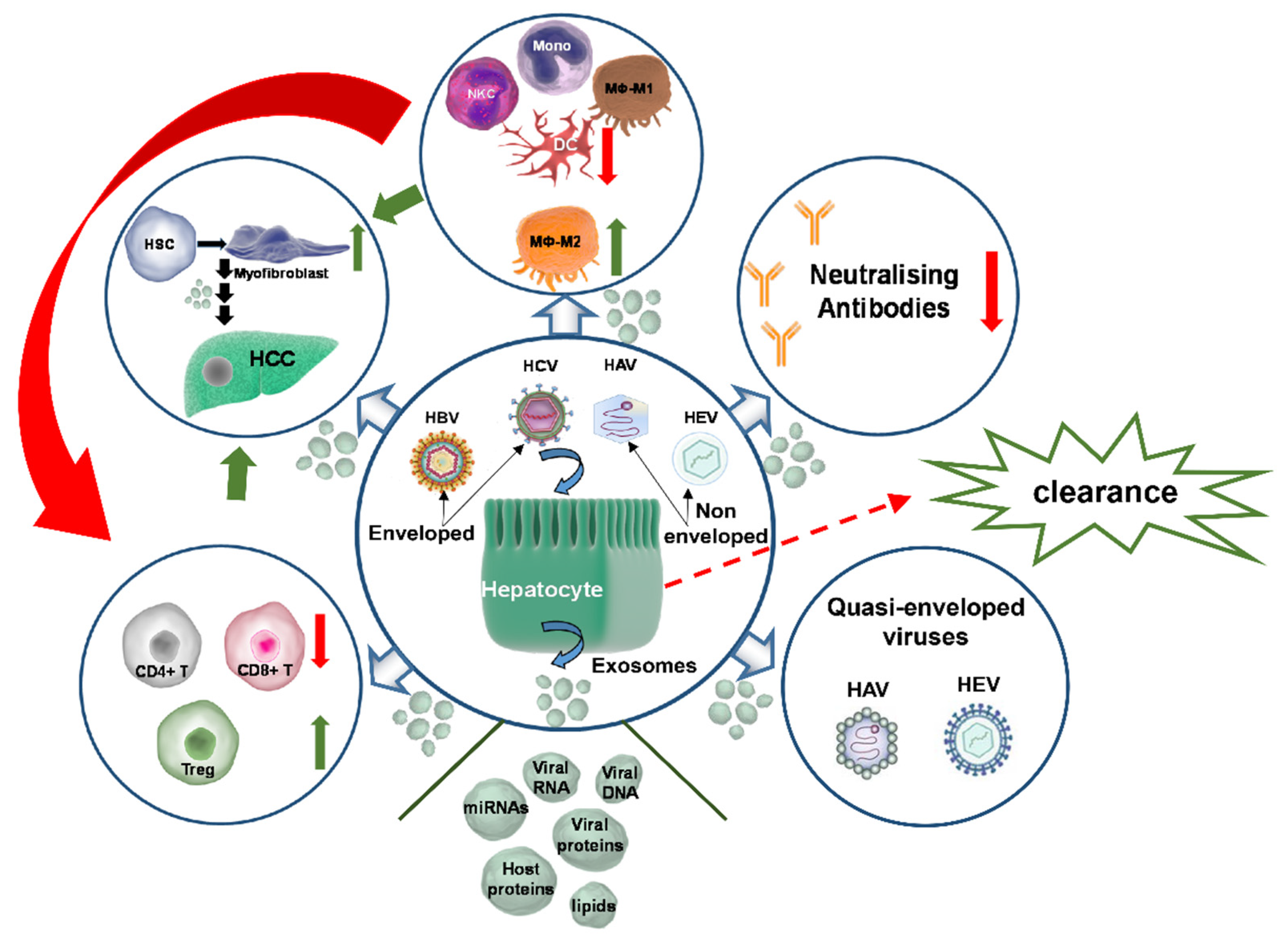
3. Exosome-Driven Regulation of the Host Immune Responses in Viral Hepatitis
3.1. Exosome-Mediated Immunomodulation of the Host Cellular Environment in Viral Hepatitis
3.2. Exosome-Mediated Immune Evasion of Hepatitis Viruses
3.3. Exosome-Mediated Immunosuppression in Viral Hepatitis
4. Exosome-Mediated Disease Progression on Viral Hepatitis Background
5. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melaram, R. Environmental Risk Factors Implicated in Liver Disease: A Mini-Review. Front. Public Health 2021, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Sofi, A.A. The Discovery of Hepatitis Viruses: Agents and Disease. J. Clin. Exp. Hepatol. 2020, 10, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Trepo, C. A brief history of hepatitis milestones. Liver Int. 2014, 34, 29–37. [Google Scholar] [CrossRef]
- World Health Organization. WHO Immunological Basis for Immunization Series: Module 18: Hepatitis A, Update 2019. Available online: https://apps.who.int/iris/handle/10665/326501 (accessed on 11 August 2022).
- Migueres, M.; Lhomme, S.; Izopet, J. Hepatitis A: Epidemiology, High-Risk Groups, Prevention and Research on Antiviral Treatment. Viruses 2021, 13, 1900. [Google Scholar] [CrossRef]
- Pallerla, S.R.; Harms, D.; Johne, R.; Todt, D.; Steinmann, E.; Schemmerer, M.; Wenzel, J.J.; Hofmann, J.; Shih, J.W.K.; Wedemeyer, H.; et al. Hepatitis E Virus Infection: Circulation, Molecular Epidemiology, and Impact on Global Health. Pathogens 2020, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; de Man, R.A.; Kamar, N.; Pan, Q. Chronic hepatitis E: Advancing research and patient care. J. Hepatol. 2022. [Google Scholar] [CrossRef]
- Westbrook, R.H.; Dusheiko, G. Natural history of hepatitis C. J. Hepatol. 2014, 61, S58–S68. [Google Scholar] [CrossRef]
- World Health Organisation. Hepatitis C. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 13 September 2022).
- World Health Organization. Hepatitis B. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 13 September 2022).
- Kanda, T.; Goto, T.; Hirotsu, Y.; Moriyama, M.; Omata, M. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections: A Review. Int. J. Mol. Sci. 2019, 20, 1358. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Saraceni, C.; Birk, J. A Review of Hepatitis B Virus and Hepatitis C Virus Immunopathogenesis. J. Clin. Transl. Hepatol. 2021, 9, 409–418. [Google Scholar] [CrossRef]
- Burns, G.S.; Thompson, A.J. Viral Hepatitis B: Clinical and Epidemiological Characteristics. Cold Spring Harb. Perspect. Med. 2014, 4, a024935. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.C. Clinical Manifestations of Hepatitis C. Clin. Liver Dis. 1997, 1, 569–585. [Google Scholar] [CrossRef]
- Dalton, H.R.; Bendall, R.; Ijaz, S.; Banks, M. Hepatitis E: An emerging infection in developed countries. Lancet Infect. Dis. 2008, 8, 698–709. [Google Scholar] [CrossRef]
- Izquierdo-Useros, N.; Puertas, M.C.; Borràs, F.E.; Blanco, J.; Martinez-Picado, J. Exosomes and retroviruses: The chicken or the egg? Cell. Microbiol. 2010, 13, 10–17. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Porcellati, S.; Emiliani, C. The Role of Extracellular Vesicles in Viral Infection and Transmission. Vaccines 2019, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotic, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitas, A.; Peterlin, B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef]
- Flanagan, J.; Middeldorp, J.; Sculley, T. Localization of the Epstein–Barr virus protein LMP 1 to exosomes. J. Gen. Virol. 2003, 84, 1871–1879. [Google Scholar] [CrossRef]
- Phillips, W.; Willms, E.; Hill, A.F. Understanding extracellular vesicle and nanoparticle heterogeneity: Novel methods and considerations. Proteomics 2021, 21, 2000118. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Metzner, C.; Zaruba, M. On the Relationship of Viral Particles and Extracellular Vesicles: Implications for Viral Vector Technology. Viruses 2021, 13, 1238. [Google Scholar] [CrossRef]
- Nolte-’t Hoen, E.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular vesicles and viruses: Are they close relatives? Proc. Natl. Acad. Sci. USA. 2016, 113, 9155–9161. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Fordjour, F.K.; Daaboul, G.G.; Gould, S.J. A shared pathway of exosome biogenesis operates at plasma and endosome membranes. bioRxiv 2019, 545228. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef]
- Hanson, P.I.; Cashikar, A. Multivesicular Body Morphogenesis. Annu. Rev. Cell Dev. Biol. 2012, 28, 337–362. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Ludwig, N.; Whiteside, T.L.; Reichert, T.E. Challenges in Exosome Isolation and Analysis in Health and Disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.A.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; van Eijndhoven, M.A.J.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Würdinger, T.; Middeldorp, J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Perez-Hernandez, D.; Gutiérrez-Vázquez, C.; Jorge, I.; López-Martín, S.; Ursa, A.; Sánchez-Madrid, F.; Vázquez, J.; Yáñez-Mó, M. The Intracellular Interactome of Tetraspanin-enriched Microdomains Reveals Their Function as Sorting Machineries toward Exosomes. J. Biol. Chem. 2013, 288, 11649–11661. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef]
- Azmi, A.S.; Bao, B.; Sarkar, F.H. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metastasis Rev. 2013, 32, 623–642. [Google Scholar] [CrossRef]
- Shifrin, D.A.; Beckler, M.D.; Coffey, R.J.; Tyska, M.J. Extracellular vesicles: Communication, coercion, and conditioning. Mol. Biol. Cell 2013, 24, 1253–1259. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Paulaitis, M.; Agarwal, K.; Nana-Sinkam, S.P. Dynamic Scaling of Exosome Sizes. Langmuir 2018, 34, 9387–9393. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, M.; Pietrowska, M.; Matysiak, N.; Mielańczyk, Ł; Widłak, P. Proteome Profiling of Exosomes Purified from a Small Amount of Human Serum: The Problem of Co-Purified Serum Components. Proteomes 2019, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Eng, W.S.; Colquhoun, D.R.; Dinglasan, R.R.; Graham, D.R.; Mahal, L.K. Complex N-Linked Glycans Serve as a Determinant for Exosome/Microvesicle Cargo Recruitment. J. Biol. Chem. 2014, 289, 32526–32537. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.; Nicco, C.; Lombard, B.; Véron, P.; Raposo, G.; Batteux, F.; Amigorena, S.; Théry, C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 2005, 106, 216–223. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; DeGeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Rieu, S.; Géminard, C.; Rabesandratana, H.; Sainte-Marie, J.; Vidal, M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin α4β1. Eur. J. Biochem. 2000, 267, 583–590. [Google Scholar] [CrossRef]
- Verweij, F.J.; van Eijndhoven, M.A.; Hopmans, E.S.; Vendrig, T.; Wurdinger, T.; Cahir-McFarland, E.; Kieff, E.; Geerts, D.; van der Kant, R.; Neefjes, J.; et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO 2011, 30, 2115–2129. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Jarad, M.; Kuczynski, E.A.; Morrison, J.; Viloria-Petit, A.M.; Coomber, B.L. Release of endothelial cell associated VEGFR2 during TGF-β modulated angiogenesis in vitro. BMC Cell Biol. 2017, 18, 10. [Google Scholar] [CrossRef]
- DeRita, R.M.; Zerlanko, B.; Singh, A.; Lu, H.; Iozzo, R.V.; Benovic, J.L.; Languino, L.R. c-Src, Insulin-Like Growth Factor I Receptor, G-Protein-Coupled Receptor Kinases and Focal Adhesion Kinase are Enriched Into Prostate Cancer Cell Exosomes. J. Cell. Biochem. 2017, 118, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, N.; Lankar, D.; Faure, F.; Regnault, A.; Dumont, C.; Raposo, G.; Hivroz, C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/ζ complex. J. Immunol. 2002, 168, 3235–3241. [Google Scholar] [CrossRef]
- Nager, A.R.; Goldstein, J.S.; Herranz-Pérez, V.; Portran, D.; Ye, F.; Garcia-Verdugo, J.M.; Nachury, M.V. An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell 2016, 168, 252–263.e14. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-P.; Lin, Z.-X.; Jiang, X.-Y.; Yu, X.-Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018, 39, 542–551. [Google Scholar] [CrossRef]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2014, 1841, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Ekroos, K.; Kauhanen, D.; Simolin, H.; Seierstad, T.; Berge, V.; Sandvig, K.; Llorente, A. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur. J. Cancer 2017, 70, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef]
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; De Medina, P.; Monsarrat, B.; Perret, B.; Silvente-Poirot, S.; et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 2010, 51, 2105–2120. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, N.; Yan, T.; Shi, Y.-N.; Chen, J.; Zhang, C.-J.; Xie, X.-J.; Liao, D.-F.; Qin, L. The crosstalk: Exosomes and lipid metabolism. Cell Commun. Signal. 2020, 18, 119. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kang, B.; Kim, O.Y.; Choi, D.-S.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20384. [Google Scholar] [CrossRef]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT Pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Saric, A.; Freeman, S.A. Endomembrane Tension and Trafficking. Front. Cell Dev. Biol. 2021, 8, 611326. [Google Scholar] [CrossRef]
- Skryabin, G.O.; Komelkov, A.V.; Savelyeva, E.E.; Tchevkina, E.M. Lipid Rafts in Exosome Biogenesis. Biochemistry 2020, 85, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, T.; Herczeg, K.; Onódi, Z.; Voszka, I.; Módos, K.; Marton, N.; Nagy, G.; Mäger, I.; Wood, M.J.; El Andaloussi, S.; et al. Isolation of Exo-somes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatog-raphy Methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef]
- Street, J.M.; Koritzinsky, E.H.; Glispie, D.M.; Yuen, P.S.T. Urine Exosome Isolation and Characterization. Drug Saf. Eval. 2017, 1641, 413–423. [Google Scholar] [CrossRef]
- Mourtzi, N.; Siahanidou, T.; Tsifintaris, M.; Karamichali, E.; Tasiopoulou, A.; Sertedaki, A.; Pesmatzoglou, M.; Kapetanaki, A.; Liosis, G.; Baltatzis, G.; et al. lncRNA NORAD is consistently detected in breastmilk exosomes and its expression is downregulated in mothers of preterm infants. Int. J. Mol. Med. 2021, 48, 216. [Google Scholar] [CrossRef]
- Prada, I.; Meldolesi, J. Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets. Int. J. Mol. Sci. 2016, 17, 1296. [Google Scholar] [CrossRef]
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef]
- Cho, Y.-E.; Im, E.-J.; Moon, P.-G.; Mezey, E.; Song, B.-J.; Baek, M.-C. Increased liver-specific proteins in circulating extracellular vesicles as potential biomarkers for drug- and alcohol-induced liver injury. PLoS ONE 2017, 12, e0172463. [Google Scholar] [CrossRef]
- Beckler, M.D.; Higginbotham, J.N.; Franklin, J.L.; Ham, A.-J.; Halvey, P.J.; Imasuen, I.E.; Whitwell, C.; Li, M.; Liebler, D.C.; Coffey, R.J. Proteomic Analysis of Exosomes from Mutant KRAS Colon Cancer Cells Identifies Intercellular Transfer of Mutant KRAS. Mol. Cell. Proteom. 2013, 12, 343–355. [Google Scholar] [CrossRef]
- Ji, H.; Greening, D.W.; Barnes, T.W.; Lim, J.W.; Tauro, B.J.; Rai, A.; Xu, R.; Adda, C.; Mathivanan, S.; Zhao, W.; et al. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics 2013, 13, 1672–1686. [Google Scholar] [CrossRef]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Y.; Sun, S.; Wang, T.; Qiao, X.; Run, X.; Liang, Z. Tau Phosphorylation and μ-Calpain Activation Mediate the Dexamethasone-Induced Inhibition on the Insulin-Stimulated Akt Phosphorylation. PLoS ONE 2012, 7, e35783. [Google Scholar] [CrossRef] [PubMed]
- Vella, L.J.; Hill, A.F.; Cheng, L. Focus on Extracellular Vesicles: Exosomes and Their Role in Protein Trafficking and Biomarker Potential in Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s Dement. 2015, 11, 600–607.e1. [Google Scholar] [CrossRef] [PubMed]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef]
- Danzer, K.M.; Kranich, L.R.; Ruf, W.P.; Cagsal-Getkin, O.; Winslow, A.R.; Zhu, L.; Vanderburg, C.R.; McLean, P.J. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol. Neurodegener. 2012, 7, 42. [Google Scholar] [CrossRef]
- den Boon, J.A.; Ahlquist, P. Organelle-Like Membrane Compartmentalization of Positive-Strand RNA Virus Replication Factories. Annu. Rev. Microbiol. 2010, 64, 241–256. [Google Scholar] [CrossRef]
- den Boon, J.A.; Diaz, A.; Ahlquist, P. Cytoplasmic Viral Replication Complexes. Cell Host Microbe 2010, 8, 77–85. [Google Scholar] [CrossRef]
- Gosert, R.; Kanjanahaluethai, A.; Egger, D.; Bienz, K.; Baker, S.C. RNA Replication of Mouse Hepatitis Virus Takes Place at Double-Membrane Vesicles. J. Virol. 2002, 76, 3697–3708. [Google Scholar] [CrossRef]
- Snijder, E.J.; van der Meer, Y.; Zevenhoven-Dobbe, J.; Onderwater, J.J.M.; van der Meulen, J.; Koerten, H.K.; Mommaas, A.M. Ultrastructure and Origin of Membrane Vesicles Associated with the Severe Acute Respiratory Syndrome Coronavirus Replication Complex. J. Virol. 2006, 80, 5927–5940. [Google Scholar] [CrossRef] [PubMed]
- Ulasli, M.; Verheije, M.H.; de Haan, C.A.; Reggiori, F. Qualitative and quantitative ultrastructural analysis of the membrane rearrangements induced by coronavirus. Cell. Microbiol. 2010, 12, 844–861. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.; Melia, C.E.; Snijder, E.J.; Bárcena, M. Double-Membrane Vesicles as Platforms for Viral Replication. Trends Microbiol. 2020, 28, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Bunz, M.; Ritter, M.; Schindler, M. HCV egress-unconventional secretion of assembled viral particles. Trends Microbiol. 2021, 30, 364–378. [Google Scholar] [CrossRef]
- Hsu, N.-Y.; Ilnytska, O.; Belov, G.; Santiana, M.; Chen, Y.-H.; Takvorian, P.M.; Pau, C.; van der Schaar, H.; Kaushik-Basu, N.; Balla, T.; et al. Viral Reorganization of the Secretory Pathway Generates Distinct Organelles for RNA Replication. Cell 2010, 141, 799–811. [Google Scholar] [CrossRef]
- V’Kovski, P.; Al-Mulla, H.; Thiel, V.; Neuman, B.W. New insights on the role of paired membrane structures in coronavirus replication. Virus Res. 2014, 202, 33–40. [Google Scholar] [CrossRef]
- Hassan, Z.; Kumar, N.D.; Reggiori, F.; Khan, G. How Viruses Hijack and Modify the Secretory Transport Pathway. Cells 2021, 10, 2535. [Google Scholar] [CrossRef]
- Tabata, K.; Arimoto, M.; Arakawa, M.; Nara, A.; Saito, K.; Omori, H.; Arai, A.; Ishikawa, T.; Konishi, E.; Suzuki, R.; et al. Unique Requirement for ESCRT Factors in Flavivirus Particle Formation on the Endoplasmic Reticulum. Cell Rep. 2016, 16, 2339–2347. [Google Scholar] [CrossRef]
- Feng, Z.; Hirai-Yuki, A.; McKnight, K.L.; Lemon, S.M. Naked Viruses That Aren’t Always Naked: Quasi-Enveloped Agents of Acute Hepatitis. Annu. Rev. Virol. 2014, 1, 539–560. [Google Scholar] [CrossRef]
- McKnight, K.L.; Xie, L.; González-López, O.; Rivera-Serrano, E.E.; Chen, X.; Lemon, S.M. Protein composition of the hepatitis A virus quasi-envelope. Proc. Natl. Acad. Sci. USA 2017, 114, 6587–6592. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamada, K.; Hoshino, Y.; Takahashi, H.; Ichiyama, K.; Tanaka, T.; Okamoto, H. Monoclonal antibodies raised against the ORF3 protein of hepatitis E virus (HEV) can capture HEV particles in culture supernatant and serum but not those in feces. Arch. Virol. 2008, 153, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Gauss-Muller, V.; Deinhardt, F. Effect of Hepatitis A Virus Infection on Cell Metabolism in Vitro. Proc. Soc. Exp. Biol. Med. 1984, 175, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.Y.; Madden, V.; Ping, L.F.; Jeong, S.-H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.L.; Murphy, P.C.; Asher, L.V.S.; LeDuc, J.W.; Lemon, S.M. Attenuation Phenotype of a Cell Culture-Adapted Variant of Hepatitis A Virus (HM175/p16) in Susceptible New World Owl Monkeys. J. Infect. Dis. 1993, 168, 592–601. [Google Scholar] [CrossRef]
- Chapuy-Regaud, S.; Dubois, M.; Plisson-Chastang, C.; Bonnefois, T.; Lhomme, S.; Bertrand-Michel, J.; You, B.; Simoneau, S.; Gleizes, P.-E.; Flan, B.; et al. Characterization of the lipid envelope of exosome encapsulated HEV particles protected from the immune response. Biochimie 2017, 141, 70–79. [Google Scholar] [CrossRef]
- Nagashima, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Nishiyama, T.; Primadharsini, P.P.; Okamoto, H. Characterization of the Quasi-Enveloped Hepatitis E Virus Particles Released by the Cellular Exosomal Pathway. J. Virol. 2017, 91, e00822-17. [Google Scholar] [CrossRef]
- Nagashima, S.; Takahashi, M.; Jirintai, S.; Tanggis; Kobayashi, T.; Nishizawa, T.; Okamoto, H. The membrane on the surface of hepatitis E virus particles is derived from the intracellular membrane and contains trans-Golgi network protein 2. Arch. Virol. 2014, 159, 979–991. [Google Scholar] [CrossRef]
- Nagashima, S.; Jirintai, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Kouki, T.; Yashiro, T.; Okamoto, H. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J. Gen. Virol. 2014, 95, 2166–2175. [Google Scholar] [CrossRef]
- Glitscher, M.; Hildt, E. Hepatitis E virus egress and beyond-the manifold roles of the viral ORF3 protein. Cell. Microbiol. 2021, 23, e13379. [Google Scholar] [CrossRef]
- Okamoto, H. Hepatitis E virus cell culture models. Virus Res. 2011, 161, 65–77. [Google Scholar] [CrossRef]
- Masciopinto, F.; Giovani, C.; Campagnoli, S.; Galli-Stampino, L.; Colombatto, P.; Brunetto, M.; Yen, T.S.B.; Houghton, M.; Pileri, P.; Abrignani, S. Association of hepatitis C virus envelope proteins with exosomes. Eur. J. Immunol. 2004, 34, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Tamai, K.; Shiina, M.; Tanaka, N.; Nakano, T.; Yamamoto, A.; Kondo, Y.; Kakazu, E.; Inoue, J.; Fukushima, K.; Sano, K.; et al. Regulation of hepatitis C virus secretion by the Hrs-dependent exosomal pathway. Virology 2012, 422, 377–385. [Google Scholar] [CrossRef]
- Ramakrishnaiah, V.; Thumann, C.; Fofana, I.; Habersetzer, F.; Pan, Q.; de Ruiter, P.E.; Willemsen, R.; Demmers, J.A.A.; Raj, V.S.; Jenster, G.; et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13109–13113. [Google Scholar] [CrossRef] [PubMed]
- Blondot, M.-L.; Bruss, V.; Kann, M. Intracellular transport and egress of hepatitis B virus. J. Hepatol. 2016, 64, S49–S59. [Google Scholar] [CrossRef] [PubMed]
- Bruss, V.; Gerhardt, E.; Vieluf, K.; Wunderlich, G. Functions of the Large Hepatitis B Virus Surface Protein in Viral Particle Morphogenesis. Intervirology 1996, 39, 23–31. [Google Scholar] [CrossRef]
- Yang, Y.; Han, Q.; Hou, Z.; Zhang, C.; Tian, Z.; Zhang, J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell. Mol. Immunol. 2017, 14, 465–475. [Google Scholar] [CrossRef]
- Kapoor, N.R.; Chadha, R.; Kumar, S.; Choedon, T.; Reddy, V.S.; Kumar, V. The HBx gene of hepatitis B virus can influence hepatic microenvironment via exosomes by transferring its mRNA and protein. Virus Res. 2017, 240, 166–174. [Google Scholar] [CrossRef]
- Jia, X.; Chen, J.; Megger, D.A.; Zhang, X.; Kozlowski, M.; Zhang, L.; Fang, Z.; Li, J.; Chu, Q.; Wu, M.; et al. Label-free Proteomic Analysis of Exosomes Derived from Inducible Hepatitis B Virus-Replicating HepAD38 Cell Line. Mol. Cell. Proteom. 2017, 16, S144–S160. [Google Scholar] [CrossRef]
- Ninomiya, M.; Inoue, J.; Krueger, E.W.; Chen, J.; Cao, H.; Masamune, A.; McNiven, M.A. The Exosome-Associated Tetraspanin CD63 Contributes to the Efficient Assembly and Infectivity of the Hepatitis B Virus. Hepatol. Commun. 2021, 5, 1238–1251. [Google Scholar] [CrossRef]
- Rosmorduc, O.; Petit, M.A.; Pol, S.; Capel, F.; Bortolotti, F.; Berthelot, P.; Brechot, C.; Kremsdorf, D. In vivo and in vitro expression of defective hepatitis B virus particles generated by spliced hepatitis B virus RNA. Hepatology 1995, 22, 10–19. [Google Scholar]
- Soussan, P.; Pol, J.; Garreau, F.; Schneider, V.; Le Pendeven, C.; Nalpas, B.; Lacombe, K.; Bonnard, P.; Pol, S.; Kremsdorf, D. Expression of Defective Hepatitis B Virus Particles Derived from Singly Spliced RNA Is Related to Liver Disease. J. Infect. Dis. 2008, 198, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Cheroni, C.; Donnici, L.; Aghemo, A.; Balistreri, F.; Bianco, A.; Zanoni, V.; Pagani, M.; Soffredini, R.; D’Ambrosio, R.; Rumi, M.G.; et al. Hepatitis C Virus Deletion Mutants Are Found in Individuals Chronically Infected with Genotype 1 Hepatitis C Virus in Association with Age, High Viral Load and Liver Inflammatory Activity. PLoS ONE 2015, 10, e0138546. [Google Scholar] [CrossRef] [PubMed]
- Karamichali, E.; Chihab, H.; Kakkanas, A.; Marchio, A.; Karamitros, T.; Pogka, V.; Varaklioti, A.; Kalliaropoulos, A.; Martinez-Gonzales, B.; Foka, P.; et al. HCV Defective Genomes Promote Persistent Infection by Modulating the Viral Life Cycle. Front. Microbiol. 2018, 9, 2942. [Google Scholar] [CrossRef] [PubMed]
- Nuesch, J.P.F.; De Chastonay, J.; Siegl, G. Detection of Defective Genomes in Hepatitis A Virus Particles Present in Clinical Specimens. J. Gen. Virol. 1989, 70, 3475–3480. [Google Scholar] [CrossRef]
- Shen, S.; Xie, Z.; Cai, D.; Yu, X.; Zhang, H.; Kim, E.S.; Zhou, B.; Hou, J.; Zhang, X.; Huang, Q.; et al. Biogenesis and molecular characteristics of serum hepatitis B virus RNA. PLoS Pathog. 2020, 16, e1008945. [Google Scholar] [CrossRef]
- Jenne, C.N.; Kubes, P. Immune surveillance by the liver. Nat. Immunol. 2013, 14, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef]
- Dreyer, F.; Baur, A. Biogenesis and Functions of Exosomes and Extracellular Vesicles. In Lentiviral Vectors and Exosomes as Gene and Protein Delivery Tools; Springer: Berlin/Heidelberg, Germany, 2016; pp. 201–216. [Google Scholar] [CrossRef]
- Kapustin, A.N.; Shanahan, C.M. Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation. J. Physiol. 2016, 594, 2905–2914. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta BBA-Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, X.; Hao, J.; Xu, G.; Zhang, W. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tissue Regeneration. Cell Transplant. 2020, 29, 963689720908500. [Google Scholar] [CrossRef]
- Stefanius, K.; Servage, K.; Orth, K. Exosomes in cancer development. Curr. Opin. Genet. Dev. 2021, 66, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xu, Y.; Deng, W.; Zhang, L.; Zhu, H.; Yu, P.; Qu, Y.; Zhao, W.; Han, Y.; Qin, C. Brain Derived Exosomes Are a Double-Edged Sword in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- de Vrij, J.; Maas, S.N.; Kwappenberg, K.M.; Schnoor, R.; Kleijn, A.; Dekker, L.; Luider, T.M.; de Witte, L.D.; Litjens, M.; van Strien, M.E.; et al. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int. J. Cancer 2015, 137, 1630–1642. [Google Scholar] [CrossRef] [PubMed]
- Lundholm, M.; Schröder, M.; Nagaeva, O.; Baranov, V.; Widmark, A.; Mincheva-Nilsson, L.; Wikström, P. Prostate Tumor-Derived Exosomes Down-Regulate NKG2D Expression on Natural Killer Cells and CD8+ T Cells: Mechanism of Immune Evasion. PLoS ONE 2014, 9, e108925. [Google Scholar] [CrossRef]
- Wang, M.; Cai, Y.; Peng, Y.; Xu, B.; Hui, W.; Jiang, Y. Exosomal LGALS9 in the cerebrospinal fluid of glioblastoma patients suppressed dendritic cell antigen presentation and cytotoxic T-cell immunity. Cell Death Dis. 2020, 11, 896. [Google Scholar] [CrossRef]
- Yamada, N.; Kuranaga, Y.; Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Akao, Y. Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-β1-mediated suppression. Oncotarget 2016, 7, 27033–27043. [Google Scholar] [CrossRef]
- Devhare, P.B.; Sasaki, R.; Shrivastava, S.; Di Bisceglie, A.M.; Ray, R.; Ray, R.B. Exosome-Mediated Intercellular Communication between Hepatitis C Virus-Infected Hepatocytes and Hepatic Stellate Cells. J. Virol. 2017, 91, e02225-16. [Google Scholar] [CrossRef]
- Karamichali, E.; Foka, P.; Valiakou, V.; Eiliadis, P.; Loukaki-Gkountara, D.; Andresaki, K.; Papadopoulou, G.; Georgopoulou, U.; Koskinas, I.-G. Exosomal cargo as a key player of the immune response after direct-acting antiviral treatment in chronic hepatitis C patients. J. Hepatol. 2022, 77, S255–S256. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Wu, S.; Chen, L. Exosomes Modulate the Viral Replication and Host Immune Responses in HBV Infection. BioMed Res. Int. 2019, 2019, 2103943. [Google Scholar] [CrossRef]
- Saad, M.H.; Badierah, R.; Redwan, E.M.; El-Fakharany, E.M. A Comprehensive Insight into the Role of Exosomes in Viral Infection: Dual Faces Bearing Different Functions. Pharmaceutics 2021, 13, 1405. [Google Scholar] [CrossRef]
- Santangelo, L.; Bordoni, V.; Montaldo, C.; Cimini, E.; Zingoni, A.; Battistelli, C.; D’Offizi, G.; Capobianchi, M.R.; Santoni, A.; Tripodi, M.; et al. Hepatitis C virus direct-acting antivirals therapy impacts on extracellular vesicles microRNAs content and on their immunomodulating properties. Liver Int. 2018, 38, 1741–1750. [Google Scholar] [CrossRef]
- Itami-Matsumoto, S.; Hayakawa, M.; Uchida-Kobayashi, S.; Enomoto, M.; Tamori, A.; Mizuno, K.; Toyoda, H.; Tamura, T.; Akutsu, T.; Ochiya, T.; et al. Circulating Exosomal miRNA Profiles Predict the Occurrence and Recurrence of Hepatocellular Carcinoma in Patients with Direct-Acting Antiviral-Induced Sustained Viral Response. Biomedicines 2019, 7, 87. [Google Scholar] [CrossRef]
- Alao, H.; Cam, M.; Keembiyehetty, C.; Zhang, F.; Serti, E.; Suarez, D.; Park, H.; Fourie, N.H.; Wright, E.C.; Henderson, W.A.; et al. Baseline Intrahepatic and Peripheral Innate Immunity are Associated with Hepatitis C Virus Clearance During Direct-Acting Antiviral Therapy. Hepatology 2018, 68, 2078–2088. [Google Scholar] [CrossRef]
- Kakizaki, M.; Yamamoto, Y.; Yabuta, S.; Kurosaki, N.; Kagawa, T.; Kotani, A. The immunological function of extracellular vesicles in hepatitis B virus-infected hepatocytes. PLoS ONE 2018, 13, e0205886. [Google Scholar] [CrossRef]
- Ortega-Prieto, A.M.; Dorner, M. Immune Evasion Strategies during Chronic Hepatitis B and C Virus Infection. Vaccines 2017, 5, 24. [Google Scholar] [CrossRef]
- Lemon, S.M.; Binn, L.N. Incomplete Neutralization of Hepatitis A Virus in vitro due to Lipid-associated Virions. J. Gen. Virol. 1985, 66, 2501–2505. [Google Scholar] [CrossRef]
- Zhou, L.; Irani, S.; Sirwi, A.; Hussain, M.M. MicroRNAs regulating apolipoprotein B-containing lipoprotein production. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2016, 1861, 2062–2068. [Google Scholar] [CrossRef]
- Bukong, T.N.; Momen-Heravi, F.; Kodys, K.; Bala, S.; Szabo, G. Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90. PLoS Pathog. 2014, 10, e1004424. [Google Scholar] [CrossRef]
- Conrad, K.D.; Giering, F.; Erfurth, C.; Neumann, A.; Fehr, C.; Meister, G.; Niepmann, M. microRNA-122 Dependent Binding of Ago2 Protein to Hepatitis C Virus RNA Is Associated with Enhanced RNA Stability and Translation Stimulation. PLoS ONE 2013, 8, e56272. [Google Scholar] [CrossRef]
- Roberts, A.P.E.; Lewis, A.P.; Jopling, C.L. miR-122 activates hepatitis C virus translation by a specialized mechanism requiring particular RNA components. Nucleic Acids Res. 2011, 39, 7716–7729. [Google Scholar] [CrossRef]
- Grünvogel, O.; Colasanti, O.; Lee, J.-Y.; Klöss, V.; Belouzard, S.; Reustle, A.; Esser-Nobis, K.; Hesebeck-Brinckmann, J.; Mutz, P.; Hoffmann, K.; et al. Secretion of Hepatitis C Virus Replication Intermediates Reduces Activation of Toll-Like Receptor 3 in Hepatocytes. Gastroenterology 2018, 154, 2237–2251.e16. [Google Scholar] [CrossRef]
- McKeating, J.A.; Zhang, L.Q.; Logvinoff, C.; Flint, M.; Zhang, J.; Yu, J.; Butera, D.; Ho, D.D.; Dustin, L.B.; Rice, C.M.; et al. Diverse Hepatitis C Virus Glycoproteins Mediate Viral Infection in a CD81-Dependent Manner. J. Virol. 2004, 78, 8496–8505. [Google Scholar] [CrossRef]
- Malik, M.A.; Mirza, J.I.A.; Umar, M.; Manzoor, S. CD81+ Exosomes Play a Pivotal Role in the Establishment of Hepatitis C Persistent Infection and Contribute Toward the Progression of Hepatocellular Carcinoma. Viral Immunol. 2019, 32, 453–462. [Google Scholar] [CrossRef]
- Bhattarai, N.; McLinden, J.H.; Xiang, J.; Kaufman, T.M.; Stapleton, J.T. Conserved Motifs within Hepatitis C Virus Envelope (E2) RNA and Protein Independently Inhibit T Cell Activation. PLoS Pathog. 2015, 11, e1005183. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Ren, Y.; Tong, Y.; Hua, X.; Zhu, F.; Huang, L.; Liu, Y.; Luo, Y.; Lu, W.; et al. Hepatitis C Virus Protects Human B Lymphocytes from Fas-Mediated Apoptosis via E2-CD81 Engagement. PLoS ONE 2011, 6, e18933. [Google Scholar] [CrossRef]
- Crotta, S.; Stilla, A.; Wack, A.; D’Andrea, A.; Nuti, S.; D’Oro, U.; Mosca, M.; Filliponi, F.; Brunetto, R.M.; Bonino, F.; et al. Inhibition of Natural Killer Cells through Engagement of CD81 by the Major Hepatitis C Virus Envelope Protein. J. Exp. Med. 2002, 195, 35–42. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Yu, Q.; He, J.J. Exosome-associated hepatitis C virus in cell cultures and patient plasma. Biochem. Biophys. Res. Commun. 2014, 455, 218–222. [Google Scholar] [CrossRef]
- Drummer, H.E. Challenges to the development of vaccines to hepatitis C virus that elicit neutralizing antibodies. Front. Microbiol. 2014, 5, 329. [Google Scholar] [CrossRef]
- Deng, L.; Jiang, W.; Wang, X.; Merz, A.; Hiet, M.-S.; Chen, Y.; Pan, X.; Jiu, Y.; Yang, Y.; Yu, B.; et al. Syntenin regulates hepatitis C virus sensitivity to neutralizing antibody by promoting E2 secretion through exosomes. J. Hepatol. 2019, 71, 52–61. [Google Scholar] [CrossRef]
- Kouwaki, T.; Fukushima, Y.; Daito, T.; Sanada, T.; Yamamoto, N.; Mifsud, E.J.; Leong, C.R.; Tsukiyama-Kohara, K.; Kohara, M.; Matsumoto, M.; et al. Extracellular Vesicles Including Exosomes Regulate Innate Immune Responses to Hepatitis B Virus Infection. Front. Immunol. 2016, 7, 335. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, H.; Sun, H.; Fan, H.; Hu, Y.; Liu, M.; Li, X.; Tang, H. Hepatitis B Virus-Encoded MicroRNA Controls Viral Replication. J. Virol. 2017, 91, e01919-16. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.S.; Decaens, T. Liver immunotolerance and hepatocellular carcinoma: Patho-physiological mechanisms and therapeutic perspectives. Eur. J. Cancer 2017, 87, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L.; Diergaarde, B.; Hong, C.-S. Tumor-Derived Exosomes (TEX) and Their Role in Immuno-Oncology. Int. J. Mol. Sci. 2021, 22, 6234. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, M.; Peng, M. Progress of exosome research in systemic lupus erythematosus. Cytokine X 2022, 4, 100066. [Google Scholar] [CrossRef]
- Sasaki, Y.T.; Sano, M.; Kin, T.; Asai, K.; Hirose, T. Coordinated expression of ncRNAs and HOX mRNAs in the human HOXA locus. Biochem. Biophys. Res. Commun. 2007, 357, 724–730. [Google Scholar] [CrossRef]
- Zhang, X.; Lian, Z.; Padden, C.; Gerstein, M.B.; Rozowsky, J.; Snyder, M.; Gingeras, T.R.; Kapranov, P.; Weissman, S.M.; Newburger, P.E. A myelopoiesis-associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood 2009, 113, 2526–2534. [Google Scholar] [CrossRef]
- Bowers, N.L.; Helton, E.S.; Huijbregts, R.P.H.; Goepfert, P.A.; Heath, S.L.; Hel, Z. Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway. PLoS Pathog. 2014, 10, e1003993. [Google Scholar] [CrossRef]
- Dai, J.; El Gazzar, M.; Li, G.Y.; Moorman, J.P.; Yao, Z.Q. Myeloid-Derived Suppressor Cells: Paradoxical Roles in Infection and Immunity. J. Innate Immun. 2015, 7, 116–126. [Google Scholar] [CrossRef]
- Thakuri, B.K.C.; Zhang, J.; Zhao, J.; Nguyen, L.N.; Khanal, S.; Cao, D.; Dang, X.; Schank, M.; Wu, X.Y.; Morrison, Z.D.; et al. LncRNA HOTAIRM1 promotes MDSC expansion and suppressive functions through the HOXA1-miR124 axis during HCV infection. Sci. Rep. 2020, 10, 22033. [Google Scholar] [CrossRef]
- Thakuri, B.K.C.; Zhang, J.; Zhao, J.; Nguyen, L.N.T.; Nguyen, L.; Schank, M.; Khanal, S.; Dang, X.; Cao, D.; Lu, Z.; et al. HCV-Associated Exosomes Upregulate RUNXOR and RUNX1 Expressions to Promote MDSC Expansion and Suppressive Functions through STAT3–miR124 Axis. Cells 2020, 9, 2715. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.-C.; Sung, P.S.; Park, S.-H. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat. Rev. Immunol. 2016, 16, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Schulze zur Wiesch, J.; Ciuffreda, D.; Lewis-Ximenez, L.; Kasprowicz, V.; Nolan, B.E.; Streeck, H.; Aneja, J.; Reyor, L.L.; Allen, T.M.; Lohse, A.W.; et al. Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J. Exp. Med. 2012, 209, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Thimme, R.; Binder, M.; Bartenschlager, R. Failure of innate and adaptive immune responses in controlling hepatitis C virus infection. FEMS Microbiol. Rev. 2012, 36, 663–683. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L.; El-Badawy, A. Programmed death-1/programmed death-L1 signaling pathway and its blockade in hepatitis C virus immunotherapy. World J. Hepatol. 2015, 7, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kang, H.; Lee, H.H.; Kim, C.W. Programmed Cell Death 1 (PD-1) and Cytotoxic T Lymphocyte-Associated Antigen 4 (CTLA-4) in Viral Hepatitis. Int. J. Mol. Sci. 2017, 18, 1517. [Google Scholar] [CrossRef] [PubMed]
- McKinney, E.F.; Smith, K.G. T cell exhaustion and immune-mediated disease—the potential for therapeutic exhaustion. Curr. Opin. Immunol. 2016, 43, 74–80. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Rushbrook, S.M.; Ward, S.M.; Unitt, E.; Vowler, S.L.; Lucas, M.; Klenerman, P.; Alexander, G.J.M. Regulatory T Cells Suppress In Vitro Proliferation of Virus-Specific CD8 + T Cells during Persistent Hepatitis C Virus Infection. J. Virol. 2005, 79, 7852–7859. [Google Scholar] [CrossRef]
- Boettler, T.; Spangenberg, H.C.; Neumann-Haefelin, C.; Panther, E.; Urbani, S.; Ferrari, C.; Blum, H.E.; von Weizsacker, F.; Thimme, R. T Cells with a CD4 + CD25 + Regulatory Phenotype Suppress In Vitro Proliferation of Virus-Specific CD8 + T Cells during Chronic Hepatitis C Virus Infection. J. Virol. 2005, 79, 7860–7867. [Google Scholar] [CrossRef]
- Cobb, D.A.; Kim, O.-K.; Golden-Mason, L.; Rosen, H.R.; Hahn, Y.S. Hepatocyte-derived exosomes promote T follicular regulatory cell expansion during hepatitis C virus infection. Hepatology 2017, 67, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Wang, J.; Kim, E.S.; Mao, R.; Dong, M.; Liu, Y.; Zhang, J.; Guo, H. Hepatitis B Virus Precore Protein p22 Inhibits Alpha Interferon Signaling by Blocking STAT Nuclear Translocation. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, K.; Liu, Y.; Xu, Y.; Zhang, F.; Yang, H.; Liu, J.; Pan, T.; Chen, J.; Wu, M.; et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat. Immunol. 2013, 14, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Winkler, C.A.; An, P.; Guo, J.-T. IFITM Genes, Variants, and Their Roles in the Control and Pathogenesis of Viral Infections. Front. Microbiol. 2019, 9, 3228. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Du, L.; Lv, D.; Li, H.; Shang, J.; Lu, J.; Zhou, L.; Bai, L.; Tang, H. Exosomal Interferon-Induced Transmembrane Protein 2 Transmitted to Dendritic Cells Inhibits Interferon Alpha Pathway Activation and Blocks Anti–Hepatitis B Virus Efficacy of Exogenous Interferon Alpha. Hepatology 2019, 69, 2396–2413. [Google Scholar] [CrossRef]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef]
- D’Souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef]
- El-Mokhtar, M.A.; Sayed, I.M. Model systems for studying extrahepatic pathogenesis of hepatitis E virus. Current knowledge and future directions. Rev. Med. Virol. 2021, 31, E2218. [Google Scholar] [CrossRef]
- Ferri, C.; Ramos-Casals, M.; Zignego, A.L.; Arcaini, L.; Roccatello, D.; Antonelli, A.; Saadoun, D.; Desbois, A.C.; Sebastiani, M.; Casato, M.; et al. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun. Rev. 2016, 15, 1145–1160. [Google Scholar] [CrossRef]
- El-Awady, M.K.; Tabll, A.A.; Redwan, E.-R.M.; Youssef, S.; Omran, M.H.; Thakeb, F.; El-Demellawy, M. Flow cytometric detection of hepatitis C virus antigens in infected peripheral blood leukocytes: Binding and entry. World J. Gastroenterol. 2005, 11, 5203–5208. [Google Scholar] [CrossRef]
- Foka, P.; Dimitriadis, A.; Karamichali, E.; Kyratzopoulou, E.; Giannimaras, D.; Koskinas, J.; Varaklioti, A.; Mamalaki, A.; Georgopoulou, U. Alterations in the iron homeostasis network: A driving force for macrophage-mediated hepatitis C virus persistency. Virulence 2016, 7, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yan, Q.; Fan, R.; Song, S.; Ren, H.; Li, Y.; Lan, Y. Hepatitis B Virus Replication in CD34+ Hematopoietic Stem Cells From Umbilical Cord Blood. Med. Sci. Monit. 2016, 22, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Coffin, C.S. Hepatitis B virus lymphotropism: Emerging details and challenges. Biotechnol. Genet. Eng. Rev. 2018, 34, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Sayed, I.M.; Abd Elhameed, Z.A.; Abd El-Kareem, D.M.; Abdel-Malek, M.A.Y.; Ali, M.E.; Ibrahim, M.A.; Sayed, A.A.; Khalaf, K.A.B.; Abdel-Wahid, L.; El-Mokhtar, M.A. Hepatitis E Virus Persistence and/or Replication in the Peripheral Blood Mononuclear Cells of Acute HEV-Infected Patients. Front. Microbiol. 2021, 12, 696680. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Duan, Z.-P.; Chen, Y.; van der Meer, F.; Lee, S.S.; Osiowy, C.; van Marle, G.; Coffin, C.S. Compartmental HBV evolution and replication in liver and extrahepatic sites after nucleos/tide analogue therapy in chronic hepatitis B carriers. J. Clin. Virol. 2017, 94, 8–14. [Google Scholar] [CrossRef]
- Jung, S.; Seo, D.J.; Yeo, D.; Wang, Z.; Min, A.; Zhao, Z.; Song, M.; Choi, I.-S.; Myoung, J.; Choi, C. Experimental infection of hepatitis E virus induces pancreatic necroptosis in miniature pigs. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Cacoub, P.; Comarmond, C.; Vieira, M.; Régnier, P.; Saadoun, D. HCV-related lymphoproliferative disorders in the direct-acting antiviral era: From mixed cryoglobulinaemia to B-cell lymphoma. J. Hepatol. 2021, 76, 174–185. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes and tumor-mediated immune suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef]
- Whiteside, T.L. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Futur. Oncol. 2017, 13, 2583–2592. [Google Scholar] [CrossRef]
- Fu, X.-H.; Li, J.-P.; Li, X.-Y.; Tan, Y.; Zhao, M.; Zhang, S.-F.; Wu, X.-D.; Xu, J.-G. M2-Macrophage-Derived Exosomes Promote Meningioma Progression through TGF-β Signaling Pathway. J. Immunol. Res. 2022, 2022, 8326591. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, J.; Tian, J.; Wang, S. Role of T cell-derived exosomes in immunoregulation. Immunol. Res. 2018, 66, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Zhao, Y.; Guo, Q.; Mi, X.; Cheng, M.; Hou, W.; Zheng, H.; Hua, B. Exosomes in the lung cancer microenvironment: Biological functions and potential use as clinical biomarkers. Cancer Cell Int. 2021, 21, 333. [Google Scholar] [CrossRef]
- Waller, L.P.; Deshpande, V.; Pyrsopoulos, N. Hepatocellular carcinoma: A comprehensive review. World J. Hepatol. 2015, 7, 2648–2663. [Google Scholar] [CrossRef] [PubMed]
- Schietroma, I.; Scheri, G.C.; Pinacchio, C.; Statzu, M.; Petruzziello, A.; Vullo, V. Hepatitis C Virus and Hepatocellular Carcinoma: Pathogenetic Mechanisms and Impact of Direct-Acting Antivirals. Open Virol. J. 2018, 12, 16–25. [Google Scholar] [CrossRef]
- Leslie, J.; Geh, D.; Elsharkawy, A.M.; Mann, D.A.; Vacca, M. Metabolic dysfunction and cancer in HCV: Shared pathways and mutual interactions. J. Hepatol. 2022, 77, 219–236. [Google Scholar] [CrossRef]
- Fu, N.; Yao, H.; Nan, Y.; Qiao, L. Role of Oxidative Stress in Hepatitis C Virus Induced Hepatocellular Carcinoma. Curr. Cancer Drug Targets 2017, 17, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zheng, B.; Goswami, S.; Meng, L.; Zhang, D.; Cao, C.; Li, T.; Zhu, F.; Ma, L.; Zhang, Z.; et al. PD1Hi CD8+ T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 331. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, C.H.; Lee, S.-W. Exosomal Transmission of MicroRNA from HCV Replicating Cells Stimulates Transdifferentiation in Hepatic Stellate Cells. Mol. Ther.-Nucleic Acids 2019, 14, 483–497. [Google Scholar] [CrossRef]
- Sadri Nahand, J.; Bokharaei-Salim, F.; Salmaninejad, A.; Nesaei, A.; Mohajeri, F.; Moshtzan, A.; Tabibzadeh, A.; Karimzadeh, M.; Moghoofei, M.; Marjani, A.; et al. microRNAs: Key players in virus-associated hepatocellular carcinoma. J. Cell. Physiol. 2019, 234, 12188–12225. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.M. Primary malignant tumours in the non-cirrhotic liver. Eur. J. Radiol. 2017, 95, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yang, W.; Song, J.; Wu, Y.; Ni, B. Hepatitis B Virus X Protein-Induced Aberrant Epigenetic Modifications Contributing to Human Hepatocellular Carcinoma Pathogenesis. Mol. Cell. Biol. 2013, 33, 2810–2816. [Google Scholar] [CrossRef]
- Geng, M.; Xin, X.; Bi, L.-Q.; Zhou, L.-T.; Liu, X.-H. Molecular mechanism of hepatitis B virus X protein function in hepatocarcinogenesis. World J. Gastroenterol. 2015, 21, 10732–10738. [Google Scholar] [CrossRef] [PubMed]
- Lefeuvre, C.; Le Guillou-Guillemette, H.; Ducancelle, A. A Pleiotropic Role of the Hepatitis B Virus Core Protein in Hepatocarcinogenesis. Int. J. Mol. Sci. 2021, 22, 13651. [Google Scholar] [CrossRef]
- Stella, L.; Santopaolo, F.; Gasbarrini, A.; Pompili, M.; Ponziani, F.R. Viral hepatitis and hepatocellular carcinoma: From molecular pathways to the role of clinical surveillance and antiviral treatment. World J. Gastroenterol. 2022, 28, 2251–2281. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Han, Q.; Zhao, H.; Zhang, J. The Mechanisms of HBV-Induced Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, ume 8, 435–450. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Z. HBV-Induced Immune Imbalance in the Development of HCC. Front. Immunol. 2019, 10, 2048. [Google Scholar] [CrossRef]
- Hu, Z.; Yin, Y.; Jiang, J.; Yan, C.; Wang, Y.; Wang, D.; Li, L. Exosomal miR-142-3p secreted by hepatitis B virus (HBV)-hepatocellular carcinoma (HCC) cells promotes ferroptosis of M1-type macrophages through SLC3A2 and the mechanism of HCC progression. J. Gastrointest. Oncol. 2022, 13, 754–767. [Google Scholar] [CrossRef]
- Tao, L.; Li, D.; Mu, S.; Tian, G.; Yan, G. LncRNA MAPKAPK5_AS1 facilitates cell proliferation in hepatitis B virus -related hepatocellular carcinoma. Lab. Investig. 2022, 102, 494–504. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamichali, E.; Foka, P.; Papadopoulou, G.; Loukaki-Gkountara, D.; Andresaki, K.; Koskinas, I.; Georgopoulou, U. Hepatitis Viruses Control Host Immune Responses by Modifying the Exosomal Biogenesis Pathway and Cargo. Int. J. Mol. Sci. 2022, 23, 10862. https://doi.org/10.3390/ijms231810862
Karamichali E, Foka P, Papadopoulou G, Loukaki-Gkountara D, Andresaki K, Koskinas I, Georgopoulou U. Hepatitis Viruses Control Host Immune Responses by Modifying the Exosomal Biogenesis Pathway and Cargo. International Journal of Molecular Sciences. 2022; 23(18):10862. https://doi.org/10.3390/ijms231810862
Chicago/Turabian StyleKaramichali, Eirini, Pelagia Foka, Georgia Papadopoulou, Domniki Loukaki-Gkountara, Konstantina Andresaki, Ioannis Koskinas, and Urania Georgopoulou. 2022. "Hepatitis Viruses Control Host Immune Responses by Modifying the Exosomal Biogenesis Pathway and Cargo" International Journal of Molecular Sciences 23, no. 18: 10862. https://doi.org/10.3390/ijms231810862
APA StyleKaramichali, E., Foka, P., Papadopoulou, G., Loukaki-Gkountara, D., Andresaki, K., Koskinas, I., & Georgopoulou, U. (2022). Hepatitis Viruses Control Host Immune Responses by Modifying the Exosomal Biogenesis Pathway and Cargo. International Journal of Molecular Sciences, 23(18), 10862. https://doi.org/10.3390/ijms231810862







