Aegle marmelos (L.) Correa: An Underutilized Fruit with High Nutraceutical Values: A Review
Abstract
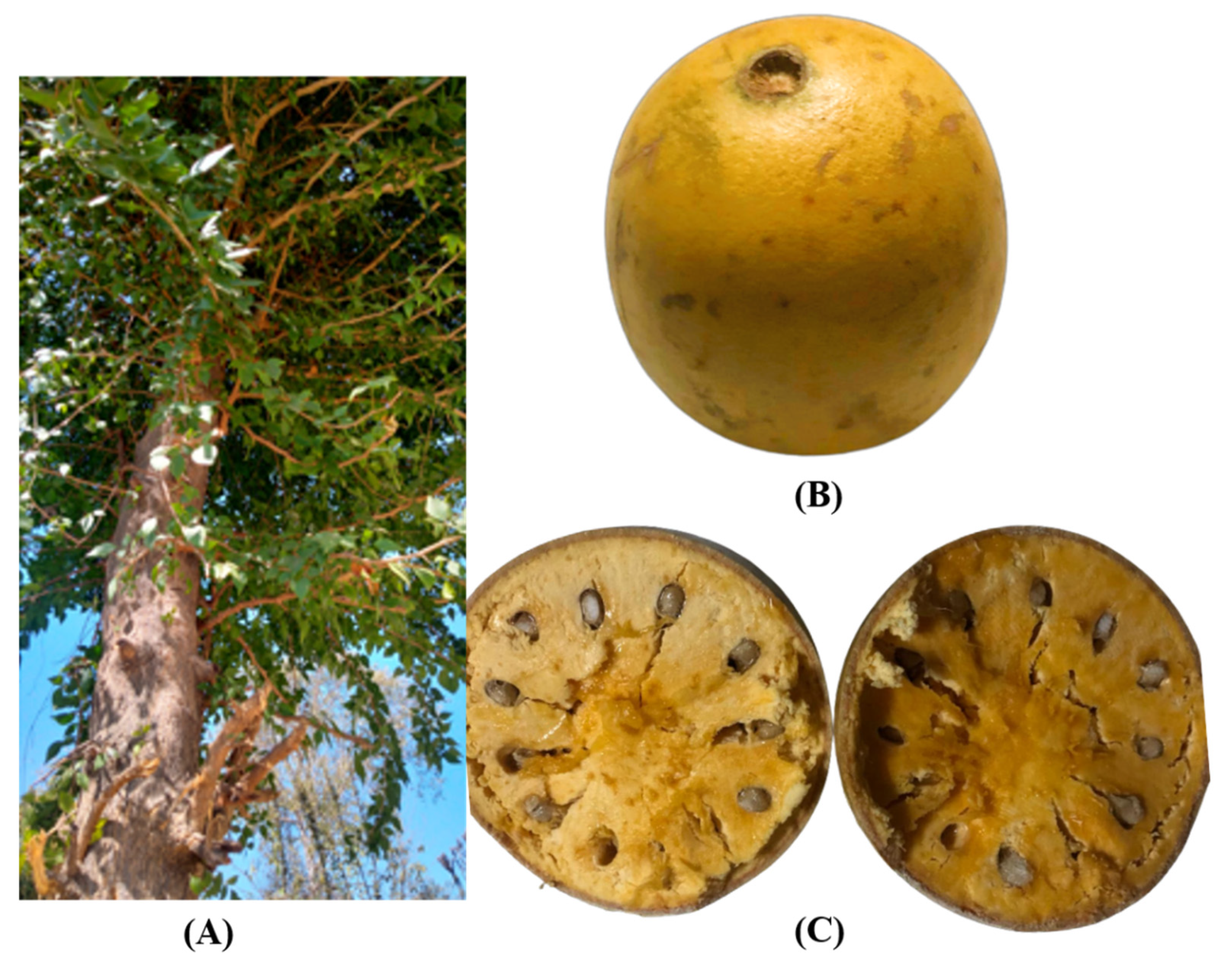
:1. Introduction
2. Nutritional Composition
2.1. Polysaccharides
2.2. Proteins
2.3. Essential Oils/Lipid Profile
2.4. Minerals and Vitamins
3. Phytochemicals
3.1. Polyphenol/Phenolic Compounds
3.2. Coumarins
3.3. Carotenes
3.4. Other Phytochemicals
4. Pharmacological Activities of Aegle marmelos
4.1. Antidiarrheal Activity
4.2. Antioxidant Activity
4.3. Antidiabetic Activity
4.4. Hepatoprotective Activity
4.5. Radio Protective Activity
4.6. Anticancer Activity
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.; Sharma, H.K.; Kaushal, P.; Upadhyay, A. Bael (Aegle marmelos Correa) products processing: A review. Afr. J. Food Sci. 2014, 8, 204–215. [Google Scholar]
- Jhajhria, A.; Kumar, K. Tremendous pharmacological values of Aegle marmelos. Int. J. Pharm. Sci. Rev. Res. 2016, 36, 121–127. [Google Scholar]
- Neeraj, V.B.; Johar, V. Bael (Aegle marmelos) extraordinary species of India: A review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1870–1887. [Google Scholar]
- Shaikh, R.U.; Ahmed, A.A. Medicinal Flora of Poona College; Empyreal Publishing House India: Guwahati, India, 2021; pp. 1–88. [Google Scholar]
- Lakht-e-Zehra, A.; Dar, N.G.; Saleem, N.; Soomro, U.A.; Afzal, W.; Naqvi, B.; Jamil, K. Nutritional exploration of leaves, seed and fruit of bael (Aegle marmelos L.) grown in Karachi region. Pak. J. Biochem. Mol. Biol. 2015, 48, 61–65. [Google Scholar]
- Kaur, A.; Kalia, M. Physico chemical analysis of bael (Aegle Marmelos) fruit pulp, seed and pericarp. Chem. Sci. Rev. Lett. 2017, 6, 1213–1218. [Google Scholar]
- Sarkar, T.; Salauddin, M.; Chakraborty, R. In-depth pharmacological and nutritional properties of bael (Aegle marmelos): A critical review. J. Agric. Food Res. 2020, 2, 100081. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.; Saroj, P.L.; Singh, G.P. Improvement and production technology of bael (Aegle marmelos) in India—A review. Curr. Hortic. 2021, 9, 3–14. [Google Scholar] [CrossRef]
- Maity, P.; Hansda, D.; Bandyopadhyay, U.; Mishra, D.K. Biological activities of crude extracts and chemical constituents of bael, Aegle marmelos (L.) Correa. Ind. J. Exp. Bio. 2009, 47, 849–861. [Google Scholar]
- Asghar, N.; Mushtaq, Z.; Arshad, M.U.; Imran, M.; Ahmad, R.S.; Hussain, S.M. Phytochemical composition, antilipidemic and antihypercholestrolemic perspectives of Bael leaf extracts. Lipids Health Dis. 2018, 17, 68. [Google Scholar] [CrossRef]
- Tagad, V.B.; Sahoo, A.K.; Annapure, U.S. Phytochemical study and GC-MS analysis of bael (Aegle marmelos) fruit pulp. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2018, 4, 779–791. [Google Scholar]
- Hazra, S.K.; Sarkar, T.; Salauddin, M.; Sheikh, H.I.; Pati, S.; Chakraborty, R. Characterization of phytochemicals, minerals and in vitro medicinal activities of bael (Aegle marmelos L.) pulp and differently dried edible leathers. Heliyon 2020, 6, e05382. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Kumar, M. Litchi (Litchi chinenis) seed: Nutritional profile, bioactivities, and its industrial applications. Trends Food Sci. Technol. 2021, 108, 58–70. [Google Scholar] [CrossRef]
- Dutta, A.; Lal, N.; Naaz, M.; Ghosh, A.; Verma, R. Ethnological and Ethno-medicinal importance of Aegle marmelos (L.) Corr (Bael) among indigenous people of India. Am. J. Ethnomed. 2014, 1, 290–312. [Google Scholar]
- Roy, S.K.; Saran, S.; Kitinoja, L. Bael (Aegle marmelos (L.) Corr. Serr.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing: Sawston, UK, 2011; pp. 186–216e. [Google Scholar]
- Baliga, M.S.; Bhat, H.P.; Joseph, N.; Fazal, F. Phytochemistry and medicinal uses of the bael fruit (Aegle marmelos Correa): A concise review. Food Res. Int. 2011, 44, 1768–1775. [Google Scholar] [CrossRef]
- Bel-Rhlid, R.; Berger, R.G.; Blank, I. Bio-mediated generation of food flavors–Towards sustainable flavor production inspired by nature. Trends Food Sci. Technol. 2018, 78, 134–143. [Google Scholar] [CrossRef]
- Sonawane, A.; Pathak, S.; Pradhan, R.C. Bioactive compounds in bael fruit pulp waste: Ultrasound-assisted extraction, characterization, modeling, and optimization approaches. Biointerface Res. Appl. Chem. 2020, 11, 9318–9334. [Google Scholar]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Shewry, P.R. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef]
- Lee, C.H.; Chen, K.T.; Lin, J.A.; Chen, Y.T.; Chen, Y.A.; Wu, J.T.; Hsieh, C.W. Recent advances in processing technology to reduce 5-hydroxymethylfurfural in foods. Trends Food Sci. Technol. 2019, 93, 271–280. [Google Scholar] [CrossRef]
- Sarkar, A.; Rashid, M.; Musarrat, M.; Billah, M. Phytochemicals and Nutritional Constituent Evaluation of Bael (Aegle marmelos) Fruit Pulp at Different Development Stage. Asian Food Sci. J. 2021, 20, 78–86. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, P.; Mehrotra, R. Determination of some ethnomedicinally important constituents of Aegle marmelos fruit during different stages of ripening. Chin. J. Nat. Med. 2011, 9, 204–209. [Google Scholar]
- Singh, U.; Kocher, A.; Boora, R. Proximate composition, available carbohydrates, dietary fibres and anti-Nutritional factors in Bael (Aegle marmelos L.) leaf, pulp and seed powder. Int. J. Sci. Res. Publ. 2012, 2, 1–4. [Google Scholar]
- Rathore, M. Nutrient content of important fruit trees from arid zone of Rajasthan. J. Hortic. For. 2009, 1, 103–108. [Google Scholar]
- Sharma, K.; Chauhan, E.S. Nutritional and phytochemical evaluation of fruit pulp powder of Aegle marmelos (Bael). J. Chem. Pharm. Res. 2016, 10, 809–814. [Google Scholar]
- Kumar, K.S.; Umadevi, M.; Bhowmik, D.; Singh, D.M.; Dutta, A.S. Recent trends in medicinal uses and health benefits of Indian traditional herbs Aegle marmelos. Pharma Innov. 2012, 1, 57–65. [Google Scholar]
- Apou, A.C.; Farzana, T.; Suzauddula, M.; Hossain, M. The Physicochemical Characteristics and Quality Evaluation of Bael Fruit (Limoniaacidissima L.) Pulp Powder for the Product of Functional Food. Daffodil Int. Univ. J. Allied Health Sci. 2019, 6, 82–88. [Google Scholar]
- Gopalan, C.; Rama Sastri, B.V.; Balasubramanian, S.C. Nutritive Value of Indian Foods; Indian Council of Medical Research: New Delhi, India, 1971.
- Ullikashi, K.Y.; Kammar, M.R.; Lokapure, S.R. Development of value added products from bael fruit (Aegle marmelos). Int. J. Curr. Micro Appli. Sci. 2017, 6, 2652–2659. [Google Scholar] [CrossRef]
- Murthy, H.N.; Bhat, M.A.; Dalawai, D. Bioactive compounds of bael (Aegle marmelos (L.) correa). In Bioactive Compounds in Underutilized Fruits and Nuts; Springer: Cham, Switzerland, 2020; pp. 459–486. [Google Scholar]
- Venthodika, A.; Chhikara, N.; Mann, S.; Garg, M.K.; Sofi, S.A.; Panghal, A. Bioactive compounds of Aegle marmelos L., medicinal values and its food applications: A critical review. Phyther. Res. 2021, 35, 1887–1907. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef]
- Rejman, K.; Górska-Warsewicz, H.; Kaczorowska, J.; Laskowski, W. Nutritional Significance of Fruit and Fruit Products in the Average Polish Diet. Nutrients 2021, 13, 2079. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Yahia, E.M.; González-León, A.; Ifie, I.; Robles-Zepeda, R.E.; Domínguez-Avila, J.A.; González-Aguilar, G.A. Ripening of ‘Hass’ avocado mesocarp alters its phytochemical profile and the in vitro cytotoxic activity of its methanolic extracts. S. Afr. J. Bot. 2020, 128, 1–8. [Google Scholar] [CrossRef]
- Rios, J.L. Essential Oils: What They Are and How the Terms Are Used and Defined. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, UK, 2016; pp. 3–10. [Google Scholar]
- Alabi, K.P.; Zhu, Z.; Sun, D.W. Transport phenomena and their effect on microstructure of frozen fruits and vegetables. Trends Food Sci. Technol. 2020, 101, 63–72. [Google Scholar] [CrossRef]
- Bazaid, S.A.; El-Amoudi, M.S.; Ali, E.F.; Abdel-Hameed, E.S. Volatile oil studies of some aromatic plants in Taif region. J. Med. Plants Stud. 2013, 1. [Google Scholar]
- Mahato, H.; Kumar, B. Medicinal Uses with Immense Economic Potential and Nutritional Properties of Aegle marmelos: A Concise Review. In Biocomposites; Books on Demand: Norderstedt, Germany, 2022; p. 133. [Google Scholar]
- Gupta, D.; John, P.P.; Kumar, P.; Jain, J. Evaluation of antioxidant activity of unripe Aegle marmelos Corr. Fruits. J. Appl. Pharm. Sci. Res. 2018, 1, 4–7. [Google Scholar]
- Bhardwaj, R.L.; Nandal, U. Nutritional and therapeutic potential of bael (Aegle marmelos Corr.) fruit juice: A review. Nutr. Food Sci. 2015, 45, 895–919. [Google Scholar] [CrossRef]
- Rahman, S.; Parvin, R. Therapeutic potential of Aegle marmelos (L.)—An overview. Asian Pac. J. Trop. Dis. 2014, 4, 71–77. [Google Scholar] [CrossRef]
- Manandhar, B.; Paudel, K.R.; Sharma, B.; Karki, R. Phytochemical profile and pharmacological activity of Aegle marmelos Linn. J. Integr. Med. 2018, 16, 153–163. [Google Scholar] [CrossRef]
- Wali, A.; Gupta, M.; Mallick, S.A.; Gupta, S.; Jaglan, S. Antioxidant Potential and Phenolic Contents of Leaf, Bark And Fruit Of" Aegle marmelos". J. Trop. For. Sci. 2016, 28, 268–274. [Google Scholar]
- Charoensiddhi, S.; Anprung, P. Bioactive compounds and volatile compounds of Thai bael fruit (Aegle marmelos (L.) Correa) as a valuable source for functional food ingredients. Int. Food Res. J. 2008, 15, 287–295. [Google Scholar]
- Gurjar, P.S.; Bhattacherjee, A.K.; Singh, A.; Dikshit, A.; Singh, V.K. Characterization of nutraceuticals in bael powder prepared from fruits harvested at different developmental stages. Indian J. Tradit. Knowl. 2019, 18, 724–730. [Google Scholar]
- Prakash, D.; Upadhyay, G.; Pushpangadan, P.; Gupta, C. Antioxidant and free radical scavenging activities of some fruits. J. Complement. Integr. Med. 2011, 8, 1–16. [Google Scholar] [CrossRef]
- Bhar, K.; Mondal, S.; Suresh, P. An eye-catching review of Aegle marmelos L. (Golden Apple). Pharmacogn. J. 2019, 11, 207–224. [Google Scholar] [CrossRef]
- Poonkodi, K.; Vimaladevi, K.; Suganthi, M.; Gayathri, N. Essential oil composition and biological activities of Aegle marmelos (L.) correa grown in Western Ghats Region-South India. J. Essent. Oil Bear. Plants 2019, 22, 1013–1021. [Google Scholar] [CrossRef]
- Pathirana, C.K.; Madhujith, T.; Eeswara, J. Bael (Aegle Marmelos L. Corrêa), a Medicinal Tree with Immense Economic Potentials. Adv. Agric. 2020, 2020, 8814018. [Google Scholar] [CrossRef]
- Rajan, S.; Gokila, M.; Jency, P.; Brindha, P.; Sujatha, R.K. Antioxidant and phytochemical properties of Aegle marmelos fruit pulp. Int. J. Curr. Pharm. Res. 2011, 3, 65–70. [Google Scholar]
- Panda, S.K.; Sahu, U.C.; Behera, S.K.; Ray, R.C. Bio-processing of bael [Aegle marmelos L.] fruits into wine with antioxidants. Food Biosci. 2014, 5, 34–41. [Google Scholar] [CrossRef]
- Farooq, S. 555 medicinal plants. In Field and Laboratory Manual (Identification with Its Phytochemical and In Vitro Studies Data); International Book Distributors: Dehradun, India, 2005. [Google Scholar]
- Kokate, C.K.; Purohit, A.P.; Gokhale, S.B. Text Book of Pharmacognosy, 18th ed.; Nirali Prakashan: Pune, India, 2002. [Google Scholar]
- Sharma, P.C.; Bhatia, V.; Bansal, N.; Sharma, A. A review on Bael Tree. Nat. Prod. Radiance 2007, 6, 171–178. [Google Scholar]
- Bramhachari, P.V.; Reddy, Y.; Kotresha, D.; Varaprasad, B. Phytochemical examination, antioxidant and radical scavenging activity of Aegle marmelos (L.) Correa extracts. J. Pharm. Res. 2010, 3, 3023–3025. [Google Scholar]
- Rishabha, M.; Ajay, K.; Anupama, S.; GT, K. Pharmacological screening, Ayurvedic values and commercial utility of Aegle marmelos. Int. J. Drug Dev. Res. 2012, 4, 28–37. [Google Scholar]
- Patel, P.K.; Sahu, J.; Sahu, L.; Prajapati, N.K.; Dubey, B.K. Aegle marmelos: A review on its medicinal properties. Int. J. Pharm. Phytopharm. Res. 2012, 1, 332–341. [Google Scholar]
- Joshi, Y.; Chaudhary, R.K.; Teotia, U.V.S. Formulation and evaluation of diclofenac sodium sustained release matrix tablets using Aegle marmelos gum. Int. J. Curr. Trends Pharm. Res. 2013, 1, 174–180. [Google Scholar]
- Mehesare, S.S.; Waghmare, S.P.; Thorat, M.G.; Hajare, S.W.; Itankar, P.R.; Ali, S.S. Evaluation of the antidiarrhoeal activity of extract of unripe fruit of Aegle marmelos. J. Pharmacogn. Phytochem. 2019, 8, 2390–2392. [Google Scholar]
- Ghorai, S.; Sarma, K.; Choudhury, P.R.; Das, G.; Singh, D.; Kalita, G.; Choudhury, J.; Arya, R.S. Anti-Diarrhoeal Activity and Toxicity Trial of Methanolic Fruit-Pulp Extract of Aegle Marmelos (L.) Correa in Sprague-Dawle Rats. Int. J. Livest. Res. 2018, 8, 326–337. [Google Scholar] [CrossRef]
- Taqvi, S.I.H.; Rahman, A.; Versiani, M.A.; Imran, H.; Khatoon, A.; Sohail, T. Studies to determine antidiarrhoeal and spasmolytic activities of extract of Aegle marmelos. L fruit. Bangladesh. J. Med. Sci. 2018, 17, 205–211. [Google Scholar] [CrossRef]
- Brijesh, S.; Daswani, P.; Tetali, P.; Antia, N.; Birdi, T. Studies on the antidiarrhoeal activity of Aegle marmelos unripe fruit: Validating its traditional usage. BMC Complement. Altern. Med. 2009, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, R.; Ijaz, M.U.; Rafique, A.; Ashraf, A.; Bano, N.; Zafar, N.; Ahmedah, H.T. Biological Activities of Methanolic Extract of Aegle marmelos against HN Protein of Newcastle Disease Virus. Agronomy 2021, 11, 1784. [Google Scholar] [CrossRef]
- Wijewardana, R.M.N.A.; Nawarathne, S.B.; Wickramasinghe, I.; Gunawardane, C.R.; Wasala, W.M.C.B.; Thilakarathne, B.M.K.S. Retention of physicochemical and antioxidant properties of dehydrated bael (Aegle marmelos) and palmyra (Borassus flabellifer) fruit powders. Procedia Food Sci. 2016, 6, 170–175. [Google Scholar] [CrossRef]
- Abdallah, I.Z.; Salem, I.; El-Salam, A.; Nayrouz, A.S. Evaluation of antidiabetic and antioxidant activity of Aegle marmelos L. Correa fruit extract in diabetic rats. Egypt. J. Hosp. Med. 2017, 67, 731–741. [Google Scholar] [CrossRef]
- Kamalakkanan, N.; Rajadurai, M.; Prince, P.; Stanely, M. Effect of Aegle marmelos Fruits on Normal and Streptozotocin-Diabetic Wistar Rats. J. Med. Food 2003, 6, 93–98. [Google Scholar] [CrossRef]
- Rajasekaran, C.; Kalaivani, T.; Ramya, S.; Jayakumararaj, R. Studies on hepatoprotective activity of ethanolic extracts of fruit pulp of Aegle marmelos (L.) Corr. J. Pharm. Res. 2009, 2, 1419–1423. [Google Scholar]
- Chandel, S.S.; Shirsat, M.; Sahu, R.K.; Nayak, S.S. Modulatory effect of dietary inclusion of Aegle marmelos fruits against cisplatin-induced hepatotoxicity in Wistar rats. Ann. Hepatol. 2018, 17, 482–489. [Google Scholar] [CrossRef]
- Sastry, A.V.S.; Girija, S.V.; Naga, S.G.; Srinivas, K. Phytochemical investigations and hepatoprotective effects of Aqueous fruit extract of Aegle marmelos Corr. Int. J. Chem. Sci. 2011, 9, 900–910. [Google Scholar]
- Baliga, M.S.; Bhat, H.P.; Pereira, M.M.; Mathias, N.; Venkatesh, P. Radioprotective effects of Aegle marmelos (L.) Correa (Bael): A concise review. J. Altern. Complement. Med. 2010, 16, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Venkatesh, P.; Baliga, M.S. Fruit extract of Aegle marmelos protects mice against radiation-induced lethality. Integr. Cancer Ther. 2004, 3, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Akhouri, V.; Kumari, M.; Kumar, A. Therapeutic effect of Aegle marmelos fruit extract against DMBA induced breast cancer in rats. Sci. Rep. 2020, 10, 18016. [Google Scholar] [CrossRef]
- Vardhini, S.P.; Sivaraj, C.; Arumugam, P.; Ranjan, H.; Kumaran, T.; Baskar, M. Antioxidant, anticancer, antibacterial activities and GC-MS analysis of aqueous extract of pulps of Aegle marmelos (L.) Correa. J. Phytopharmacol. 2018, 7, 72–78. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Kosem, N.; Luanratana, O.; Jongsomboonkusol, S.; Pongpan, N. Antiproliferative activity of Thai medicinal plant extracts on human breast adenocarcinoma cell line. Fitoterapia 2004, 75, 375–377. [Google Scholar] [CrossRef]
- Agrawal, A.; Verma, P.; Goyal, P.K. Chemomodulatory effects of Aegle marmelos against DMBA-induced skin tumorigenesis in Swiss albino mice. Asian Pac. J. Cancer Prev. 2010, 11, 1311–1314. [Google Scholar]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Guyton, A.; Hall, J.E. Dietary Balances; Regulation of Feeding; Obesity and Starvation; Vitamins and Minerals. In Guyton and Hall Textbook of Medical Physiology, 12th ed.; Hall, J.E., Ed.; Elsevier: Philadelphia, PA, USA, 2011; pp. 845–857. [Google Scholar]
- Mohi-Ud-Din, R.; Mir, R.H.; Sawhney, G.; Dar, M.A.; Bhat, Z.A. Possible pathways of hepatotoxicity caused by chemical agents. Curr. Drug Metab. 2019, 20, 867–879. [Google Scholar] [CrossRef]
- Jetter, A.; Kullak-Ublick, G.A. Drugs and hepatic transporters: A review. Pharmacol. Res. 2020, 154, 104234. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.N.; Dubey, S.K.; Sharma, P.; Sati, N. Medicinal values of bael (Aegle marmelos) (L.) Corr.: A review. Int. J. Curr. Pharm. Rev. Res. 2011, 2, 12–22. [Google Scholar]
- Benni, J.M.; Jayanthi, M.K.; Suresha, R.N. Evaluation of the anti-inflammatory activity of Aegle marmelos (Bilwa) root. Indian J. Pharmacol. 2011, 43, 393. [Google Scholar] [CrossRef] [PubMed]
| Source/Region | Type | Compound | Yield/Concentration | Reference |
|---|---|---|---|---|
| Bael fruit pulp (Bangladesh) | Polysaccharide (%) | Carbohydrate | 36.80–41.70 | [21] |
| Total sugar | 3.08–6.94 | |||
| Bael fruit (North India) | Polysaccharide (%) | Fructose | 1.01–1.55 | [22] |
| Glucose | 1.15–1.88 | |||
| Sucrose | 2.45–12.01 | |||
| Bael fruit pericarp (Ludhiana, Punjab) | Polysaccharide (%) | Total sugars | 1.83 | [6] |
| Non-reducing sugars | 0.91 | |||
| Reducing sugars | 0.92 | |||
| Bael fruit pulp (Ludhiana, Punjab) | Polysaccharide (%) | Total sugars | 14.35 | |
| Non-reducing sugars | 9.93 | |||
| Reducing sugars | 4.42 | |||
| Bael fruit pulp (Karachi, Pakistan) | Polysaccharide (%) | Carbohydrate | 34.35 | [5] |
| Bael fruit pulp (Patiala, Punjab) | Polysaccharide (g/100 g) | Total soluble sugars | 7.6 | [23] |
| Reducing sugars | 6.2 | |||
| Non-reducing sugars | 1.4 | |||
| Starch | 3.6 | |||
| Bael fruit (Rajasthan, India) | Polysaccharide (%) | Carbohydrate | 31.8 | [24] |
| Bael fruit pulp (Bangladesh) | Protein (%) | - | 7.52–8.81 | [21] |
| Bael fruit pericarp (Ludhiana, Punjab) | Protein (%) | - | 1.31 | [6] |
| Bael fruit pulp (Ludhiana, Punjab) | Protein (%) | - | 3.64 | |
| Bael fruit pulp (Rajasthan, India) | Protein (%) | - | 4.35 | [25] |
| Bael fruit pulp (Karachi, Pakistan) | Protein (%) | - | 1.87 | [5] |
| Bael fruit Faizabad (U.P.) Nadia, West Bengal | Protein (%) | - | 3.6 | [1] |
| Protein (%) | - | 8.8 | ||
| Bael fruit pulp (Patiala, Punjab) | Protein (g/100 g) | - | 4.7 | [23] |
| Bael fruit | Protein (%) | - | 1.8 | [26] |
| Bael fruit (Rajasthan, India) | Protein (%) | - | 1.8 | [24] |
| Bael fruit pulp (Karachi) | Fat (%) | - | 45.6 | [5] |
| Bael edible portion | Fat (%) | - | 0.6 | [27] |
| Bael fruit pulp | Fat (%) | - | 3.7 | [28] |
| Bael (unripe fruit) | Minerals (mg) | P | 52 | [1,7] |
| K | 610 | |||
| Ca | 80 | |||
| Bael fruit pulp | Minerals (mg/100 g) | Cu | 0.21 | [1] |
| K | 610 | |||
| Ca | 80 | |||
| P | 52 | |||
| Fe | 0.60 | |||
| Bael fruit (Bellary district) | Minerals (mg) | Ca | 85 | [29] |
| P | 50 | |||
| K | 600 | |||
| Vitamins (mg) | Vitamin A | 55 | ||
| Vitamin C | 8–60 | |||
| Riboflavin | 1.19 | |||
| Niacin | 1.1 | |||
| Bael fruit | Vitamins (mg) | Vitamin A | 55 | [30] |
| Vitamin C | 8 | |||
| Bael fruit | Vitamins (mg/100 g) | Vitamin A | 55 | [30,31] |
| Vitamin B1 | 0.13 | |||
| Vitamin B2 | 1200 | |||
| Vitamin C | 8 | |||
| Riboflavin | 1190–1200 | |||
| Thiamine | 0.13 | |||
| Ascorbic acid | 8 | |||
| Bael fruit pulp (Karachi) | Vitamins (mg%) | Vitamin B1 | 0.16 | [5] |
| Vitamin B2 | 0.18 | |||
| Vitamin B3 | 0.87 | |||
| Vitamin C | 73.2 |
| Variety/Region | Type | Compound | Concentration/Yield | Reference |
|---|---|---|---|---|
| A. marmelos | TPC (mg GAE/g) | - | 10.6–87.34 | [43,44] |
| A. marmelos (Lucknow) | Polyphenol | Tannic acid (g 100/g) | 2.81–4.84 | [45] |
| Marmelosin (μg/g) | 415.75–737.00 | |||
| A. marmelos | Polyphenol (µg/g) | Chlorogenic acid | 136.8 | [46] |
| Ferulic acid | 98.3 | |||
| Ellagic acid | 248.5 | |||
| Gallic acid | 873.6 | |||
| Quercetin | 56.9 | |||
| Protocatechuic acid | 47.9 | |||
| A. marmelos (Kolkata, West Bengal, India) | TPC (GAE mg/g) | - | 16.23–25.14 | [12] |
| TFC (g CE/100 g) | - | 9.74–18.17 | ||
| Phenolic acid (mg/100 g) | Gallic acid | 580.27–617.17 | ||
| 2,3-dihydroxy benzoic acid | 10.35–35.94 | |||
| Chlorogenic acid | 0.38–56.31 | |||
| p-Coumaric acid | 233.54–361.42 | |||
| Vanillic acid | 52.80–102.40 | |||
| Flavonoid (mg/100 g) | Rutin | 32.25–59.90 | ||
| Carotenes (μg/100 g) | α-carotene (alpha C) | 42.76–1698.22 | ||
| β-carotene (beta C) | 51.67–153.43 | |||
| γ-carotene (gamma C) | 18.43–467.17 | |||
| δ-carotene (delta C) | 43.74–45.03 | |||
| A. marmelos (Phichit, Thailand) | Total flavonoids (mg CE/g dw) | - | 15.20 ± 0.51 | [44] |
| Total carotenoids (μg/g dw) | - | 32.98 ± 0.51 | [44] | |
| A. marmelos | Alkaloids | Aegelenine, Halfordinol, Aegeline, Ethyl cinnamate, Aegelinosides A, Ethyl cinnamamide, Aegelinosides B, Dictamine, Fragrine | - | [7,47,48,49] |
| Terpenoids | Caryophyllene, Valencene, Cineol, Terpinolene, cis-Limonene oxide, P-cymene, cis-Linalool oxide, Methyl perilate, Cubedol, Isosylvestrene, Elemol, Myrcene, Epi-cubebal, Humulene, Hexanylhexanoate, Linalool, Limonene | - | [49] | |
| Coumarins | Alloimperatorin, Zanthotoxol, Imperatorin, Xanthotoxol, Isoimperatorin, Umbelliferone, Marmelide, Scopoletin, Marmelosin, Scopolentin, Marmesin, Psoralen-a, Scoparone, Marmin, Methyl ether, Psoralen | - | ||
| Tannins | Skimminianine, 4,7,8-trimethoxyfuro-quinoline, | - | [6] | |
| Amino acids | Methionine, Phenyl alanine, Leucine, Tyrosine, Isoleucine, Alanine, Aspartic acid, and Arginine | - | [50] |
| Variety/Region | Type of Study/Activity | Type of Extract | Organism or Cell Line/Assay | Dosage | Key Findings | Reference |
|---|---|---|---|---|---|---|
| A. marmelos (LallubhaiVrajalal Gandhi, Ahmedabad, India) | Antidiarrheal (in vitro) | Ethanolic extract of dried fruit pulp | Shigella boydii, S. dysenteriae, S. sonnei and S. flexner | 0.5–4 mg/mL | MIC:MBC Shigella sonnei—250:500, Shigella flexner—400:500, Shigella boydii—500:500, Shigella dysenteriae—250:400. Maximum inhibition was observed for Shigella sonnei while minimum value of inhibition was observed for Shigella dysenteriae | [58] |
| A. marmelos | Antidiarrheal (in vivo) | Ethanolic extract of unripe fruit | Animal model: mice | 400–800 mg/kg | Extract of unripe fruits of Aegle marmelos show inhibition of 67.44% and 70.93% at dosages of 400 mg/kg and 800 mg/kg, respectively | [59] |
| A. marmelos | Antidiarrheal (in vivo) | Methanolic extract of unripe fruit | Animal model: SD rat | 15–1600 mg/kg orally | Significant inhibition against diarrhea induced by administration of castor oil in rats. No wet feaces were observed after 1 h. | [60] |
| A. marmelos (Tiruchirappalli, Tamilnadu, India) | Antioxidant | Aqueous and alcoholic (ethanol) extract of fruit pulp | - | 100 µg/mL | DPPH assay—44.36–40.12% (IC50 = 92.648–106.15 μg/mL); ferric-reducing assay—28.7–50.33% (IC50 = 158.99–283.06 µg/mL); NO scavenging = 52.02–63.74%; H2O2 scavenging = 69.0–73.77% (IC50 = 52.19–56.53 μg/ mL) | [50] |
| A. marmelos | Antioxidant | Methanolic extract of fruit | - | - | DPPH assay—IC50 = 52.06 µg/mL DW; FRAP assay—IC50 = 59.32 µmol/g DW | [63] |
| A. marmelos | Antioxidant | Methanolic extract of fruit | - | 200–1000 μg/mL | DPPH assay: 24.31–81.33% | [64] |
| A. marmelos (Delhi, India) | Antioxidant | Methanolic extract of unripe fruit | - | - | IC50 = 62.59 μg/mL (DPPH assay) | [39] |
| A. marmelos | Antioxidant | Chloroform and aqueous extract of dry and ripe fruit | - | 5–0.15μ/mL | Scavenging activity = 88–65% | [41] |
| A. marmelos | Antidiabetic (in vivo) | Ethanolic extract of fruit | Animal model: Alloxan (120 mg/kg) induced diabetic rats (180–195 g) | Alloxan: 120 mg/kg; Ethanolic extract: 125–500 mg/kg/day | Glucose—97.48–78.82 (mg/dL); Insulin—6.58–15.64 (μIU/mL) Reduction in glucose level while increase in insulin level in serum with increase in dosage of A. marmelos fruit extract | [65] |
| A. marmelos (Vijayawada, Andhra Pradesh, India) | Antidiabetic (in vivo) | Aqueous extract of fruit | Female albino Streptozotocin (STZ) induced diabetic Wistar rats | AMFEt: 250 mg/kg; STZ: 45 mg/kg | Decrease in blood glucose level (p < 0.05, 280.0–61.4 mgdL–1) and increase in plasma insulin level (17.9–21.6 µUmL–1) | [66] |
| A. marmelos (Vellore, Tamil Nadu, India) | Hepatoprotective (in vivo) | Ethanolic extract of fruit pulp | CCl4-induced liver damage mice | Ethanolic extract—500 mg/kg | Reduction in SGOT: 81.3 U/mL; SGPT: 64.5 U/mL and ALP: 8.1 KA units levels in CCl4-treated animals | [67] |
| A. marmelos (Bilaspur, Chhattisgarh, India) | Hepatoprotective (in vivo) | Fruit pulp | Cisplatin-induced liver damage Wistar albino rat (91–129 g) | Cisplatin (6 mg/kg); Fruit extract concentration—2–4% | AST: 69.84–110.19 U/L ALT: 89.26–133.25 U/L ALP: 129.81–161.73 U/L ACP: 131.61–182.54 U/L (p < 0.05). Increasing activity of antioxidant enzymes, and significant change in physiological parameters | [68] |
| A. marmelos (Vijayawada, Andhra Pradesh, India) | Hepatoprotective (in vivo) | Aqueous fruit extract | Paracetamol-induced liver damage in Wistar albino rats | Paracetamol: 2 g/kg; Aqueous fruit extract 100–400 mg/kg | Reduction in level (p < 0.001) of ALP (123–168 IU/L), Bilirubin (BLN; 1.5–1.22 mg/dL), SGPT/ALT (43–54.33 IU/L) and SGOT/AST (176–218.3 IU/L) | [69] |
| A. marmelos | Radioprotective (in vivo) | Hydroalcoholic fruit extract | Age-matched Swiss albino mice | Dosage: 5–80 mg/kg | LD50/30 value recorded for the group administered with bael extract before exposure to radiation was 8.8 Gy. Mice administered with dosage of 20 mg/kg bael extract showed increase in survival with 50% and 29% survival after 10 and 30 days, respectively | [70] |
| A. marmelos | Radioprotective (in vivo) | Hydroalcoholic fruit extract | Mice | Dosage: 20 mg/kg | LD50/30 was recorded to be 8.8 Gy for mice administered with bael fruit extract. For 10 Gy (p < 0.001) and 9 Gy (p < 0.05) irradiation, pretreatment with AME reduced 10-day mortality by 2- and 1.4-fold, respectively | [71] |
| A. marmelos | Anti-cancerous (in vivo) | Ethanolic fruit pulp extract | Female Charles Foster rats (~150 g) | Dosage: 200 mg/kg BW/day | Decrease in breast tumor volume (p < 0.05), as well as a significant decrease (p < 0.0001) in serum biomarkers- serum malondialdehyde (MDA), TNF-α), and glucose levels | [72] |
| A. marmelos | Anti-cancerous (in vitro) | Aqueous fruit pulp extract | MCF7 cell line | Dosage: 100 g/mL | Maximal MCF7 cell death was at a rate of 66.514.65%, with IC50 value of 47.92 µg/mL | [73] |
| A. marmelos | Anti-cancerous (in vitro) | Methanolic extract of the fruit | Human breast cancer cells (SKBR3) | - | IC50 of 144.00 ± 1.21 μg/mL when bael fruit extract was tested against SKBR3 | [74] |
| A. marmelos | Anti-cancerous (in vivo) | Methanolic extract of the bael fruit pulp | Swiss albino mice | Dosage: 50 mg/kg BW | Administration of bael fruit extract (orally) results in a 70% inhibition of tumor, with 50% reduction in tumor incidence during post-initiation phase | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Radha; Kumar, M.; Zhang, B.; Kumari, N.; Singh, D.; Chandran, D.; Sarkar, T.; Dhumal, S.; Sheri, V.; et al. Aegle marmelos (L.) Correa: An Underutilized Fruit with High Nutraceutical Values: A Review. Int. J. Mol. Sci. 2022, 23, 10889. https://doi.org/10.3390/ijms231810889
Sharma N, Radha, Kumar M, Zhang B, Kumari N, Singh D, Chandran D, Sarkar T, Dhumal S, Sheri V, et al. Aegle marmelos (L.) Correa: An Underutilized Fruit with High Nutraceutical Values: A Review. International Journal of Molecular Sciences. 2022; 23(18):10889. https://doi.org/10.3390/ijms231810889
Chicago/Turabian StyleSharma, Niharika, Radha, Manoj Kumar, Baohong Zhang, Neeraj Kumari, Daljeet Singh, Deepak Chandran, Tanmay Sarkar, Sangram Dhumal, Vijay Sheri, and et al. 2022. "Aegle marmelos (L.) Correa: An Underutilized Fruit with High Nutraceutical Values: A Review" International Journal of Molecular Sciences 23, no. 18: 10889. https://doi.org/10.3390/ijms231810889
APA StyleSharma, N., Radha, Kumar, M., Zhang, B., Kumari, N., Singh, D., Chandran, D., Sarkar, T., Dhumal, S., Sheri, V., Dey, A., Rajalingam, S., Viswanathan, S., Mohankumar, P., Vishvanathan, M., Sathyaseelan, S. K., & Lorenzo, J. M. (2022). Aegle marmelos (L.) Correa: An Underutilized Fruit with High Nutraceutical Values: A Review. International Journal of Molecular Sciences, 23(18), 10889. https://doi.org/10.3390/ijms231810889











