Skeletal Muscle Dysfunction in Experimental Pulmonary Hypertension
Abstract
:1. Introduction
2. Results
2.1. Reduced Exercise Endurance in Rats with SU/Hx-Induced PH
2.2. Increased Levels of Type II Markers in Soleus of Animals with SU/Hx-Induced PH
2.3. Sarcomeric Aggregates in Skeletal Muscles and Hearts from Animals with SU/Hx-Induced PH
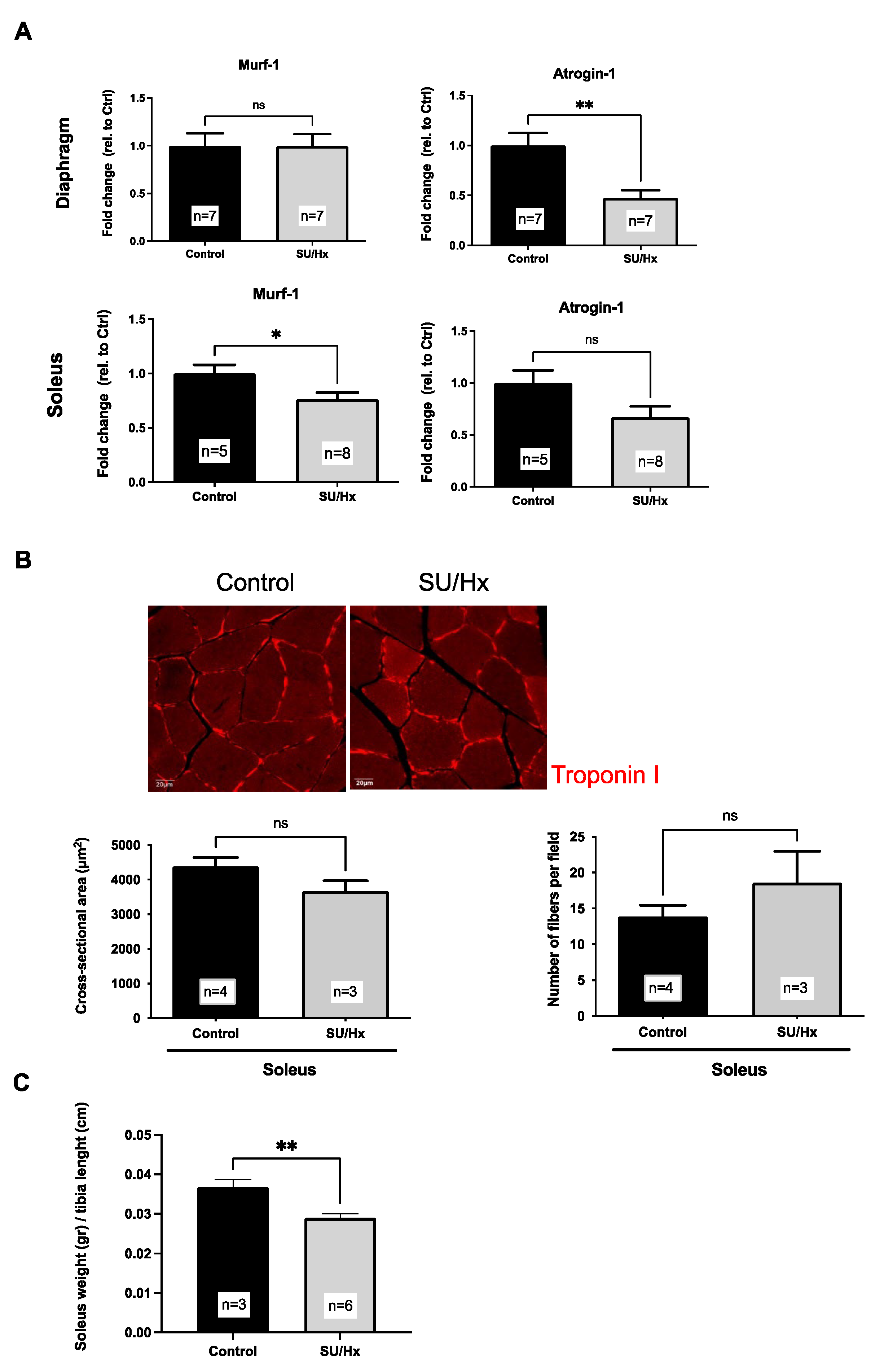
2.4. Muscle Atrophy in Rats with SU/Hx-Induced PH
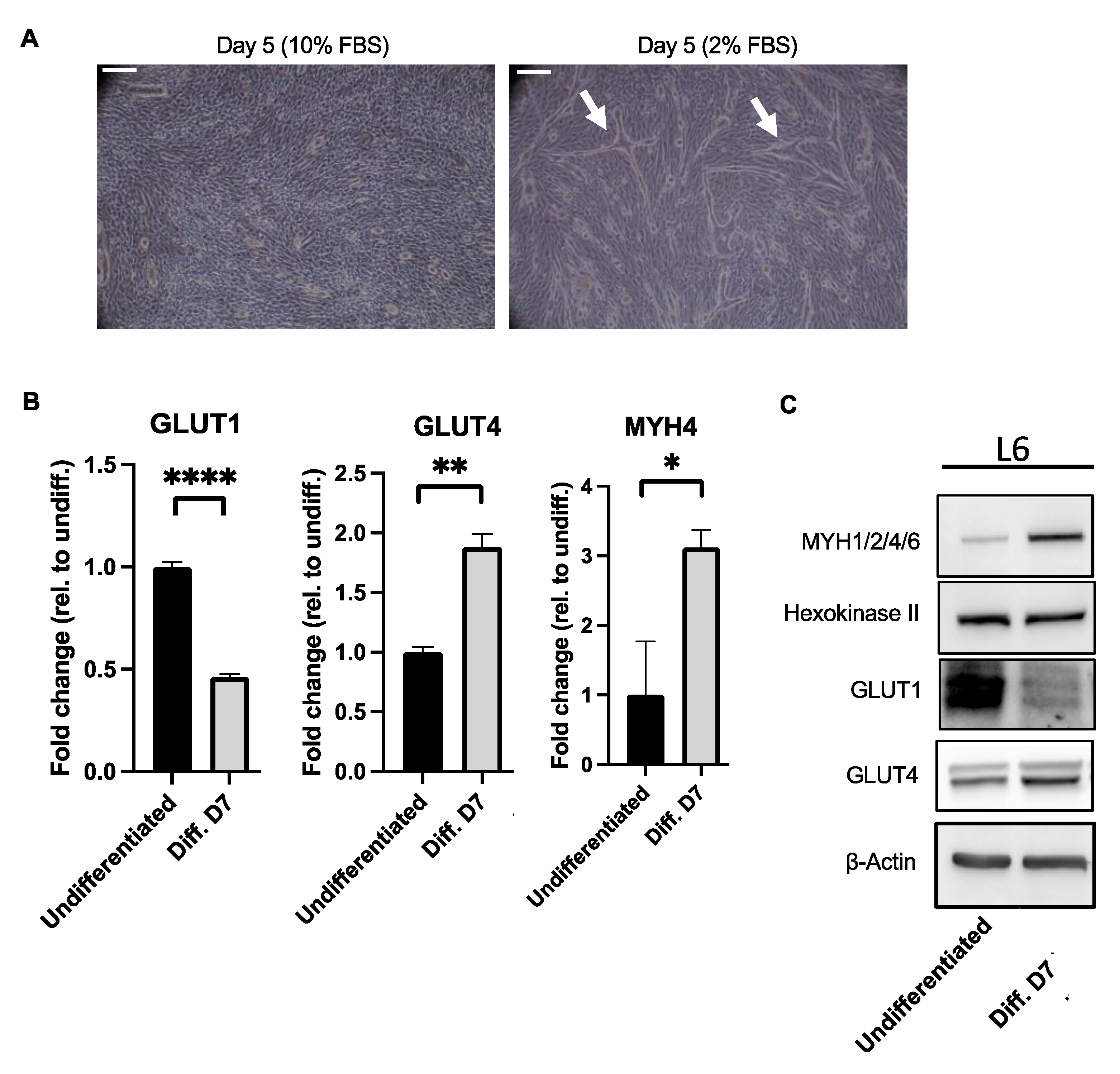
2.5. Increased Levels of Type II Markers in L6 Myotubes
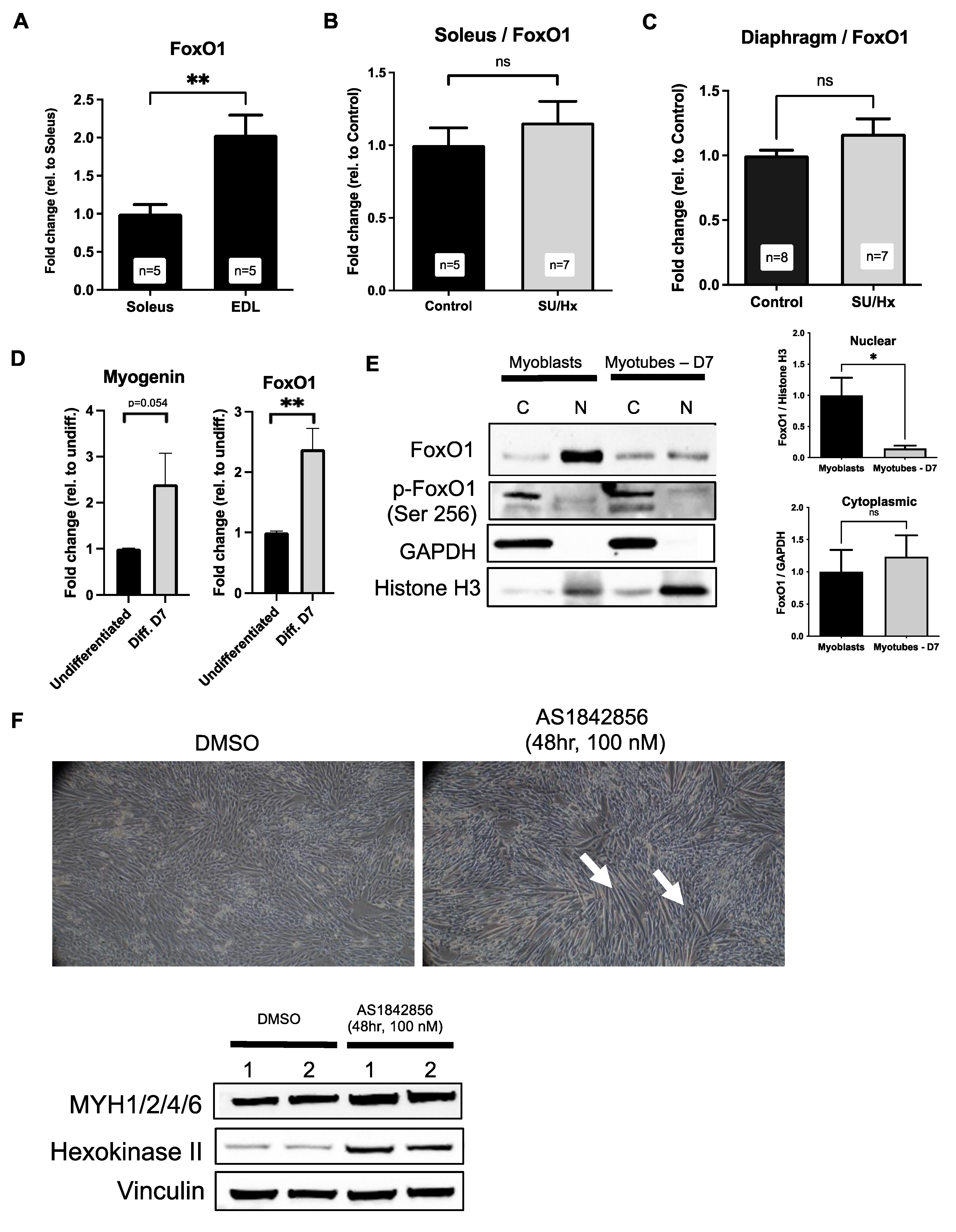
2.6. Increased Levels of FoxO1 in Soleus Muscles of Rats with SU/Hx-Induced PH, and FoxO1 Inhibition Increases Type I Markers in L6 Myotubes
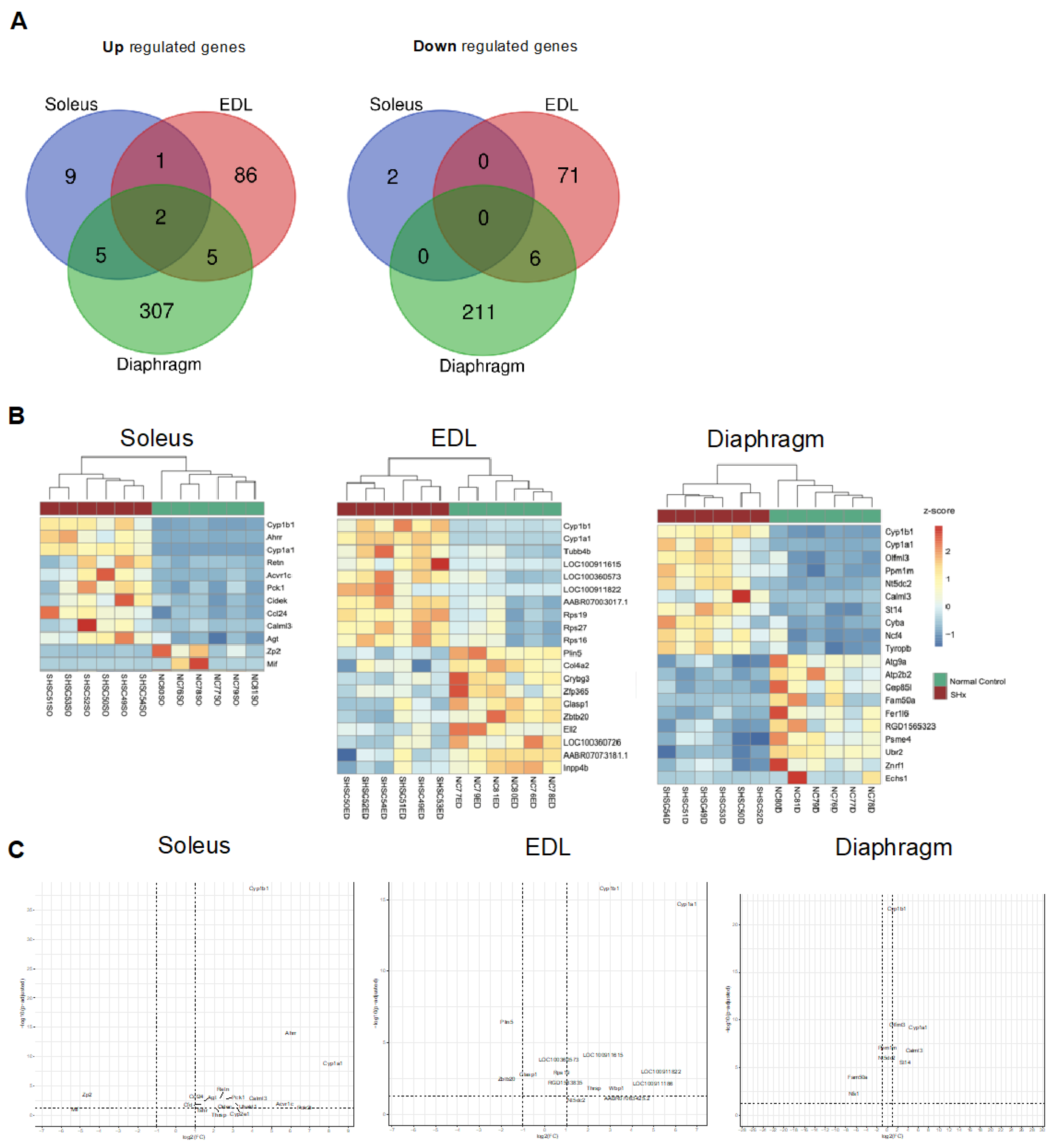
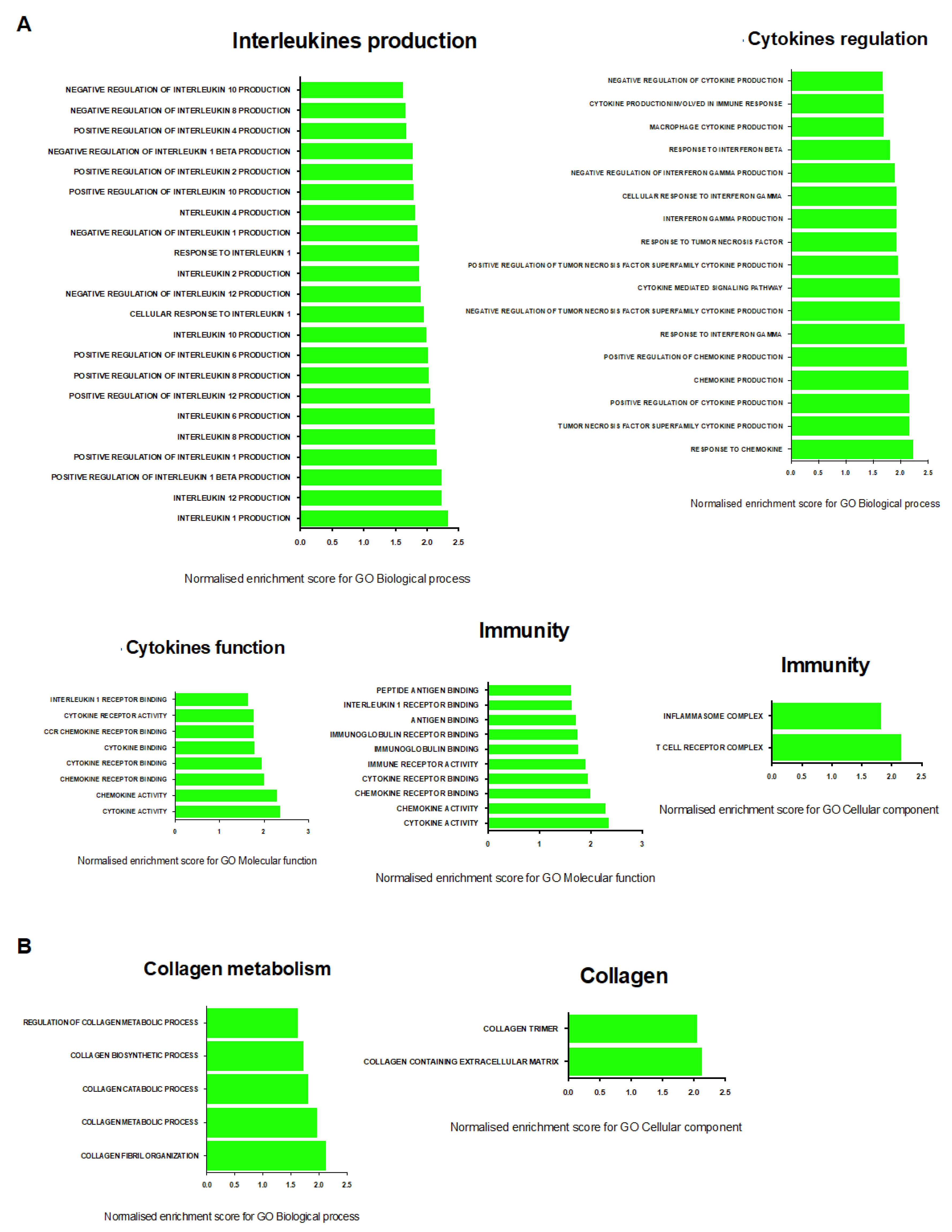
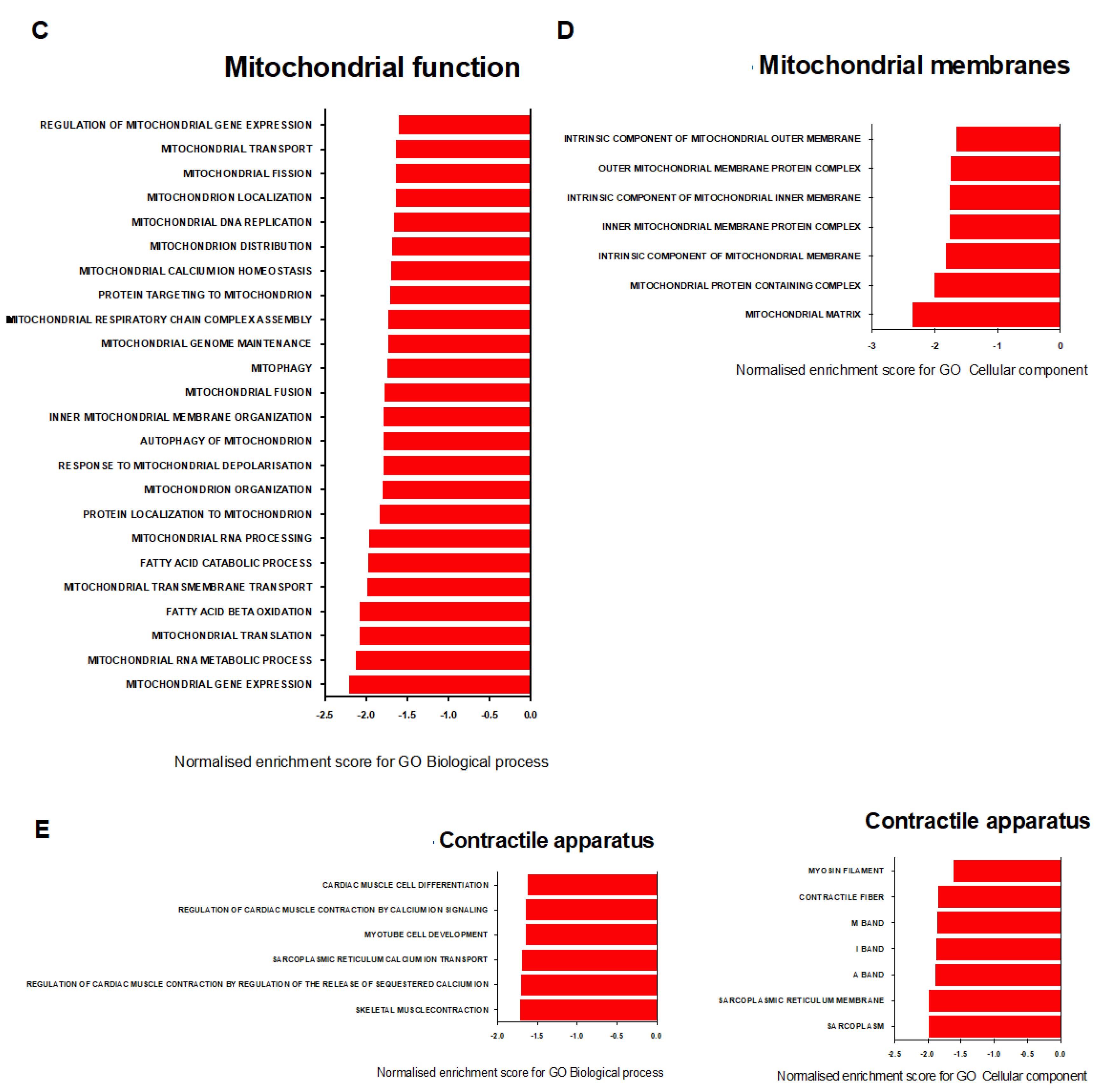
2.7. Transcriptional Signature of Skeletal Muscle Dysfunction in SU/Hx-Induced PH
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Models of Pulmonary Hypertension
4.2. Exercise Endurance Test
4.3. Hemodynamic Measurements
4.4. Rat Tissue Isolation
4.5. Immunohistochemistry
4.6. Cell Lines and Culture Conditions
4.7. Isolation of mRNA and Transcript Expression Analysis
4.8. RNA Sequencing and Bioinformatics Analysis
4.9. Cellular Fractionation
4.10. Western Blot Analysis
4.11. Antibodies and Reagents
4.12. Confocal Microscopy
4.13. Statistical ANALYSIS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Actn2 | Actinin alpha 2 |
| CSA | Cross-sectional area |
| Cyp1a1 | Cytochrome P450 family 1 subfamily A member 1 |
| Cyp1b1 | Cytochrome P450 family 1 subfamily B member 1 |
| DMSO | Dimethyl sulfoxide |
| Fbxo32 (Atrogin-1) | F-box protein 32 |
| FI | Fulton’s Index |
| FoxO1 | Forkhead box O1 |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| Glut1 | Glucose transporter type 1 |
| Glut4 | Glucose transporter type 4 |
| LVSP | Left Ventricular Systolic Pressure |
| Myh1 | Myosin heavy chain 1 |
| Myh1/2/4/6 | Myosin heavy chain 1/2/4/6 |
| Myh4 | Myosin heavy chain 4 |
| Nup133 | Nucleoporin 133 |
| PAH | Pulmonary Arterial Hypertension |
| PGC-1a (Ppargc1a) | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PH | Pulmonary Hypertension |
| RVSP | Right Ventricular Systolic Pressure |
| Sirt1 | Sirtuin 1 |
| SU/Hx | Sugen/Hypoxia |
| Tfam | Transcription factor A, mitochondrial |
| Tnni1 | Troponin I1, slow skeletal type |
| Trim63 (Murf-1) | Tripartite motif containing 63 |
References
- Batt, J.; Ahmed, S.S.; Correa, J.; Bain, A.; Granton, J. Skeletal muscle dysfunction in idiopathic pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2014, 50, 74–86. [Google Scholar] [CrossRef]
- Enache, I.; Charles, A.-L.; Bouitbir, J.; Favret, F.; Zoll, J.; Metzger, D.; Oswald-Mammosser, M.; Geny, B.; Charloux, A. Skeletal muscle mitochondrial dysfunction precedes right ventricular impairment in experimental pulmonary hypertension. Mol. Cell. Biochem. 2013, 373, 161–170. [Google Scholar] [CrossRef]
- Malenfant, S.; Potus, F.; Fournier, F.; Breuils-Bonnet, S.; Pflieger, A.; Bourassa, S.; Tremblay, E.; Nehmé, B.; Droit, A.; Bonnet, S.; et al. Skeletal muscle proteomic signature and metabolic impairment in pulmonary hypertension. J. Mol. Med. 2015, 93, 573–584. [Google Scholar] [CrossRef]
- Gonçalves, D.; Padrão, A.I.; Ferreira, R.; Justino, J.; Neuparth, M.J.; Vitorino, R.; Fonseca, H.; Silva, A.F.; Duarte, J.A.; Leite-Moreira, A.; et al. Signaling pathways underlying skeletal muscle wasting in experimental pulmonary arterial hypertension. Biochim. Biophys. Acta 2015, 1852, 2722–2731. [Google Scholar] [CrossRef]
- de Man, F.S.; Van Hees, H.W.; Handoko, M.L.; Niessen, H.W.; Schalij, I.; Humbert, M.; Dorfmüller, P.; Mercier, O.; Bogaard, H.J.; Postmus, P.E.; et al. Diaphragm muscle fiber weakness in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2011, 183, 1411–1418. [Google Scholar] [CrossRef]
- Breda, A.P.; De Albuquerque, A.L.P.; Jardim, C.; Morinaga, L.K.; Suesada, M.M.; Fernandes, C.; Dias, B.; Lourenço, R.B.; Salge, J.M.; Souza, R. Skeletal muscle abnormalities in pulmonary arterial hypertension. PLoS ONE 2014, 9, e114101. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Graves, D.T. FOXO transcription factors: Their clinical significance and regulation. BioMed Res. Int. 2014, 2014, 925350. [Google Scholar] [CrossRef]
- Kitamura, T.; Kitamura, Y.I.; Funahashi, Y.; Shawber, C.J.; Castrillon, D.H.; Kollipara, R.; DePinho, R.; Kitajewski, J.; Accili, D. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J. Clin. Investig. 2007, 117, 2477–2485. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, X.-E.; Liu, Y.-G.; Yang, G.-S. FoxO1 regulates muscle fiber-type specification and inhibits calcineurin signaling during C2C12 myoblast differentiation. Mol. Cell. Biochem. 2011, 348, 77–87. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Chen, D.; Yu, B.; Huang, Z. FoxO1: A novel insight into its molecular mechanisms in the regulation of skeletal muscle differentiation and fiber type specification. Oncotarget 2017, 8, 10662–10674. [Google Scholar] [CrossRef] [Green Version]
- Hudalla, H.; Michael, Z.; Christodoulou, N.; Willis, G.R.; Fernandez-Gonzalez, A.; Filatava, E.J.; Dieffenbach, P.; Fredenburgh, L.E.; Stearman, R.S.; Geraci, M.W.; et al. Carbonic anhydrase inhibition ameliorates inflammation and experimental pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2019, 61, 512–524. [Google Scholar] [CrossRef]
- Spyropoulos, F.; Michael, Z.; Finander, B.; Vitali, S.; Kosmas, K.; Zymaris, P.; Kalish, B.T.; Kourembanas, S.; Christou, H. Acetazolamide improves right ventricular function and metabolic gene dysregulation in experimental pulmonary arterial hypertension. Front. Cardiovasc. Med. 2021, 8, 662870. [Google Scholar] [CrossRef]
- Papathanasiou, A.; Spyropoulos, F.; Michael, Z.; Joung, K.; Briana, D.; Malamitsi-Puchner, A.; Mantzoros, C.; Christou, H. Adipokines and metabolic regulators in human and experimental pulmonary arterial hypertension. Int. J. Mol. Sci. 2021, 22, 1435. [Google Scholar] [CrossRef]
- Konieczny, P.; Fuchs, P.; Reipert, S.; Kunz, W.S.; Zelöd, A.; Fischer, I.; Paulin, D.; Schröder, R.; Wiche, G. Myofiber integrity depends on desmin network targeting to Z-disks and costameres via distinct plectin isoforms. J. Cell Biol. 2008, 181, 667–681. [Google Scholar] [CrossRef]
- Fischer, D.; Clemen, C.S.; Olive, M.; Ferrer, I.; Goudeau, B.; Roth, U.; Badorf, P.; Wattjes, M.P.; Lutterbey, G.; Kral, T.; et al. Different early pathogenesis in myotilinopathy compared to primary desminopathy. Neuromuscul. Disord. 2006, 16, 361–367. [Google Scholar] [CrossRef]
- Beharry, A.W.; Sandesara, P.B.; Roberts, B.; Ferreira, L.F.; Senf, S.M.; Judge, S. HDAC1 activates FoxO and is both sufficient and required for skeletal muscle atrophy. J. Cell Sci. 2014, 127, 1441–1453. [Google Scholar] [CrossRef]
- Guillet-Deniau, I.; Leturque, A.; Girard, J. Expression and cellular localization of glucose transporters (GLUT1, GLUT3, GLUT4) during differentiation of myogenic cells isolated from rat foetuses. J. Cell Sci. 1994, 107, 487–496. [Google Scholar] [CrossRef]
- Savai, R.; Al-Tamari, H.M.; Sedding, D.; Kojonazarov, B.; Muecke, C.; Teske, R.; Capecchi, M.R.; Weissmann, N.; Grimminger, F.; Seeger, W.; et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat. Med. 2014, 20, 1289–1300. [Google Scholar] [CrossRef]
- Iovino, S.; Oriente, F.; Botta, G.; Cabaro, S.; Iovane, V.; Paciello, O.; Viggiano, D.; Perruolo, G.; Formisano, P.; Beguinot, F. PED/PEA-15 induces autophagy and mediates TGF-beta1 effect on muscle cell differentiation. Cell Death Differ. 2012, 19, 1127–1138. [Google Scholar] [CrossRef]
- Albers, P.H.; Pedersen, A.J.; Birk, J.B.; Kristensen, D.E.; Vind, B.F.; Baba, O.; Nøhr, J.; Højlund, K.; Wojtaszewski, J.F. Human muscle fiber type-specific insulin signaling: Impact of obesity and type 2 diabetes. Diabetes 2015, 64, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Malenfant, S.; Lebret, M.; Breton-Gagnon, É.; Potus, F.; Paulin, R.; Bonnet, S.; Provencher, S. Exercise intolerance in pulmonary arterial hypertension: Insight into central and peripheral pathophysiological mechanisms. Eur. Respir. Rev. 2021, 30, 200284. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.; Li, Y.; Zhao, L.; Lu, M.; Yao, X.; Xia, H.; Wang, Y.-C.; Liu, M.-F.; Jiang, J.; et al. Thyroid hormone regulates muscle fiber type conversion via miR-133a1. J. Cell Biol. 2014, 207, 753–766. [Google Scholar] [CrossRef]
- Cannon, D.T.; Nogueira, L.; Gutierrez-Gonzalez, A.K.; Gilmore, N.K.; Bigby, T.D.; Breen, E.C. Role of IL-33 receptor (ST2) deletion in diaphragm contractile and mitochondrial function in the Sugen5416/hypoxia model of pulmonary hypertension. Respir. Physiol. Neurobiol. 2021, 295, 103783. [Google Scholar] [CrossRef]
- Langer, H.T.; Mossakowski, A.A.; Willis, B.J.; Grimsrud, K.N.; Wood, J.A.; Lloyd, K.C.; Zbinden-Foncea, H.; Baar, K. Generation of desminopathy in rats using CRISPR-Cas9. J. Cachexia Sarcopenia Muscle 2020, 11, 1364–1376. [Google Scholar] [CrossRef]
- Li, H.; Zheng, L.; Mo, Y.; Gong, Q.; Jiang, A.; Zhao, J. Voltage-dependent anion channel 1(VDAC1) participates the apoptosis of the mitochondrial dysfunction in desminopathy. PLoS ONE 2016, 11, e0167908. [Google Scholar] [CrossRef]
- Bär, H.; Fischer, D.; Goudeau, B.; Kley, R.A.; Clemen, C.S.; Vicart, P.; Herrmann, H.; Vorgerd, M.; Schröder, R. Pathogenic effects of a novel heterozygous R350P desmin mutation on the assembly of desmin intermediate filaments in vivo and in vitro. Hum. Mol. Genet. 2005, 14, 1251–1260. [Google Scholar] [CrossRef]
- Clemen, C.S.; Fischer, D.; Reimann, J.; Eichinger, L.; Müller, C.R.; Müller, H.D.; Goebel, H.H.; Schröder, R. How much mutant protein is needed to cause a protein aggregate myopathy in vivo? Lessons from an exceptional desminopathy. Hum. Mutat. 2009, 30, E490–E499. [Google Scholar] [CrossRef]
- Clemen, C.S.; Herrmann, H.; Strelkov, S.V.; Schröder, R. Desminopathies: Pathology and mechanisms. Acta Neuropathol. 2013, 125, 47–75. [Google Scholar] [CrossRef]
- Clemen, C.S.; Stöckigt, F.; Strucksberg, K.-H.; Chevessier, F.; Winter, L.; Schütz, J.; Bauer, R.; Thorweihe, J.-M.; Wenzel, D.; Schlötzer-Schrehardt, U.; et al. The toxic effect of R350P mutant desmin in striated muscle of man and mouse. Acta Neuropathol. 2015, 129, 297–315. [Google Scholar] [CrossRef]
- Xiang, G.; Ying, K.; Jiang, P.; Jia, M.; Sun, Y.; Li, S.; Wu, X.; Hao, S. Growth differentiation factor 11 induces skeletal muscle atrophy via a STAT3-dependent mechanism in pulmonary arterial hypertension. Skelet. Muscle 2022, 12, 10. [Google Scholar] [CrossRef]
- Smuder, A.J.; Sollanek, K.J.; Min, K.; Nelson, W.B.; Powers, S.K. Inhibition of forkhead boxO-specific transcription prevents mechanical ventilation-induced diaphragm dysfunction. Crit. Care Med. 2015, 43, e133–e142. [Google Scholar] [CrossRef]
- Kamei, Y.; Miura, S.; Suzuki, M.; Kai, Y.; Mizukami, J.; Taniguchi, T.; Mochida, K.; Hata, T.; Matsuda, J.; Aburatani, H.; et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J. Biol. Chem. 2004, 279, 41114–41123. [Google Scholar] [CrossRef]
- Wu, A.-L.; Kim, J.-H.; Zhang, C.; Unterman, T.G.; Chen, J. Forkhead box protein O1 negatively regulates skeletal myocyte differentiation through degradation of mammalian target of rapamycin pathway components. Endocrinology 2008, 149, 1407–1414. [Google Scholar] [CrossRef]
- Hakuno, F.; Yamauchi, Y.; Kaneko, G.; Yoneyama, Y.; Nakae, J.; Chida, K.; Kadowaki, T.; Yamanouchi, K.; Nishihara, M.; Takahashi, S.-I. Constitutive expression of insulin receptor substrate (IRS)-1 inhibits myogenic differentiation through nuclear exclusion of Foxo1 in L6 myoblasts. PLoS ONE 2011, 6, e25655. [Google Scholar] [CrossRef]
- Dean, A.; Gregorc, T.; Docherty, C.K.; Harvey, K.Y.; Nilsen, M.; Morrell, N.W.; MacLean, M.R. Role of the aryl hydrocarbon receptor in sugen 5416-induced experimental pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2018, 58, 320–330. [Google Scholar] [CrossRef]
- Handoko, M.; Man, F.H.-D.; Happé, C.; Schalij, I.; Musters, R.; Westerhof, N.; Postmus, P.; Paulus, W.; van der Laarse, W.; Vonk-Noordegraaf, A. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 2009, 120, 42–49. [Google Scholar] [CrossRef]
- Bogaard, H.J.; Natarajan, R.; Mizuno, S.; Abbate, A.; Chang, P.J.; Chau, V.Q.; Hoke, N.N.; Kraskauskas, D.; Kasper, M.; Salloum, F.N.; et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am. J. Respir. Crit. Care Med. 2010, 182, 652–660. [Google Scholar] [CrossRef]
- Christou, H.; Reslan, O.M.; Mam, V.; Tanbe, A.F.; Vitali, S.H.; Touma, M.; Arons, E.; Mitsialis, S.A.; Kourembanas, S.; Khalil, R.A. Improved pulmonary vascular reactivity and decreased hypertrophic remodeling during nonhypercapnic acidosis in experimental pulmonary hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2012, 302, L875–L890. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Cornwell, M.; Vangala, M.; Taing, L.; Herbert, Z.; Köster, J.; Li, B.; Sun, H.; Li, T.; Zhang, J.; Qiu, X.; et al. VIPER: Visualization Pipeline for RNA-seq, a Snakemake workflow for efficient and complete RNA-seq analysis. BMC Bioinform. 2018, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| RNA Sequencing | PCR Validation | |||
|---|---|---|---|---|
| Soleus | ||||
| Gene | Fold Change | p-Value adj. | Mean Fold Change | p-Value |
| Cyp1a1 | 371.05 | 2.49 × 10−10 | 11.56 | 0.0635 |
| Cyp1b1 | 16.68 | 1.28 × 10−38 | 11.01 | 0.0151 |
| EDL | ||||
| Gene | Fold Change | p-Value adj. | Mean Fold Change | p-Value |
| Cyp1a1 | 111.05 | 9.47 × 10−16 | 128.4 | 0.0155 |
| Cyb1b1 | 6.54 | 3.43 × 10−16 | 0.8 | 0.7593 |
| Diaphragm | ||||
| Gene | Fold Change | p-Value adj. | Mean Fold Change | p-Value |
| Cyp1a1 | 88.95 | 2.19 × 10−10 | 123.2 | 0.014 |
| Cyp1b1 | 7.76 | 8.02 × 10−23 | 0.53 | 0.4874 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmas, K.; Michael, Z.; Papathanasiou, A.E.; Spyropoulos, F.; Adib, E.; Jasuja, R.; Christou, H. Skeletal Muscle Dysfunction in Experimental Pulmonary Hypertension. Int. J. Mol. Sci. 2022, 23, 10912. https://doi.org/10.3390/ijms231810912
Kosmas K, Michael Z, Papathanasiou AE, Spyropoulos F, Adib E, Jasuja R, Christou H. Skeletal Muscle Dysfunction in Experimental Pulmonary Hypertension. International Journal of Molecular Sciences. 2022; 23(18):10912. https://doi.org/10.3390/ijms231810912
Chicago/Turabian StyleKosmas, Kosmas, Zoe Michael, Aimilia Eirini Papathanasiou, Fotios Spyropoulos, Elio Adib, Ravi Jasuja, and Helen Christou. 2022. "Skeletal Muscle Dysfunction in Experimental Pulmonary Hypertension" International Journal of Molecular Sciences 23, no. 18: 10912. https://doi.org/10.3390/ijms231810912






