Prokaryotic Expression of Eimeria magna SAG10 and SAG11 Genes and the Preliminary Evaluation of the Effect of the Recombinant Protein on Immune Protection in Rabbits
Abstract
:1. Introduction
2. Results
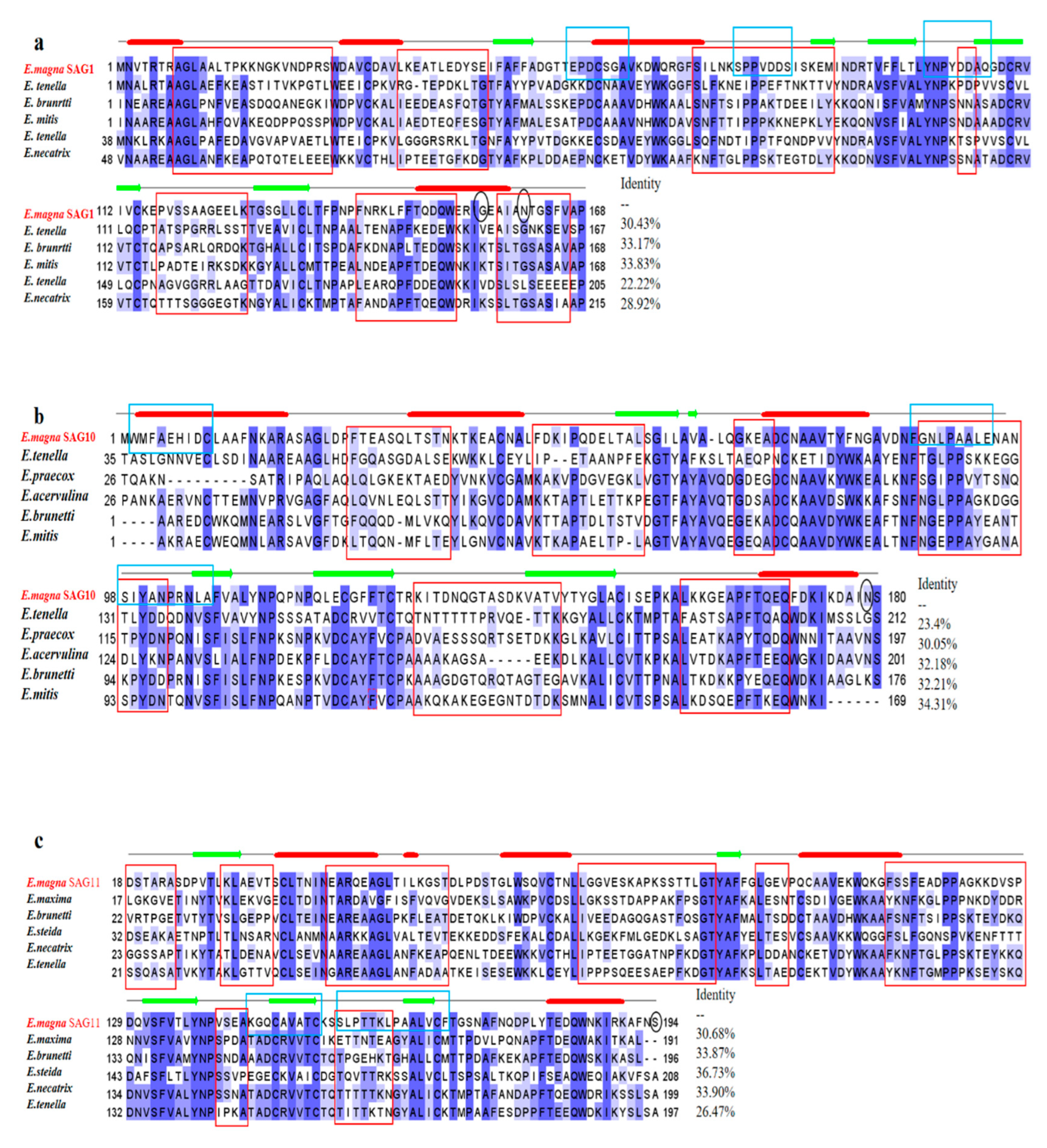
2.1. Cloning and Bioinformatics Analysis of EmSAGs Genes
2.2. Bioinformatics Analysis
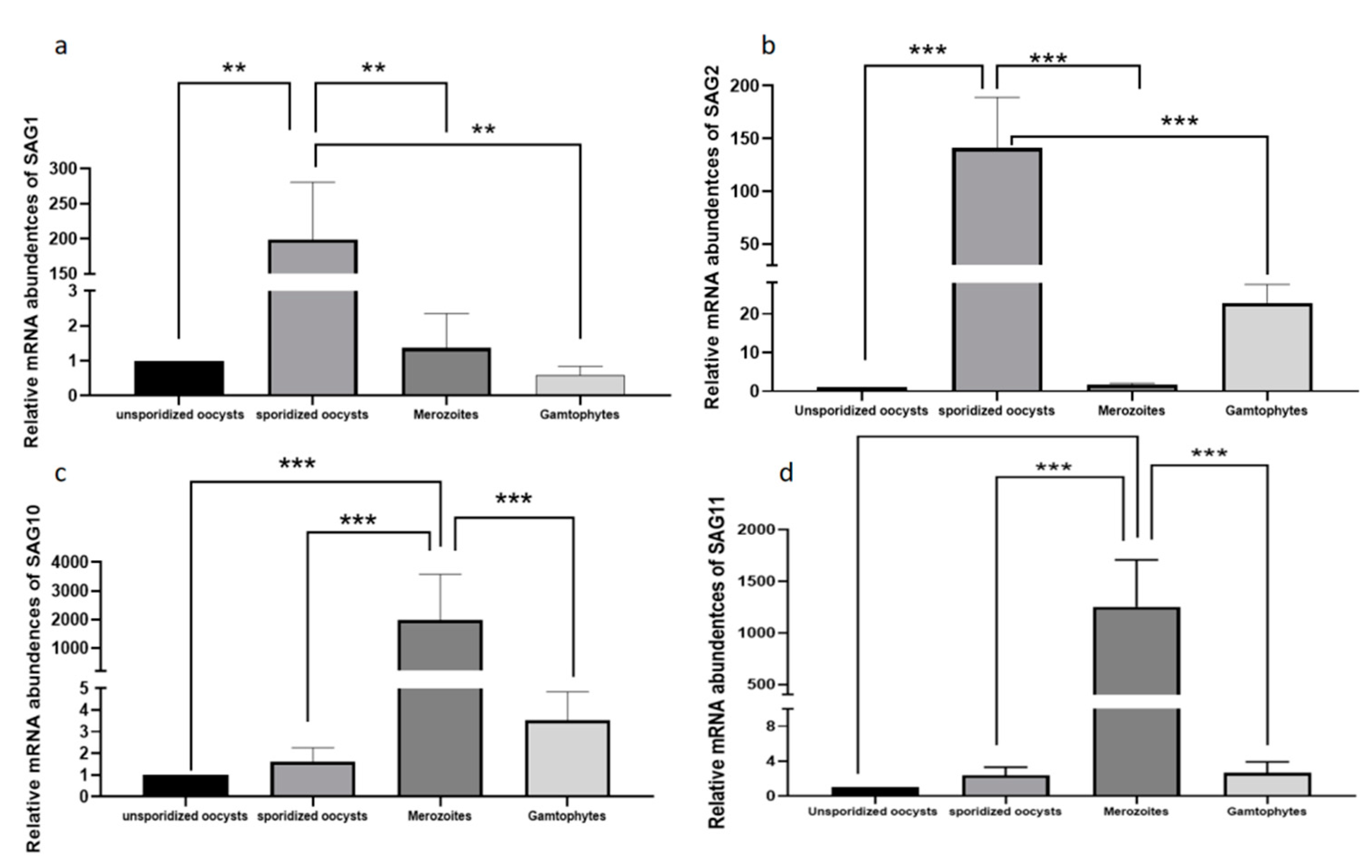
2.3. Analysis of the Transcription Level Differences in Different Development Stages of EmSAGs
2.4. Expression of rEmSAG10 and rEmSAG11
2.5. Western Blotting of rEmSAG10 and rEmSAG11
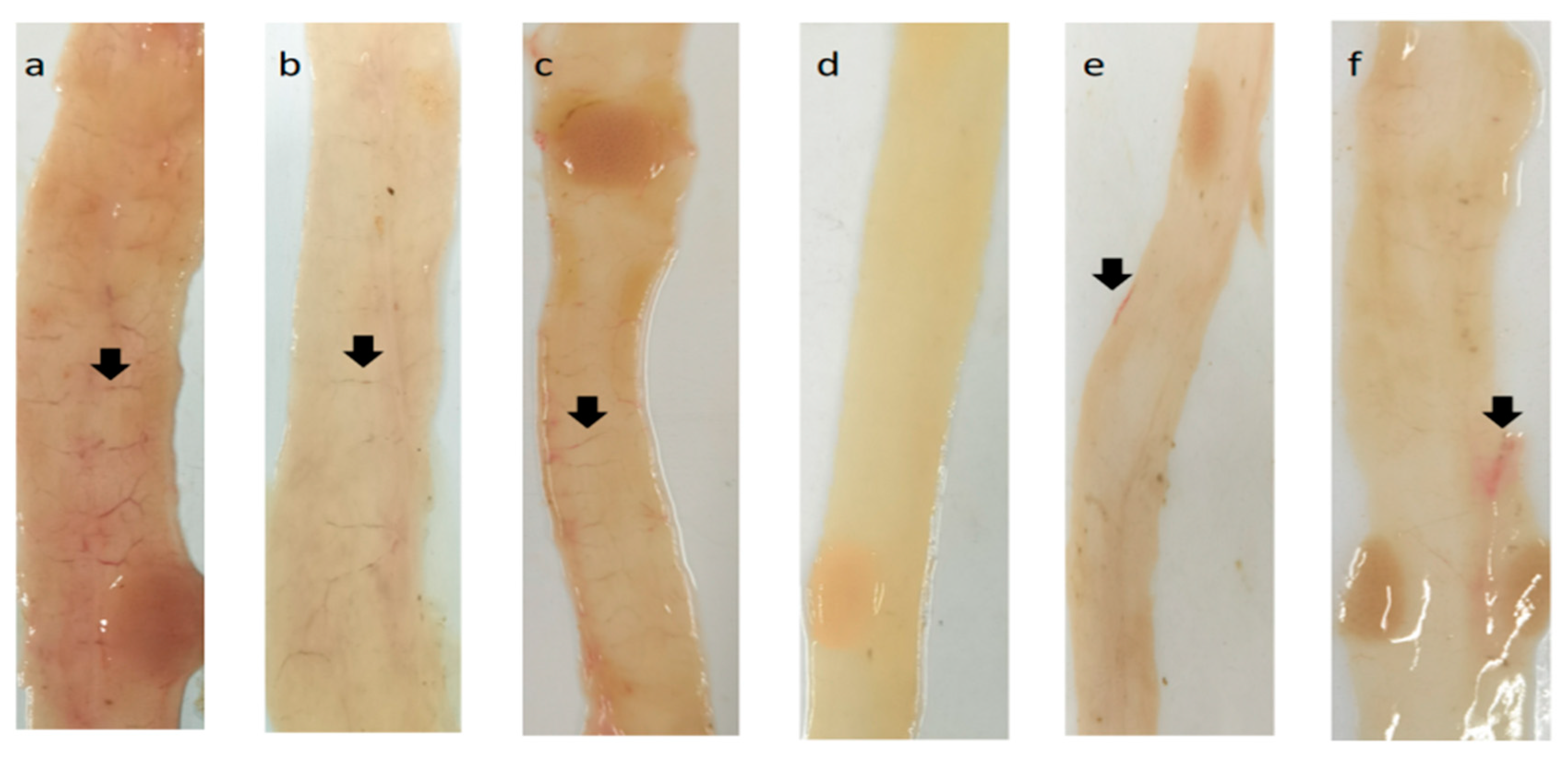
2.6. Protective Efficacy of rEmSAG10 and rEmSAG11
2.7. Detection of Specific Antibody Levels
2.8. Cytokine Detection
3. Discussion
4. Materials and Methods
4.1. Animals, Parasites, and Serum
4.2. Total RNA Extraction and Reverse Transcription
4.3. Cloning and Bioinformatics Analysis of the EmSAGs Genes
4.4. Transcription Level of the SAGs Genes
4.5. Prokaryotic Expression and Purification
4.6. Western Blot Analysis of rEmSAG10 and rEmSAG11
4.7. Immunization Procedure and Experimental Grouping
4.8. Evaluation of the Protective Efficacy of rEmSAG10 and rEmSAG11
4.9. Detection of the Serum Antibody IgG
4.10. Cytokine Detection
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd El-Ghany, W.A. Coccidiosis: A Parasitic Disease of Significant Importance in Rabbits. World’s Vet. J. 2020, 10, 499–507. [Google Scholar] [CrossRef]
- Pakandl, M.; Ahmed, N.E.; Licois, D.; Coudert, P. Eimeria magna Pérard, 1925: Study of the endogenous development of parental and precocious strains. Vet. Parasitol. 1996, 65, 213–222. [Google Scholar] [CrossRef]
- Tao, G.; Wang, Y.; Li, C.; Gu, X.; Cui, P.; Fang, S.; Liu, X. High pathogenicity and strong immunogenicity of a Chinese isolate of Eimeria magna Pérard, 1925. Parasitol. Int. 2017, 66, 207. [Google Scholar] [CrossRef]
- Song, J.K.; Hu, R.S.; Fan, X.C.; Wang, S.S.; Zhang, H.J.; Zhao, G.H. Molecular characterization of Blastocystis from pigs in Shaanxi province of China. Acta Trop. 2017, 173, 130–135. [Google Scholar] [CrossRef]
- Akpo, Y.; Kpodékon, M.T.; Djago, Y.; Licois, D.; Youssao, I.A. Vaccination of rabbits against coccidiosis using precocious lines of Eimeria magna and Eimeria media in Benin. Vet. Parasitol. 2012, 184, 73–76. [Google Scholar] [CrossRef]
- Pilarczyk, B.; Tomza-Marciniak, A.; Pilarczyk, R.; Januś, E.; Stanek, P.; Seremak, B.; Sablik, P. The effect of the sex, age, and breed of farmed rabbits and the choice of management system on the extensity and intensity of Eimeria infection. Vet. World. 2020, 13, 1654. [Google Scholar] [CrossRef]
- Drouet-Viard, F.; Coudert, P.; Licois, D.; Boivin, M. Acquired protection of the rabbit (Oryctolagus cuniculus) against coccidiosis using a precocious line of Eimeria magna: Effect of vaccine dose and age at vaccination. Vet. Parasitol. 1997, 69, 197–201. [Google Scholar] [CrossRef]
- Akpo, Y.; Kpodékon, M.T.; Djago, Y.; Licois, D.; Youssao, I.A. Evaluation de l’innocuité des souches précoces de Eimeria magna et de Eimeria media issues du Bénin en vue de leur utilisation comme souches vaccinales. Int. J. Biol. Sci. 2011, 5, 1682–1687. [Google Scholar] [CrossRef]
- Ojimelukwe, A.E.; Emedhem, D.E.; Agu, G.O. Populations of Eimeria tenella express resistance to commonly used anticoccidial drugs in southern Nigeria. J. Vet. Med. Sci. 2018, 6, 192–200. [Google Scholar] [CrossRef]
- Luo, Y.; Ren, Y.; Bai, X. Preliminary observation on immune protective effect of recombinant surface antigens sag13 and sag14 of Eimeria skrjabini on rabbits. J. Vet. Med. Health 2022, 53, 11. [Google Scholar]
- Chow, Y.P.; Wan, K.L.; Blake, D.P.; Tomley, F.; Nathan, S. Immunogenic Eimeria tenella glycosylphosphatidylinositol-anchored surface antigens (SAGs) induce inflammatory responses in avian macrophages. PLoS ONE 2011, 6, e25233. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Zheng, H.; Xu, Z.; Roellig, D.M.; Li, N.; Xiao, L. Comparative genomics reveals Cyclospora cayetanensis possesses coccidia-like metabolism and invasion components but unique surface antigens. BMC Genom. 2016, 17, 316. [Google Scholar] [CrossRef]
- Ramly, N.Z.; Dix, S.R.; Ruzheinikov, S.N.; Sedelnikova, S.E.; Baker, P.J.; Chow, Y.P.; Rice, D.W. The structure of a major surface antigen SAG19 from Eimeria tenella unifies the Eimeria SAG family. Commun. Biol. 2021, 4, 376. [Google Scholar] [CrossRef]
- Song, X.; Gao, Y.; Xu, L.; Yan, R.; Li, X. Partial protection against four species of chicken coccidia induced by multivalent subunit vaccine. Vet. Parasitol. 2015, 212, 80–85. [Google Scholar] [CrossRef]
- Liu, T.; Huang, J.; Li, Y.; Ehsan, M.; Wang, S.; Zhou, Z.; Li, X. Molecular characterisation and the protective immunity evaluation of Eimeria maxima surface antigen gene. Parasites Vectors 2018, 11, 325. [Google Scholar] [CrossRef]
- Zhao, P.; Li, Y.; Zhou, Y.; Zhao, J.; Fang, R. In vivo immunoprotective comparison between recombinant protein and DNA vaccine of Eimeria tenella surface antigen 4. Vet. Parasitol. 2020, 278, 109032. [Google Scholar] [CrossRef]
- Licois, D.; Coudert, P.; Drouet-Viard, F.; Boivin, M. Eimeria magna: Pathogenicity, immunogenicity and selection of a precocious line. Vet. Parasitol. 1995, 60, 27–35. [Google Scholar] [CrossRef]
- Pakandl, M.; Licois, D.; Coudert, P. Electron microscopic study on sporocysts and sporozoites of parental strains and precocious lines of rabbit coccidia Eimeria intestinalis, E. media and E. magna. Parasitol. Res. 2001, 87, 63–66. [Google Scholar] [CrossRef]
- Bachene, M.S.; Temim, S.; Ainbaziz, H.; Bachene, A.; Suo, X. A vaccination trial with a precocious line of Eimeria magna in Algerian local rabbits Oryctolagus cuniculus. Vet. Parasitol. 2018, 261, 73–76. [Google Scholar] [CrossRef]
- Fang, S.; Gu, X.; El-Ashram, S.; Li, X.; Yu, X.; Guo, B.; Suo, X. Immune protection provided by a precocious line trivalent vaccine against rabbit Eimeria. Vet. Parasitol. 2019, 275, 108927. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, X.; Yang, S.; Zhong, S.; Yang, T.; Zhou, Y.; Li, Y. Fecal metabolomic analysis of rabbits infected with Eimeria intestinalis and Eimeria magna based on LC-MS/MS technique. Microb. Pathog. 2022, 162, 105357. [Google Scholar] [CrossRef] [PubMed]
- Meunier, M.; Chemaly, M.; Dory, D. DNA vaccination of poultry: The current status in 2015. Vaccine 2016, 34, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, W.; Huang, X.; Tian, D.; Liu, J.; Yang, X.; Song, X. Protective efficacy of coccidial common antigen glyceraldehyde 3-phosphate dehydrogenase (GAPDH) against challenge with three Eimeria species. Front. Microbiol. 2017, 8, 1245. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, D.; Xu, L.; Yan, R.; Li, X.; Shah, M.A.; Song, X. Rhomboid protein 2 of Eimeria maxima provided partial protection against infection by homologous species. Vet. Res. 2021, 52, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, L.; Huang, J.; Wang, S.; Lu, M.; Song, X.; Li, X. The molecular characterization and immune protection of microneme 2 of Eimeria acervulina. Vet. Parasitol. 2016, 215, 96–105. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, S.; Zhao, Q.; Dong, H.; Huang, B.; Zhao, H.; Han, H. Molecular characterization of surface antigen 10 of Eimeria tenella. Parasitol. Res. 2019, 118, 2989–2999. [Google Scholar] [CrossRef]
- Wei, W.R.; Shen, N.X.; Xiao, J.; Tao, Y.Y.; Luo, Y.J.; Angel, C.; Yang, G. Expression analysis and serodiagnostic potential of microneme proteins 1 and 3 in Eimeria stiedai. Genes 2020, 11, 725. [Google Scholar] [CrossRef]
- Song, X.; Huang, X.; Yan, R.; Xu, L.; Li, X. Efficacy of chimeric DNA vaccines encoding Eimeria tenella 5401 and chicken IFN-γor IL-2 against coccidiosis in chickens. Exp. Parasitol. 2015, 156, 19–25. [Google Scholar] [CrossRef]
- Reid, A.J.; Blake, D.P.; Ansari, H.R.; Billington, K.; Browne, H.P.; Bryant, J.; Pain, A. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. 2014, 24, 1676–1685. [Google Scholar] [CrossRef]
- Jahn, D.; Matros, A.; Bakulina, A.Y.; Tiedemann, J.; Schubert, U.; Giersberg, M.; Kipriyanov, S.M. Model structure of the immunodominant surface antigen of Eimeria tenella identified as a target for sporozoite-neutralizing monoclonal antibody. Parasitol. Res. 2009, 105, 655–668. [Google Scholar] [CrossRef]
- Song, X.; Xu, L.; Yan, R.; Huang, X.; Li, X. Construction of Eimeria tenella multi-epitope DNA vaccines and their protective efficacies against experimental infection. Vet. Immunol. Immunopathol. 2015, 166, 79–87. [Google Scholar] [CrossRef]
- Song, X.; Ren, Z.; Yan, R.; Xu, L.; Li, X. Induction of protective immunity against Eimeria tenella, Eimeria necatrix, Eimeria maxima and Eimeria acervulina infections using multivalent epitope DNA vaccines. Vaccine 2015, 33, 2764–2770. [Google Scholar] [CrossRef]
- Tabarés, E.; Ferguson, D.; Clark, J.; Soon, P.E.; Wan, K.L.; Tomley, F. Eimeria tenella sporozoites and merozoites differentially express glycosylphosphatidylinositol-anchored variant surface proteins. Mol. Biochem. Parasitol. 2004, 135, 123–132. [Google Scholar] [CrossRef]
- Kundu, K.; Garg, R.; Kumar, S.; Mandal, M.; Tomley, F.M.; Blake, D.P.; Banerjee, P.S. Humoral and cytokine response elicited during immunisation with recombinant Immune Mapped protein-1 (EtIMP-1) and oocysts of Eimeria tenella. Vet. Parasitol. 2017, 244, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Karakus, U.; Sahin, D.; Mittl, P.R.; Mooij, P.; Koopman, G.; Boyman, O. Receptor-gated IL-2 delivery by an anti-human IL-2 antibody activates regulatory T cells in three different species. Sci. Transl. Med. 2020, 12, 574. [Google Scholar] [CrossRef]
- Hirai, T.; Ramos, T.L.; Lin, P.Y.; Simonetta, F.; Su, L.L.; Picton, L.K.; Negrin, R.S. Selective expansion of regulatory T cells using an orthogonal IL-2/IL-2 receptor system facilitates transplantation tolerance. J. Clin. Invest. 2021, 131, e139991. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Panelli, M.C.; Wang, E.; Nagorsen, D.; Marincola, F.M. The dual role of IL-10. Trends Immunol. 2003, 24, 36–40. [Google Scholar] [CrossRef]
- Gazzinelli, R.T.; Oswald, I.P.; James, S.L.; Sher, A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J. Immunol. 1992, 148, 1792–1796. [Google Scholar] [PubMed]
- Rothwell, L.; Young, J.R.; Zoorob, R.; Whittaker, C.A.; Hesketh, P.; Archer, A.; Kaiser, P. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J. Immunol. 2004, 173, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Inagaki-Ohara, K.; Dewi, F.N.; Hisaeda, H.; Smith, A.L.; Jimi, F.; Miyahira, M.; Nawa, Y. Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection. Infect. Immun. 2006, 74, 5292–5301. [Google Scholar] [CrossRef]
- Hoan, T.D.; Thao, D.T.; Gadahi, J.A.; Song, X.; Xu, L.; Yan, R.; Li, X. Analysis of humoral immune response and cytokines in chickens vaccinated with Eimeria brunetti apical membrane antigen-1 (EbAMA1) DNA vaccine. Exp. Parasitol. 2014, 144, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Lillehoj, H.S.; Lee, S.H.; Dalloul, R.A.; Lillehoj, E.P. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunop. 2006, 114, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Liu, X.; Li, X.; Xu, L.; Yan, R.; Song, X. Eimeria maxima Rhomboid-like Protein 5 Provided Partial Protection against Homologous Challenge in Forms of Recombinant Protein and DNA Plasmid in Chickens. Vaccines 2021, 10, 32. [Google Scholar] [CrossRef]
- Liu, M.; Kuo, F.; Capistrano, K.J.; Kang, D.; Nixon, B.G.; Shi, W.; Li, M.O. TGF-β suppresses type 2 immunity to cancer. Nature 2020, 587, 115–120. [Google Scholar] [CrossRef]
- Dalloul, R.A.; Lillehoj, H.S. Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Rev. Vaccines 2006, 5, 143–163. [Google Scholar] [CrossRef]
- Jakowlew, S.B.; Mathias, A.; Lillehoj, H.S. Transforming growth factor-β isoforms in the developing chicken intestine and spleen: Increase in transforming growth factor-β4 with coccidia infection. Vet. Immunol. Immunop. 1997, 55, 321–339. [Google Scholar] [CrossRef]
- Omer, F.M.; Kurtzhals, J.A.; Riley, E.M. Maintaining the immunological balance in parasitic infections: A role for TGF-β? Parasitol. Today 2000, 16, 18–23. [Google Scholar] [CrossRef]
- Arendt, M.K.; Sand, J.M.; Marcone, T.M.; Cook, M.E. Interleukin-10 neutralizing antibody for detection of intestinal luminal levels and as a dietary additive in Eimeria challenged broiler chicks. Poult. Sci. 2016, 95, 430–438. [Google Scholar] [CrossRef]
- Ip, W.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Knoke, K.; Rongisch, R.R.; Grzes, K.M.; Schwarz, R.; Lorenz, B.; Yogev, N.; Fabri, M. Tofacitinib Suppresses IL-10/IL-10R Signaling and Modulates Host Defense Responses in Human Macrophages. J. Investig. Dermatol. 2022, 142, 559–570. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Liu, X.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. Diverse role of TGF-β in kidney disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, T.; Tao, G.; Bao, G.; Suo, J.; Hao, L.; Fu, Y.; Suo, X. Stable transfection of Eimeria intestinalis and investigation of its life cycle, reproduction and immunogenicity. Front. Microbiol. 2016, 7, 807. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]




| Genes | Number of Amino Acids | Molecular Weight | Isoelectric Point | Signal Peptide | Transmembrane Area | GPI Anchor Point | Linear Epitope of B Antigen |
|---|---|---|---|---|---|---|---|
| SAG1 | 201 | 21.76 | 4.48 | - | - | 174 | 5–33, 38–55, 63–72, 79–102, 114–118, 127–146, 157–184 |
| SAG2 | 41 | 4.58 | 10.66 | - | - | - | 6–14, 16–18, 21, 23–38 |
| SAG10 | 215 | 22.81 | 4.84 | - | 195–214 | 193 | 6–9, 18–37, 45–58, 68–72, 85–106, 119–123, 133–156, 168–186, 189–197 |
| SAG11 | 247 | 26.07 | 4.94 | 1–23 | 224–246 | 225 | 17–34, 43–77, 86–101, 108–126, 129–156, 167–171, 180–190, 202–217, 220–228 |
| Group | Weight Gain after Immunization and before Challenge | Gain Weight after Challenge | Relative Weight Gain Rate (%) | Feed Meat Ratio | Output of Oocysts | Oocyst Decrease Ratio |
|---|---|---|---|---|---|---|
| Unchallenged control | 579.3 ± 117.7 a | 503.1 ± 126 b | 100 | 2.7:1 | 0 | 0 |
| Challenged Control | 626.8 ± 133.4 a | 226.8 ± 147.8 a | 50.3 | 6.1:1 | 18,491.6 ± 14,467.0 a | 0 |
| Quil-A Saponin | 678.7 ± 200.1 a | 236.8 ± 81.5 a | 45.0 | 5.9:1 | 18,216 ± 106.8 a | 13.1% |
| recombinant pET-32a tag protein | 629.3 ± 156.6 a | 232.5 ± 104.1 a | 46.2 | 6:1 | 16,483.3 ± 8410.4 a | −11.6% |
| rEmSAG10 immunized | 602.5 ± 62.7 a | 325.4 ± 78.8 b | 62.7 | 3.8:1 | 5400 ± 78.9 b | 70.8% |
| rEmSAG11 immunized | 646.2 ± 103.5 a | 307.5 ± 90.7 b | 61.1 | 4.5:1 | 3483.3 ± 2104.1 b | 81.2% |
| Name | Primer | Primer Sequence (5′→3′) |
|---|---|---|
| β-tubulin | Forward | TCACTTTCGTCGGCAACTCAACC |
| Reverse | AACTCCATCTCGTCCATACCCTCTC | |
| GAPDH | Forward | GTGTAGCGGGCGTTTGAGGATG |
| Reverse | GTCGTTCACAGCCACCACTTCC | |
| SAG1 | Forward | CGTGAAAGACTGGCAGAGAGGATTC |
| Reverse | GGCGTCGTCGTAAGGATTGTAGAG | |
| SAG2 | Forward | CAGTGTAGTGACGCCGTGGAAG |
| Reverse | ACTTGCTTGGGGTTCGCTTGTG | |
| SAG10 | Forward | CGCAACGGTTTATACATACGGTTTGG |
| Reverse | CGGCATAGGCTGAGTTCTTGATGG | |
| SAG11 | Forward | CTCTACAATCCCGTCAGCGAAGC |
| Reverse | TTTGTGGTATTGCGTGAGGGTAGC |
| Name | Primer | Primer Sequence (5′→3′) |
|---|---|---|
| SAG10 | Forward | ATGTGGATGTTCGCAGAA |
| Reverse | TTAAAACAGAGCAAGTCCT | |
| SAG11 | Forward | ATGAGCGACCCTGTAACA |
| Reverse | CTAGAATAGAAACGCAGCC |
| Group | Number of Rabbits | Inoculation Treatment (1 mL in Total) | Insect Attack (×105) |
|---|---|---|---|
| Unchallenged control | 8 | Sterile PBS | -- |
| Challenged control | 8 | Sterile PBS | 1 × 105 sporulated oocysts/week 4/oral |
| Quil-A saponin | 8 | Saponin (2 mg/mL) | 1 × 105 sporulated oocysts/week 4/oral |
| Recombinant pET-32a tag protein | 8 | recombinant pET-32a tag protein (150 μg) + saponin (2 mg/mL) | 1 × 105 sporulated oocysts/week 4/oral |
| rEmSAG10 immunized | 8 | SAG10 protein (150 μg) + saponin (2 mg/mL) | 1 × 105 sporulated oocysts/week 4/oral |
| rEmSAG11 immunized | 8 | SAG11 protein (150 μg) + saponin (2 mg/mL) | 1 × 105 sporulated oocysts/week 4/oral |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, J.; Xiao, J.; Bai, X.; Chen, H.; Zheng, R.; Gu, X.; Xie, Y.; He, R.; Xu, J.; Jing, B.; et al. Prokaryotic Expression of Eimeria magna SAG10 and SAG11 Genes and the Preliminary Evaluation of the Effect of the Recombinant Protein on Immune Protection in Rabbits. Int. J. Mol. Sci. 2022, 23, 10942. https://doi.org/10.3390/ijms231810942
Pu J, Xiao J, Bai X, Chen H, Zheng R, Gu X, Xie Y, He R, Xu J, Jing B, et al. Prokaryotic Expression of Eimeria magna SAG10 and SAG11 Genes and the Preliminary Evaluation of the Effect of the Recombinant Protein on Immune Protection in Rabbits. International Journal of Molecular Sciences. 2022; 23(18):10942. https://doi.org/10.3390/ijms231810942
Chicago/Turabian StylePu, Jiayan, Jie Xiao, Xin Bai, Hao Chen, Ruoyu Zheng, Xiaobin Gu, Yue Xie, Ran He, Jing Xu, Bo Jing, and et al. 2022. "Prokaryotic Expression of Eimeria magna SAG10 and SAG11 Genes and the Preliminary Evaluation of the Effect of the Recombinant Protein on Immune Protection in Rabbits" International Journal of Molecular Sciences 23, no. 18: 10942. https://doi.org/10.3390/ijms231810942






