Molecular Approaches and Echocardiographic Deformation Imaging in Detecting Myocardial Fibrosis
Abstract
1. Introduction
2. Diagnosis of MF
2.1. The Gold Standard for Diagnosis of MF
2.2. Other Techniques for Diagnosing MF
3. Cellular and Molecular Pathways Involved in Myocardial Fibrosis
4. The Role of MicroRNAs in Myocardial Fibrosis
5. The Advantages and Limitations of Echocardiographic Deformation Imaging in the Assessment of Myocardial Fibrosis
5.1. Clinical Utility of Strain Deformation for Tissue Characterization
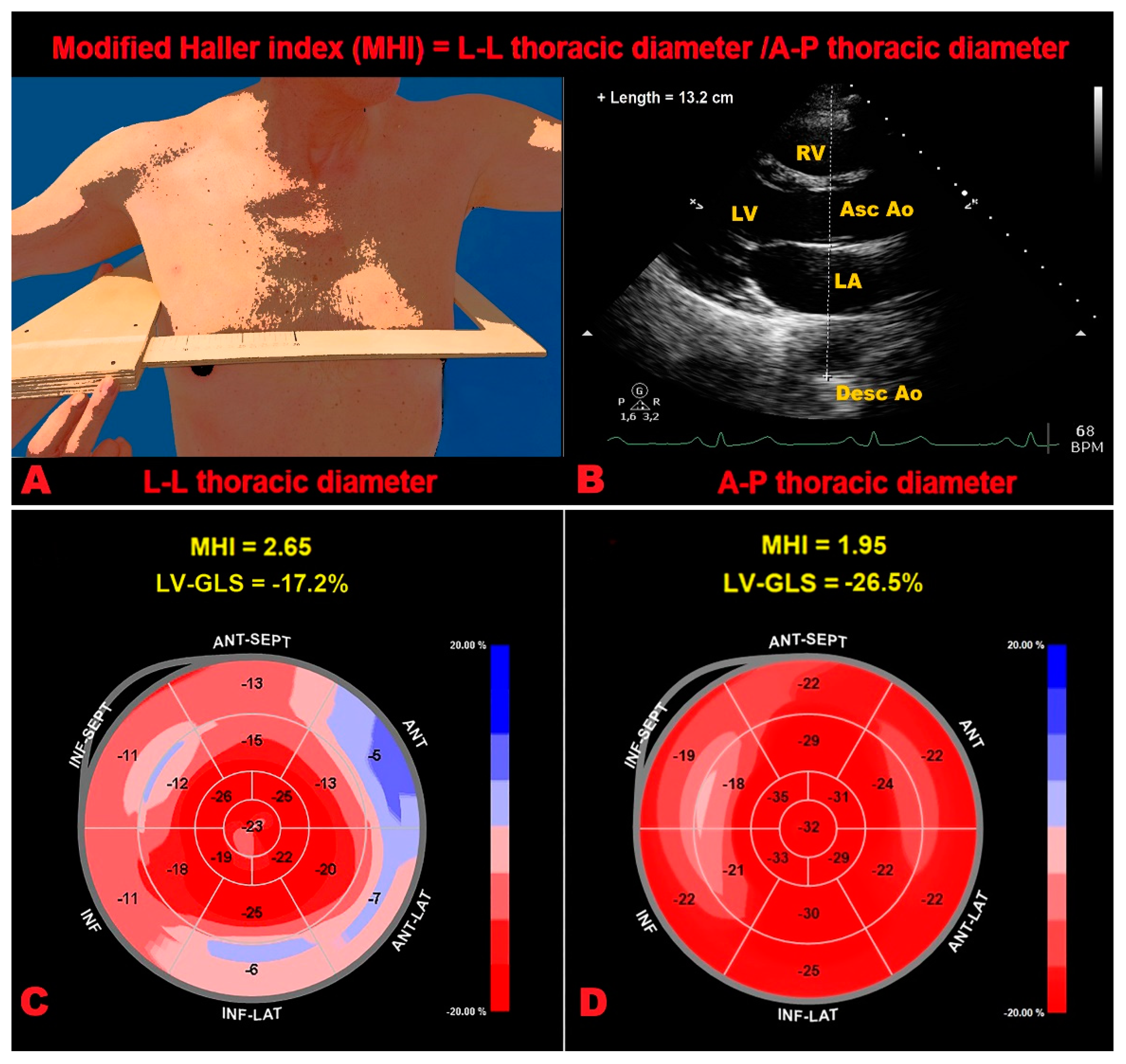
5.2. Technical Limitation of 2D-STE Analysis
6. The Interplay between Deformation Imaging and Molecular Approaches in the Clinical Decision-Making Process
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jellis, C.; Martin, J.; Narula, J.; Marwick, T.H. Assessment of nonischemic myocardial fibrosis. J. Am. Coll Cardiol. 2010, 56, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Murtha, L.A.; Schuliga, M.J.; Mabotuwana, N.S.; Hardy, S.A.; Waters, D.W.; Burgess, J.K.; Knight, D.A.; Boyle, A.J. The Processes and Mechanisms of Cardiac and Pulmonary Fibrosis. Front. Physiol. 2017, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Disertori, M.; Mase, M.; Ravelli, F. Myocardial fibrosis predicts ventricular tachyarrhythmias. Trends Cardiovasc. Med. 2017, 27, 363–372. [Google Scholar] [CrossRef]
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis—A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82. [Google Scholar] [CrossRef]
- de Boer, R.A.; De Keulenaer, G.; Bauersachs, J.; Brutsaert, D.; Cleland, J.G.; Diez, J.; Du, X.J.; Ford, P.; Heinzel, F.R.; Lipson, K.E.; et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 272–285. [Google Scholar] [CrossRef]
- Bacmeister, L.; Schwarzl, M.; Warnke, S.; Stoffers, B.; Blankenberg, S.; Westermann, D.; Lindner, D. Inflammation and fibrosis in murine models of heart failure. Basic Res. Cardiol. 2019, 114, 19. [Google Scholar] [CrossRef]
- Borer, J.S.; Truter, S.; Herrold, E.M.; Falcone, D.J.; Pena, M.; Carter, J.N.; Dumlao, T.F.; Lee, J.A.; Supino, P.G. Myocardial fibrosis in chronic aortic regurgitation: Molecular and cellular responses to volume overload. Circulation 2002, 105, 1837–1842. [Google Scholar] [CrossRef]
- Raman, B.; Ariga, R.; Spartera, M.; Sivalokanathan, S.; Chan, K.; Dass, S.; Petersen, S.E.; Daniels, M.J.; Francis, J.; Smillie, R.; et al. Progression of myocardial fibrosis in hypertrophic cardiomyopathy: Mechanisms and clinical implications. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 157–167. [Google Scholar] [CrossRef]
- Vergaro, G.; Del Franco, A.; Giannoni, A.; Prontera, C.; Ripoli, A.; Barison, A.; Masci, P.G.; Aquaro, G.D.; Solal, A.C.; Padeletti, L.; et al. Galectin-3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. Int. J. Cardiol. 2015, 184, 96–100. [Google Scholar] [CrossRef]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy revisited. Circulation 2007, 115, 3213–3223. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Zuchi, C.; Bardelli, G.; Murrone, A.; Lauciello, R.; D’Addario, S.; Mengoni, A.; Alunni, G.; Ambrosio, G. Fibrosis assessment by integrated backscatter and its relationship with longitudinal deformation and diastolic function in heart failure with preserved ejection fraction. Int. J. Cardiovasc. Imaging 2016, 32, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Graham-Brown, M.P.; Patel, A.S.; Stensel, D.J.; March, D.S.; Marsh, A.M.; McAdam, J.; McCann, G.P.; Burton, J.O. Imaging of Myocardial Fibrosis in Patients with End-Stage Renal Disease: Current Limitations and Future Possibilities. Biomed. Res. Int. 2017, 2017, 5453606. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.B.; Inoue, Y.; Mehra, M.R. Amyloidosis and the heart: A comprehensive review. Arch. Intern. Med. 2006, 166, 1805–1813. [Google Scholar] [CrossRef]
- Zarate, Y.A.; Hopkin, R.J. Fabry’s disease. Lancet 2008, 372, 1427–1435. [Google Scholar] [CrossRef]
- Martos, R.; Baugh, J.; Ledwidge, M.; O’Loughlin, C.; Conlon, C.; Patle, A.; Donnelly, S.C.; McDonald, K. Diastolic heart failure: Evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation 2007, 115, 888–895. [Google Scholar] [CrossRef]
- Zheng, L.; Ding, X.; Liu, K.; Feng, S.; Tang, B.; Li, Q.; Huang, D.; Yang, S. Molecular imaging of fibrosis using a novel collagen-binding peptide labelled with (99m)Tc on SPECT/CT. Amino Acids 2017, 49, 89–101. [Google Scholar] [CrossRef]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef]
- Daly, K.P.; Marshall, A.C.; Vincent, J.A.; Zuckerman, W.A.; Hoffman, T.M.; Canter, C.E.; Blume, E.D.; Bergersen, L. Endomyocardial biopsy and selective coronary angiography are low-risk procedures in pediatric heart transplant recipients: Results of a multicenter experience. J. Heart Lung Transplant. 2012, 31, 398–409. [Google Scholar] [CrossRef]
- Hassan, S.; Barrett, C.J.; Crossman, D.J. Imaging tools for assessment of myocardial fibrosis in humans: The need for greater detail. Biophys. Rev. 2020, 12, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Parsai, C.; O’Hanlon, R.; Prasad, S.K.; Mohiaddin, R.H. Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J. Cardiovasc. Magn. Reson. 2012, 14, 54. [Google Scholar] [CrossRef]
- Captur, G.; Manisty, C.; Moon, J.C. Cardiac MRI evaluation of myocardial disease. Heart 2016, 102, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Piechnik, S.K.; Jerosch-Herold, M. Myocardial T1 mapping and extracellular volume quantification: An overview of technical and biological confounders. Int. J. Cardiovasc. Imaging 2018, 34, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.S.; Morcos, S.K.; Almén, T.; Bellin, M.-F.; Bertolotto, M.; Bongartz, G.; Clement, O.; Leander, P.; Heinz-Peer, G.; Reimer, P.; et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: Updated ESUR Contrast Medium Safety Committee guidelines. Eur. Radiol. 2012, 23, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Blissett, S.; Chetrit, M.; Kovacina, B.; Mardigyan, V.; Afilalo, J. Performing Cardiac Magnetic Resonance Imaging in Patients with Cardiac Implantable Electronic Devices: A Contemporary Review. Can. J. Cardiol. 2018, 34, 1682–1686. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Zhang, W.; Jia, Q.; Wang, X.; Li, Y.; Lv, S.; Zhang, J. Roles of Biomarkers in Myocardial Fibrosis. Aging Dis. 2020, 11, 1157–1174. [Google Scholar] [CrossRef]
- Muhl, L.; Genove, G.; Leptidis, S.; Liu, J.; He, L.; Mocci, G.; Sun, Y.; Gustafsson, S.; Buyandelger, B.; Chivukula, I.V.; et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat. Commun. 2020, 11, 3953. [Google Scholar] [CrossRef]
- Kanisicak, O.; Khalil, H.; Ivey, M.J.; Karch, J.; Maliken, B.D.; Correll, R.N.; Brody, M.J.; Lin, S.-C.J.; Aronow, B.J.; Tallquist, M.D.; et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016, 7, 12260. [Google Scholar] [CrossRef]
- Moore-Morris, T.; Guimarães-Camboa, N.; Banerjee, I.; Zambon, A.C.; Kisseleva, T.; Velayoudon, A.; Stallcup, W.B.; Gu, Y.; Dalton, N.D.; Cedenilla, M.; et al. Resident fibroblast lineages mediate pressure overload–induced cardiac fibrosis. J. Clin. Investig. 2014, 124, 2921–2934. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The role of transforming growth factor (TGF)-beta in the infarcted myocardium. J. Thorac. Dis. 2017, 9 (Suppl. 1), S52–S63. [Google Scholar] [CrossRef] [PubMed]
- Cucoranu, I.; Clempus, R.; Dikalova, A.; Phelan, P.J.; Ariyan, S.; Dikalov, S.; Sorescu, D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005, 97, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Macias, M.J.; Martin-Malpartida, P.; Massague, J. Structural determinants of Smad function in TGF-beta signaling. Trends Biochem. Sci. 2015, 40, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.L. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am. J. Pathol. 2009, 175, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Lefer, A.M.; Tsao, P.; Aoki, N.; Palladino, M.A., Jr. Mediation of cardioprotection by transforming growth factor-beta. Science 1990, 249, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Baxter, G.F.; Mocanu, M.M.; Brar, B.K.; Latchman, D.S.; Yellon, D.M. Cardioprotective effects of transforming growth factor-beta1 during early reoxygenation or reperfusion are mediated by p42/p44 MAPK. J. Cardiovasc. Pharmacol. 2001, 38, 930–939. [Google Scholar] [CrossRef]
- Piccoli, M.T.; Bar, C.; Thum, T. Non-coding RNAs as modulators of the cardiac fibroblast phenotype. J. Mol. Cell Cardiol. 2016, 92, 75–81. [Google Scholar] [CrossRef]
- Lee, Y.; Jeon, K.; Lee, J.T.; Kim, S.; Kim, V.N. MicroRNA maturation: Stepwise processing and subcellular localization. EMBO J. 2002, 21, 4663–4670. [Google Scholar] [CrossRef]
- Lund, E.; Guttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear export of microRNA precursors. Science 2004, 303, 95–98. [Google Scholar] [CrossRef]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef]
- Zeng, Y.; Cullen, B.R. Structural requirements for pre-microRNA binding and nuclear export by Exportin 5. Nucleic Acids Res. 2004, 32, 4776–4785. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Monteys, A.M.; Spengler, R.M.; Wan, J.; Tecedor, L.; Lennox, K.A.; Xing, Y.; Davidson, B.L. Structure and activity of putative intronic miRNA promoters. RNA 2010, 16, 495–505. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef] [PubMed]
- Duisters, R.F.; Tijsen, A.J.; Schroen, B.; Leenders, J.J.; Lentink, V.; van der Made, I.; Herias, V.; van Leeuwen, R.E.; Schellings, M.W.; Barenbrug, P.; et al. miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009, 104, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Villar, A.V.; Garcia, R.; Merino, D.; Llano, M.; Cobo, M.; Montalvo, C.; Martin-Duran, R.; Hurle, M.A.; Nistal, J.F. Myocardial and circulating levels of microRNA-21 reflect left ventricular fibrosis in aortic stenosis patients. Int. J. Cardiol. 2013, 167, 2875–2881. [Google Scholar] [CrossRef]
- Zhou, X.L.; Xu, H.; Liu, Z.B.; Wu, Q.C.; Zhu, R.R.; Liu, J.C. miR-21 promotes cardiac fibroblast-to-myofibroblast transformation and myocardial fibrosis by targeting Jagged1. J. Cell Mol. Med. 2018, 22, 3816–3824. [Google Scholar] [CrossRef]
- Gupta, S.K.; Itagaki, R.; Zheng, X.; Batkai, S.; Thum, S.; Ahmad, F.; Van Aelst, L.N.; Sharma, A.; Piccoli, M.-T.; Weinberger, F.; et al. miR-21 promotes fibrosis in an acute cardiac allograft transplantation model. Cardiovasc. Res. 2016, 110, 215–226. [Google Scholar] [CrossRef]
- Li, Q.; Yao, Y.; Shi, S.; Zhou, M.; Zhou, Y.; Wang, M.; Chiu, J.; Huang, Z.; Zhang, W.; Liu, M.; et al. Inhibition of miR-21 alleviated cardiac perivascular fibrosis via repressing EndMT in T1DM. J. Cell. Mol. Med. 2019, 24, 910–920. [Google Scholar] [CrossRef]
- Xu, H.F.; Ding, Y.J.; Zhang, Z.X.; Wang, Z.F.; Luo, C.L.; Li, B.X.; Shen, Y.W.; Tao, L.Y.; Zhao, Z.Q. MicroRNA21 regulation of the progression of viral myocarditis to dilated cardiomyopathy. Mol. Med. Rep. 2014, 10, 161–168. [Google Scholar] [CrossRef]
- Sayed, D.; Rane, S.; Lypowy, J.; He, M.; Chen, I.Y.; Vashistha, H.; Yan, L.; Malhotra, A.; Vatner, D.; Abdellatif, M. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol. Biol. Cell 2008, 19, 3272–3282. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Tagliabue, E.; Mrakic-Sposta, S.; Uccellatore, A.C.; Senesi, P.; Terruzzi, I.; Trabucchi, E.; Rossi-Bernardi, L.; Luzi, L. Lower miR-21/ROS/HNE levels associate with lower glycemia after habit-intervention: DIAPASON study 1-year later. Cardiovasc. Diabetol. 2022, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Mrakic-Sposta, S.; Tagliabue, E.; Prattichizzo, F.; Micheloni, S.; Sangalli, E.; Specchia, C.; Uccellatore, A.C.; Lupini, S.; Spinetti, G.; et al. Circulating microRNA-21 is an early predictor of ROS-mediated damage in subjects with high risk of developing diabetes and in drug-naive T2D. Cardiovasc. Diabetol. 2019, 18, 18. [Google Scholar] [CrossRef]
- La Sala, L.; Mrakic-Sposta, S.; Micheloni, S.; Prattichizzo, F.; Ceriello, A. Glucose-sensing microRNA-21 disrupts ROS homeostasis and impairs antioxidant responses in cellular glucose variability. Cardiovasc. Diabetol. 2018, 17, 105. [Google Scholar] [CrossRef]
- Lin, R.; Rahtu-Korpela, L.; Szabo, Z.; Kemppi, A.; Skarp, S.; Kiviniemi, A.M.; Lepojärvi, E.S.; Halmetoja, E.; Kilpiö, T.; Porvari, K.; et al. MiR-185-5p regulates the development of myocardial fibrosis. J. Mol. Cell. Cardiol. 2021, 165, 130–140. [Google Scholar] [CrossRef]
- La Sala, L.; Cattaneo, M.; De Nigris, V.; Pujadas, G.; Testa, R.; Bonfigli, A.R.; Genovese, S.; Ceriello, A. Oscillating glucose induces microRNA-185 and impairs an efficient antioxidant response in human endothelial cells. Cardiovasc. Diabetol. 2016, 15, 71. [Google Scholar] [CrossRef]
- Mavrogeni, S.I.; Sfikakis, P.P.; Koutsogeorgopoulou, L.; Markousis-Mavrogenis, G.; Dimitroulas, T.; Kolovou, G.; Kitas, G.D. Cardiac Tissue Characterization and Imaging in Autoimmune Rheumatic Diseases. JACC Cardiovasc. Imaging 2017, 10, 1387–1396. [Google Scholar] [CrossRef]
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef]
- Madry, W.; Karolczak, M.A. Physiological basis in the assessment of myocardial mechanics using speckle-tracking echocardiography 2D. Part I. J. Ultrason. 2016, 16, 135–144. [Google Scholar]
- Lisi, M.; Cameli, M.; Mandoli, G.E.; Pastore, M.C.; Righini, F.M.; D’Ascenzi, F.; Focardi, M.; Rubboli, A.; Mondillo, S.; Henein, M.Y. Detection of myocardial fibrosis by speckle-tracking echocardiography: From prediction to clinical applications. Heart Fail. Rev. 2022, 27, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.-U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Stohr, E.J.; Shave, R.E.; Baggish, A.L.; Weiner, R.B. Left ventricular twist mechanics in the context of normal physiology and cardiovascular disease: A review of studies using speckle tracking echocardiography. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H633–H644. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.J.; Altman, M.; Stanton, T.; Thomas, L. Echocardiographic Strain in Clinical Practice. Heart Lung Circ. 2019, 28, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Scully, P.R.; Bastarrika, G.; Moon, J.C.; Treibel, T.A. Myocardial Extracellular Volume Quantification by Cardiovascular Magnetic Resonance and Computed Tomography. Curr. Cardiol. Rep. 2018, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Moharram, M.A.; Lamberts, R.R.; Whalley, G.; Williams, M.J.A.; Coffey, S. Myocardial tissue characterisation using echocardiographic deformation imaging. Cardiovasc. Ultrasound 2019, 17, 27. [Google Scholar] [CrossRef]
- Janicki, J.S.; Brower, G.L. The role of myocardial fibrillar collagen in ventricular remodeling and function. J. Card Fail. 2002, 8 (Suppl. 6), S319–S325. [Google Scholar] [CrossRef]
- Baicu, C.F.; Stroud, J.D.; Livesay, V.A.; Hapke, E.; Holder, J.; Spinale, F.G.; Zile, M.R. Changes in extracellular collagen matrix alter myocardial systolic performance. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H122–H132. [Google Scholar] [CrossRef]
- Narayan, S.; Janicki, J.S.; Shroff, S.G.; Pick, R.; Weber, K.T. Myocardial collagen and mechanics after preventing hypertrophy in hypertensive rats. Am. J. Hypertens 1989, 2, 675–682. [Google Scholar] [CrossRef]
- Dhillon, A.; Sweet, W.; Popovic, Z.; Smedira, N.G.; Thamilarasan, M.; Lytle, B.W.; Tan, C.; Starling, R.C.; Lever, H.M.; Moravec, C.S.; et al. Association of Noninvasively Measured Left Ventricular Mechanics With In Vitro Muscle Contractile Performance: A Prospective Study in Hypertrophic Cardiomyopathy Patients. J. Am. Heart Assoc. 2014, 3, e001269. [Google Scholar] [CrossRef]
- Kobayashi, T.; Popovic, Z.; Bhonsale, A.; Smedira, N.G.; Tan, C.; Rodriguez, E.R.; Thamilarasan, M.; Lytle, B.W.; Lever, H.M.; Desai, M.Y. Association between septal strain rate and histopathology in symptomatic hypertrophic cardiomyopathy patients undergoing septal myectomy. Am. Heart J. 2013, 166, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Reyes, A.M.; Youker, K.; Estep, J.D.; Torre-Amione, G.; Nagueh, S.F. Molecular and cellular correlates of cardiac function in end-stage DCM: A study using speckle tracking echocardiography. JACC Cardiovasc. Imaging 2014, 7, 441–452. [Google Scholar] [CrossRef]
- Her, A.Y.; Choi, E.Y.; Shim, C.Y.; Song, B.W.; Lee, S.; Ha, J.W.; Rim, S.J.; Hwang, K.C.; Chang, B.C.; Chung, N. Prediction of left atrial fibrosis with speckle tracking echocardiography in mitral valve disease: A comparative study with histopathology. Korean Circ. J. 2012, 42, 311–318. [Google Scholar] [CrossRef]
- Cameli, M.; Mondillo, S.; Righini, F.M.; Lisi, M.; Dokollari, A.; Lindqvist, P.; Maccherini, M.; Henein, M. Left Ventricular Deformation and Myocardial Fibrosis in Patients with Advanced Heart Failure Requiring Transplantation. J. Card Fail. 2016, 22, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Vanzzini, N.; Fritche-Salazar, J.F.; Vázquez-Castro, N.M.; Rivera-Lara, P.; Pérez-Méndez, O.; Martínez-Herrera, H.; Gómez-Sánchez, M.; Aranda-Frausto, A.; Herrera-Bello, H.; Luna-Luna, M.; et al. Echocardiographic and Histologic Correlations in Patients with Severe Aortic Stenosis: Influence of Overweight and Obesity. J. Cardiovasc. Ultrasound 2016, 24, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, I.; Scatena, C.; Mazzanti, C.M.; Conte, L.; Pugliese, N.R.; Franceschi, S.; Lessi, F.; Menicagli, M.; De Martino, A.; Pratali, S.; et al. Micro-RNA-21 (biomarker) and global longitudinal strain (functional marker) in detection of myocardial fibrotic burden in severe aortic valve stenosis: A pilot study. J. Transl. Med. 2016, 14, 248. [Google Scholar] [CrossRef][Green Version]
- Park, S.J.; Cho, S.W.; Kim, S.M.; Ahn, J.; Carriere, K.; Jeong, D.S.; Lee, S.C.; Park, S.W.; Choe, Y.H.; Park, P.W.; et al. Assessment of Myocardial Fibrosis Using Multimodality Imaging in Severe Aortic Stenosis: Comparison with Histologic Fibrosis. JACC Cardiovasc. Imaging 2019, 12, 109–119. [Google Scholar] [CrossRef]
- Escher, F.; Kasner, M.; Kuhl, U.; Heymer, J.; Wilkenshoff, U.; Tschope, C.; Schultheiss, H.P. New echocardiographic findings correlate with intramyocardial inflammation in endomyocardial biopsies of patients with acute myocarditis and inflammatory cardiomyopathy. Mediat. Inflamm. 2013, 2013, 875420. [Google Scholar] [CrossRef]
- Kasner, M.; Sinning, D.; Escher, F.; Lassner, D.; Kuhl, U.; Schultheiss, H.P.; Tschope, C. The utility of speckle tracking imaging in the diagnostic of acute myocarditis, as proven by endomyocardial biopsy. Int. J. Cardiol. 2013, 168, 3023–3024. [Google Scholar] [CrossRef]
- Mehta, P.; Chapel, D.B.; Goyal, N.; Yu, D.B.; Mor-Avi, V.; Narang, A.; Addetia, K.; Sarswat, N.; Lang, R.M.; Husain, A.N.; et al. A histopathologic schema to quantify the burden of cardiac amyloidosis: Relationship with survival and echocardiographic parameters. Echocardiography 2018, 36, 285–291. [Google Scholar] [CrossRef]
- Phelan, D.; Collier, P.; Thavendiranathan, P.; Popovic, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012, 98, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Pagourelias, E.D.; Mirea, O.; Duchenne, J.; Van Cleemput, J.; Delforge, M.; Bogaert, J.; Kuznetsova, T.; Voigt, J.U. Echo Parameters for Differential Diagnosis in Cardiac Amyloidosis: A Head-to-Head Comparison of Deformation and Nondeformation Parameters. Circ. Cardiovasc. Imaging 2017, 10, e005588. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.P.; Stewart, W.J.; Yang, X.S.; Donnell, R.O.; Leon, A.R.; Felner, J.M.; Thomas, J.D.; Merlino, J.D. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am. J. Cardiol. 2009, 103, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.J.; Emami, M.; Mereles, D.; Korosoglou, G.; Kristen, A.V.; Voss, A.; Schellberg, D.; Zugck, C.; Galuschky, C.; Giannitsis, E.; et al. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: Incremental value compared with clinical and biochemical markers. J. Am. Coll Cardiol. 2012, 60, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Reant, P.; Reynaud, A.; Pillois, X.; Dijos, M.; Arsac, F.; Touche, C.; Landelle, M.; Rooryck, C.; Roudaut, R.; Lafitte, S. Comparison of resting and exercise echocardiographic parameters as indicators of outcomes in hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2015, 28, 194–203. [Google Scholar] [CrossRef]
- Reant, P.; Mirabel, M.; Lloyd, G.; Peyrou, J.; Lopez-Ayala, J.M.; Dickie, S.; Bulluck, H.; Captur, G.; Rosmini, S.; Guttmann, O.; et al. Global longitudinal strain is associated with heart failure outcomes in hypertrophic cardiomyopathy. Heart 2016, 102, 741–747. [Google Scholar] [CrossRef]
- Kearney, L.G.; Lu, K.; Ord, M.; Patel, S.K.; Profitis, K.; Matalanis, G.; Burrell, L.M.; Srivastava, P.M. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 827–833. [Google Scholar] [CrossRef]
- Le, T.T.; Huang, W.; Singh, G.K.; Toh, D.F.; Ewe, S.H.; Tang, H.C.; Loo, G.; Bryant, J.A.; Ang, B.; Tay, E.L.; et al. Echocardiographic Global Longitudinal Strain Is Associated with Myocardial Fibrosis and Predicts Outcomes in Aortic Stenosis. Front. Cardiovasc. Med. 2021, 8, 750016. [Google Scholar]
- Grenne, B.; Eek, C.; Sjoli, B.; Dahlslett, T.; Uchto, M.; Hol, P.K.; Skulstad, H.; Smiseth, O.A.; Edvardsen, T.; Brunvand, H. Acute coronary occlusion in non-ST-elevation acute coronary syndrome: Outcome and early identification by strain echocardiography. Heart 2010, 96, 1550–1556. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Migliori, C.; Bianchi, S.; Lombardo, M. Usefulness of second trimester left ventricular global longitudinal strain for predicting adverse maternal outcome in pregnant women aged 35 years or older. Int. J. Cardiovasc. Imaging 2021. online ahead of print. [Google Scholar] [CrossRef]
- Phelan, D.; Thavendiranathan, P.; Popovic, Z.; Collier, P.; Griffin, B.; Thomas, J.D.; Marwick, T.H. Application of a parametric display of two-dimensional speckle-tracking longitudinal strain to improve the etiologic diagnosis of mild to moderate left ventricular hypertrophy. J. Am. Soc. Echocardiogr. 2014, 27, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Kraigher-Krainer, E.; Shah, A.M.; Gupta, D.K.; Santos, A.; Claggett, B.; Pieske, B.; Zile, M.; Voors, A.A.; Lefkowitz, M.P.; Packer, M.; et al. Impaired Systolic Function by Strain Imaging in Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2013, 63, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Ternacle, J.; Bodez, D.; Guellich, A.; Audureau, E.; Rappeneau, S.; Lim, P.; Radu, C.; Guendouz, S.; Couetil, J.-P.; Benhaiem, N.; et al. Causes and Consequences of Longitudinal LV Dysfunction Assessed by 2D Strain Echocardiography in Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2016, 9, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.H. Pondering the Prognosis and Pathology of Cardiac Amyloidosis: Answers Breed Questions. JACC Cardiovasc. Imaging 2016, 9, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Popovic, Z.B.; Kwon, D.H.; Mishra, M.; Buakhamsri, A.; Greenberg, N.L.; Thamilarasan, M.; Flamm, S.D.; Thomas, J.D.; Lever, H.M.; Desai, M.Y. Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J. Am. Soc. Echocardiogr. 2008, 21, 1299–1305. [Google Scholar] [CrossRef]
- Kim, E.K.; Lee, S.C.; Hwang, J.W.; Chang, S.A.; Park, S.J.; On, Y.K.; Park, K.M.; Choe, Y.H.; Kim, S.M.; Park, S.W.; et al. Differences in apical and non-apical types of hypertrophic cardiomyopathy: A prospective analysis of clinical, echocardiographic, and cardiac magnetic resonance findings and outcome from 350 patients. Eur Heart J. Cardiovasc. Imaging 2016, 17, 678–686. [Google Scholar] [CrossRef]
- Afonso, L.; Kondur, A.; Simegn, M.; Niraj, A.; Hari, P.; Kaur, R.; Ramappa, P.; Pradhan, J.; Bhandare, D.; Williams, K.A.; et al. Two-dimensional strain profiles in patients with physiological and pathological hypertrophy and preserved left ventricular systolic function: A comparative analyses. BMJ Open 2012, 2, e001390. [Google Scholar] [CrossRef]
- Dulgheru, R.; Pibarot, P.; Sengupta, P.P.; Pierard, L.A.; Rosenhek, R.; Magne, J.; Donal, E.; Bernard, A.; Fattouch, K.; Cosyns, B.; et al. Multimodality Imaging Strategies for the Assessment of Aortic Stenosis: Viewpoint of the Heart Valve Clinic International Database (HAVEC) Group. Circ. Cardiovasc. Imaging 2016, 9, e004352. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Cerini, F.; Cerrone, A.; Argiento, L.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; Rumi, M.G.; Vigano, M. Liver stiffness measurement identifies subclinical myocardial dysfunction in non-advanced non-alcoholic fatty liver disease patients without overt heart disease. Intern. Emerg Med. 2022, 17, 1425–1438. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Zaid, R.R.; Barker, C.M.; Little, S.H.; Nagueh, S.F. Pre- and post-operative diastolic dysfunction in patients with valvular heart disease: Diagnosis and therapeutic implications. J. Am. Coll. Cardiol. 2013, 62, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.; Everett, R.J.; Kwiecinski, J.; Vesey, A.T.; Yeung, E.; Esson, G.; Jenkins, W.; Koo, M.; Mirsadraee, S.; White, A.C.; et al. Myocardial Fibrosis and Cardiac Decompensation in Aortic Stenosis. JACC Cardiovasc. Imaging 2016, 10, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- Aalaei-Andabili, S.H.; Bavry, A.A. Left Ventricular Diastolic Dysfunction and Transcatheter Aortic Valve Replacement Outcomes: A Review. Cardiol. Ther. 2019, 8, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. Incremental prognostic role of left atrial reservoir strain in asymptomatic patients with moderate aortic stenosis. Int. J. Cardiovasc. Imaging 2021, 37, 1913–1925. [Google Scholar] [CrossRef]
- Salas-Pacheco, J.L.; Avila-Vanzzini, N.; Eugenia, R.M.; Arias-Godinez, J.A. Left atrium function by 2D speckle tracking in aortic valve disease. Echocardiography 2016, 33, 1828–1834. [Google Scholar] [CrossRef]
- Calin, A.; Mateescu, A.D.; Rosca, M.; Beladan, C.C.; Enache, R.; Botezatu, S.; Cosei, I.; Calin, C.; Simion, M.; Ginghina, C.; et al. Left atrial dysfunction as a determinant of pulmonary hypertension in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Int. J. Cardiovasc. Imaging 2017, 33, 1939–1947. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Vincenti, A.; Baravelli, M.; Rigamonti, E.; Tagliabue, E.; Bassi, P.; Nicolosi, G.L.; Anza, C.; Lombardo, M. Prognostic value of global left atrial peak strain in patients with acute ischemic stroke and no evidence of atrial fibrillation. Int. J. Cardiovasc. Imaging 2019, 35, 603–613. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Cara, M.D.; Nicolosi, G.L.; Eusebio, A.; Bordonali, M.; Santalucia, P.; Lombardo, M. Rapid Risk Stratification of Acute Ischemic Stroke Patients in the Emergency Department: The Incremental Prognostic Role of Left Atrial Reservoir Strain. J. Stroke Cerebrovasc. Dis. 2021, 30, 106100. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Lonati, C.; Lombardo, M.; Rigamonti, E.; Binda, G.; Vincenti, A.; Nicolosi, G.L.; Bianchi, S.; Harari, S.; Anza, C. Incremental prognostic value of global left atrial peak strain in women with new-onset gestational hypertension. J. Hypertens 2019, 37, 1668–1675. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Caminati, A.; Lipsi, R.; Nicolosi, G.L.; Lombardo, M.; Anza, C.; Harari, S. Early left atrial dysfunction in idiopathic pulmonary fibrosis patients without chronic right heart failure. Int. J. Cardiovasc. Imaging 2020, 36, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Guichard, J.B.; Nattel, S. Atrial Cardiomyopathy: A Useful Notion in Cardiac Disease Management or a Passing Fad? J. Am. Coll. Cardiol. 2017, 70, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Fabritz, L.; Guasch, E.; Antoniades, C.; Bardinet, I.; Benninger, G.; Betts, T.R.; Brand, E.; Breithardt, G.; Bucklar-Suchankova, G.; Camm, A.J.; et al. Expert consensus document: Defining the major health modifiers causing atrial fibrillation: A roadmap to underpin personalized prevention and treatment. Nat. Rev. Cardiol. 2016, 13, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Lombardo, M.; Nicolosi, G.L.; Rigamonti, E.; Anza, C. Incremental diagnostic role of left atrial strain analysis in thrombotic risk assessment of nonvalvular atrial fibrillation patients planned for electrical cardioversion. Int. J. Cardiovasc. Imaging 2021, 37, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Lombardo, M.; Nicolosi, G.L.; Gensini, G.F.; Ambrosio, G. Mechanical concordance between left atrium and left atrial appendage in nonvalvular atrial fibrillation: Can it be exploited to avoid transesophageal echocardiography prior to electrical cardioversion during COVID-19 pandemic? Int. J. Cardiovasc. Imaging 2022, 38, 351–362. [Google Scholar] [CrossRef]
- Lisi, M.; Mandoli, G.E.; Cameli, M.; Pastore, M.C.; Righini, F.M.; Benfari, G.; Rubboli, A.; D’Ascenzi, F.; Focardi, M.; Tsioulpas, C.; et al. Left atrial strain by speckle tracking predicts atrial fibrosis in patients undergoing heart transplantation. Eur. Hear. J. Cardiovasc. Imaging 2021, 23, 829–835. [Google Scholar] [CrossRef]
- Kuppahally, S.S.; Akoum, N.; Burgon, N.S.; Badger, T.J.; Kholmovski, E.G.; Vijayakumar, S.; Rao, S.N.; Blauer, J.; Fish, E.N.; Dibella, E.V.; et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: Relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ. Cardiovasc. Imaging 2010, 3, 231–239. [Google Scholar] [CrossRef]
- Hopman, L.; Mulder, M.J.; van der Laan, A.M.; Demirkiran, A.; Bhagirath, P.; van Rossum, A.C.; Allaart, C.P.; Gotte, M.J.W. Impaired left atrial reservoir and conduit strain in patients with atrial fibrillation and extensive left atrial fibrosis. J. Cardiovasc. Magn. Reson. 2021, 23, 131. [Google Scholar] [CrossRef]
- Lisi, M.; Cameli, M.; Righini, F.M.; Malandrino, A.; Tacchini, D.; Focardi, M.; Tsioulpas, C.; Bernazzali, S.; Tanganelli, P.; Maccherini, M.; et al. RV Longitudinal Deformation Correlates with Myocardial Fibrosis in Patients with End-Stage Heart Failure. JACC Cardiovasc. Imaging 2015, 8, 514–522. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, L.; Xie, Y.; Zhang, Y.; Zhu, S.; Wu, C.; Sun, W.; Li, M.; Gao, Y.; Wang, B.; et al. 3-Dimensional Versus 2-Dimensional STE for Right Ventricular Myocardial Fibrosis in Patients with End-Stage Heart Failure. JACC Cardiovasc. Imaging 2021, 14, 1309–1320. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Lauciello, R.; Zuchi, C.; Mengoni, A.; Bardelli, G.; Alunni, G.; Gronda, E.G.; Ambrosio, G. Superior Prognostic Value of Right Ventricular Free Wall Compared to Global Longitudinal Strain in Patients with Heart Failure. J. Am. Soc. Echocardiogr. 2019, 32, 836-844.e1. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Pieske-Kraigher, E.; Cuspidi, C.; Morris, D.A.; Burkhardt, F.; Baudisch, A.; Hassfeld, S.; Tschope, C.; Pieske, B. Right ventricular strain in heart failure: Clinical perspective. Arch. Cardiovasc. Dis. 2017, 110, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, M.; Citarelli, G.; Antoncecchi, V.; Romito, R.; Monitillo, F.; Leone, M.; Puzzovivo, A.; Lattarulo, M.S.; Rizzo, C.; Caldarola, P.; et al. Right Ventricular Longitudinal Strain Measures Independently Predict Chronic Heart Failure Mortality. Echocardiography 2016, 33, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Negishi, K.; Thavendiranathan, P.; Cho, G.Y.; Popescu, B.A.; Vinereanu, D.; Kurosawa, K.; Penicka, M.; Marwick, T.H. Effect of Experience and Training on the Concordance and Precision of Strain Measurements. JACC Cardiovasc. Imaging 2017, 10, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Rosner, A.; Barbosa, D.; Aarsaether, E.; Kjonas, D.; Schirmer, H.; D’Hooge, J. The influence of frame rate on two-dimensional speckle-tracking strain measurements: A study on silico-simulated models and images recorded in patients. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1137–1147. [Google Scholar] [CrossRef]
- Nicolosi, G.L. The strain and strain rate imaging paradox in echocardiography: Overabundant literature in the last two decades but still uncertain clinical utility in an individual case. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e297–e305. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Daraban, A.M.; Unlu, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181.e1172. [Google Scholar] [CrossRef]
- Mirea, O.; Pagourelias, E.D.; Duchenne, J.; Bogaert, J.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Force EA-A-IST: Variability and Reproducibility of Segmental Longitudinal Strain Measurement: A Report From the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc. Imaging 2018, 11, 15–24. [Google Scholar] [CrossRef]
- Mirea, O.; Pagourelias, E.D.; Duchenne, J.; Bogaert, J.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Force EA-A-IST: Intervendor Differences in the Accuracy of Detecting Regional Functional Abnormalities: A Report From the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc. Imaging 2018, 11, 25–34. [Google Scholar] [CrossRef]
- Zghal, F.; Bougteb, H.; Reant, P.; Lafitte, S.; Roudaut, R. Assessing global and regional left ventricular myocardial function in elderly patients using the bidimensional strain method. Echocardiography 2011, 28, 978–982. [Google Scholar] [CrossRef]
- Takigiku, K.; Takeuchi, M.; Izumi, C.; Yuda, S.; Sakata, K.; Ohte, N.; Tanabe, K.; Nakatani, S. Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ. J. 2012, 76, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Innocenti, F.; Guzzo, A.; Guerrini, E.; Vignaroli, D.; Pini, R. Left Ventricular Systolic Longitudinal Function as Predictor of Outcome in Patients with Sepsis. Circ. Cardiovasc. Imaging 2015, 8, e003865. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, T.; Agarwal, S.; Popovic, Z.B.; Marwick, T.H. Normal ranges of left ventricular strain: A meta-analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.A.; Mota, C.C.; Simoes, E.S.A.C.; Nunes Mdo, C.; Barbosa, M.M. Assessing pre-clinical ventricular dysfunction in obese children and adolescents: The value of speckle tracking imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Kaier, T.E.; Morgan, D.; Grapsa, J.; Demir, O.M.; Paschou, S.A.; Sundar, S.; Hakky, S.; Purkayastha, S.; Connolly, S.; Fox, K.F.; et al. Ventricular remodelling post-bariatric surgery: Is the type of surgery relevant? A prospective study with 3D speckle tracking. Eur. Hear. J. Cardiovasc. Imaging 2014, 15, 1256–1262. [Google Scholar] [CrossRef]
- Vitarelli, A.; Martino, F.; Capotosto, L.; Martino, E.; Colantoni, C.; Ashurov, R.; Ricci, S.; Conde, Y.; Maramao, F.; Vitarelli, M.; et al. Early myocardial deformation changes in hypercholesterolemic and obese children and adolescents: A 2D and 3D speckle tracking echocardiography study. Medicine (Baltimore) 2014, 93, e71. [Google Scholar] [CrossRef]
- Holland, D.J.; Marwick, T.H.; Haluska, B.A.; Leano, R.; Hordern, M.D.; Hare, J.L.; Fang, Z.Y.; Prins, J.B.; Stanton, T. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart 2015, 101, 1061–1066. [Google Scholar] [CrossRef]
- Cvijic, M.; Voigt, J.U. Application of strain echocardiography in valvular heart diseases. Anatol. J. Cardiol. 2020, 23, 244–253. [Google Scholar] [CrossRef]
- Voigt, J.U.; Cvijic, M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc. Imaging 2019, 12, 1849–1863. [Google Scholar] [CrossRef]
- Li, S.; Yang, J.; Zhu, L.; Li, Y.; Peng, H.; Lin, Y.; Li, X.; Huang, Z.; Wang, H. Left Ventricular Mechanics Assessed by 2-dimensional Speckle Tracking Echocardiography in Children and Adolescents with Idiopathic Scoliosis. Clin. Spine Surg. 2017, 30, E381–E389. [Google Scholar] [CrossRef]
- Chao, C.-J.; Jaroszewski, D.; Gotway, M.; Ewais, M.; Wilansky, S.; Lester, S.; Unzek, S.; Appleton, C.P.; Chaliki, H.P.; Gaitan, B.D.; et al. Effects of Pectus Excavatum Repair on Right and Left Ventricular Strain. Ann. Thorac. Surg. 2018, 105, 294–301. [Google Scholar] [CrossRef]
- Jaroszewski, D.E.; Velazco, C.S.; Pulivarthi, V.; Arsanjani, R.; Obermeyer, R.J. Cardiopulmonary Function in Thoracic Wall Deformities: What Do We Really Know? Eur. J. Pediatr. Surg. 2018, 28, 327–346. [Google Scholar] [PubMed]
- Sonaglioni, A.; Baravelli, M.; Vincenti, A.; Trevisan, R.; Zompatori, M.; Nicolosi, G.L.; Lombardo, M.; Anza, C. A New modified anthropometric haller index obtained without radiological exposure. Int. J. Cardiovasc. Imaging 2018, 34, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Archer, J.E.; Gardner, A.; Berryman, F.; Pynsent, P. The measurement of the normal thorax using the Haller index methodology at multiple vertebral levels. J. Anat. 2016, 229, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Lombardo, M.; Anza, C.; Ambrosio, G. Reduced Myocardial Strain Parameters in Subjects with Pectus Excavatum: Impaired Myocardial Function or Methodological Limitations Due to Chest Deformity? Semin. Thorac. Cardiovasc. Surg. 2021, 33, 251–262. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Braga, M.; Villa, M.C.; Migliori, C.; Lombardo, M. Does chest wall conformation influence myocardial strain parameters in infants with pectus excavatum? J. Clin. Ultrasound 2021, 49, 918–928. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M.; Gensini, G.F.; Ambrosio, G. Influence of chest conformation on myocardial strain parameters in healthy subjects with mitral valve prolapse. Int. J. Cardiovasc. Imaging 2021, 37, 1009–1022. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Esposito, V.; Caruso, C.; Nicolosi, G.L.; Bianchi, S.; Lombardo, M.; Gensini, G.F.; Ambrosio, G. Chest conformation spuriously influences strain parameters of myocardial contractile function in healthy pregnant women. J. Cardiovasc. Med. 2021, 22, 767–779. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Trevisan, R.; Granato, A.; Zompatori, M.; Lombardo, M. Modified Haller index validation and correlation with left ventricular strain in a cohort of subjects with obesity and without overt heart disease. Intern. Emerg. Med. 2022. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Rigamonti, E.; Nicolosi, G.L.; Lombardo, M. Prognostic Value of Modified Haller Index in Patients with Suspected Coronary Artery Disease Referred for Exercise Stress Echocardiography. J. Cardiovasc. Echogr. 2021, 31, 85–95. [Google Scholar]
- Sonaglioni, A.; Rigamonti, E.; Nicolosi, G.L.; Lombardo, M. Appropriate use criteria implementation with modified Haller index for predicting stress echocardiographic results and outcome in a population of patients with suspected coronary artery disease. Int. J. Cardiovasc. Imaging 2021, 37, 2917–2930. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. Impact of Chest Wall Conformation on the Outcome of Primary Mitral Regurgitation due to Mitral Valve Prolapse. J. Cardiovasc. Echogr. 2022, 32, 29–37. [Google Scholar] [PubMed]
- Raafs, A.G.; Verdonschot, J.A.; Henkens, M.T.; Adriaans, B.P.; Wang, P.; Derks, K.; Hamid, M.A.A.; Knackstedt, C.; Empel, V.P.; Díez, J.; et al. The combination of carboxy-terminal propeptide of procollagen type I blood levels and late gadolinium enhancement at cardiac magnetic resonance provides additional prognostic information in idiopathic dilated cardiomyopathy—A multilevel assessment of myocardial fibrosis in dilated cardiomyopathy. Eur. J. Heart Fail. 2021, 23, 933–944. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Radmilovic, J.; Ballo, P.; Mele, D.; Agricola, E.; Cameli, M.; Rossi, A.; Esposito, R.; Novo, G.; Mondillo, S.; et al. Left ventricular hypertrophy or storage disease? the incremental value of speckle tracking strain bull’s-eye. Echocardiography 2017, 34, 746–759. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Rev. Esp. Cardiol. 2016, 69, 1167. [Google Scholar] [PubMed]
- Braunwald, E. The war against heart failure: The Lancet lecture. Lancet 2015, 385, 812–824. [Google Scholar] [CrossRef]
- Berra, C.; Manfrini, R.; Regazzoli, D.; Radaelli, M.G.; Disoteo, O.; Sommese, C.; Fiorina, P.; Ambrosio, G.; Folli, F. Blood pressure control in type 2 diabetes mellitus with arterial hypertension. The important ancillary role of SGLT2-inhibitors and GLP1-receptor agonists. Pharmacol. Res. 2020, 160, 105052. [Google Scholar] [CrossRef]
- Berra, C.C.; Resi, V.; Mirani, M.; Folini, L.; Rossi, A.; Solerte, S.B.; Fiorina, P. Clinical efficacy and predictors of response to dulaglutide in type-2 diabetes. Pharmacol. Res. 2020, 159, 104996. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11 Pt 1, 260–274. [Google Scholar] [CrossRef]
- Dahlslett, T.; Karlsen, S.; Grenne, B.; Eek, C.; Sjoli, B.; Skulstad, H.; Smiseth, O.A.; Edvardsen, T.; Brunvand, H. Early assessment of strain echocardiography can accurately exclude significant coronary artery stenosis in suspected non-ST-segment elevation acute coronary syndrome. J. Am. Soc. Echocardiogr. 2014, 27, 512–519. [Google Scholar] [CrossRef]
- Kansal, M.M.; Mansour, I.N.; Ismail, S.; Bress, A.; Wu, G.; Mirza, O.; Marpadga, R.; Gheith, H.; Kim, Y.; Li, Y.; et al. Left ventricular global longitudinal strain predicts mortality and heart failure admissions in African American patients. Clin. Cardiol. 2017, 40, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Thellier, N.; Altes, A.; Appert, L.; Binda, C.; Leman, B.; Marsou, W.; Debry, N.; Joly, C.; Ennezat, P.-V.; Tribouilloy, C.; et al. Prognostic Importance of Left Ventricular Global Longitudinal Strain in Patients with Severe Aortic Stenosis and Preserved Ejection Fraction. J. Am. Soc. Echocardiogr. 2020, 33, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; La Sala, L. Molecular Approaches and Echocardiographic Deformation Imaging in Detecting Myocardial Fibrosis. Int. J. Mol. Sci. 2022, 23, 10944. https://doi.org/10.3390/ijms231810944
Sonaglioni A, Nicolosi GL, Rigamonti E, Lombardo M, La Sala L. Molecular Approaches and Echocardiographic Deformation Imaging in Detecting Myocardial Fibrosis. International Journal of Molecular Sciences. 2022; 23(18):10944. https://doi.org/10.3390/ijms231810944
Chicago/Turabian StyleSonaglioni, Andrea, Gian Luigi Nicolosi, Elisabetta Rigamonti, Michele Lombardo, and Lucia La Sala. 2022. "Molecular Approaches and Echocardiographic Deformation Imaging in Detecting Myocardial Fibrosis" International Journal of Molecular Sciences 23, no. 18: 10944. https://doi.org/10.3390/ijms231810944
APA StyleSonaglioni, A., Nicolosi, G. L., Rigamonti, E., Lombardo, M., & La Sala, L. (2022). Molecular Approaches and Echocardiographic Deformation Imaging in Detecting Myocardial Fibrosis. International Journal of Molecular Sciences, 23(18), 10944. https://doi.org/10.3390/ijms231810944







