The Role of Plant Progesterone in Regulating Growth, Development, and Biotic/Abiotic Stress Responses
Abstract
:1. Introduction
2. Metabolism of Progesterone in Plants
3. Research on Progesterone Receptors in Plants
4. The Regulation of Progesterone on Plant Growth and Development
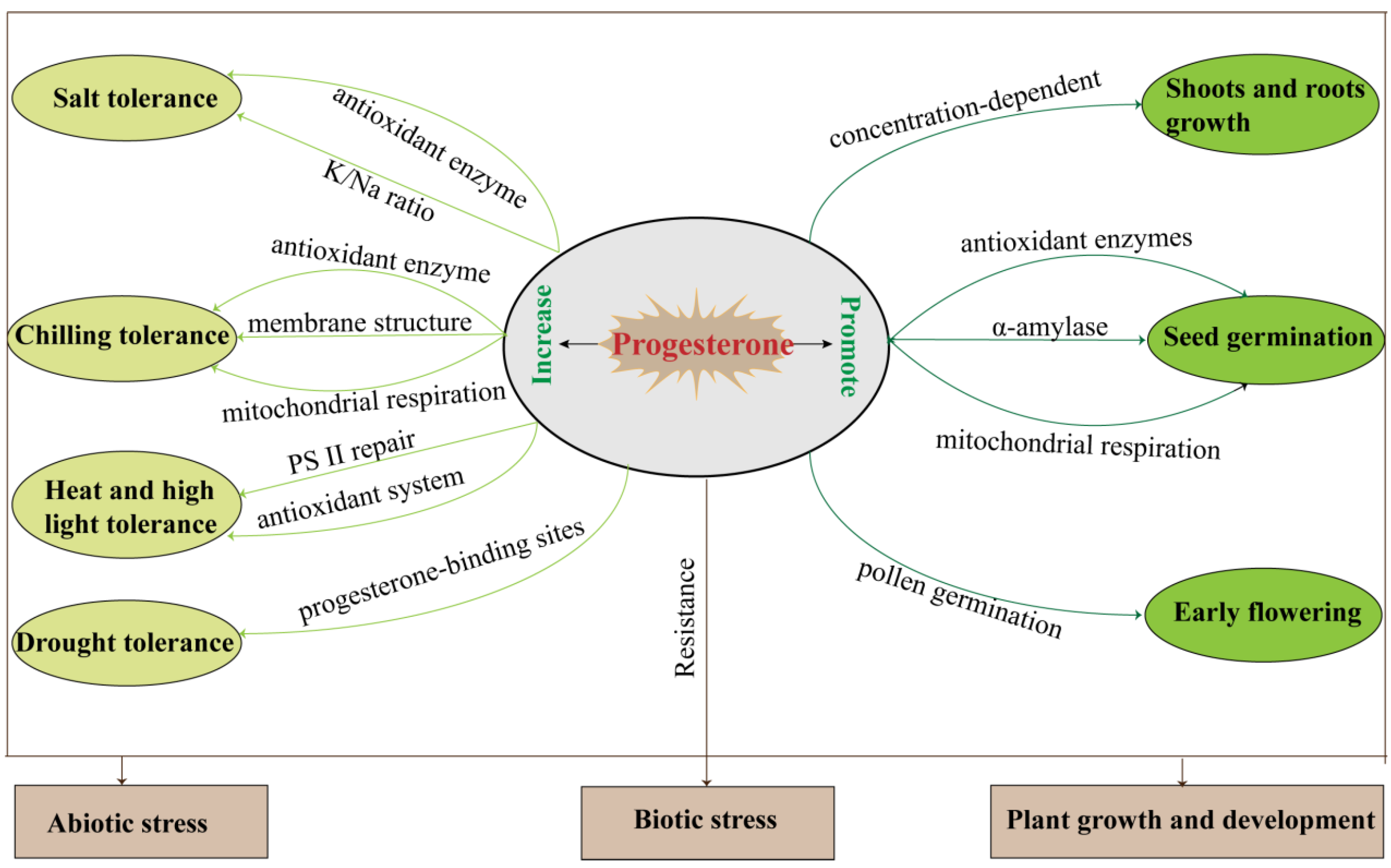
4.1. Shoot and Root Growth
4.2. Seed Germination
4.3. Reproductive Development
5. Plant Biotic and Abiotic Stress Regulation by Progesterone
5.1. Salt Stress
5.2. Chilling Stress
5.3. Drought Stress
5.4. Heat and High Light
5.5. Biotic Stresses
6. The Regulation of Progesterone on Photosynthesis
7. Correlation between Progesterone and Brassinosteroid
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gompel, A. Progesterone and endometrial cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Sherman, M.E.; Kannan, N.; Stanczyk, F.Z. Progesterone and breast cancer. Endocr. Rev. 2020, 41, 320–344. [Google Scholar] [CrossRef] [PubMed]
- González-Orozco, J.C.; Camacho-Arroyo, I. Progesterone actions during central nervous system development. Front. Neurosci. 2019, 13, 503. [Google Scholar] [CrossRef]
- Guennoun, R. Progesterone in the brain: Hormone, neurosteroid and neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. [Google Scholar] [CrossRef] [PubMed]
- Brotfain, E.; Gruenbaum, S.E.; Boyko, M.; Kutz, R.; Zlotnik, A.; Klein, M. Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr. Neuropharmacol. 2016, 14, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.W.; Yeatts, S.D.; Silbergleit, R. Progesterone in traumatic brain injury. N. Engl. J. Med. 2015, 372, 1766–1767. [Google Scholar] [CrossRef] [PubMed]
- Gawienowski, A.M.; Gibbs, C.C. Identification of cholesterol and progesterone in apple seeds. Steroids 1968, 12, 545–550. [Google Scholar] [CrossRef]
- Šaden-Krehula, M.; Tajić, M.; Kolbah, D. Sex hormones and corticosteroids in pollen of Pinus nigra. Phytochemistry 1979, 18, 345–346. [Google Scholar] [CrossRef]
- Carson, J.D.; Jenkins, R.L.; Wilson, E.M.; Howell, W.M.; Moore, R. Naturally occurring progesterone in loblolly pine (Pinus taeda L.): A major steroid precursor of environmental androgens. Environ. Toxicol. Chem. Int. J. 2008, 27, 1273–1278. [Google Scholar] [CrossRef]
- Simons, R.; Grinwich, D. Immunoreactive detection of four mammalian steroids in plants. Can. J. Bot. 1989, 67, 288–296. [Google Scholar] [CrossRef]
- Iino, M.; Nomura, T.; Tamaki, Y.; Yamada, Y.; Yoneyama, K.; Takeuchi, Y.; Mori, M.; Asami, T.; Nakano, T.; Yokota, T. Progesterone: Its occurrence in plants and involvement in plant growth. Phytochemistry 2007, 68, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Simerský, R.; Novák, O.; Morris, D.A.; Pouzar, V.; Strnad, M. Identification and quantification of several mammalian steroid hormones in plants by UPLC-MS/MS. J. Plant Growth Regul. 2009, 28, 125–136. [Google Scholar] [CrossRef]
- Pauli, G.F.; Friesen, J.B.; Gödecke, T.; Farnsworth, N.R.; Glodny, B. Occurrence of progesterone and related animal steroids in two higher plants. J. Nat. Prod. 2010, 73, 338–345. [Google Scholar] [CrossRef]
- Bennett, R.; Heftmann, E.; Winter, B.J. Conversion of sitosterol to progesterone by Digitalis lanata. Die Nat. 1969, 56, 463. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.; El-Olemy, M. Pregnenolone and progesterone from 20α-hydroxycholesterol by Cheiranthus cheiri leaf homogenates. Phytochemistry 1971, 10, 3053–3056. [Google Scholar] [CrossRef]
- Lindemann, P.; Luckner, M. Biosynthesis of pregnane derivatives in somatic embryos of Digitalis lanata. Phytochemistry 1997, 46, 507–513. [Google Scholar] [CrossRef]
- Sundararaman, P.; Djerassi, C. A convenient synthesis of progesterone from stigmasterol. J. Org. Chem. 1977, 42, 3633–3634. [Google Scholar] [CrossRef]
- Janeczko, A. The presence and activity of progesterone in the plant kingdom. Steroids 2012, 77, 169–173. [Google Scholar] [CrossRef]
- Aringer, L.; Eneroth, P.; Nordström, L. Side-chain cleavage of 4-cholesten-3-one, 5-cholesten-3α-ol, β-sitosterol, and related steroids in endocrine tissues from rat and man. J. Steroid Biochem. 1979, 11, 1271–1285. [Google Scholar] [CrossRef]
- Hoyte, R.; RB, H. Enzymatic side chain cleavage of c-20 alkyl and aryl analogs of (20-s)-20-hydroxycholesterol: Implications for the biosynthesis of pregnenolone. J. Biol. Chem. 1979, 254, 2278–2286. [Google Scholar] [CrossRef]
- Lindemann, P. Steroidogenesis in plants–Biosynthesis and conversions of progesterone and other pregnane derivatives. Steroids 2015, 103, 145–152. [Google Scholar] [CrossRef]
- Seidel, S.; Kreis, W.; Reinhard, E. D5-3b-Hydroxysteroid dehydrogenase/D5-D4-ketosteroid isomerase (3b-HSD), a possible enzyme of cardiac glycoside biosynthesis, in cell cultures and plants of Digitalis lanata EHRH. Plant Cell Rep. 1990, 8, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, D.E.; Keilholz, W.; Seitz, H.U. Purification, characterization and partial peptide microsequencing of progesterone 5β-reductase from shoot cultures of Digitalis purpurea. Eur. J. Biochem. 1994, 225, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Herl, V.; Fischer, G.; Müller-Uri, F.; Kreis, W. Molecular cloning and heterologous expression of progesterone 5β-reductase from Digitalis lanata Ehrh. Phytochemistry 2006, 67, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, D.E.; Wendroth, S.; Seitz, H.U. A stereospecific enzyme of the putative biosynthetic pathway of cardenolides: Characterization of a progesterone 5β-reductase from leaves of Digitalis purpurea L. FEBS Lett. 1990, 271, 239–242. [Google Scholar] [CrossRef]
- Caspi, E.; Lewis, D. Progesterone: Its possible role in the biosynthesis of cardenolides in Digitalis lanata. Science 1967, 156, 519–520. [Google Scholar] [CrossRef]
- Klein, J.; Horn, E.; Ernst, M.; Leykauf, T.; Leupold, T.; Dorfner, M.; Wolf, L.; Ignatova, A.; Kreis, W.; Munkert, J. RNAi-mediated gene knockdown of progesterone 5β-reductases in Digitalis lanata reduces 5β-cardenolide content. Plant Cell Rep. 2021, 40, 1631–1646. [Google Scholar] [CrossRef]
- Herl, V.; Fischer, G.; Reva, V.; Stiebritz, M.; Muller, Y.; Müller-Uri, F.; Kreis, W. The VEP1 gene (At4g24220) encodes a short-chain dehydrogenase/reductase with 3-oxo-Δ4, 5-steroid 5β-reductase activity in Arabidopsis thaliana L. Biochimie 2009, 91, 517–525. [Google Scholar] [CrossRef]
- Kairuz, E.; Pérez-Alonso, N.; Capote-Pérez, A.; Pérez-Pérez, A.; Espinosa-Antón, A.A.; Angenon, G.; Jiménez, E.; Chong-Pérez, B. Enhancement of cardenolide production in transgenic Digitalis purpurea L. by expressing a progesterone-5β-reductase from Arabidopsis thaliana L. Ind. Crops Prod. 2020, 146, 112166. [Google Scholar] [CrossRef]
- Bauer, P.; Munkert, J.; Brydziun, M.; Burda, E.; Muller-Uri, F.; Groger, H.; Muller, Y.; Kreis, W. Highly conserved progesterone 5b-reductase genes (P5bR) from 5b-cardenolide-free and 5b-cardenolide-producing angiosperms. Phytochemistry 2010, 71, 1495–1505. [Google Scholar] [CrossRef]
- Pérez-Bermúdez, P.; Moya Garcia, A.A.; Tuñón, I.; Gavidia, I. Digitalis purpurea P5βR2, encoding steroid 5β-reductase, is a novel defense-related gene involved in cardenolide biosynthesis. New Phytol. 2010, 185, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Wendroth, S.; Seitz, H.U. Characterization and localization of progesterone 5 α-reductase from cell cultures of foxglove (Digitalis lanata EHRH). Biochem. J. 1990, 266, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Biswas, M.G.; Chao, A.; Russell, D.W.; Chory, J. Conservation of function between mammalian and plant steroid 5α-reductases. Proc. Natl. Acad. Sci. USA 1997, 94, 3554–3559. [Google Scholar] [CrossRef] [PubMed]
- Rosati, F.; Bardazzi, I.; De Blasi, P.; Simi, L.; Scarpi, D.; Guarna, A.; Serio, M.; Racchi, M.L.; Danza, G. 5α-Reductase activity in Lycopersicon esculentum: Cloning and functional characterization of LeDET2 and evidence of the presence of two isoenzymes. J. Steroid Biochem. Mol. Biol. 2005, 96, 287–299. [Google Scholar] [CrossRef]
- Rosati, F.; Danza, G.; Guarna, A.; Cini, N.; Racchi, M.L.; Serio, M. New evidence of similarity between human and plant steroid metabolism: 5α-reductase activity in Solanum malacoxylon. Endocrinology 2003, 144, 220–229. [Google Scholar] [CrossRef]
- Yang, X.-H.; Xu, Z.-H.; Xue, H.-W. Arabidopsis membrane steroid binding protein 1 is involved in inhibition of cell elongation. Plant Cell 2005, 17, 116–131. [Google Scholar] [CrossRef]
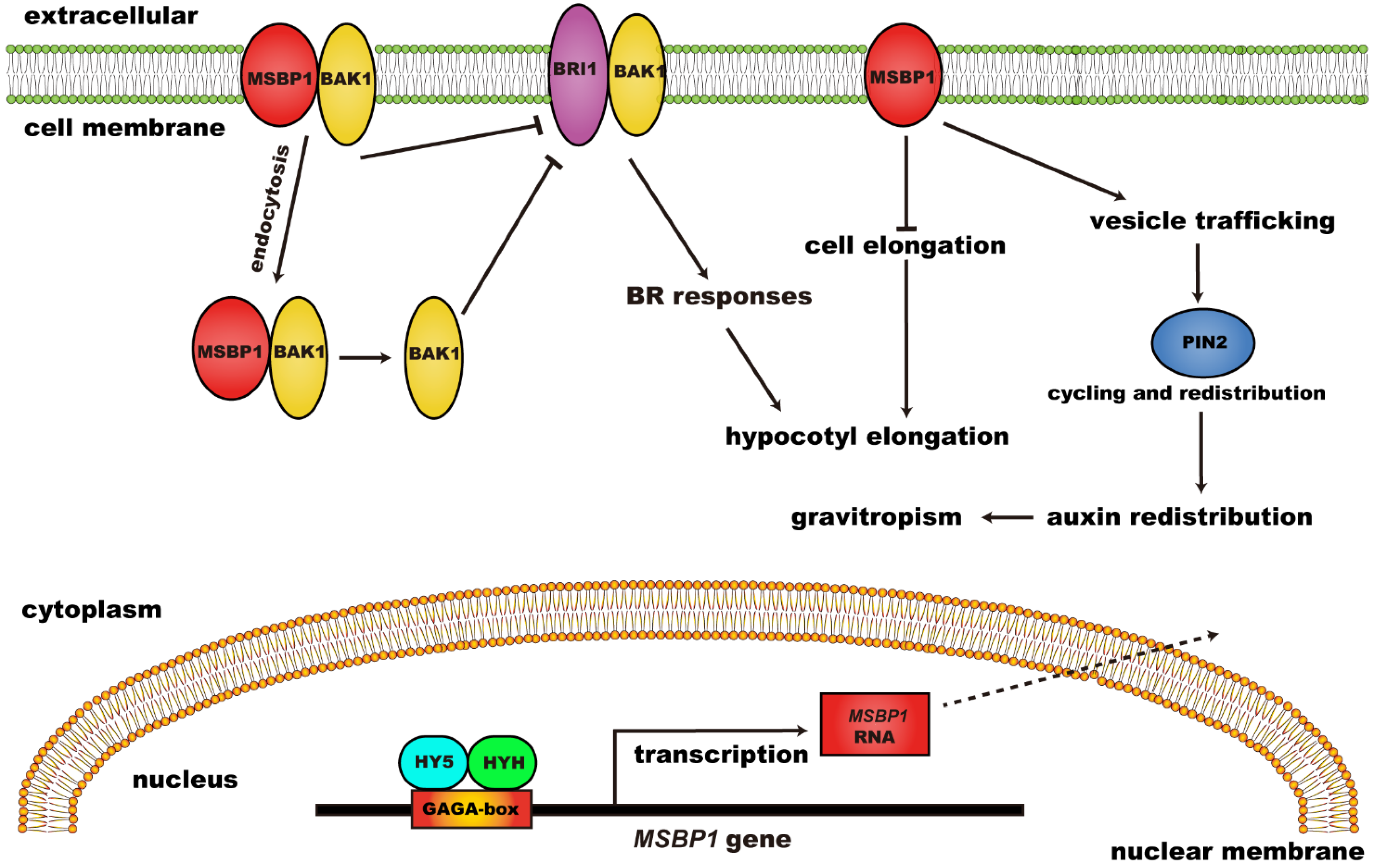
- Song, L.; Shi, Q.-M.; Yang, X.-H.; Xu, Z.-H.; Xue, H.-W. Membrane steroid-binding protein 1 (MSBP1) negatively regulates brassinosteroid signaling by enhancing the endocytosis of BAK1. Cell Res. 2009, 19, 864–876. [Google Scholar] [CrossRef]
- Shi, Q.-M.; Yang, X.; Song, L.; Xue, H.-W. Arabidopsis MSBP1 is activated by HY5 and HYH and is involved in photomorphogenesis and brassinosteroid sensitivity regulation. Mol. Plant 2011, 4, 1092–1104. [Google Scholar] [CrossRef]
- Yang, X.; Song, L.; Xue, H.-W. Membrane steroid binding protein 1 (MSBP1) stimulates tropism by regulating vesicle trafficking and auxin redistribution. Mol. Plant 2008, 1, 1077–1087. [Google Scholar] [CrossRef]
- Janeczko, A.; Budziszewska, B.; Skoczowski, A.; Dybała, M. Specific binding sites for progesterone and 17beta-estradiol in cells of Triticum aestivum L. Acta Biochim. Pol. 2008, 55, 707–711. [Google Scholar] [CrossRef]
- Janeczko, A.; Oklešťková, J.; Siwek, A.; Dziurka, M.; Pociecha, E.; Kocurek, M.; Novák, O. Endogenous progesterone and its cellular binding sites in wheat exposed to drought stress. J. Steroid Biochem. Mol. Biol. 2013, 138, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, B.; Gupta, K. Steroid hormone effects on growth and apical dominance of sunflower. Phytochemistry 1981, 20, 989–991. [Google Scholar] [CrossRef]
- Erdal, S.; Dumlupinar, R. Mammalian sex hormones stimulate antioxidant system and enhance growth of chickpea plants. Acta Physiol. Plant. 2011, 33, 1011–1017. [Google Scholar] [CrossRef]
- Dadaşoğlu, E.; Tosun, M. The effect of mammalian sex hormones on in vitro properties of sainfoin (Onobrychis sativa L.). Fresenius Environ. Bull. 2018, 27, 7222–7229. [Google Scholar]
- Turkoglu, A. Effects of mammalian sex hormones on regeneration capacity, retrotransposon polymorphism and genomic instability in wheat (Triticum aestivum L.). 2022; preprints. [Google Scholar]
- Erdal, S.; Dumlupinar, R. Progesterone and β-estradiol stimulate seed germination in chickpea by causing important changes in biochemical parameters. Z. Nat. C 2010, 65, 239–244. [Google Scholar] [CrossRef]
- Erdal, S. Effects of mammalian sex hormones on antioxidant enzyme activities, H2O2 content and lipid peroxidation in germinating bean seeds. J. Fac. Agric. 2009, 40, 79–85. [Google Scholar]
- Erdal, S.; Dumlupinar, R.; Cakmak, T.; Genisel, M. Mammalian sex hormones influence germination velocity and enzyme activities in germinating maize seeds. Fresenius Environ. Bull. 2010, 19, 1458–1465. [Google Scholar]
- Turk, H. Progesterone Promotes Mitochondrial Respiration at the Biochemical and Molecular Level in Germinating Maize Seeds. Plants 2021, 10, 1326. [Google Scholar] [CrossRef]
- Speranza, A.; Crosti, P.; Malerba, M.; Stocchi, O.; Scoccianti, V. The environmental endocrine disruptor, bisphenol A, affects germination, elicits stress response and alters steroid hormone production in kiwifruit pollen. Plant Biol. 2011, 13, 209–217. [Google Scholar] [CrossRef]
- Ylstra, B.; Touraev, A.; Brinkmann, A.O.; Heberle-Bors, E.; Tunen, A. Steroid hormones stimulate germination and tube growth of in vitro matured tobacco pollen. Plant Physiol. 1995, 107, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Spivak, S.; Berdichevets, I.; Yarmolinsky, D.; Maneshina, T.; Shpakovski, G.; Kartel, N. Construction and characteristics of transgenic tobacco Nicotiana tabacum L. plants expressing CYP11A1 cDNA encoding cytochrome P450SCC. Russ. J. Genet. 2009, 45, 1067–1073. [Google Scholar] [CrossRef]
- Janeczko, A.; Filek, W. Stimulation of generative development in partly vernalized winter wheat by animal sex hormones. Acta Physiol. Plant. 2002, 24, 291–295. [Google Scholar] [CrossRef]
- Janeczko, A.; Oklestkova, J.; Novak, O.; Śniegowska-Świerk, K.; Snaczke, Z.; Pociecha, E. Disturbances in production of progesterone and their implications in plant studies. Steroids 2015, 96, 153–163. [Google Scholar] [CrossRef]
- Janeczko, A.; Filek, W.; Biesaga-Kościelniak, J.; Marcińska, I.; Janeczko, Z. The influence of animal sex hormones on the induction of flowering in Arabidopsis thaliana: Comparison with the effect of 24-epibrassinolide. Plant Cell Tissue Organ Cult. 2003, 72, 147–151. [Google Scholar] [CrossRef]
- Shpakovski, G.V.; Spivak, S.G.; Berdichevets, I.N.; Babak, O.G.; Kubrak, S.V.; Kilchevsky, A.V.; Aralov, A.V.; Slovokhotov, I.Y.; Shpakovski, D.G.; Baranova, E.N. A key enzyme of animal steroidogenesis can function in plants enhancing their immunity and accelerating the processes of growth and development. BMC Plant Biol. 2017, 17, 189. [Google Scholar] [CrossRef]
- Erdal, S. Alleviation of salt stress in wheat seedlings by mammalian sex hormones. J. Sci. Food Agric. 2012, 92, 1411–1416. [Google Scholar] [CrossRef]
- Erdal, S.; Genisel, M.; Turk, H.; Gorcek, Z. Effects of progesterone application on antioxidant enzyme activities and K+/Na+ ratio in bean seeds exposed to salt stress. Toxicol. Ind. Health 2012, 28, 942–946. [Google Scholar] [CrossRef]
- Erdal, S. Exogenous mammalian sex hormones mitigate inhibition in growth by enhancing antioxidant activity and synthesis reactions in germinating maize seeds under salt stress. J. Sci. Food Agric. 2012, 92, 839–843. [Google Scholar] [CrossRef]
- Sabzmeydani, E.; Sedaghathoor, S.; Hashemabadi, D. Effect of salicylic acid and progesterone on physiological characteristics of Kentucky bluegrass under salinity stress. Rev. Cienc. Agrícolas 2021, 38, 111–124. [Google Scholar] [CrossRef]
- Genisel, M.; Turk, H.; Erdal, S. Exogenous progesterone application protects chickpea seedlings against chilling-induced oxidative stress. Acta Physiol. Plant. 2013, 35, 241–251. [Google Scholar] [CrossRef]
- Erdal, S.; Genisel, M. The property of progesterone to mitigate cold stress in maize is linked to a modulation of the mitochondrial respiratory pathway. Theor. Exp. Plant Physiol. 2016, 28, 385–393. [Google Scholar] [CrossRef]
- Hao, J.; Li, X.; Xu, G.; Huo, Y.; Yang, H. Exogenous progesterone treatment alleviates chilling injury in postharvest banana fruit associated with induction of alternative oxidase and antioxidant defense. Food Chem. 2019, 286, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, S.; Li, X.; Yang, H. Exogenous progesterone alleviates chilling injury by upregulating IbAOX1 to mediate redox homeostasis and proline accumulation in postharvest sweetpotato tuberous root. Postharvest Biol. Technol. 2022, 183, 111738. [Google Scholar] [CrossRef]
- Filek, M.; Rudolphi-Skórska, E.; Sieprawska, A.; Kvasnica, M.; Janeczko, A. Regulation of the membrane structure by brassinosteroids and progesterone in winter wheat seedlings exposed to low temperature. Steroids 2017, 128, 37–45. [Google Scholar] [CrossRef]
- Xue, R.; Wang, S.; Xu, H.; Zhang, P.; Li, H.; Zhao, H. Progesterone increases photochemical efficiency of photosystem II in wheat under heat stress by facilitating D1 protein phosphorylation. Photosynthetica 2017, 55, 664–670. [Google Scholar] [CrossRef]
- Su, X.; Wu, S.; Yang, L.; Xue, R.; Li, H.; Wang, Y.; Zhao, H. Exogenous progesterone alleviates heat and high light stress-induced inactivation of photosystem II in wheat by enhancing antioxidant defense and D1 protein stability. Plant Growth Regul. 2014, 74, 311–318. [Google Scholar] [CrossRef]
- Janeczko, A.; Tóbiás, I.; Skoczowski, A.; Dubert, F.; Gullner, G.; Barna, B. Progesterone moderates damage in Arabidopsis thaliana caused by infection with Pseudomonas syringae or P. fluorescens. Biol. Plant. 2013, 57, 169–173. [Google Scholar] [CrossRef]
- Fujioka, S.; Li, J.; Choi, Y.-H.; Seto, H.; Takatsuto, S.; Noguchi, T.; Watanabe, T.; Kuriyama, H.; Yokota, T.; Chory, J. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell 1997, 9, 1951–1962. [Google Scholar]
- Li, J.; Nagpal, P.; Vitart, V.; McMorris, T.C.; Chory, J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science 1996, 272, 398–401. [Google Scholar] [CrossRef]
- Davies, P.J. The plant hormones: Their nature, occurrence, and functions. In Plant Hormones; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–15. [Google Scholar]

| Plant Growth and Development | Progesterone Action | Plant Species | Reference |
|---|---|---|---|
| Shoot and root growth | Progesterone regulated plant growth in a concentration dependent manner. | Arabidopsis thaliana | [11] |
| Helianthus annuus | [42] | ||
| Cicer arietinum | [43] | ||
| Tissue culture | Progesterone improved shoot and callus formation. | Onobrychis sativa | [44] |
| Progesterone regulated responded embryogenic callus and regenerable callus induction. | Triticum aestivum | [45] | |
| Seed germination | Progesterone accelerated seed germination. | Cicer arietinum | [46] |
| Phaseolus vulgaris | [47] | ||
| Zea mays | [48,49] | ||
| Reproductive development | Progesterone increased gradually with pollen germination. | Actinidia deliciosa | [50] |
| Progesterone stimulated pollen germination and tube elongation. | Nicotiana tabacum | [51,52] | |
| Progesterone induced plant flowering and promoted reproductive growth. | Triticum aestivum | [53,54] | |
| Arabidopsis thaliana | [55] |
| Biotic/Abiotic Stress | Progesterone Action | Plant Species | Reference |
|---|---|---|---|
| Salt | Progesterone stimulated enzymatic and non-enzymatic antioxidant mechanisms and increased the levels of osmoprotectants. | Triticum aestivum | [57] |
| Progesterone stimulated SOD, POX, and CAT activities and mitigated the salt-reduced K/Na ratio. | Phaseolus vulgaris | [58] | |
| Progesterone stimulated antioxidant activity and osmoprotectants accumulation. | Zea mays | [59] | |
| Progesterone improved salinity tolerance and increased pigments and antioxidant enzyme activities. | Poa pratensis | [60] | |
| Chilling | Progesterone improved relative leaf water content, chlorophyll content, and antioxidative activity. | Cicer arietinum | [61] |
| Progesterone enhanced mitochondrial respiratory pathway, and upregulated the transcript level and protein accumulation of alternative oxidase (AOX). | Zea mays | [62] | |
| Progesterone induced AOX and improved enzyme and non-enzymatic antioxidant defenses. | Musa nana Lour | [63] | |
| Progesterone enhanced the transcription level of IbAOX1 and the activity of AOX, inhibited the formation of chilling injury, reduced membrane permeability, malonaldehyde levels, and ROS production, and enhanced the antioxidant protection system. | Ipomoea batatas | [64] | |
| Progesterone increased the area per lipid molecule in monolayers, resulting in formation of more flexible surface structures. | Triticum aestivum | [65] | |
| Drought | Progesterone-binding sites on the cell membrane were increased by drought stress in drought-sensitive cultivar (Katoda) but not in drought-tolerant cultivar (Monsun), while progesterone-binding sites in the cytoplasm were increased by drought in Monsun but not in Katoda. | Triticum aestivum | [41] |
| Overexpressing mammalian CYP11A1 in tomato can significantly increase tolerance to drought and long-term dehydration. | Solanum lycopersicum | [56] | |
| Heat and high light | Progesterone alleviated heat-stress-induced hydrogen peroxide, malondialdehyde, and relative electrolytic leakage, improved the activities of superoxide dismutase, catalase, and peroxidase, and reduced PSII injury by promoting D1 protein phosphorylation. | Triticum aestivum | [66] |
| Progesterone enhanced antioxidant defense system and facilitated D1 protein stability under heat and high light cross-stress. | Triticum aestivum | [67] | |
| Biotic stresses | Progesterone diminished the necrotic symptoms and the electrolyte leakage, and improved the efficiency of photosystem II caused by Pseudomonas bacteria. | Arabidopsis thaliana | [68] |
| CYP11A1-overexpressing transgenic tobacco exhibited resistance to infection by fungal pathogens Botrytis cinerea. | Nicotiana tabacum | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Chen, L.; Chen, H.; Xue, R.; Wang, Y.; Song, J. The Role of Plant Progesterone in Regulating Growth, Development, and Biotic/Abiotic Stress Responses. Int. J. Mol. Sci. 2022, 23, 10945. https://doi.org/10.3390/ijms231810945
Li H, Chen L, Chen H, Xue R, Wang Y, Song J. The Role of Plant Progesterone in Regulating Growth, Development, and Biotic/Abiotic Stress Responses. International Journal of Molecular Sciences. 2022; 23(18):10945. https://doi.org/10.3390/ijms231810945
Chicago/Turabian StyleLi, Hua, Lulu Chen, Hongyu Chen, Ruili Xue, Yuexia Wang, and Jianbo Song. 2022. "The Role of Plant Progesterone in Regulating Growth, Development, and Biotic/Abiotic Stress Responses" International Journal of Molecular Sciences 23, no. 18: 10945. https://doi.org/10.3390/ijms231810945
APA StyleLi, H., Chen, L., Chen, H., Xue, R., Wang, Y., & Song, J. (2022). The Role of Plant Progesterone in Regulating Growth, Development, and Biotic/Abiotic Stress Responses. International Journal of Molecular Sciences, 23(18), 10945. https://doi.org/10.3390/ijms231810945





