Recent Advances of Chitosan Formulations in Biomedical Applications
Abstract
1. Introduction
2. Properties of Chitosan
2.1. Physical Properties
2.1.1. Solubility
2.1.2. Viscosity
2.2. Chemical Properties
2.3. Biodegradability
2.4. Toxicity
3. Methods of Preparation
4. Extraction
4.1. Deproteinization
4.2. Desulfurization
4.3. Decolorization
4.4. Deacetylation
5. Modifications of Chitosan
6. Antimicrobial Properties of Chitosan
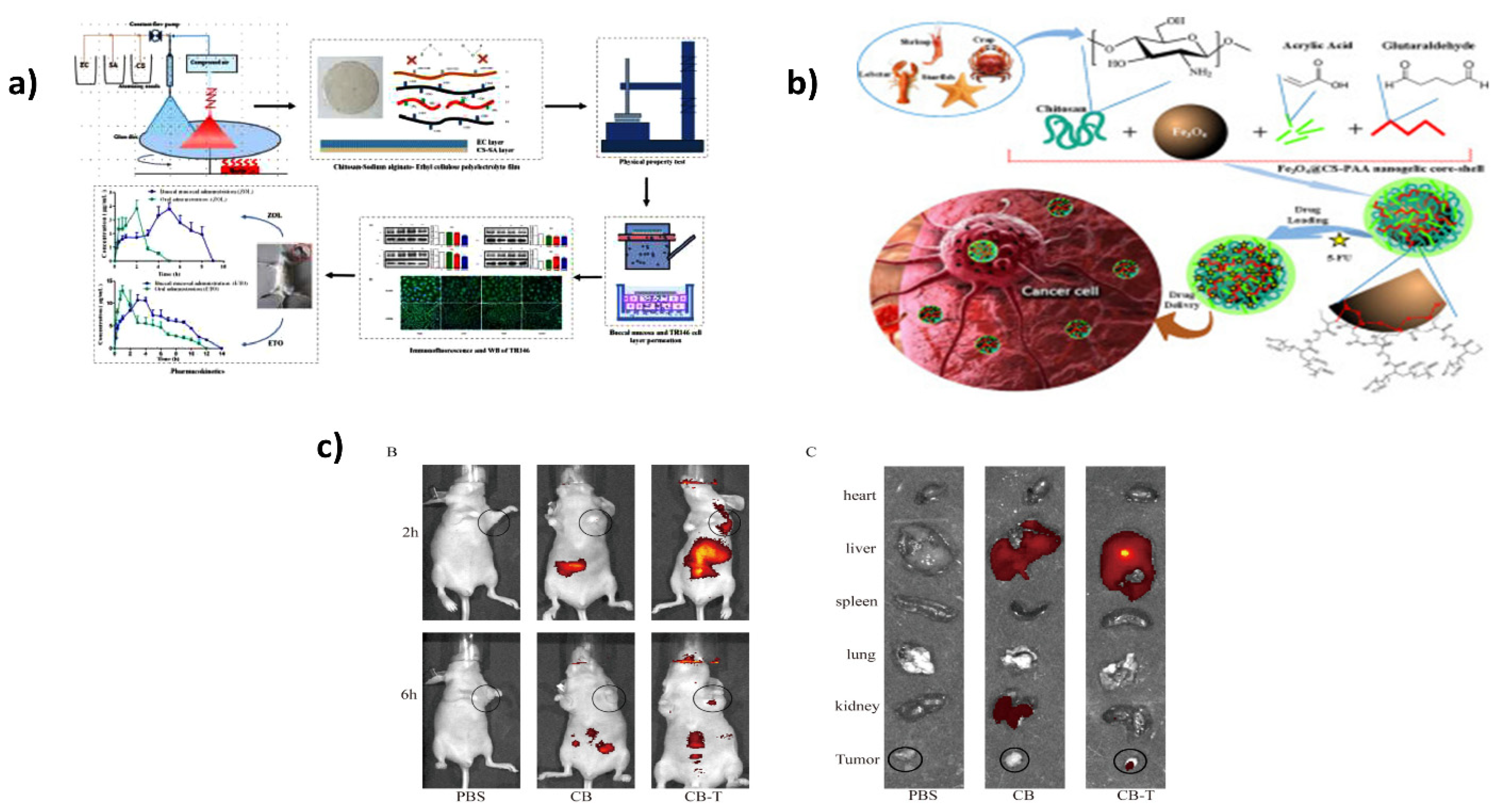
7. Applications in Drug Delivery
8. Applications in Gene Delivery

9. Applications in Protein Delivery

10. Vaccine Delivery
11. Tissue Engineering
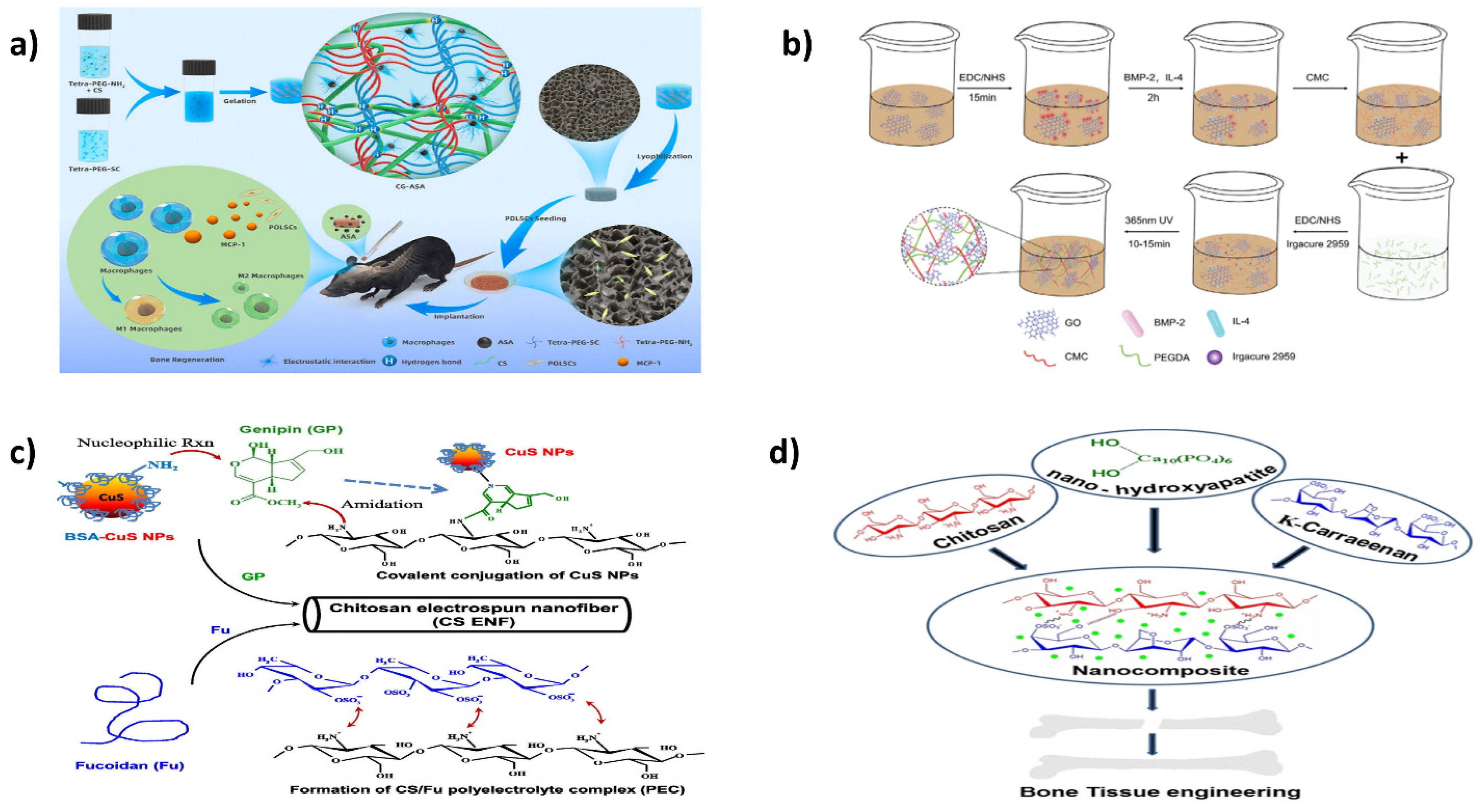
11.1. Bone
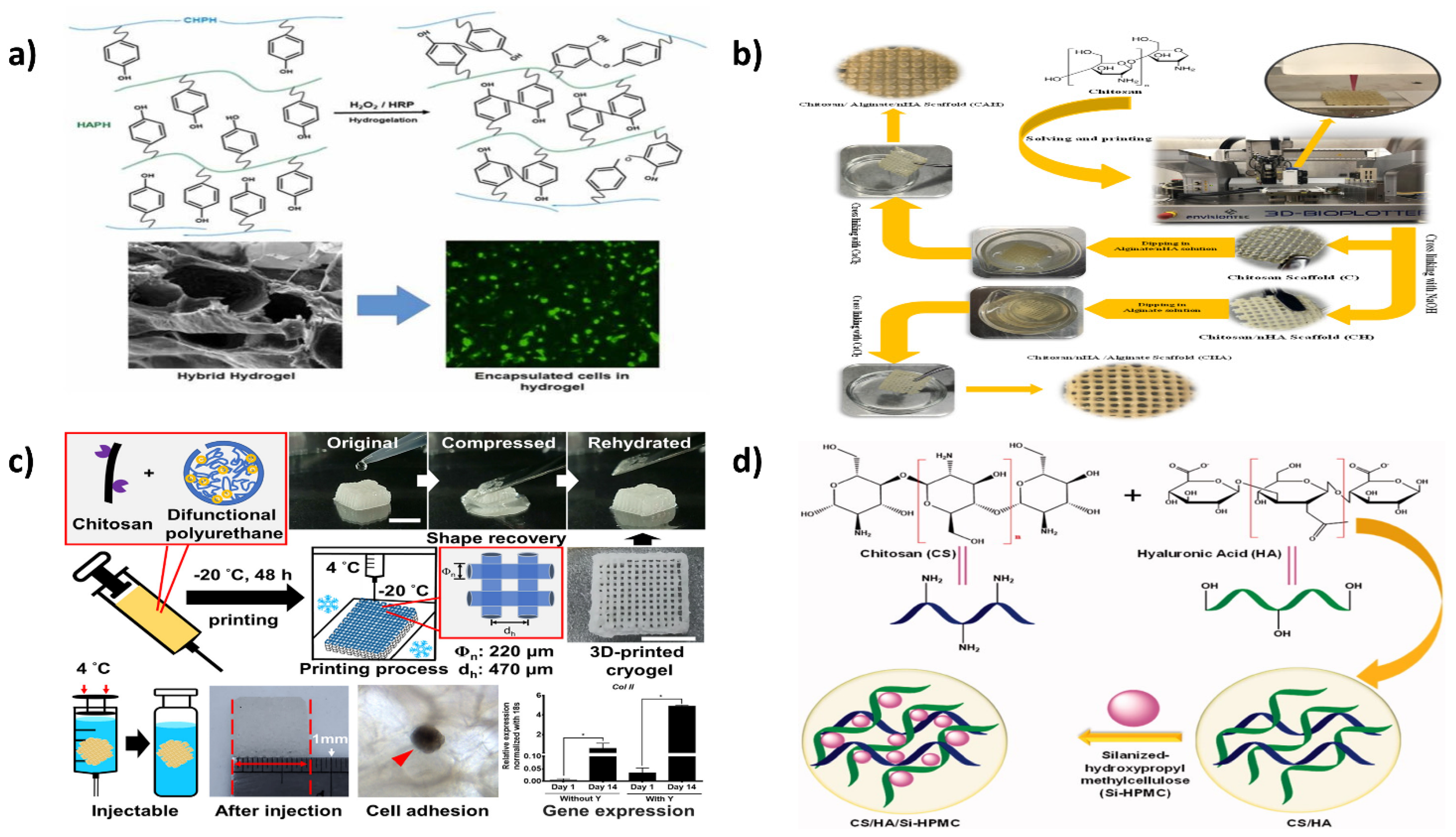
11.2. Cartilage
11.3. Blood Vessel
11.4. Corneal
11.5. Periodontal
11.6. Miscellaneous
11.6.1. Skin
11.6.2. Cardiac Tissue
11.6.3. Connective Tissue
| Device Type | Model Drug/Drug | Polymer Formulation | Preparation Method | Tissue | Effects/Results | Reference |
|---|---|---|---|---|---|---|
| 3D-Nanofibrous scaffold | - | Poly(vinyl alcohol)/keratin/chitosan | Layer-by-layer electrospinning | - | 5% w/v keratin and 2% w/v chitosan electrospun with 10% w/v PVA showed remarkable properties such as high tensile strength, which doubled with increasing polymer concentration from 10 wt.% to 50 wt.%, swelling ratio (over 100%), porosity (82% to 86%). However, after 4 weeks of incubation, the scaffolds degraded significantly (50–66%). | [266] |
| Modified halloysite nanotube-based nanocomposite films | - | Chitosan/PVA/PVP | Solution casting | - | The synthesized nanocomposite films demonstrated enhanced thermal and mechanical attributes, uniform size distribution, surface topology, and enzymatic breakdown, with low swelling ratio and hydrophilic properties. In vitro, MTT and AO-EB assay revealed superior cell proliferation and adhesiveness as compared to neat PVA/PVP films ((118.31 ± 0.68% proliferation by 5 wt.%), and their hemocompatibility with RBCs was low (0.46 ± 0.05%). | [267] |
| Membranes | - | Chitosan/collagen/hydroxyapatite | Solvent casting | Bone/cartilage | Micro- and nanoporous membranes had excellent hydroxyapatite dispersion in the matrix. Thermally stable composites due to the incorporation of hydroxyapatite and collagen. No cytotoxicity and the highest adhesion were found in the membrane with 1.5% w/v Cs. 0.75% w/v collagen and 0.75% w/v hydroxyapatite. | [268] |
| Multilayer scaffold | Chitosan/gelatin/nano-hydroxyapatite | Iterative hierarchical method | Bone/cartilage | Adipose mesenchymal stem cells (ADSCs) differentiated into osteoblasts and chondrocytes similar in morphology to natural tissues, facilitating the expression of both osteogenic genes (OCN, Col I, and Runx2) and chondrogenic genes (ACAN, Sox9, and Col II). | [269] | |
| Electrospun nanofibers | - | Chitosan/polypyrrole/collagen | Electrospinning | Heart/nerve/cardiovascular/skin | 10% w/w polypyrrole-containing scaffolds exhibited optimum mechanical properties, good cell attachment, growth, and differentiation. | [270] |
| Composite scaffold | Chitosan/polyvinyl alcohol/cellulose nanocrystals (CNC)/β-Tricalcium Phosphate | Freeze drying | Bone | 5% and 10% of CNC-based scaffolds exhibited significant calcium deposition after 72 h of culture. | [271] | |
| Hydrogels | Chitosan oxidized quince seed gum/curcumin loaded-halloysite nanotubes | Sonication | - | CS/O-QSG (25:75) exhibited rapid gelation and compression strength, and with 10–30%, CUR-HNTs enhanced cellular growth and proliferation by 150%. | [272] | |
| Hydrogels | Chitosan/mucin/Montmorillonite/hydroxyethyl methacrylate | Freeze drying | - | Good material characteristics such as porosity and water uptake as well as biocompatible with C2C12 and MC3T3E1 cell lines. | [273] | |
| Films | Chitosan/collagen | Dual crosslinking with genipin and tannic acid | Cornea/skin | 80% film retention after 2 weeks of incubating with lipase and lysozyme and biocompatible with mouse fibroblast cells. | [274] | |
| Films | Chitosan/silk fibroin | Solvent casting | Bone/adipose/cartilage/skin | Excellent adhesion, growth, and proliferation of rat bone-marrow-derived mesenchymal stem cells, while promoting osteogenic and adipose differentiation. | [275] | |
| Hydrogels | Chitosan/decellularized annulus fibrosis matrix (DAFM) | Freeze drying | Intervertebral disc | Sustained release of fibroblast growth factors as a stimulus for AFSC growth and expression of ECM factors. | [276] |
12. Wound Healing

13. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5 | FU-5-fluorouracil |
| AC | Adenine-functionalized chitosan |
| ADSCs | Adipose mesenchymal stem cells |
| BP | QCS-quaternized chitosan-modified black phosphorus nanosheets |
| BSA | Bovine serum albumin |
| CMCS | Carboxymethyl chitosan |
| CMCTS | Carboxymethyl chitosan |
| CNC | Cellulose nanocrystals |
| CNs | Chitosan nanoparticles |
| COS | Chitosan-oligosaccharides |
| CPO | Calcium peroxide |
| CS | Chitosan |
| CS/DA | Chitosan/dicarboxylic acid |
| CS-HA | Hydrocaffeic acid-modified chitosan |
| CUR | Curcumin |
| DAFM | Decellularized annulus fibrosis matrix |
| DCs | Dendronized chitosans |
| DD | Degree of deacetylation |
| DDS | Drug delivery systems |
| DTX | Docetaxel |
| EC | Ethylcellulose |
| ECM | Extracellular matrix |
| EPCs | Endothelial progenitor cells |
| F/C | Fucoidan/chitosan |
| GA | Glycyrrhetinic acid |
| GelMA | Gelatin methacryloyl |
| H/Al-MSN | Aluminum-modified mesoporous silica nanoparticles |
| HA | Hyaluronic acid |
| hmCS | Hydrophobically modified chitosan |
| HPLCs | Human periodontal ligament cells |
| HUVECs | Human umbilical vein endothelial cells |
| iSur - | pDNA-survivin shRNA-expressing plasmids |
| LA | Lactobionic acid |
| MACS | Micro-channeled alkylated chitosan sponge |
| MCS | Maleic chitosan |
| MT NPs | p-mercaptobenzoic acid-embedded N, N, N-trimethyl chitosan nanoparticles |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| nHA | Nano-hydroxyapatite |
| NP | Nanoparticle |
| OEG | Oligoethylene glycol |
| OQGG@CMCS | Carboxymethyl chitosan/oxidized quaternized guar gum |
| PAA | Polyacrylic acid |
| PCADK | Poly (cyclohexane-1, 4-diylacetone dimethylene ketal) |
| PCL | Poly(-caprolactone) |
| PEC | Polyelectrolyte complex |
| PEGDA | Poly(ethylene glycol) diacrylate |
| PEM | Polyelectrolyte multilayer |
| PTX | Paclitaxel |
| RHC-CHI | Recombinant human collagen-chitosan |
| rhIL-2 | Recombinant human interleukin-2 |
| ROS | Reactive oxygen species |
| SA | Sodium alginate |
| SAD | Sodium alginate dialdehyde |
| SCO | Splice correction oligonucleotides |
| SHED | Stem cells from human exfoliated deciduous teeth |
| Si-HPMC | Silanized-hydroxypropyl methylcellulose |
| siRNA | Small interfering RNA |
| TPEG | Thiol-terminated poly(ethylene glycol) |
References
- Mady, F.M.; Ibrahim, S.R.; Abourehab, M.A. Development and evaluation of alginate-gum blend mucoadhesive microspheres for controlled release of metformin hydrochloride. J. Adv. Biomed. Pharm. Sci. 2021, 4, 111–118. [Google Scholar] [CrossRef]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan films and scaffolds for regenerative medicine applications: A review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D. Recovery and utilization of chitin and chitosan in food processing waste management. Food Technol. 1991, 45, 114–122. [Google Scholar]
- Knorr, D.; Teutonico, R.A. Chitosan immobilization and permeabilization of amaranthus tricolor cells. J. Agric. Food Chem. 1986, 34, 96–97. [Google Scholar] [CrossRef]
- Hadwinger, L.A.; Fristensky, B.; Riggleman, R.C.I. Chitin, Chitosan and Related Enzymes; Zikakis, J.P., Ed.; Academic Press: New York, NY, USA, 1984; pp. 291–302. [Google Scholar]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Synowiecki, J.; Abdul, N.A.; Al-Khateeb, Q. Mycelia of LMIlcor Rouxii as a source of chitin and chitosan. Food Chem. 1997, 60, 605–610. [Google Scholar] [CrossRef]
- Kohlhoff, M.; Niehues, A.; Wattjes, J.; Bénéteau, J.; Cord-Landwehr, S.; El Gueddari, N.E.; Bernard, F.; Rivera-Rodriguez, G.R.; Moerschbacher, B.M. Chitinosanase: A fungal chitosan hydrolyzing enzyme with a new and unusually specific cleavage pattern. Carbohydr. Polym. 2017, 174, 1121–1128. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Pramanik, S.; Sali, V. Connecting the dots in drug delivery: A tour d’horizon of chitosan-based nanocarriers system. Int. J. Biol. Macromol. 2021, 169, 103–121. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Islam, M.S.; Haque, P.; Rashid, T.U.; Khan, M.N.; Mallik, A.K.; Khan, M.N.I.; Khan, M.; Rahman, M.M. Core–shell drug carrier from folate conjugated chitosan obtained from prawn shell for targeted doxorubicin delivery. J. Mater. Sci. Mater. Med. 2017, 28, 55. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Taylor, P.; Dutta, P.K.; Ravikumar, M.N.V.; Dutta, J. Chitin and chitosan for versatile applications. J. Macromol. Sci. Part C 2002, 42, 37–41. [Google Scholar] [CrossRef]
- Gorantla, S.; Dabholkar, N.; Sharma, S.; Krishna, V.; Alexander, A.; Singhvi, G. Chitosan-based microneedles as a potential platform for drug delivery through the skin: Trends and regulatory aspects. Int. J. Biol. Macromol. 2021, 184, 438–453. [Google Scholar] [CrossRef] [PubMed]
- Martau, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector-biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Mehrban, S.F.; Aliabadi, H.A.M.; Karimi, M.; Mohammadi, A.; Maleki, A.; Mahdavi, M.; Larijani, B.; Shalan, A.E. Review: The latest advances in biomedical applications of chitosan hydrogel as a powerful natural structure with eye-catching biological properties. J. Mater. Sci. 2022, 57, 3855–3891. [Google Scholar] [CrossRef]
- Daraghmeh, N.H.; Chowdhry, B.Z.; Leharne, S.A.; Al Omari, M.M.; Badwan, A.A. Chitin. Profiles Drug Subst. Excip. Relat. Methodol. 2011, 36, 35–102. [Google Scholar] [CrossRef] [PubMed]
- Knidri, H.E.L.; Belaabed, R.; El, R.; Laajeb, A.; Addaou, A.; Lahsini, A. Physicochemical characterization of chitin and chitosan producted from parapenaeus longirostris shrimp shell wastes. J. Mater. Environ. Sci. 2017, 8, 3648–3653. [Google Scholar]
- Hashemi, M.; Nazari, Z.; Noshirvani, N. Synthesis of chitosan based magnetic molecularly imprinted polymers for selective separation and spectrophotometric determination of histamine in tuna fish. Carbohydr. Polym. 2017, 177, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Shajahan, A.; Shankar, S.; Sathiyaseelan, A.; Narayan, K.S.; Narayanan, V.; Kaviyarasan, V.; Ignacimuthu, S. Comparative studies of chitosan and its nanoparticles for the adsorption efficiency of various dyes. Int. J. Biol. Macromol. 2017, 104, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A. Solubility of chitin: Solvents, solution behaviors and their related mechanisms. In Solubility of Polysaccharides; IntechOpen: Rijeka, Croatia, 2017; Chapter 7; ISBN 978-953-51-3650-7. [Google Scholar]
- Kurita, K.; Kamiya, M.; Nishimura, S.I. Solubilization of a rigid polysaccharide: Controlled partial N-acetylation of chitosan to develop solubility. Carbohydr. Polym. 1991, 16, 83–92. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Kubota, N.; Tatsumoto, N.; Sano, T.; Toya, K. A simple preparation of half N-acetylated chitosan highly soluble in water and aqueous organic solvents. Carbohydr. Res. 2000, 324, 268–274. [Google Scholar] [CrossRef]
- Kasaai, M.R.; Arul, J. Intrinsic viscosity—Molecular weight relationship for chitosan. J. Polym. Sci. B Polym. Phys. 2000, 38, 2591–2598. [Google Scholar] [CrossRef]
- Zhai, X.; Li, C.; Ren, D.; Wang, J.; Ma, C.; Abd El-Aty, A.M. The impact of chitooligosaccharides and their derivatives on the in vitro and in vivo antitumor activity: A comprehensive review. Carbohydr. Polym. 2021, 266, 118132. [Google Scholar] [CrossRef]
- Onishi, H.; Machida, Y. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials 1999, 20, 175–182. [Google Scholar] [CrossRef]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Verheul, R.J.; Amidi, M.; van Steenbergen, M.J.; van Riet, E.; Jiskoot, W.; Hennink, W.E. Influence of the degree of acetylation on the enzymatic degradation and in vitro biological properties of trimethylated chitosans. Biomaterials 2009, 30, 3129–3135. [Google Scholar] [CrossRef]
- Kittur, F.S.; Vishu Kumar, A.B.; Varadaraj, M.C.; Tharanathan, R.N. Chitooligosaccharides—Preparation with the aid of pectinase isozyme from Aspergillus Niger and their antibacterial activity. Carbohydr. Res. 2005, 340, 1239–1245. [Google Scholar] [CrossRef]
- Kittur, F.S.; Vishu Kumar, A.B.; Tharanathan, R.N. Low molecular weight Chitosans—Preparation by depolymerization with Aspergillus Niger pectinase, and characterization. Carbohydr. Res. 2003, 338, 1283–1290. [Google Scholar] [CrossRef]
- McConnell, E.L.; Murdan, S.; Basit, A.W. An investigation into the digestion of chitosan (noncrosslinked and crosslinked) by human colonic bacteria. J. Pharm. Sci. 2008, 97, 3820–3829. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Aspden, T.J.; Mason, J.D.; Jones, N.S.; Lowe, J.; Skaugrud, O.; Illum, L. Chitosan as a nasal delivery system: The effect of chitosan solutions on in vitro and in vivo mucociliary transport rates in human turbinates and volunteers. J. Pharm. Sci. 1997, 86, 509–513. [Google Scholar] [CrossRef]
- Aspden, T.J.; Adler, J.; Davis, S.S.; Skaugrud, Ø.; Illum, L. Chitosan as a nasal delivery system: Evaluation of the effect of chitosan on mucociliary clearance rate in the frog palate model. Int. J. Pharm. 1995, 122, 69–78. [Google Scholar] [CrossRef]
- Dyer, A.M.; Hinchcliffe, M.; Watts, P.; Castile, J.; Jabbal-Gill, I.; Nankervis, R.; Smith, A.; Illum, L. Nasal delivery of insulin using novel chitosan based formulations: A comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm. Res. 2002, 19, 998–1008. [Google Scholar] [CrossRef]
- Ribeiro, M.P.; Espiga, A.; Silva, D.; Baptista, P.; Henriques, J.; Ferreira, C.; Silva, J.C.; Borges, J.P.; Pires, E.; Chaves, P.; et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009, 17, 817–824. [Google Scholar] [CrossRef]
- Carreño-Gómez, B.; Duncan, R. Evaluation of the biological properties of soluble chitosan and chitosan microspheres. Int. J. Pharm. 1997, 148, 231–240. [Google Scholar] [CrossRef]
- Gades, M.D.; Stern, J.S. Chitosan supplementation and fecal fat excretion in men. Obes. Res. 2003, 11, 683–688. [Google Scholar] [CrossRef]
- Tapola, N.S.; Lyyra, M.L.; Kolehmainen, R.M.; Sarkkinen, E.S.; Schauss, A.G. Safety aspects and cholesterol-lowering efficacy of chitosan tablets. J. Am. Coll. Nutr. 2008, 27, 22–30. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Costa, M.R.; Pereira, M.; Campos, D.A.; Odila, J.; Madureira, A.R.; Cardelle-Cobas, A.; Tavaria, F.K.; Rodrigues, A.S.; et al. Chitosan mouthwash: Toxicity and in vivo validation. Carbohydr. Polym. 2014, 111, 385–392. [Google Scholar] [CrossRef]
- Ravindranathan, S.; Koppolu, B.P.; Smith, S.G.; Zaharoff, D.A. Effect of chitosan properties on immunoreactivity. Mar. Drugs 2016, 14, 91. [Google Scholar] [CrossRef]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef]
- Dowling, M.B.; Kumar, R.; Keibler, M.A.; Hess, J.R.; Bochicchio, G.V.; Raghavan, S.R. A self-assembling hydrophobically modified chitosan capable of reversible hemostatic action. Biomaterials 2011, 32, 3351–3357. [Google Scholar] [CrossRef]
- Horio, T.; Ishihara, M.; Fujita, M.; Kishimoto, S.; Kanatani, Y.; Ishizuka, T.; Nogami, Y.; Nakamura, S.; Tanaka, Y.; Morimoto, Y.; et al. Effect of photocrosslinkable chitosan hydrogel and its sponges to stop bleeding in a rat liver injury model. Artif. Organs 2010, 34, 342–347. [Google Scholar] [CrossRef]
- Valentine, R.; Athanasiadis, T.; Moratti, S.; Hanton, L.; Robinson, S.; Wormald, P.-J. The efficacy of a novel chitosan gel on hemostasis and wound healing after endoscopic sinus surgery. Am. J. Rhinol. Allergy 2010, 24, 70–75. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F.A. Applications and properties of chitosan. J. Bioact. Compat. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Tolaimate, A.; Desbrieres, J.; Rhazi, M.; Alagui, A. Contribution to the preparation of chitins and chitosans with controlled physico-chemical properties. Polymer 2003, 44, 7939–7952. [Google Scholar] [CrossRef]
- Rashid, T.U.; Rahman, M.M.; Kabir, S.; Shamsuddin, S.M.; Khan, M.A. A new approach for the preparation of chitosan from γ-irradiation of prawn shell: Effects of radiation on the characteristics of chitosan. Polym. Int. 2012, 61, 1302–1308. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.K. Extraction and characterization of chitin and chitosan from (Labeo Rohit) fish scales. Procedia Mater. Sci. 2014, 6, 482–489. [Google Scholar] [CrossRef]
- Methacanon, P.; Prasitsilp, M.; Pothsree, T.; Pattaraarchachai, J. Heterogeneous N-deacetylation of squid chitin in alkaline solution. Carbohydr. Polym. 2003, 52, 119–123. [Google Scholar] [CrossRef]
- Pelletier, A.; Lemire, I.; Sygusch, J.; Chornet, E.; Overend, R.P. Chitin/chitosan transformation by thermo-mechano-chemical treatment including characterization by enzymatic depolymerization. Biotechnol. Bioeng. 1990, 36, 310–315. [Google Scholar] [CrossRef]
- Focher, B.; Beltrame, P.L.; Naggi, A.; Torri, G. Alkaline N-deacetylation of chitin enhanced by flash treatments. reaction kinetics and structure modifications. Carbohydr. Polym. 1990, 12, 405–418. [Google Scholar] [CrossRef]
- Domard, A.; Roberts, G.A.F.; Varum, K.M. Advances in chitin science. Domard A 1997, 410. [Google Scholar]
- Lertwattanaseri, T.; Ichikawa, N.; Mizoguchi, T.; Tanaka, Y.; Chirachanchai, S. Microwave technique for efficient deacetylation of chitin nanowhiskers to a chitosan nanoscaffold. Carbohydr. Res. 2009, 344, 331–335. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2012, 3, 203–230. [Google Scholar] [CrossRef]
- Mima, S.; Miya, M.; Iwamoto, R.; Yoshikawa, S. Highly deacetylated chitosan and its properties. J. Appl. Polym. Sci. 1983, 28, 1909–1917. [Google Scholar] [CrossRef]
- Isa, M.H.M.; Yasir, M.S.; Hasan, A.B.; Fadilah, N.I.M.; Hassan, A.R. The effect of gamma irradiation on chitosan and its application as a plant growth promoter in Chinese kale (Brassica Alboglabra). AIP Conf. Proc. 2016, 1704, 030003. [Google Scholar] [CrossRef]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and sulfated chitosan derivatives for biomedical applications: A review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P.; Guo, Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef]
- Snyman, D.; Hamman, J.H.; Kotze, J.S.; Rollings, J.E.; Kotzé, A.F. The relationship between the absolute molecular weight and the degree of quaternisation of N-trimethyl chitosan chloride. Carbohydr. Polym. 2002, 50, 145–150. [Google Scholar] [CrossRef]
- Wang, T.; Farajollahi, M.; Choi, Y.S.; Lin, I.-T.; Marshall, J.E.; Thompson, N.M.; Kar-Narayan, S.; Madden, J.D.W.; Smoukov, S.K. Electroactive polymers for sensing. Interface Focus 2016, 6, 20160026. [Google Scholar] [CrossRef]
- Sarmento, B.; Goycoolea, F.M.; Sosnik, A.; das Neves, J. Chitosan and chitosan derivatives for biological applications: Chemistry and functionalization. Int. J. Carbohydr. Chem. 2011, 2011, 802693. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, X.F.; Zhu, C.L.; Yang, H.J.; Lu, C.H.; Wang, W.L.; Pang, Y.; Yang, C.; Chen, L.J.; Li, X.F. A stable quaternized chitosan-black phosphorus nanocomposite for synergetic disinfection of antibiotic-resistant pathogens. ACS Appl. Bio. Mater. 2021, 4, 4821–4832. [Google Scholar] [CrossRef]
- Yadav, A.V.; Bhise, S.B. Chitosan: A potential biomaterial effective against typhoid. Curr. Sci. 2004, 87, 1176–1178. [Google Scholar]
- Dilamian, M.; Montazer, M.; Masoumi, J. Antimicrobial electrospun membranes of chitosan/poly(ethylene oxide) incorporating poly(hexamethylene biguanide) hydrochloride. Carbohydr. Polym. 2013, 94, 364–371. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan biomaterials for current and potential dental applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Park, P.-J.; Kim, S.-K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 2001, 44, 71–76. [Google Scholar] [CrossRef]
- Fatima, I.; Rasul, A.; Shah, S.; Saadullah, M.; Islam, N.; Khames, A.; Salawi, A.; Ahmed, M.M.; Almoshari, Y.; Abbas, G.; et al. Novasomes as nano-vesicular carriers to enhance topical delivery of fluconazole: A new approach to treat fungal infections. Molecules 2022, 27, 2936. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Khames, A.; Genedy, S.; Mostafa, S.; Khaleel, M.A.; Omar, M.M.; el Sisi, A.M. Sesame oil-based nanostructured lipid carriers of nicergoline, intranasal delivery system for brain targeting of synergistic cerebrovascular protection. Pharmaceutics 2021, 13, 581. [Google Scholar] [CrossRef]
- Abd El-Aziz, E.A.E.D.; Elgayar, S.F.; Mady, F.M.; Abourehab, M.A.S.; Hasan, O.A.; Reda, L.M.; Alaaeldin, E. The potential of optimized liposomes in enhancement of cytotoxicity and apoptosis of encapsulated Egyptian propolis on hep-2 cell line. Pharmaceutics 2021, 13, 2184. [Google Scholar] [CrossRef]
- Ashfaq, M.; Shah, S.; Rasul, A.; Hanif, M.; Khan, H.U.; Khames, A.; Abdelgawad, M.A.; Ghoneim, M.M.; Ali, M.Y.; Abourehab, M.A.S.; et al. Enhancement of the solubility and bioavailability of pitavastatin through a self-nanoemulsifying drug delivery system (SNEDDS). Pharmaceutics 2022, 14, 482. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Ahmed, O.A.A.; Balata, G.F.; Almalki, W.H. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood–brain barrier. Int. J. Nanomed. 2018, 13, 3679–3687. [Google Scholar] [CrossRef]
- Dong, J.; Tao, L.; Abourehab, M.A.S.; Hussain, Z. Design and development of novel hyaluronate-modified nanoparticles for combo-delivery of curcumin and alendronate: Fabrication, Characterization, and cellular and molecular evidences of enhanced bone regeneration. Int. J. Biol. Macromol. 2018, 116, 1268–1281. [Google Scholar] [CrossRef]
- Zhuo, F.; Abourehab, M.A.S.; Hussain, Z. Hyaluronic acid decorated tacrolimus-loaded nanoparticles: Efficient approach to maximize dermal targeting and anti-dermatitis efficacy. Carbohydr. Polym. 2018, 197, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, M.; Abourehab, M.A.S.; Abdou, R. Monoolein cubic nanoparticles as novel carriers for docetaxel. J. Drug Deliv. Sci. Technol. 2020, 56, 101501. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Y.; Chen, L. Chitosan and its derivatives as vehicles for drug delivery. Drug Deliv. 2017, 24, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Mohanto, S.; Manne, R.; Rajendran, R.R.; Deepak, A.; Edapully, S.J.; Patil, T.; Katari, O. Nanoparticle-based drug delivery system: The magic bullet for the treatment of chronic pulmonary diseases. Mol. Pharm. 2021, 18, 3671–3718. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Ansari, M.J.; Singh, A.; Hassan, A.; Abdelgawad, M.A.; Shrivastav, P.; Abualsoud, B.M.; Amaral, L.S.; Pramanik, S. Cubosomes as an emerging platform for drug delivery: A review of the state of the art. J. Mater. Chem. B 2022, 10, 2781–2819. [Google Scholar] [CrossRef]
- Abdel Mouez, M.; Zaki, N.M.; Mansour, S.; Geneidi, A.S. Bioavailability enhancement of verapamil HCl via intranasal chitosan microspheres. Eur. J. Pharm. Sci. 2014, 51, 59–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, W.; Lv, P.; Wang, L.; Ma, G. Preparation and evaluation of alginate-chitosan microspheres for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2011, 77, 11–19. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Du, J.; Sun, Y.; Li, F.; Duan, Y. Targeted SiRNA delivery by anti-HER2 antibody-modified nanoparticles of MPEG-chitosan diblock copolymer. J. Biomater. Sci. Polym. Ed. 2013, 24, 1219–1232. [Google Scholar] [CrossRef]
- Kalsoom Khan, A.; Saba, A.U.; Nawazish, S.; Akhtar, F.; Rashid, R.; Mir, S.; Nasir, B.; Iqbal, F.; Afzal, S.; Pervaiz, F.; et al. Carrageenan based bionanocomposites as drug delivery tool with special emphasis on the influence of ferromagnetic nanoparticles. Oxid. Med. Cell Longev. 2017, 2017, 8158315. [Google Scholar] [CrossRef]
- Saeed, R.M.; Dmour, I.; Taha, M.O. Stable chitosan-based nanoparticles using polyphosphoric acid or hexametaphosphate for tandem ionotropic/covalent crosslinking and subsequent investigation as novel vehicles for drug delivery. Front. Bioeng. Biotechnol. 2020, 8, 4. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, L.; Ye, S.; Li, G.; Zhang, M. Construction of an environmentally friendly octenylsuccinic anhydride modified PH-sensitive chitosan nanoparticle drug delivery system to alleviate inflammation and oxidative stress. Carbohydr. Polym. 2020, 236, 115972. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Application of PH-responsive fucoidan/chitosan nanoparticles to improve oral quercetin delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Z.; Liu, L.; Li, M.; Zuo, A.; Guo, J. Preparation, in vitro and in vivo evaluation of chitosan-sodium alginate-ethyl cellulose polyelectrolyte film as a novel buccal mucosal delivery vehicle. Eur. J. Pharm. Sci. 2022, 168, 106085. [Google Scholar] [CrossRef]
- Hussain, Z.; Rahim, M.A.; Jan, N.; Shah, H.; Rawas-Qalaji, M.; Khan, S.; Sohail, M.; Thu, H.E.; Ramli, N.A.; Sarfraz, R.M.; et al. Cell membrane cloaked nanomedicines for bio-imaging and immunotherapy of cancer: Improved pharmacokinetics, cell internalization and anticancer efficacy. J. Control. Release 2021, 335, 130–157. [Google Scholar] [CrossRef]
- Kheiri, K.; Sohrabi, N.; Mohammadi, R.; Amini-Fazl, M.S. Preparation and characterization of magnetic nanohydrogel based on chitosan for 5-fluorouracil drug delivery and kinetic study. Int. J. Biol. Macromol. 2022, 202, 191–198. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ansari, M.J.; Das, S.S.; Alshahrani, S.M. Development and characterization of ethyl cellulose nanosponges for sustained release of brigatinib for the treatment of non-small cell lung cancer. J. Polym. Eng. 2020, 40, 823–832. [Google Scholar] [CrossRef]
- Ansari, M.J.; Alshetaili, A.; Aldayel, I.A.; Alablan, F.M.; Alsulays, B.; Alshahrani, S.; Alalaiwe, A.; Ansari, M.N.; Ur Rehman, N.; Shakeel, F. Formulation, characterization, in vitro and in vivo evaluations of self-nanoemulsifying drug delivery system of luteolin. J. Taibah Univ. Sci. 2020, 14, 1386–1401. [Google Scholar] [CrossRef]
- Ansari, M.J. Factors affecting preparation and properties of nanoparticles by nanoprecipitation method. Indo Am. J. Pharm. Sci. 2017, 2017, 4854–4858. [Google Scholar]
- Ouerghi, O.; Geesi, M.H.; Ibnouf, E.O.; Ansari, M.J.; Alam, P.; Elsanousi, A.; Kaiba, A.; Riadi, Y. Sol-gel synthesized rutile TiO2 nanoparticles loaded with cardamom essential oil: Enhanced antibacterial activity. J. Drug Deliv. Sci. Technol. 2021, 64, 102581. [Google Scholar] [CrossRef]
- Ansari, M.J. An overview of techniques for multifold enhancement in solubility of poorly soluble drugs. Curr. Issues Pharm. Med. Sci. 2019, 32, 203–209. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, Z.; Feng, L.; Deng, L.; Fang, Z.; Liu, Z.; Li, Y.; Wu, X.; Qin, L.; Guo, R.; et al. Chitosan-based nanoparticle co-delivery of docetaxel and curcumin ameliorates anti-tumor chemoimmunotherapy in lung cancer. Carbohydr. Polym. 2021, 268, 118237. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Chen, J.; Tang, C.; Yin, C. Enhanced antitumor efficacy of glutathione-responsive chitosan based nanoparticles through co-delivery of chemotherapeutics, genes, and immune agents. Carbohydr. Polym. 2021, 270, 118384. [Google Scholar] [CrossRef]
- Amiry, F.; Sazegar, M.R.; Mahmoudi, A. Smart polymeric nanocomposite based on protonated aluminosilicate, curcumin, and chitosan for mesalamine drug delivery as an anti-inflammatory nanocarrier. Microporous Mesoporous Mater. 2022, 330, 111533. [Google Scholar] [CrossRef]
- Grant, J.J.; Pillai, S.C.; Perova, T.S.; Hehir, S.; Hinder, S.J.; McAfee, M.; Breen, A. Electrospun fibres of chitosan/PVP for the effective chemotherapeutic drug delivery of 5-fluorouracil. Chemosensors 2021, 9, 70. [Google Scholar] [CrossRef]
- Jin, Z.; Hu, G.; Zhao, K. Mannose-anchored quaternized chitosan/thiolated carboxymethyl chitosan composite NPs as mucoadhesive carrier for drug delivery. Carbohydr. Polym. 2022, 283, 119174. [Google Scholar] [CrossRef] [PubMed]
- Lohiya, G.; Katti, D.S. Carboxylated chitosan-mediated improved efficacy of mesoporous silica nanoparticle-based targeted drug delivery system for breast cancer therapy. Carbohydr. Polym. 2022, 277, 118822. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Jo, S.H.; Phan, Q.T.; Park, H.; Park, S.H.; Oh, C.W.; Lim, K.T. Dual PH-/thermo-responsive chitosan-based hydrogels prepared using “click” chemistry for colon-targeted drug delivery applications. Carbohydr. Polym. 2021, 260, 117812. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, S.; Meng, Y.; Guo, Z.; Cheng, M.; Li, J. Fabrication of self-healing pectin/chitosan hybrid hydrogel via diels-alder reactions for drug delivery with high swelling property, PH-responsiveness, and cytocompatibility. Carbohydr. Polym. 2021, 268, 118244. [Google Scholar] [CrossRef]
- Rahnama, H.; Nouri Khorasani, S.; Aminoroaya, A.; Molavian, M.R.; Allafchian, A.; Khalili, S. Facile preparation of chitosan-dopamine-inulin aldehyde hydrogel for drug delivery application. Int. J. Biol. Macromol. 2021, 185, 716–724. [Google Scholar] [CrossRef]
- Yang, D.; Gao, K.; Bai, Y.; Lei, L.; Jia, T.; Yang, K.; Xue, C. Microfluidic synthesis of chitosan-coated magnetic alginate microparticles for controlled and sustained drug delivery. Int. J. Biol. Macromol. 2021, 182, 639–647. [Google Scholar] [CrossRef]
- Gerami, S.E.; Pourmadadi, M.; Fatoorehchi, H.; Yazdian, F.; Rashedi, H.; Nigjeh, M.N. Preparation of PH-sensitive chitosan/polyvinylpyrrolidone/α-Fe2O3 nanocomposite for drug delivery application: Emphasis on ameliorating restrictions. Int. J. Biol. Macromol. 2021, 173, 409–420. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, J.; Li, S.; Ma, T.; Deng, L.; Kong, Y. Construction of a PH-responsive drug delivery platform based on the hybrid of mesoporous silica and chitosan. J. Saudi Chem. Soc. 2021, 25, 101174. [Google Scholar] [CrossRef]
- Aigner, A. Applications of RNA interference: Current state and prospects for SiRNA-based strategies in vivo. Appl. Microbiol. Biotechnol. 2007, 76, 9–21. [Google Scholar] [CrossRef]
- Debus, H.; Baumhof, P.; Probst, J.; Kissel, T. Delivery of messenger RNA using poly(ethylene imine)-poly(ethylene glycol)-copolymer blends for polyplex formation: Biophysical characterization and in vitro transfection properties. J. Control. Release 2010, 148, 334–343. [Google Scholar] [CrossRef]
- Plianwong, S.; Opanasopit, P.; Ngawhirunpat, T.; Rojanarata, T. Fast, facile and ethidium bromide-free assay based on the use of adsorption indicator for the estimation of polyethylenimine to nucleic acid ratio of complete polyplex assembly for gene delivery. Talanta 2013, 115, 241–245. [Google Scholar] [CrossRef]
- Ghanbari, P.; Mohseni, M.; Tabasinezhad, M.; Yousefi, B.; Saei, A.A.; Sharifi, S.; Rashidi, M.R.; Samadi, N. Inhibition of survivin restores the sensitivity of breast cancer cells to docetaxel and vinblastine. Appl. Biochem. Biotechnol. 2014, 174, 667–681. [Google Scholar] [CrossRef]
- Pennati, M.; Folini, M.; Zaffaroni, N. Targeting survivin in cancer therapy. Expert Opin. Ther. Targets 2008, 12, 463–476. [Google Scholar] [CrossRef]
- Jere, D.; Jiang, H.-L.; Kim, Y.-K.; Arote, R.; Choi, Y.-J.; Yun, C.-H.; Cho, M.-H.; Cho, C.-S. Chitosan-graft-polyethylenimine for Akt1 SiRNA delivery to lung cancer cells. Int. J. Pharm. 2009, 378, 194–200. [Google Scholar] [CrossRef]
- Kami, D.; Takeda, S.; Itakura, Y.; Gojo, S.; Watanabe, M.; Toyoda, M. Application of magnetic nanoparticles to gene delivery. Int. J. Mol. Sci. 2011, 12, 3705–3722. [Google Scholar] [CrossRef]
- Wang, J.; Dou, B.; Bao, Y. Efficient targeted PDNA/SiRNA delivery with folate-low-molecular-weight polyethyleneimine-modified pullulan as non-viral carrier. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 98–109. [Google Scholar] [CrossRef]
- Singha, K.; Namgung, R.; Kim, W.J. Polymers in small-interfering RNA delivery. Nucleic Acid Ther. 2011, 21, 133–147. [Google Scholar] [CrossRef]
- Arami, S.; Mahdavi, M.; Fathi, M.; Entezami, A.A. Synthesis and characterization of Fe3O4-PEG-LAC-chitosan-PEI nanoparticle as a survivin SiRNA delivery system. Hum. Exp. Toxicol. 2016, 36, 227–237. [Google Scholar] [CrossRef]
- Suarato, G.; Li, W.; Meng, Y. Role of PH-responsiveness in the design of chitosan-based cancer nanotherapeutics: A review. Biointerphases 2016, 11, 04B201. [Google Scholar] [CrossRef]
- van der Meel, R.; Vehmeijer, L.J.C.; Kok, R.J.; Storm, G.; van Gaal, E.V.B. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: Current status. Adv. Drug Deliv. Rev. 2013, 65, 1284–1298. [Google Scholar] [CrossRef]
- Mei, L.; Fu, L.; Shi, K.; Zhang, Q.; Liu, Y.; Tang, J.; Gao, H.; Zhang, Z.; He, Q. Increased tumor targeted delivery using a multistage liposome system functionalized with RGD, TAT and cleavable PEG. Int. J. Pharm. 2014, 468, 26–38. [Google Scholar] [CrossRef]
- Zheng, Q.C.; Jiang, S.; Wu, Y.Z.; Shang, D.; Zhang, Y.; Hu, S.B.; Cheng, X.; Zhang, C.; Sun, P.; Gao, Y.; et al. Dual-targeting nanoparticle-mediated gene therapy strategy for hepatocellular carcinoma by delivering small interfering RNA. Front. Bioeng. Biotechnol. 2020, 8, 512. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, T.; Chen, J.; Cui, Y.; Zhang, X.; Lee, R.J.; Sun, F.; Li, Y.; Teng, L. PLGA/PCADK composite microspheres containing hyaluronic acid–chitosan SiRNA nanoparticles: A rational design for rheumatoid arthritis therapy. Int. J. Pharm. 2021, 596, 120204. [Google Scholar] [CrossRef]
- Baghdan, E.; Pinnapireddy, S.R.; Strehlow, B.; Engelhardt, K.H.; Schäfer, J.; Bakowsky, U. Lipid coated chitosan-DNA nanoparticles for enhanced gene delivery. Int. J. Pharm. 2018, 535, 473–479. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Williams, R.O., III; Smyth, H.D.C. Development of PEGylated chitosan/CRISPR-Cas9 dry powders for pulmonary delivery via thin-film freeze-drying. Int. J. Pharm. 2021, 605, 120831. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Dowaidar, M.; Langel, Ü. Carbonized chitosan encapsulated hierarchical porous zeolitic imidazolate frameworks nanoparticles for gene delivery. Microporous Mesoporous Mater. 2020, 302, 110200. [Google Scholar] [CrossRef]
- Jaiswal, S.; Dutta, P.K.; Kumar, S.; Koh, J.; Pandey, S. Methyl methacrylate modified chitosan: Synthesis, characterization and application in drug and gene delivery. Carbohydr. Polym. 2019, 211, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Dowaidar, M.; Nasser Abdelhamid, H.; Hällbrink, M.; Langel, Ü.; Zou, X. Chitosan enhances gene delivery of oligonucleotide complexes with magnetic nanoparticles–cell-penetrating peptide. J. Biomater. Appl. 2018, 33, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Hakimi, S.; Esmaeily, A.; Samadi, F.Y.; Mortazavian, E.; Nazari, M.; Mohammadi, Z.; Tehrani, N.R.; Tehrani, M.R. Novel chitosan based nanoparticles as gene delivery systems to cancerous and noncancerous cells. Int. J. Pharm. 2019, 560, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, Q.; Wu, W.; Wang, J.; Chu, P.K.; Bai, H.; Tang, G. Reconstructed chitosan with alkylamine for enhanced gene delivery by promoting endosomal escape. Carbohydr. Polym. 2020, 227, 115339. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, N.; Zeng, Z.; Huang, J.; Xiang, Z.; Guan, Y.-Q. Neuroprotective effect of chitosan nanoparticle gene delivery system grafted with acteoside (ACT) in Parkinson’s Disease models. J. Mater. Sci. Technol. 2020, 43, 197–207. [Google Scholar] [CrossRef]
- Iravani Kashkouli, K.; Torkzadeh-Mahani, M.; Mosaddegh, E. Synthesis and characterization of aminotetrazole-functionalized magnetic chitosan nanocomposite as a novel nanocarrier for targeted gene delivery. Mater. Sci. Eng. C 2018, 89, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Yasar, H.; Ho, D.-K.; De Rossi, C.; Herrmann, J.; Gordon, S.; Loretz, B.; Lehr, C.-M. Starch-chitosan polyplexes: A versatile carrier system for anti-infectives and gene delivery. Polymers 2018, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, G.S.; Zayed, G.M.; Elshaier, Y.A.M.M.; Alsharif, F.M. Chitosan-gelatin hydrogel crosslinked with oxidized sucrose for the ocular delivery of timolol maleate. J. Pharm. Sci. 2018, 107, 3098–3104. [Google Scholar] [CrossRef] [PubMed]
- Rebekah, A.; Sivaselvam, S.; Viswanathan, C.; Prabhu, D.; Gautam, R.; Ponpandian, N. Magnetic nanoparticle-decorated graphene oxide-chitosan composite as an efficient nanocarrier for protein delivery. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125913. [Google Scholar] [CrossRef]
- Zhu, Y.; Marin, L.M.; Xiao, Y.; Gillies, E.R.; Siqueira, W.L. Ph-sensitive chitosan nanoparticles for salivary protein delivery. Nanomaterials 2021, 11, 1028. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-isopropylacrylamide)-based hydrogels for biomedical applications: A review of the state-of-the-art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Jang, K.J.; Lee, W.S.; Park, S.; Han, J.; Kim, J.E.; Kim, B.M.; Chung, J.H. Sulfur(VI) fluoride exchange (SuFEx)-mediated synthesis of the chitosan-PEG conjugate and its supramolecular hydrogels for protein delivery. Nanomaterials 2021, 11, 318. [Google Scholar] [CrossRef]
- Cao, J.; Cheng, J.; Xi, S.; Qi, X.; Shen, S.; Ge, Y. Alginate/chitosan microcapsules for in-situ delivery of the protein, interleukin-1 receptor antagonist (IL-1Ra), for the treatment of dextran sulfate sodium (DSS)-induced colitis in a mouse model. Eur. J. Pharm. Biopharm. 2019, 137, 112–121. [Google Scholar] [CrossRef]
- Huang, J.; Deng, Y.; Ren, J.; Chen, G.; Wang, G.; Wang, F.; Wu, X. Novel in situ forming hydrogel based on xanthan and chitosan re-gelifying in liquids for local drug delivery. Carbohydr. Polym. 2018, 186, 54–63. [Google Scholar] [CrossRef]
- Zhu, T.; Shi, L.; Ma, C.; Xu, L.; Yang, J.; Zhou, G.; Zhu, X.; Shen, L. Fluorinated chitosan-mediated intracellular catalase delivery for enhanced photodynamic therapy of oral cancer. Biomater. Sci. 2021, 9, 658–662. [Google Scholar] [CrossRef]
- Vozza, G.; Danish, M.; Byrne, H.J.; Frías, J.M.; Ryan, S.M. Application of box-behnken experimental design for the formulation and optimisation of selenomethionine-loaded chitosan nanoparticles coated with zein for oral delivery. Int. J. Pharm. 2018, 551, 257–269. [Google Scholar] [CrossRef]
- Zhang, R.; Luo, S.; Hao, L.-K.; Jiang, Y.-Y.; Gao, Y.; Zhang, N.-N.; Zhang, X.-C.; Song, Y.-M. Preparation and properties of thrombus-targeted urokinase/multi-walled carbon nanotubes (MWCNTs)-chitosan (CS)-RGD drug delivery system. J. Biomed. Nanotechnol. 2021, 17, 1711–1725. [Google Scholar] [CrossRef]
- Huang, T.-H.; Hsu, S.; Chang, S.-W. Molecular interaction mechanisms of glycol chitosan self-healing hydrogel as a drug delivery system for gemcitabine and doxorubicin. Comput. Struct. Biotechnol. J. 2022, 20, 700–709. [Google Scholar] [CrossRef]
- Shao, D.; Gao, Q.; Sheng, Y.; Li, S.; Kong, Y. Construction of a dual-responsive dual-drug delivery platform based on the hybrids of Mesoporous Silica, sodium hyaluronate, chitosan and oxidized sodium carboxymethyl cellulose. Int. J. Biol. Macromol. 2022, 202, 37–45. [Google Scholar] [CrossRef]
- Soleimanbeigi, M.; Dousti, F.; Hassanzadeh, F.; Mirian, M.; Varshosaz, J.; Kasesaz, Y.; Rostami, M. Boron phenyl alanine targeted chitosan—PNIPAAm core–shell thermo-responsive nanoparticles: Boosting drug delivery to glioblastoma in BNCT. Drug Dev. Ind. Pharm. 2021, 47, 1607–1623. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Mazinani, S.; Abdouss, M.; Kalhor, H.; Kalantari, K.; Amiri, I.S.; Ramezani, Z. Designing chitosan nanoparticles embedded into graphene oxide as a drug delivery system. Polym. Bull. 2022, 79, 541–554. [Google Scholar] [CrossRef]
- Farmanbar, N.; Mohseni, S.; Darroudi, M. Green synthesis of chitosan-coated magnetic nanoparticles for drug delivery of oxaliplatin and irinotecan against colorectal cancer cells. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Ijaz, H.; Tulain, U.R.; Minhas, M.U.; Mahmood, A.; Sarfraz, R.M.; Erum, A.; Danish, Z. Design and in vitro evaluation of PH-sensitive crosslinked chitosan-grafted acrylic acid copolymer (CS-Co-AA) for targeted drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 336–348. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yun, Y.-P.; Shim, K.-S.; Kim, H.-J.; Kim, S.E.; Park, K.; Song, H.-R. In vitro anti-inflammation and chondrogenic differentiation effects of inclusion nanocomplexes of hyaluronic acid-beta cyclodextrin and simvastatin. Tissue Eng. Regen. Med. 2018, 15, 263–274. [Google Scholar] [CrossRef]
- Wei, J.; Xue, W.; Yu, X.; Qiu, X.; Liu, Z. PH sensitive phosphorylated chitosan hydrogel as vaccine delivery system for intramuscular immunization. J. Biomater. Appl. 2017, 31, 1358–1369. [Google Scholar] [CrossRef]
- Gao, X.; Liu, N.; Wang, Z.; Gao, J.; Zhang, H.; Li, M.; Du, Y.; Gao, X.; Zheng, A. Development and optimization of chitosan nanoparticle-based intranasal vaccine carrier. Molecules 2022, 27, 204. [Google Scholar] [CrossRef]
- Zuo, Z.; Zou, Y.; Li, Q.; Guo, Y.; Zhang, T.; Wu, J.; He, C.; Eko, F.O. Intranasal immunization with inactivated chlamydial elementary bodies formulated in VCG-chitosan nanoparticles induces robust immunity against intranasal chlamydia psittaci challenge. Sci. Rep. 2021, 11, 10389. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Gao, C.; Wang, Y.; Cheng, X.; Yang, Y.; Gou, Q.; Lei, L.; Chen, Y.; Wang, X.; et al. Development of a chitosan-modified PLGA nanoparticle vaccine for protection against Escherichia Coli K1 caused meningitis in mice. J. Nanobiotechnology 2021, 19, 69. [Google Scholar] [CrossRef]
- Zewail, M. Folic acid decorated chitosan-coated solid lipid nanoparticles for the oral treatment of rheumatoid arthritis. Ther. Deliv. 2021, 12, 297–310. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Fu, Q.; Xie, X.; Song, Y.; Xu, M.; Li, J. 3D-printed polycaprolactone-chitosan based drug delivery implants for personalized administration. Mater. Des. 2022, 214, 110394. [Google Scholar] [CrossRef]
- Wei, H.; Liu, S.; Chu, Y.; Tong, Z.; Yang, M.; Guo, Y.; Chen, T.; Wu, Y.; Sun, H.; Fan, L. Hydrogel-based microneedles of chitosan derivatives for drug delivery. React. Funct. Polym. 2022, 172, 105200. [Google Scholar] [CrossRef]
- Yuan, H.; Li, W.; Song, C.; Huang, R. An injectable supramolecular nanofiber-reinforced chitosan hydrogel with antibacterial and anti-inflammatory properties as potential carriers for drug delivery. Int. J. Biol. Macromol. 2022, 205, 563–573. [Google Scholar] [CrossRef]
- Yu, Y.; Kim, D.H.; Suh, E.Y.; Jeong, S.-H.; Kwon, H.C.; Le, T.P.; Kim, Y.; Shin, S.-A.; Park, Y.-H.; Huh, K.M. Injectable glycol chitosan thermogel formulation for efficient inner ear drug delivery. Carbohydr. Polym. 2022, 278, 118969. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Alqahtani, M.S.; Mahmood, A.; Barkat, K.; Khan, M.T.; Tulain, U.R.; Rashid, A. β-cyclodextrin chitosan-based hydrogels with tunable PH-responsive properties for controlled release of acyclovir: Design, characterization, safety, and pharmacokinetic evaluation. Drug Deliv. 2021, 28, 1093–1108. [Google Scholar] [CrossRef]
- Kazemi-Andalib, F.; Mohammadikish, M.; Divsalar, A.; Sahebi, U. Hollow microcapsule with PH-sensitive chitosan/polymer shell for in vitro delivery of curcumin and gemcitabine. Eur. Polym. J. 2022, 162, 110887. [Google Scholar] [CrossRef]
- Hongsa, N.; Thinbanmai, T.; Luesakul, U.; Sansanaphongpricha, K.; Muangsin, N. A novel modified chitosan/collagen coated-gold nanoparticles for 5-fluorouracil delivery: Synthesis, characterization, in vitro drug release studies, anti-inflammatory activity and in vitro cytotoxicity assay. Carbohydr. Polym. 2022, 277, 118858. [Google Scholar] [CrossRef]
- Shah, M.K.; Azad, A.K.; Nawaz, A.; Ullah, S.; Latif, M.S.; Rahman, H.; Alsharif, K.F.; Alzahrani, K.J.; El-Kott, A.F.; Albrakati, A.; et al. Formulation development, characterization and antifungal evaluation of chitosan NPs for topical delivery of voriconazole in vitro and ex vivo. Polymers 2022, 14, 135. [Google Scholar] [CrossRef]
- Affes, S.; Aranaz, I.; Acosta, N.; Heras, Á.; Nasri, M.; Maalej, H. Chitosan derivatives-based films as PH-sensitive drug delivery systems with enhanced antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2021, 182, 730–742. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Jagga, S.; Hasnain, M.S.; Nayak, A.K. Chapter 14—Chitosan-based scaffolds in tissue engineering and regenerative medicine. In Chitosan in Biomedical Applications; Hasnain, M.S., Beg, S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 329–354. ISBN 978-0-12-821058-1. [Google Scholar]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Lin, L.; Kasper, F.K.; Qin, Y.-X.; Mikos, A.G.; Sitharaman, B. Two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites for bone tissue engineering. Biomacromolecules 2013, 14, 900–909. [Google Scholar] [CrossRef]
- Holt, B.D.; Wright, Z.M.; Arnold, A.M.; Sydlik, S.A. Graphene oxide as a scaffold for bone regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1437. [Google Scholar] [CrossRef]
- Morozowich, N.L.; Nichol, J.L.; Allcock, H.R. Investigation of apatite mineralization on antioxidant polyphosphazenes for bone tissue engineering. Chem. Mater. 2012, 24, 3500–3509. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Viji Chandran, S.; Arumugam, B.; Saravanan, S.; Devanand Venkatasubbu, G.; Selvamurugan, N. Chitosan/nano-hydroxyapatite/nano-zirconium dioxide scaffolds with MiR-590-5p for bone regeneration. Int. J. Biol. Macromol. 2018, 111, 953–958. [Google Scholar] [CrossRef]
- Dubey, N.; Bentini, R.; Islam, I.; Cao, T.; Castro Neto, A.H.; Rosa, V. Graphene: A Versatile carbon-based material for bone tissue engineering. Stem Cells Int. 2015, 2015, 804213. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Nayak, A.K. 7—Nanocomposites for improved orthopedic and bone tissue engineering applications. In Applications of Nanocomposite Materials in Orthopedics; Inamuddin, A.M.A., Ali, M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 145–177. ISBN 978-0-12-813740-6. [Google Scholar]
- Zhang, Y.; Dou, X.; Zhang, L.; Wang, H.; Zhang, T.; Bai, R.; Sun, Q.; Wang, X.; Yu, T.; Wu, D.; et al. Facile Fabrication of a biocompatible composite gel with sustained release of aspirin for bone regeneration. Bioact. Mater. 2022, 11, 130–139. [Google Scholar] [CrossRef]
- Nazeer, M.A.; Yilgör, E.; Yilgör, I. Intercalated chitosan/hydroxyapatite nanocomposites: Promising materials for bone tissue engineering applications. Carbohydr. Polym. 2017, 175, 38–46. [Google Scholar] [CrossRef]
- Pistone, A.; Iannazzo, D.; Celesti, C.; Piperopoulos, E.; Ashok, D.; Cembran, A.; Tricoli, A.; Nisbet, D. Engineering of chitosan-hydroxyapatite-magnetite hierarchical scaffolds for guided bone growth. Materials 2019, 12, 2321. [Google Scholar] [CrossRef]
- Prakash, J.; Prema, D.; Venkataprasanna, K.S.; Balagangadharan, K.; Selvamurugan, N.; Venkatasubbu, G.D. Nanocomposite chitosan film containing graphene oxide/hydroxyapatite/gold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 154, 62–71. [Google Scholar] [CrossRef]
- Zou, M.; Sun, J.; Xiang, Z. Induction of M2-type macrophage differentiation for bone defect repair via an interpenetration network hydrogel with a GO-based controlled release system. Adv. Healthc. Mater. 2021, 10, 2001502. [Google Scholar] [CrossRef]
- Lu, H.-T.; Huang, G.-Y.; Chang, W.-J.; Lu, T.-W.; Huang, T.-W.; Ho, M.-H.; Mi, F.-L. Modification of chitosan nanofibers with CuS and fucoidan for antibacterial and bone tissue engineering applications. Carbohydr. Polym. 2022, 281, 119035. [Google Scholar] [CrossRef]
- Zia, I.; Jolly, R.; Mirza, S.; Rehman, A.; Shakir, M. Nanocomposite materials developed from nano-hydroxyapatite impregnated chitosan/κ-carrageenan for bone tissue engineering. ChemistrySelect 2022, 7, e202103234. [Google Scholar] [CrossRef]
- Esmaeili, J.; Jadbabaee, S.; Far, F.M.; Lukolayeh, M.E.; Kırboğa, K.K.; Rezaei, F.S.; Barati, A. Decellularized alstroemeria flower stem modified with chitosan for tissue engineering purposes: A cellulose/chitosan scaffold. Int. J. Biol. Macromol. 2022, 204, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and PH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef] [PubMed]
- Gritsch, L.; Maqbool, M.; Mouriño, V.; Ciraldo, F.E.; Cresswell, M.; Jackson, P.R.; Lovell, C.; Boccaccini, A.R. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: Copper and strontium. J. Mater. Chem. B 2019, 7, 6109–6124. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef]
- Vaidhyanathan, B.; Vincent, P.; Vadivel, S.; Karuppiah, P.; AL-Dhabi, N.A.; Sadhasivam, D.R.; Vimalraj, S.; Saravanan, S. Fabrication and investigation of the suitability of chitosan-silver composite scaffolds for bone tissue engineering applications. Process Biochem. 2021, 100, 178–187. [Google Scholar] [CrossRef]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Irani, S.; Bakhshi, H. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int. J. Biol. Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef]
- Li, M.; Jia, W.; Zhang, X.; Weng, H.; Gu, G.; Chen, Z. Hyaluronic acid oligosaccharides modified mineralized collagen and chitosan with enhanced osteoinductive properties for bone tissue engineering. Carbohydr. Polym. 2021, 260, 117780. [Google Scholar] [CrossRef]
- Morille, M.; Van-Thanh, T.; Garric, X.; Cayon, J.; Coudane, J.; Noël, D.; Venier-Julienne, M.-C.; Montero-Menei, C.N. New PLGA–P188–PLGA matrix enhances TGF-Β3 release from pharmacologically active microcarriers and promotes chondrogenesis of mesenchymal stem cells. J. Control. Release 2013, 170, 99–110. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Balasundaram, G.; Storey, D.M.; Webster, T.J. Novel nano-rough polymers for cartilage tissue engineering. Int. J. Nanomed. 2014, 9, 1845–1853. [Google Scholar] [CrossRef]
- Kock, L.; Van Donkelaar, C.C.; Ito, K. Tissue engineering of functional articular cartilage: The current status. Cell Tissue Res. 2012, 347, 613–627. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Domalik-Pyzik, P.; Chłopek, J.; Pielichowska, K. Chitosan-based hydrogels: Preparation, properties, and applications. In Cellulose-Based Superabsorbent Hydrogels; Mondal, I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1665–1693. ISBN 978-3-319-77830-3. [Google Scholar]
- Suh, J.K.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef]
- Neves, S.C.; Moreira Teixeira, L.S.; Moroni, L.; Reis, R.L.; Van Blitterswijk, C.A.; Alves, N.M.; Karperien, M.; Mano, J.F. Chitosan/poly(ɛ-caprolactone) blend scaffolds for cartilage repair. Biomaterials 2011, 32, 1068–1079. [Google Scholar] [CrossRef]
- Shamekhi, M.A.; Mirzadeh, H.; Mahdavi, H.; Rabiee, A.; Mohebbi-Kalhori, D.; Baghaban Eslaminejad, M. Graphene oxide containing chitosan scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2019, 127, 396–405. [Google Scholar] [CrossRef]
- Kashi, M.; Baghbani, F.; Moztarzadeh, F.; Mobasheri, H.; Kowsari, E. Green synthesis of degradable conductive thermosensitive oligopyrrole/chitosan hydrogel intended for cartilage tissue engineering. Int. J. Biol. Macromol. 2018, 107, 1567–1575. [Google Scholar] [CrossRef]
- Davachi, S.M.; Haramshahi, S.M.A.; Akhavirad, S.A.; Bahrami, N.; Hassanzadeh, S.; Ezzatpour, S.; Hassanzadeh, N.; Malekzadeh Kebria, M.; Khanmohammadi, M.; Bagher, Z. Development of chitosan/hyaluronic acid hydrogel scaffolds via enzymatic reaction for cartilage tissue engineering. Mater. Today Commun. 2022, 30, 103230. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Yazdanpanah, Z.; Alizadeh Sardroud, H.; Sharma, N.K.; Wilson, L.D.; Chen, X. Fabrication of chitosan/alginate/hydroxyapatite hybrid scaffolds using 3D printing and impregnating techniques for potential cartilage regeneration. Int. J. Biol. Macromol. 2022, 204, 62–75. [Google Scholar] [CrossRef]
- Chen, T.-C.; Wong, C.-W.; Hsu, S. Three-dimensional printing of chitosan cryogel as injectable and shape recoverable scaffolds. Carbohydr. Polym. 2022, 285, 119228. [Google Scholar] [CrossRef]
- Pitrolino, K.A.; Felfel, R.M.; Pellizzeri, L.M.; MLaren, J.; Popov, A.A.; Sottile, V.; Scotchford, C.A.; Scammell, B.E.; Roberts, G.A.F.; Grant, D.M. Development and in vitro assessment of a bi-layered chitosan-nano-hydroxyapatite osteochondral scaffold. Carbohydr. Polym. 2022, 282, 119126. [Google Scholar] [CrossRef]
- Hu, M.; Yang, J.; Xu, J. Structural and biological investigation of chitosan/hyaluronic acid with silanized-hydroxypropyl methylcellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Drug Deliv. 2021, 28, 607–619. [Google Scholar] [CrossRef]
- Zuliani, C.C.; Damas, I.I.; Andrade, K.C.; Westin, C.B.; Moraes, Â.M.; Coimbra, I.B. Chondrogenesis of human amniotic fluid stem cells in chitosan-xanthan scaffold for cartilage tissue engineering. Sci. Rep. 2021, 11, 3063. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.; Li, M.; Li, J.; Wan, Y. Enhanced dual network hydrogels consisting of thiolated chitosan and silk fibroin for cartilage tissue engineering. Carbohydr. Polym. 2020, 227, 115335. [Google Scholar] [CrossRef]
- Fukunishi, T.; Best, C.A.; Sugiura, T.; Shoji, T.; Yi, T.; Udelsman, B.; Ohst, D.; Ong, C.S.; Zhang, H.; Shinoka, T.; et al. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/chitosan scaffolds in a sheep model. PLoS ONE 2016, 11, e0158555. [Google Scholar] [CrossRef]
- Chupa, J.M.; Foster, A.M.; Sumner, S.R.; Madihally, S.V.; Matthew, H.W. Vascular cell responses to polysaccharide materials: In vitro and in vivo evaluations. Biomaterials 2000, 21, 2315–2322. [Google Scholar] [CrossRef]
- Huang, C.; Chen, R.; Ke, Q.; Morsi, Y.; Zhang, K.; Mo, X. Electrospun collagen-chitosan-TPU nanofibrous scaffolds for tissue engineered tubular grafts. Colloids Surf. B Biointerfaces 2011, 82, 307–315. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Y.; Shen, Z.; Li, M.; Zhang, W.; Liu, Y.; Zhang, Y.; Duan, J.; Ma, Z.; Sang, S. 3D printed GelMA/carboxymethyl chitosan composite scaffolds for vasculogenesis. Int. J. Polym. Mater. Polym. Biomater. 2022. [Google Scholar] [CrossRef]
- Gao, H.; Hu, P.; Sun, G.; Wang, L.; Tian, Y.; Mo, H.; Liu, C.; Zhang, J.; Shen, J. Decellularized scaffold-based poly(ethylene glycol) biomimetic vascular patches modified with polyelectrolyte multilayer of heparin and chitosan: Preparation and vascular tissue engineering applications in a porcine model. J. Mater. Chem. B 2022, 10, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Fiqrianti, I.A.; Widiyanti, P.; Manaf, M.A.; Savira, C.Y.; Cahyani, N.R.; Bella, F.R. Poly-L-Lactic Acid (PLLA)-chitosan-collagen electrospun tube for vascular graft application. J. Funct. Biomater. 2018, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Soriente, A.; Amodio, S.P.; Fasolino, I.; Raucci, M.G.; Demitri, C.; Engel, E.; Ambrosio, L. Chitosan/PEGDA based scaffolds as bioinspired materials to control in vitro angiogenesis. Mater. Sci. Eng. C 2021, 118, 111420. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Liu, Z.; Li, S.; Deng, C.; Yang, X.; Deng, Q.; Sun, Y.; Zhang, Y.; Ma, Z.; et al. Evaluation of interleukin-4-loaded sodium alginate–chitosan microspheres for their support of microvascularization in engineered tissues. ACS Biomater. Sci. Eng. 2021, 7, 4946–4958. [Google Scholar] [CrossRef]
- Engelmann, K.; Bednarz, J.; Valtink, M. Prospects for endothelial transplantation. Exp. Eye Res. 2004, 78, 573–578. [Google Scholar] [CrossRef]
- World Health Organization. Universal Eye Health: A Global Action Plan 2014–2019; World Health Organization: Geneva, Switzerland, 2013; ISBN 9241506563. [Google Scholar]
- Kafle, P.A.; Singh, S.K.; Sarkar, I.; Surin, L. Amniotic membrane transplantation with and without limbal stem cell transplantation in chemical eye injury. Nepal J. Ophthalmol. 2015, 7, 52–55. [Google Scholar] [CrossRef][Green Version]
- Kreft, M.E.; Dragin, U. Amniotic membrane in tissue engineering and regenerative medicine. Slov. Med. J. 2010, 79, 707–715. [Google Scholar]
- Yousaf, S.; Keshel, S.H.; Farzi, G.A.; Momeni-Moghadam, M.; Ahmadi, E.D.; Asencio, I.O.; Mozafari, M.; Sefat, F. Scaffolds for corneal tissue engineering. In Handbook of Tissue Engineering Scaffolds: Volume Two; Elsevier: Amsterdam, Netherlands, 2019; pp. 649–672. [Google Scholar]
- Tayebi, T.; Baradaran-Rafii, A.; Hajifathali, A.; Rahimpour, A.; Zali, H.; Shaabani, A.; Niknejad, H. Biofabrication of chitosan/chitosan nanoparticles/polycaprolactone transparent membrane for corneal endothelial tissue engineering. Sci. Rep. 2021, 11, 7060. [Google Scholar] [CrossRef]
- Feng, L.; Liu, R.; Zhang, X.; Li, J.; Zhu, L.; Li, Z.; Li, W.; Zhang, A. Thermo-gelling dendronized chitosans as biomimetic scaffolds for corneal tissue engineering. ACS Appl. Mater. Interfaces 2021, 13, 49369–49379. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Z.; Liu, Y.; Wang, L.; Jiang, Z.; Li, T.; Zhang, W.; Liang, Y. Carboxymethyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohydr. Polym. 2018, 192, 240–250. [Google Scholar] [CrossRef]
- Xu, W.; Liu, K.; Li, T.; Zhang, W.; Dong, Y.; Lv, J.; Wang, W.; Sun, J.; Li, M.; Wang, M.; et al. An in situ hydrogel based on carboxymethyl chitosan and sodium alginate dialdehyde for corneal wound healing after alkali burn. J. Biomed. Mater. Res. A 2019, 107, 742–754. [Google Scholar] [CrossRef]
- Shahin, A.; Ramazani, S.A.; Mehraji, S.; Eslami, H. Synthesis and characterization of a chitosan/gelatin transparent film crosslinked with a combination of EDC/NHS for corneal epithelial cell culture scaffold with potential application in cornea implantation. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 568–578. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Young, T.-H.; Wang, T.-J. Investigating the effect of chitosan/polycaprolactone blends in differentiation of corneal endothelial cells and extracellular matrix compositions. Exp. Eye Res. 2019, 185, 107679. [Google Scholar] [CrossRef]
- Li, T.; Liang, Y.; Wang, Z.; Zhang, W.; Wang, L.; Zhou, Q.; Xu, W. Tissue-engineered scaffold based on carboxymethyl chitin or chitosan for corneal epithelial transplantation. Polym. J. 2018, 50, 511–521. [Google Scholar] [CrossRef]
- Chou, S.; Lee, C.; Lai, J. Bioengineered keratocyte spheroids fabricated on chitosan coatings enhance tissue repair in a rabbit corneal stromal defect model. J. Tissue Eng. Regen. Med. 2018, 12, 316–320. [Google Scholar] [CrossRef]
- Moradian-Oldak, J.; Qichao, R. Chitosan-Amelogenin Hydrogel for In Situ Enamel Growth. U.S. Patent Application 14/142,086, 3 July 2014. [Google Scholar]
- Gu, L.S.; Cai, X.; Guo, J.M.; Pashley, D.H.; Breschi, L.; Xu, H.H.K.; Wang, X.Y.; Tay, F.R.; Niu, L.N. Chitosan-based extrafibrillar demineralization for dentin bonding. J. Dent. Res. 2018, 98, 186–193. [Google Scholar] [CrossRef]
- Mulder, R.; Grobler, S.; Moodley, D.; Perchyonok, T. Towards bioactive dental restorative materials with chitosan and nanodiamonds: Evaluation and application. Int. J. Dent. Oral Sci. 2015, 2, 147–154. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, S.J.; Hwang, M.J.; Song, H.J.; Park, Y.J. Pulse electrodeposition of hydroxyapatite/chitosan coatings on titanium substrate for dental implant. Colloid Polym. Sci. 2017, 295, 1843–1849. [Google Scholar] [CrossRef]
- Alnufaiy, B.M.; Lambarte, R.N.A.; Alhamdan, K.S. The osteogenetic potential of chitosan coated implant: An in vitro study. J. Stem Cells Regen. Med. 2020, 16, 44–49. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Suwattanachai, P.; Everts, V.; Pimkhaokham, A.; Ampornaramveth, R.S. In vivo bone regeneration induced by a scaffold of chitosan/dicarboxylic acid seeded with human periodontal ligament cells. Int. J. Mol. Sci. 2019, 20, 4883. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Noroozi Pesyan, N.; Fathi, M.; Omidi, Y. Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 1338–1357. [Google Scholar] [CrossRef]
- Navidi, G.; Allahvirdinesbat, M.; Al-Molki, S.M.M.; Davaran, S.; Panahi, P.N.; Aghazadeh, M.; Akbarzadeh, A.; Eftekhari, A.; Safa, K.D. Design and fabrication of M-SAPO-34/chitosan scaffolds and evaluation of their effects on dental tissue engineering. Int. J. Biol. Macromol. 2021, 187, 281–295. [Google Scholar] [CrossRef]
- Soares, D.G.; Bordini, E.A.F.; Cassiano, F.B.; Bronze-Uhle, E.S.; Pacheco, L.E.; Zabeo, G.; Hebling, J.; Lisboa-Filho, P.N.; Bottino, M.C.; de Souza Costa, C.A. Characterization of novel calcium hydroxide-mediated highly porous chitosan-calcium scaffolds for potential application in dentin tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2546–2559. [Google Scholar] [CrossRef]
- Tondnevis, F.; Ketabi, M.A.; Fekrazad, R.; Sadeghi, A.; Abolhasani, M.M. Using chitosan besides nano hydroxyapatite and fluorohydroxyapatite boost dental pulp stem cell proliferation. J. Biomim. Biomater. Biomed. Eng. 2019, 42, 39–50. [Google Scholar] [CrossRef]
- Vagropoulou, G.; Trentsiou, M.; Georgopoulou, A.; Papachristou, E.; Prymak, O.; Kritis, A.; Epple, M.; Chatzinikolaidou, M.; Bakopoulou, A.; Koidis, P. Hybrid chitosan/gelatin/nanohydroxyapatite scaffolds promote odontogenic differentiation of dental pulp stem cells and in vitro biomineralization. Dent. Mater. 2021, 37, e23–e36. [Google Scholar] [CrossRef]
- Shen, R.; Xu, W.; Xue, Y.; Chen, L.; Ye, H.; Zhong, E.; Ye, Z.; Gao, J.; Yan, Y. The use of chitosan/PLA nano-fibers by emulsion eletrospinning for periodontal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 419–430. [Google Scholar] [CrossRef]
- Yang, X.; Han, G.; Pang, X.; Fan, M. Chitosan/collagen scaffold containing bone morphogenetic protein-7 DNA supports dental pulp stem cell differentiation in vitro and in vivo. J. Biomed. Mater. Res. A 2020, 108, 2519–2526. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Georgopoulou, A.; Grivas, I.; Bekiari, C.; Prymak, O.; Loza, Κ.; Epple, M.; Papadopoulos, G.C.; Koidis, P.; Chatzinikolaidou, Μ. Dental pulp stem cells in chitosan/gelatin scaffolds for enhanced orofacial bone regeneration. Dent. Mater. 2019, 35, 310–327. [Google Scholar] [CrossRef]
- Divband, B.; Aghazadeh, M.; Al-qaim, Z.H.; Samiei, M.; Hussein, F.H.; Shaabani, A.; Shahi, S.; Sedghi, R. Bioactive chitosan biguanidine-based injectable hydrogels as a novel BMP-2 and VEGF carrier for osteogenesis of dental pulp stem cells. Carbohydr. Polym. 2021, 273, 118589. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.J.; Moon, J.-H.; Kim, J.H.; Heo, D.N.; Bang, J.B.; Lim, H.-N.; Kwon, I.K. Preparation of antibacterial chitosan membranes containing silver nanoparticles for dental barrier membrane applications. J. Ind. Eng. Chem. 2018, 66, 196–202. [Google Scholar] [CrossRef]
- Ansari, M.J.; Ahmed, M.M.; Anwer, M.K.; Jamil, S.; Alalaiwe, A.; Alshetaili, A.S.; Al-Shdefat, R.; Ali, R.; Shakeel, F. Formulation and characterization of fluconazole loaded olive oil nanoemulsions. Indo Am. J. Pharm. Sci. 2017, 4, 852–860. [Google Scholar]
- Ansari, M.J.; Ahmed, M.M.; Anwer, M.K.; Jamil, S.; Al-Shdefat, R.; Ali, B. Solubility and stability enhancement of curcumin through cyclodextrin complexation. Int. J. Biol. Pharm. Allied Sci. 2014, 3, 2668–2675. [Google Scholar]
- Guo, J.L.; Kim, Y.S.; Xie, V.Y.; Smith, B.T.; Watson, E.; Lam, J.; Pearce, H.A.; Engel, P.S.; Mikos, A.G. Modular, tissue-specific, and biodegradable hydrogel cross-linkers for tissue engineering. Sci. Adv. 2019, 5, eaaw7396. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ibnouf, E.O.; Kalam, M.A.; Alshamsan, A.; Aldawsari, M.F.; Alalaiwe, A.; Ansari, M.J. Formulation and in vitro evaluation of topical nanosponge-based gel containing butenafine for the treatment of fungal skin infection. Saudi Pharm. J. 2021, 29, 467–477. [Google Scholar] [CrossRef]
- Abdel-Kader, M.; Al-Shdefat, R. Evaluation of Antifungal Activity of Olive Oil Based Nanoemulsions Evaluation of Essential Oils for Antimicrobial Activity from Some Moroccan Aromatic Plants Medicinal View Project Isoflavonoids View Project. Bull. Environ. Pharmacol. Life Sci. 2016, 5, 1–4. [Google Scholar]
- Fatima, F.; Aldawsari, M.F.; Ahmed, M.M.; Anwer, M.K.; Naz, M.; Ansari, M.J.; Hamad, A.M.; Zafar, A.; Jafar, M. Green synthesized silver nanoparticles using tridax procumbens for topical application: Excision wound model and histopathological studies. Pharmaceutics 2021, 13, 1754. [Google Scholar] [CrossRef]
- He, Y.; Lu, F. Development of synthetic and natural materials for tissue engineering applications using adipose stem cells. Stem Cells Int. 2016, 2016, 5786257. [Google Scholar] [CrossRef]
- Nagarkar, R.; Patel, J. Polyvinyl alcohol: A comprehensive study. Acta Sci. Pharm. Sci. 2019, 3, 34–44. [Google Scholar]
- Kohane, D.S.; Langer, R. Polymeric biomaterials in tissue engineering. Pediatr. Res. 2008, 63, 487–491. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Tripathi, S.; Singh, B.N.; Divakar, S.; Kumar, G.; Mallick, S.P.; Srivastava, P. Design and evaluation of ciprofloxacin loaded collagen chitosan oxygenating scaffold for skin tissue engineering. Biomed. Mater. 2021, 16, 25021. [Google Scholar] [CrossRef]
- Pezeshki, M.; Mojgan, M.; Sarah, Z. Tailoring the gelatin/chitosan electrospun scaffold for application in skin tissue engineering: An in vitro study. Prog. Biomater. 2018, 7, 207–218. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Prospects of chitosan-based scaffolds for growth factor release in tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1382–1389. [Google Scholar] [CrossRef]
- Prabaharan, M.; Rodriguez-Perez, M.A.; de Saja, J.A.; Mano, J.F. Preparation and characterization of poly(L-lactic acid)-chitosan hybrid scaffolds with drug release capability. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 427–434. [Google Scholar] [CrossRef]
- Zhang, M.; Wan, T.; Fan, P.; Shi, K.; Chen, X.; Yang, H.; Liu, X.; Xu, W.; Zhou, Y. Photopolymerizable chitosan hydrogels with improved strength and 3D printability. Int. J. Biol. Macromol. 2021, 193, 109–116. [Google Scholar] [CrossRef]
- Yang, Y.; Campbell Ritchie, A.; Everitt, N.M. Recombinant human collagen/chitosan-based soft hydrogels as biomaterials for soft tissue engineering. Mater. Sci. Eng. C 2021, 121, 111846. [Google Scholar] [CrossRef]
- Islam, M.T.; Laing, R.M.; Wilson, C.A.; McConnell, M.; Ali, M.A. Fabrication and characterization of 3-dimensional electrospun poly(vinyl alcohol)/keratin/chitosan nanofibrous scaffold. Carbohydr. Polym. 2022, 275, 118682. [Google Scholar] [CrossRef]
- Kouser, S.; Prabhu, A.; Prashantha, K.; Nagaraja, G.K.; D’souza, J.N.; Meghana Navada, K.; Qurashi, A.; Manasa, D.J. Modified halloysite nanotubes with chitosan incorporated PVA/PVP bionanocomposite films: Thermal, mechanical properties and biocompatibility for tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127941. [Google Scholar] [CrossRef]
- Becerra, J.; Rodriguez, M.; Leal, D.; Noris-Suarez, K.; Gonzalez, G. Chitosan-collagen-hydroxyapatite membranes for tissue engineering. J. Mater. Sci. Mater. Med. 2022, 33, 18. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, S.; Zhang, R.; Wang, Y.; Jiao, Z.; Li, W.; Nie, Y.; Liu, T.; Song, K. Dynamic process enhancement on chitosan/gelatin/nano-hydroxyapatite-bone derived multilayer scaffold for osteochondral tissue repair. Mater. Sci. Eng. C 2022, 133, 112662. [Google Scholar] [CrossRef]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef]
- Ali, A.; Bano, S.; Poojary, S.; Chaudhary, A.; Kumar, D.; Negi, Y.S. Effect of cellulose nanocrystals on chitosan/PVA/nano β-TCP composite scaffold for bone tissue engineering application. J. Biomater. Sci. Polym. Ed. 2022, 33, 1–19. [Google Scholar] [CrossRef]
- Yavari Maroufi, L.; Ghorbani, M. Injectable chitosan-quince seed gum hydrogels encapsulated with curcumin loaded-halloysite nanotubes designed for tissue engineering application. Int. J. Biol. Macromol. 2021, 177, 485–494. [Google Scholar] [CrossRef]
- Barik, D.; Kundu, K.; Dash, M. Montmorillonite stabilized chitosan-co-mucin hydrogel for tissue engineering applications. RSC Adv. 2021, 11, 30329–30342. [Google Scholar] [CrossRef]
- Shah, R.; Stodulka, P.; Skopalova, K.; Saha, P. Dual crosslinked collagen/chitosan film for potential biomedical applications. Polymers 2019, 11, 94. [Google Scholar] [CrossRef]
- Li, D.-W.; He, J.; He, F.-L.; Liu, Y.-L.; Liu, Y.-Y.; Ye, Y.-J.; Deng, X.; Yin, D.-C. Silk fibroin/chitosan thin film promotes osteogenic and adipogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J. Biomater. Appl. 2018, 32, 1164–1173. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Z.; Ge, X.; Zhang, Y.; Xu, H. Decellularized annulus fibrosus matrix/chitosan hybrid hydrogels with basic fibroblast growth factor for annulus fibrosus tissue engineering. Tissue Eng Part A 2019, 25, 1605–1613. [Google Scholar] [CrossRef]
- Fang, S.W.; Li, C.F.; Shih, D.Y.C. Antifungal activity of chitosan and its preservative effect on low-sugar candied kumquat. J. Food Prot. 1994, 57, 136–140. [Google Scholar] [CrossRef]
- Kim, C.H.; Choi, J.W.; Chun, H.J.; Choi, K.S. Synthesis of chitosan derivatives with quaternary ammonium salt and their antibacterial activity. Polymer. Bull. 1997, 38, 387–393. [Google Scholar] [CrossRef]
- Franklin, T.J.; Snow, G.A. Biochemistry of Antimicrobial Action; Chapman and Hall: London, UK, 1981; ISBN 9780412224409. [Google Scholar]
- Torkaman, S.; Rahmani, H.; Ashori, A.; Najafi, S.H.M. Modification of chitosan using amino acids for wound healing purposes: A review. Carbohydr. Polym. 2021, 258, 117675. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, Y.; Yan, H.; Rafique, M.; Li, S.; Shan, X.; Wu, L.; Qiao, M.; Kong, D.; Wang, L. Anti-infective and pro-coagulant chitosan-based hydrogel tissue adhesive for sutureless wound closure. Biomacromolecules 2020, 21, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Zhao, J.Y.; Hu, W.Z.; Ma, K.; Chao, Y.; Sun, P.J.; Fu, X.B.; Zhang, H. Synthetic poly(vinyl alcohol)-chitosan as a new type of highly efficient hemostatic sponge with blood-triggered swelling and high biocompatibility. J. Mater. Chem. B 2019, 7, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wu, L.; Yan, H.; Jiang, Z.; Li, S.; Li, W.; Bai, Y.; Wang, H.; Cheng, Z.; Kong, D.; et al. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021, 12, 4733. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Li, C.M. Electrospun ZnO-loaded chitosan/PCL bilayer membranes with spatially designed structure for accelerated wound healing. Carbohydr. Polym. 2022, 282, 119131. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.H.; Ouf, A.; Elshishiny, F.; Taskin, M.B.; Song, J.; Dong, M.; Chen, M.; Siam, R.; Mamdouh, W. Antimicrobial and wound-healing activities of graphene-reinforced electrospun chitosan/gelatin nanofibrous nanocomposite scaffolds. ACS Omega 2022, 7, 1838–1850. [Google Scholar] [CrossRef]
- Deng, P.; Yao, L.; Chen, J.; Tang, Z.; Zhou, J. Chitosan-based hydrogels with injectable, self-healing and antibacterial properties for wound healing. Carbohydr. Polym. 2022, 276, 118718. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Li, Q.; Liu, X.; Guo, Z. Preparation of cross-linked chitosan quaternary ammonium salt hydrogel films loading drug of gentamicin sulfate for antibacterial wound dressing. Mar. Drugs 2021, 19, 479. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Feng, Z.; Peng, K.; Wei, A.; Wang, Y.; Tong, Z.; Cheng, B. Preparation of a chitosan/carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos. B Eng. 2020, 197, 108139. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, M.; Woo, M.W.; Li, Y.; Han, W.; Dang, X. High-mechanical strength carboxymethyl chitosan-based hydrogel film for antibacterial wound dressing. Carbohydr. Polym. 2021, 256, 117590. [Google Scholar] [CrossRef]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef]
- Ghasemian Lemraski, E.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, S.; Rafiee-Moghaddam, R.; Webster, T.J. Antimicrobial double-layer wound dressing based on chitosan/polyvinyl alcohol/copper: In vitro and in vivo assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar] [CrossRef]
- Wang, S.; Yan, F.; Ren, P.; Li, Y.; Wu, Q.; Fang, X.; Chen, F.; Wang, C. Incorporation of metal-organic frameworks into electrospun chitosan/poly (vinyl alcohol) nanofibrous membrane with enhanced antibacterial activity for wound dressing application. Int. J. Biol. Macromol. 2020, 158, 9–17. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, X.; Zheng, S.; Wang, Y.; Li, Z.; Zhang, H.; Nie, L.; Zhang, Y.; Zhao, Y.; Yang, X. Bio-adhesive catechol-modified chitosan wound healing hydrogel dressings through glow discharge plasma technique. Chem. Eng. J. 2021, 427, 130843. [Google Scholar] [CrossRef]
- Zhang, D.; Ouyang, Q.; Hu, Z.; Lu, S.; Quan, W.; Li, P.; Chen, Y.; Li, S. Catechol functionalized chitosan/active peptide microsphere hydrogel for skin wound healing. Int. J. Biol. Macromol. 2021, 173, 591–606. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Pérez-Álvarez, L.; Sáez-Martínez, V.; Benito-Cid, S.; Ruiz-Rubio, L.; Pérez-González, R.; Vilas-Vilela, J.L.; Alonso, J.M. Wound healing and antibacterial chitosan-genipin hydrogels with controlled drug delivery for synergistic anti-inflammatory activity. Int. J. Biol. Macromol. 2022, 203, 679–694. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, C.; Peng, X.; Zhang, K.; Yu, X. A self-healing and injectable oxidized quaternized guar gum/carboxymethyl chitosan hydrogel with efficient hemostatic and antibacterial properties for wound dressing. Colloids Surf. B Biointerfaces 2022, 209, 112207. [Google Scholar] [CrossRef]
- Karizmeh, M.S.; Poursamar, S.A.; Kefayat, A.; Farahbakhsh, Z.; Rafienia, M. An in vitro and in vivo study of PCL/chitosan electrospun mat on polyurethane/propolis foam as a bilayer wound dressing. Mater. Sci. Eng. C 2022, 135, 112667. [Google Scholar] [CrossRef]
- Dadkhah Tehrani, F.; Shabani, I.; Shabani, A. A hybrid oxygen-generating wound dressing based on chitosan thermosensitive hydrogel and decellularized amniotic membrane. Carbohydr. Polym. 2022, 281, 119020. [Google Scholar] [CrossRef]
- Liang, H.; Mirinejad, M.S.; Asefnejad, A.; Baharifar, H.; Li, X.; Saber-Samandari, S.; Toghraie, D.; Khandan, A. Fabrication of tragacanthin gum-carboxymethyl chitosan bio-nanocomposite wound dressing with silver-titanium nanoparticles using freeze-drying method. Mater. Chem. Phys. 2022, 279, 125770. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Zhang, Y.; Cao, Q.; Wang, X.; Han, Y.; Sun, G.; Li, Y.; Zhou, J. Novel lignin–chitosan–PVA composite hydrogel for wound dressing. Mater. Sci. Eng. C 2019, 104, 110002. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Valachová, K.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Šoltés, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef]
- Zou, P.; Lee, W.-H.; Gao, Z.; Qin, D.; Wang, Y.; Liu, J.; Sun, T.; Gao, Y. Wound dressing from polyvinyl alcohol/chitosan electrospun fiber membrane loaded with OH-CATH30 nanoparticles. Carbohydr. Polym. 2020, 232, 115786. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G.; Applications, G.C. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
| Device Type | Model Drug/Drug | Polymer Formulation | Preparation Method | Administration Route | Delivered Site | Effect/Results | References |
|---|---|---|---|---|---|---|---|
| Solid–lipid nanoparticles | Leflunomide | Chitosan/Folic acid | Layer-by-layer coating | oral | joint | FA-CS-SLNs exhibited sustained release for 168 h and lowered liver toxicity and enhanced joint healing compared to leflunomide suspensions. | [161] |
| Implants | Ibuprofen | Chitosan/polycaprolactone | Hotmelt extrusion/Fused deposition modeling | - | - | Sustained release for 120 h by diffusion-erosion, 75.3% cell viability. | [162] |
| Hydrogel-based microneedles | Salvia miltiorrhiza | Carboxymethyl chitosan/oxidized pullulan | Skin | Mucosa | Simple penetration of HFM-1 into infant porcine skin was demonstrated because of its remarkable mechanical characteristics. Drug release was rapid from HFM-1, hence suitable for transdermal delivery. | [163] | |
| Hydrogel | Berberine chloride hydrate | Chitosan/puerarin | Interpenetrating network | Injectable | Increased rheological properties due to dense structure of CS/PUE18 hydrogels. Dual anti-inflammatory and antimicrobial activities with pH-dependent drug release | [164] | |
| Thermogel | Dexamethasone | Hexanoyl glycol chitosan | Injectable | Inner ear | Versatile release kinetics with no initial burst release. Excellent residual stability without any ear-related side effects and could deliver high concentrations of the drug to the inner ear. | [165] | |
| Hydrogel | Acyclovir | Chitosan/β-cyclodextrin/methacrylic acid (MAA) and N′ N′-methylenebis-acrylamide (MBA) | Free radical polymerization | Oral | Zero-order kinetics of drug release with pH-dependent swelling behavior. After acute oral toxicity studies, no significant behavioral, histopathological, and clinical changes were observed in Wistar rats. Increased bioavailability as compared to acyclovir suspension at a dose of 20 mg/kg in rabbit plasma. | [166] | |
| Hollow capsule | Gemcitabine/curcumin | Chitosan/poly(ethylene glycol dimethacrylate-co-methacrylic acid) | Layer-by-layer method | pH-dependent drug delivery at pHs 5.5 and 7.4. Encapsulation efficiency was above 84%, and release efficiency was 82%. Good cytotoxicity towards HCT-116 colorectal cancer cell lines. | [167] | ||
| Nanohybrid | 5-fluorouracil | Chitosan/collagen/gold nanoparticles/biotin-quat188-chitosan (Bi-QCS-AuNPs@collagen) | Layer-by-layer assembly | - | - | Bi-QCS-AuNPs@collagen overcame the low drug load capacity of AuNPs from 64.675 to 87.46% as well as excellent anti-inflammatory activity in macrophage cell lines (RAW264.7). Moreover, in comparison to free 5-FU, the nanohybrid improved drug activity by 3.3-fold in HeLa cell lines and 6.2-fold in A549 cell lines, respectively. | [168] |
| Nanoparticles | Voriconazole | Chitosan | Spray-drying | Topical | Skin | The drug loading in NPs ranged from 75% to 90%. Sustained-release profile in rat skin model and exhibited antifungal activity against C. albicans. | [169] |
| Films | Ciprofloxacin | Chitosan/chitosan-depolymerization products | Casting | - | - | Low acetylated and molecular weight CDP-based films exhibited reduced swelling and ciprofloxacin released in a controlled manner for up to 54% in 24 h in a pH-dependent manner. | [170] |
| Device Type | Model Drug/Drug | Polymer Formulation | Preparation Method | Wound Site | Effects/Results | References |
|---|---|---|---|---|---|---|
| Hydrogels | - | Gallic acid/chitosan | Discharge plasma technology | Traditional DPPH scavenging experiments revealed remarkable antioxidant characteristics. CS-GA formed hydrogels by cross-linking by undergoing oxidation at a physiological state. High cytocompatibility and hemocompatibility were observed in vivo rat skin-layer defects and liver hemorrhagic models (46.6% collagen fiber growth by the 7th day). CS-GA functions at the wound site without the application of prolonged pressure. | [297] | |
| Hydrogels | Catechol-modified chitosan/Oyster peptide microspheres/β-sodium glycerophosphate (β-GP) | [298] | ||||
| Hydrogels | Amoxicillin, tetracycline, cefuroxime, acetylsalicylic acid | Chitosan/genipin | Ulcer wounds/dermal tissue | Synergistic antibacterial-anti-inflammatory wound healing was observed with ASA-based antibiotic combinations with sustained drug release. | [299] | |
| Hydrogels | Carboxymethyl chitosan/oxidized quaternized guar gum OQGG@CMCS | Excellent antibacterial and hemostatic activity, self-healing in S. aureus rat model. | [300] | |||
| Electrospun mats | Chitosan/poly-ε-caprolactone fibrous mat/polyurethane foam/ethanolic extract of propolis (EEP) | Electrospinning | PCL/CS-PU/EEP bilayered wound dressing exhibited improved biocompatibility and healing potential both in vitro and in vivo. | [301] | ||
| Thermosensitive hydrogel-microparticles based hybrid | Chitosan/β-glycerophosphate thermosensitive hydrogel/decellularized amniotic membrane/polylactic acid microparticles | The hybrid oxygen-generating wound dressing material promoted cell adhesion and growth and was non-cytotoxic, and released oxygen for 7 days. | [302] | |||
| Bionanocomposite | Carboxymethyl cellulose/tragacanth gum/silver-titanium nanoparticles | Freeze drying | The wound dressing exhibited porosity between 65–79%, which increased with the addition of silver-TiO2. Higher wound dressing weight loss was observed for the highest concentration of AgO/TiO2. | [303] | ||
| Hydrogels | Chitosan/lignin/polyvinyl alcohol | Freeze thawing | Lignin-based PVA-chitosan hydrogels had good mechanical strength, protein adsorbing capacity, and wound healing with environmental regulation ability. | [304] | ||
| Membrane | Chitosan/hyaluronan/phosphatidylcholine dihydroquercetin (Ch/HA/PCDQ) | Ch/HA/PCDQ membranes displayed antibacterial, antioxidant, and anti-inflammatory activities as well as showed biocompatibility. Significantly greater wound healing potency was observed in mouse full thickness wound model. | [305] | |||
| Nanoparticles-loaded electrospun nanofibers | OH-CATH30 antibacterial peptide | Chitosan/polyvinyl alcohol | Electrospinning | Skin wounds | NP-30-NFs exhibited antibacterial activity against E.coli and S. aureus and showed wound healing in mouse skin wounds. | [306] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. https://doi.org/10.3390/ijms231810975
Abourehab MAS, Pramanik S, Abdelgawad MA, Abualsoud BM, Kadi A, Ansari MJ, Deepak A. Recent Advances of Chitosan Formulations in Biomedical Applications. International Journal of Molecular Sciences. 2022; 23(18):10975. https://doi.org/10.3390/ijms231810975
Chicago/Turabian StyleAbourehab, Mohammed A. S., Sheersha Pramanik, Mohamed A. Abdelgawad, Bassam M. Abualsoud, Ammar Kadi, Mohammad Javed Ansari, and A. Deepak. 2022. "Recent Advances of Chitosan Formulations in Biomedical Applications" International Journal of Molecular Sciences 23, no. 18: 10975. https://doi.org/10.3390/ijms231810975
APA StyleAbourehab, M. A. S., Pramanik, S., Abdelgawad, M. A., Abualsoud, B. M., Kadi, A., Ansari, M. J., & Deepak, A. (2022). Recent Advances of Chitosan Formulations in Biomedical Applications. International Journal of Molecular Sciences, 23(18), 10975. https://doi.org/10.3390/ijms231810975








