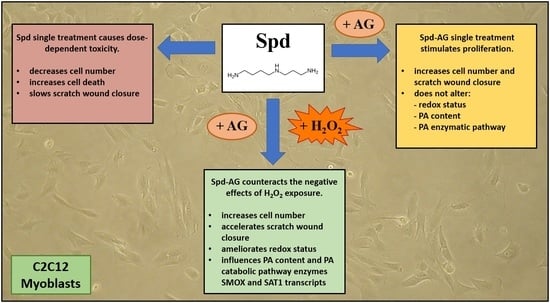
The Impact of Spermidine on C2C12 Myoblasts Proliferation, Redox Status and Polyamines Metabolism under H2O2 Exposure
Abstract
:1. Introduction
2. Results
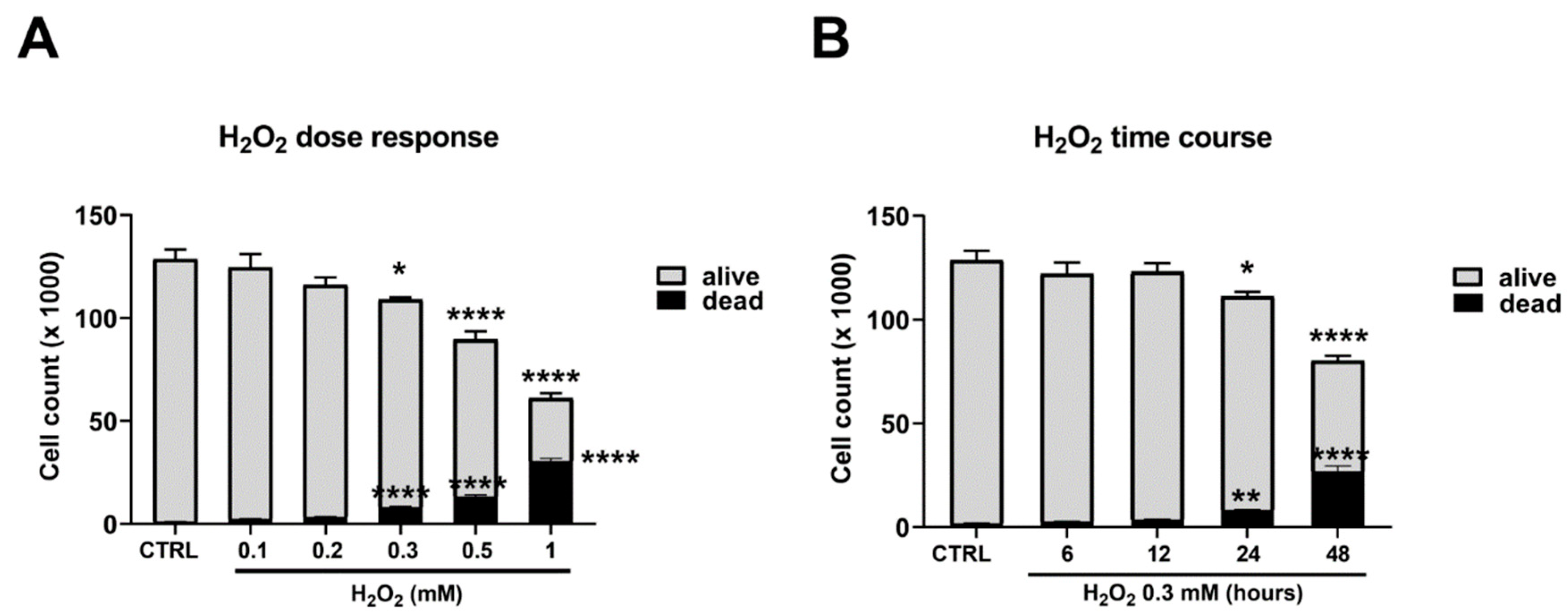
2.1. Hydrogen Peroxide Treatment Reduces C2C12 Myoblast Viability and Total Antioxidant Capacity
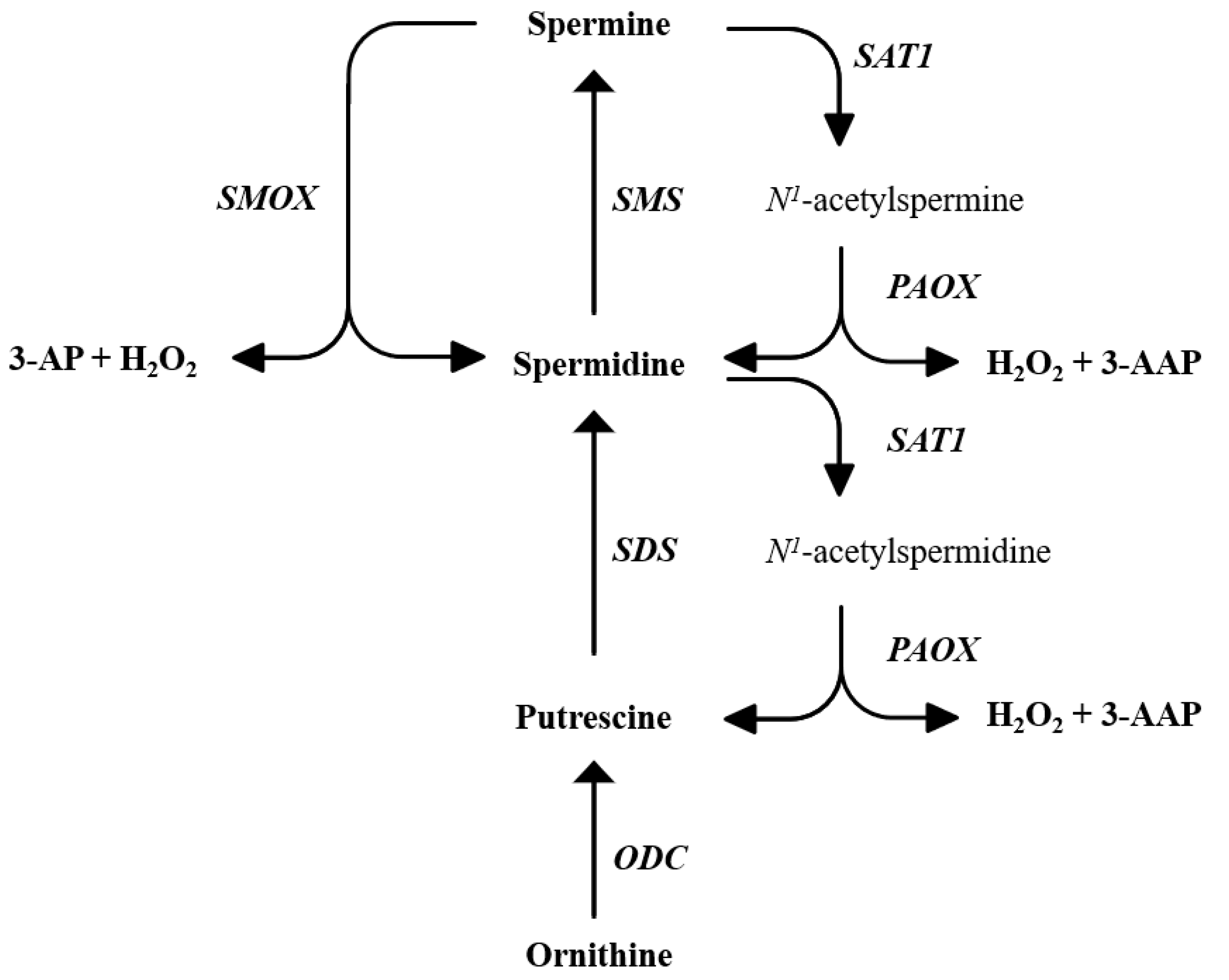
2.2. Hydrogen Peroxide Treatment Reduces Intracellular Polyamines Content
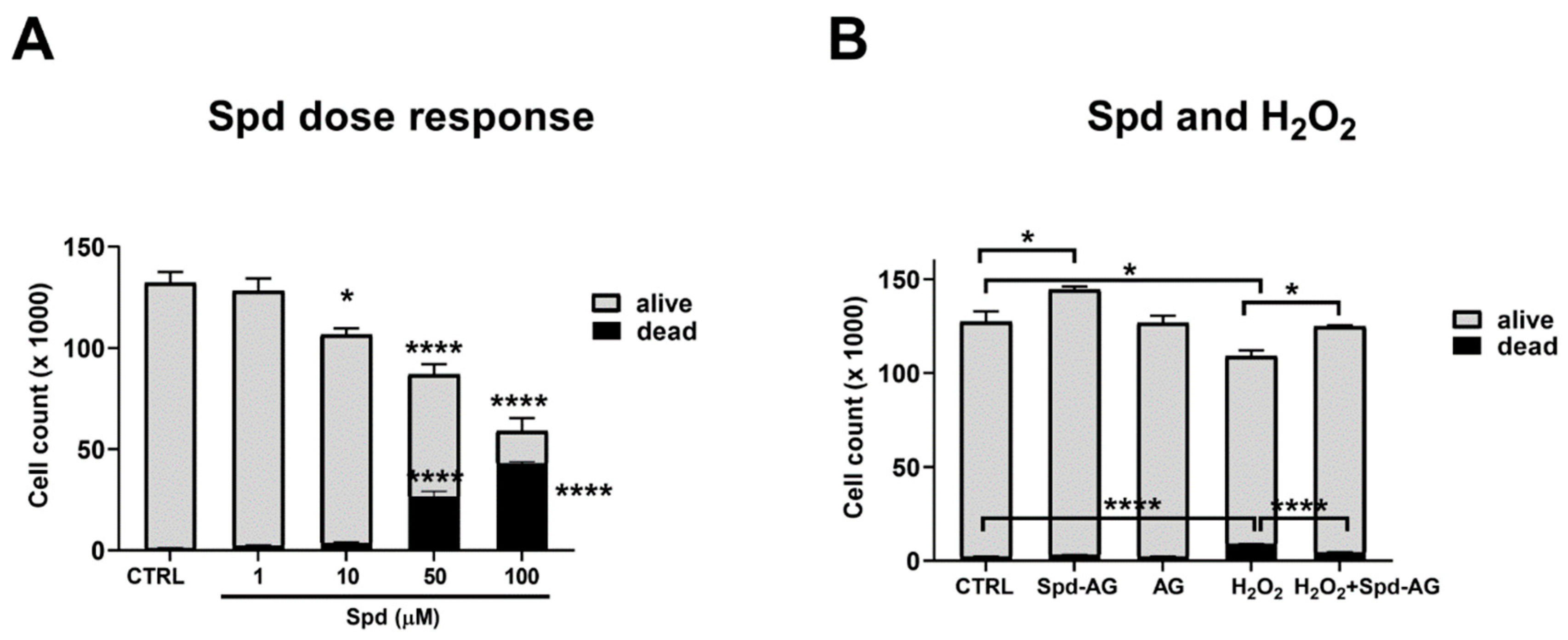
2.3. Effect of Spermidine and Aminoguanidine Treatment on C2C12 Myoblast Viability
2.4. Spermidine-AG Accelerates Scratch Wound Closure
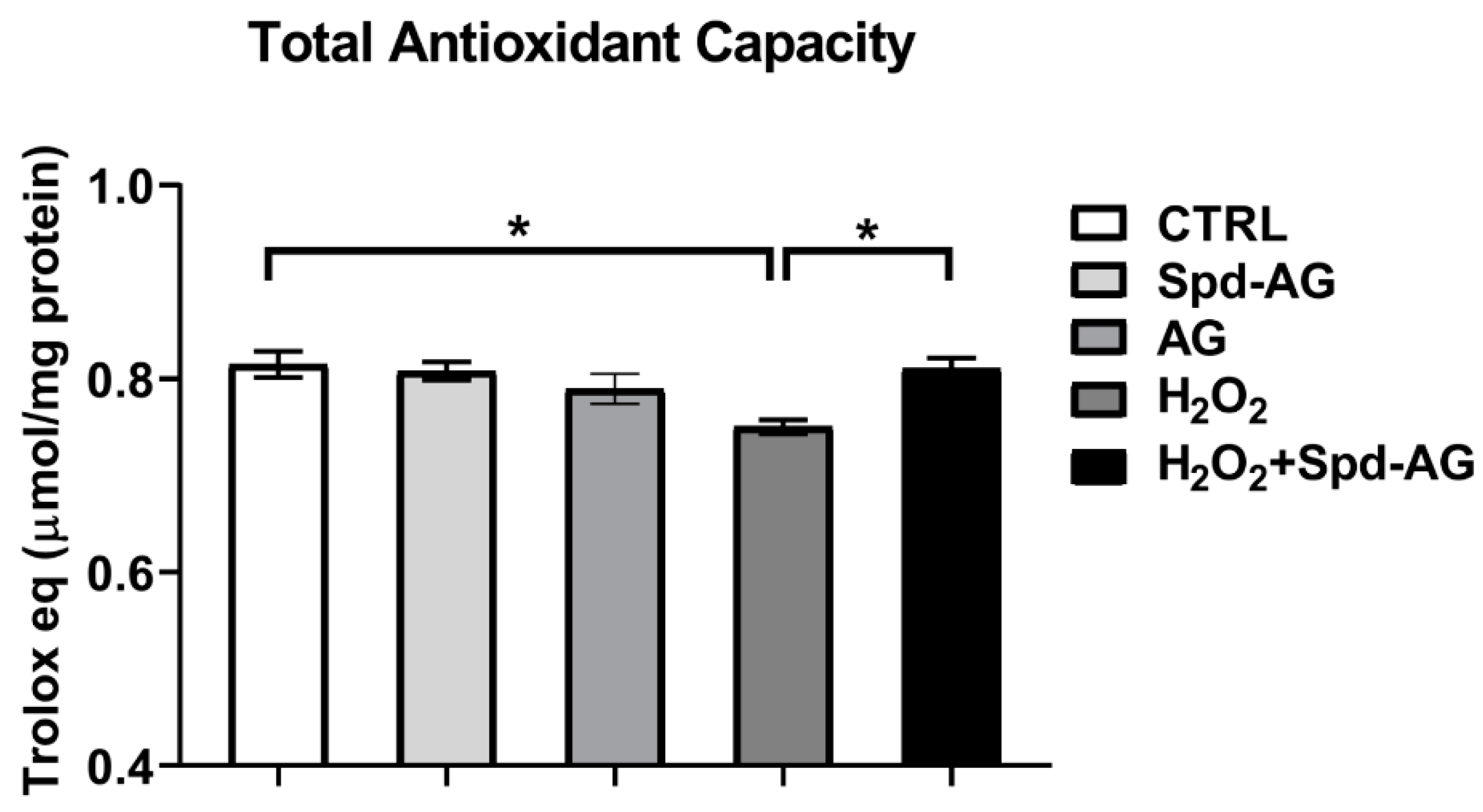
2.5. Spermidine-AG Treatment Restores Redox Status and Total Antioxidant Capacity in C2C12 Myoblasts
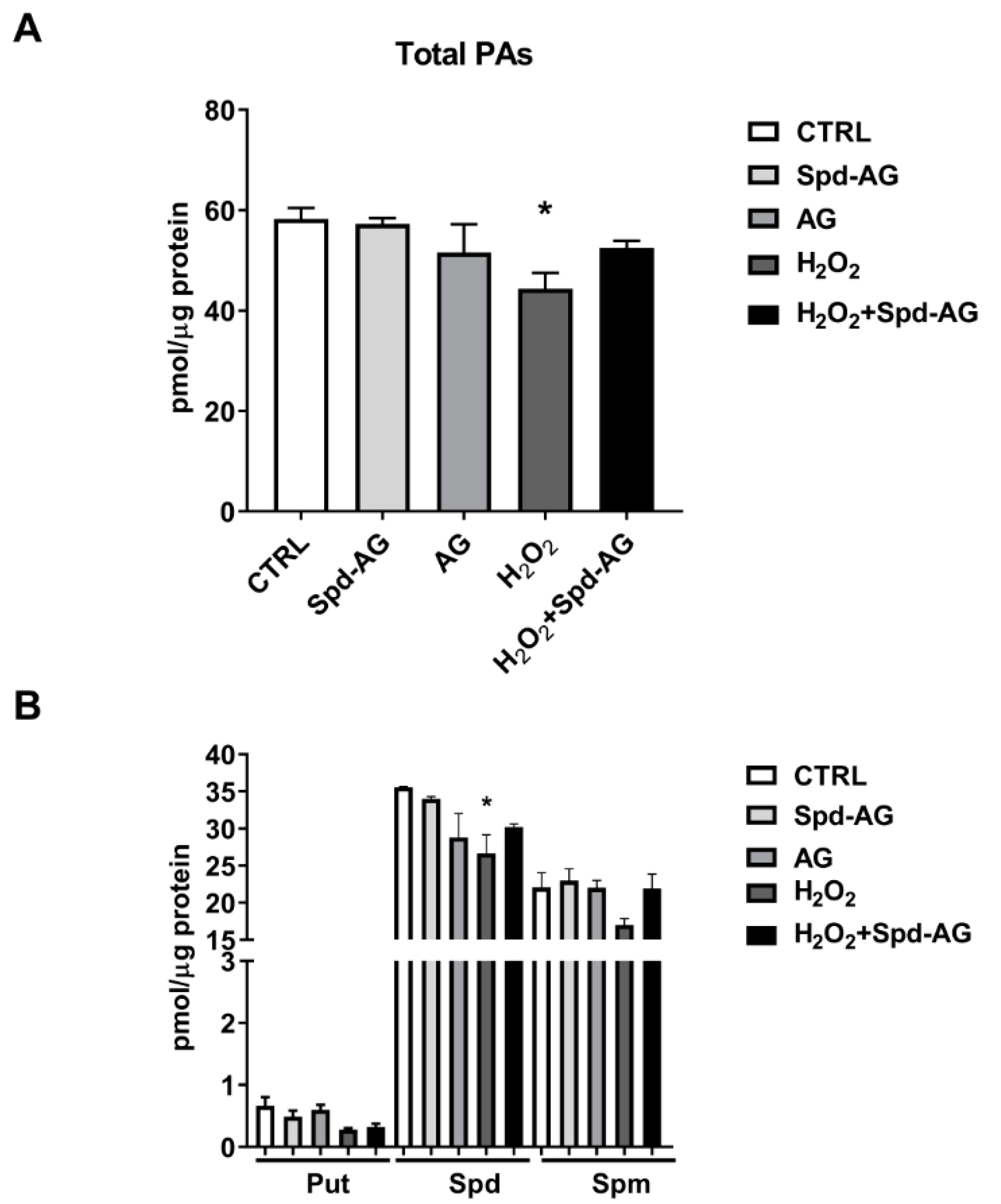
2.6. Spd-AG Treatment Counteracts H2O2-Induced Polyamines Imbalance
2.7. Effect of Spd-AG and Hydrogen Peroxide Treatment on Polyamines Enzymatic Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Trolox® Equivalents Antioxidant Capacity
4.3. Polyamines Content
4.4. Wound Closure Assay
4.5. Glutathione Homeostasis
4.6. RNA Isolation, Reverse Transcription and qReal-Time PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- García-Prat, L.; Perdiguero, E.; Alonso-Martín, S.; Dell’Orso, S.; Ravichandran, S.; Brooks, S.R.; Juan, A.H.; Campanario, S.; Jiang, K.; Hong, X.; et al. FoxO maintains a genuine muscle stem-cell quiescent state until geriatric age. Nat. Cell Biol. 2020, 22, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Leonetti, A.; Duranti, G.; Sabatini, S.; Ceci, R.; Mariottini, P. Skeletal Muscle Pathophysiology: The Emerging Role of Spermine Oxidase and Spermidine. Med. Sci. 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Miano, C.; Pelosi, L.; Musarò, A. An Overview about the Biology of Skeletal Muscle Satellite Cells. Curr. Genom. 2019, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Lehka, L.; Rędowicz, M.J. Mechanisms regulating myoblast fusion: A multilevel interplay. Semin. Cell Dev. Biol. 2020, 104, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms Regulating Muscle Regeneration: Insights into the Interrelated and Time-Dependent Phases of Tissue Healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef]
- Reinoso-Sánchez, J.F.; Baroli, G.; Duranti, G.; Scaricamazza, S.; Sabatini, S.; Valle, C.; Morlando, M.; Casero, R.A., Jr.; Bozzoni, I.; Mariottini, P.; et al. Emerging Role for Linear and Circular Spermine Oxidase RNAs in Skeletal Muscle Physiopathology. Int. J. Mol. Sci. 2020, 21, 8227. [Google Scholar] [CrossRef]
- Scaricamazza, S.; Salvatori, I.; Ferri, A.; Valle, C. Skeletal Muscle in ALS: An Unappreciated Therapeutic Opportunity? Cells 2021, 10, 525. [Google Scholar] [CrossRef]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 156. [Google Scholar] [CrossRef]
- Spasojevic, I.; Jones, D.R.; Andrades, M.E. Hydrogen Peroxide in Adaptation. Oxidative Med. Cell. Longev. 2012, 2012, 596019. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Jackson, M.J.; Stretton, C.; McArdle, A. Hydrogen peroxide as a signal for skeletal muscle adaptations to exercise: What do concentrations tell us about potential mechanisms? Redox Biol. 2020, 35, 101484. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Lian, D.; Chen, M.M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Ceci, R.; Valls, M.R.B.; Duranti, G.; Dimauro, I.; Quaranta, F.; Pittaluga, M.; Sabatini, S.; Caserotti, P.; Parisi, P.; Parisi, A.; et al. Oxidative stress responses to a graded maximal exercise test in older adults following explosive-type resistance training. Redox Biol. 2013, 2, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Dimauro, I.; Sgura, A.; Pittaluga, M.; Magi, F.; Fantini, C.; Mancinelli, R.; Sgadari, A.; Fulle, S.; Caporossi, D. Regular exercise participation improves genomic stability in diabetic patients: An exploratory study to analyse telomere length and DNA damage. Sci. Rep. 2017, 7, 4137. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Ozdemir, M.; Hyatt, H. Redox Control of Proteolysis During Inactivity-Induced Skeletal Muscle Atrophy. Antioxid. Redox Signal. 2020, 33, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Bazzucchi, I.; Patrizio, F.; Ceci, R.; Duranti, G.; Sabatini, S.; Sgrò, P.; Di Luigi, L.; Sacchetti, M. Quercetin Supplementation Improves Neuromuscular Function Recovery from Muscle Damage. Nutrients 2020, 12, 2850. [Google Scholar] [CrossRef]
- Tanabe, Y.; Fujii, N.; Suzuki, K. Dietary Supplementation for Attenuating Exercise-Induced Muscle Damage and Delayed-Onset Muscle Soreness in Humans. Nutrients 2021, 14, 70. [Google Scholar] [CrossRef]
- Hirano, R.; Shirasawa, H.; Kurihara, S. Health-Promoting Effects of Dietary Polyamines. Med. Sci. 2021, 9, 8. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [Green Version]
- Bongers, K.S.; Fox, D.K.; Kunkel, S.D.; Stebounova, L.V.; Murry, D.J.; Pufall, M.A.; Ebert, S.M.; Dyle, M.C.; Bullard, S.A.; Dierdorff, J.M.; et al. Spermine oxidase maintains basal skeletal muscle gene expression and fiber size and is strongly repressed by conditions that cause skeletal muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E144–E158. [Google Scholar] [CrossRef]
- Ni, Y.Q.; Liu, Y.S. New Insights into the Roles and Mechanisms of Spermidine in Aging and Age-Related Diseases. Aging Dis. 2021, 12, 1948–1963. [Google Scholar] [CrossRef] [PubMed]
- Polticelli, F.; Salvi, D.; Mariottini, P.; Amendola, R.; Cervelli, M. Molecular evolution of the polyamine oxidase gene family in Metazoa. BMC Evol. Biol. 2012, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Salvi, D.; Polticelli, F.; Amendola, R.; Mariottini, P. Structure-function relationships in the evolutionary framework of spermine oxidase. J. Mol. Evol. 2013, 76, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.; Dunston, T.T.; Woster, P.M.; Casero, R.A., Jr. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018, 293, 18736–18745. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray-Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Cervelli, M.; Angelucci, E.; Germani, F.; Amendola, R.; Mariottini, P. Inflammation, carcinogenesis and neurodegeneration studies in transgenic animal models for polyamine research. Amino Acids 2014, 46, 521–530. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y. Kinetic evaluation of polyamines as radical scavengers. Anticancer Res. 2005, 25, 965–969. [Google Scholar]
- Shu, S.; Kobayashi, M.; Marunaka, K.; Yoshino, Y.; Goto, M.; Katsuta, Y.; Ikari, A. Magnesium Supplementation Attenuates Ultraviolet-B-Induced Damage Mediated through Elevation of Polyamine Production in Human HaCaT Keratinocytes. Cells 2022, 11, 2268. [Google Scholar] [CrossRef]
- Tkachenko, A.G.; Akhova, A.V.; Shumkov, M.S.; Nesterova, L.Y. Polyamines reduce oxidative stress in Escherichia coli cells exposed to bactericidal antibiotics. Res. Microbiol. 2012, 163, 83–91. [Google Scholar] [CrossRef]
- Farriol, M.; Segovia-Silvestre, T.; Venereo, Y.; Orta, X. Antioxidant effect of polyamines on erythrocyte cell membrane lipoperoxidation after free-radical damage. Phytother. Res. 2003, 17, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Amendola, R.; Bellini, A.; Cervelli, M.; Degan, P.; Marcocci, L.; Martini, F.; Mariottini, P. Direct oxidative DNA damage, apoptosis and radio sensitivity by spermine oxidase activities in mouse neuroblastoma cells. Biochim. Biophys. Acta 2005, 1755, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Casero, R.A., Jr. Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: A potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006, 66, 11125–11130. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Hacker, A.; Huang, Y.; Casero, R.A., Jr. Tumor necrosis factor alpha induces spermidine/spermine N1-acetyltransferase through nuclear factor kappaB in non-small cell lung cancer cells. J. Biol. Chem. 2006, 281, 24182–24192. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Murray-Stewart, T.; Casero, R.A., Jr. Inflammation and polyamine catabolism: The good, the bad and the ugly. Biochem. Soc. Trans. 2007, 35 Pt 2, 300–304. [Google Scholar] [CrossRef]
- Bianchi, M.; Bellini, A.; Cervelli, M.; Degan, P.; Marcocci, L.; Martini, F.; Scatteia, M.; Mariottini, P.; Amendola, R. Chronic sub-lethal oxidative stress by spermine oxidase overactivity induces continuous DNA repair and hypersensitivity to radiation exposure. Biochim. Biophys. Acta 2007, 1773, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Mastrantonio, R.; Cervelli, M.; Pietropaoli, S.; Mariottini, P.; Colasanti, M.; Persichini, T. HIV-Tat Induces the Nrf2/ARE Pathway through NMDA Receptor-Elicited Spermine Oxidase Activation in Human Neuroblastoma Cells. PLoS ONE. 2016, 11, e0149802. [Google Scholar] [CrossRef]
- Fan, J.; Yang, X.; Li, J.; Shu, Z.; Dai, J.; Liu, X.; Li, B.; Jia, S.; Kou, X.; Yang, Y.; et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef]
- Nilsson, B.O.; Persson, L. Beneficial effects of spermidine on cardiovascular health and longevity suggest a cell type-specific import of polyamines by cardiomyocytes. Biochem. Soc. Trans. 2019, 47, 265–272. [Google Scholar] [CrossRef]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Sakata, K.; Kashiwagi, K.; Ueda, S.; Iwasaki, S.; Shirahata, A.; Igarashi, K. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem. Biophys. Res. Commun. 2001, 282, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Qi, C.; Shen, L.; Wang, J.; Liu, X.; Zhang, N.; Bing, T.; Shangguan, D. Oxidative degradation of polyamines by serum supplement causes cytotoxicity on cultured cells. Sci. Rep. 2018, 8, 10384. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Kensler, T.W.; Casero, R.A., Jr. Induction of phase 2 enzymes by serum oxidized polyamines through activation of Nrf2: Effect of the polyamine metabolite acrolein. Biochem. Biophys. Res. Commun. 2003, 305, 662–670. [Google Scholar] [CrossRef]
- Holbert, C.E.; Dunworth, M.; Foley, J.R.; Dunston, T.T.; Murray-Stewart, T.; Casero, R.A., Jr. Autophagy induction by exogenous polyamines is an artifact of bovine serum amine oxidase activity in culture serum. J. Biol. Chem. 2020, 295, 9061–9068. [Google Scholar] [CrossRef] [PubMed]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. J. Vis. Exp. 2014, 88, 51046. [Google Scholar] [CrossRef]
- Rea, G.; Bocedi, A.; Cervelli, M. Question: What is the biological function of the polyamines? IUBMB Life 2004, 56, 167–169. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, F.; Medina, M.Á.; Villalobos-Rueda, L.; Urdiales, J.L. Polyamines in mammalian pathophysiology. Cell Mol. Life Sci. 2019, 76, 3987–4008. [Google Scholar] [CrossRef]
- Cervelli, M.; Pietropaoli, S.; Signore, F.; Amendola, R.; Mariottini, P. Polyamines metabolism and breast cancer: State of the art and perspectives. Breast Cancer Res. Treat. 2014, 148, 233–248. [Google Scholar] [CrossRef]
- Li, J.; Meng, Y.; Wu, X.; Sun, Y. Polyamines and related signaling pathways in cancer. Cancer Cell Int. 2020, 20, 539. [Google Scholar] [CrossRef]
- Sari, I.N.; Setiawan, T.; Kim, K.S.; Wijaya, Y.T.; Cho, K.W.; Kwon, H.Y. Metabolism and function of polyamines in cancer progression. Cancer Lett. 2021, 519, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.C.; Sirisoma, N.S.; Kuppusamy, P.; Zweier, J.L.; Woster, P.M.; Casero, R.A., Jr. The natural polyamine spermine functions directly as a free radical scavenger. Pro. Natl. Acad. Sci. USA 1998, 95, 11140–11145. [Google Scholar] [CrossRef] [PubMed]
- Amendola, R.; Cervelli, M.; Fratini, E.; Sallustio, D.E.; Tempera, G.; Ueshima, T.; Mariottini, P.; Agostinelli, E. Reactive oxygen species spermine metabolites generated from amine oxidases and radiation represent a therapeutic gain in cancer treatments. Int. J. Oncol. 2013, 43, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Amendola, R.; Cervelli, M.; Tempera, G.; Fratini, E.; Varesio, L.; Mariottini, P.; Agostinelli, E. Spermine metabolism and radiation-derived reactive oxygen species for future therapeutic implications in cancer: An additive or adaptive response. Amino Acids 2014, 46, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, I.; Szeliga, A.; Moraczewski, J.; Czaplicka, I.; Brzóska, E. Comparison of satellite cell-derived myoblasts and C2C12 differentiation in two- and three-dimensional cultures: Changes in adhesion protein expression. Cell Biol. Int. 2011, 35, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Dimauro, I.; Antonioni, A.; Mercatelli, N.; Grazioli, E.; Fantini, C.; Barone, R.; Macaluso, F.; Di Felice, V.; Caporossi, D. The early response of αB-crystallin to a single bout of aerobic exercise in mouse skeletal muscles depends upon fiber oxidative features. Redox Biol. 2019, 24, 101183. [Google Scholar] [CrossRef]
- Wannenes, F.; Magni, L.; Bonini, M.; Dimauro, I.; Caporossi, D.; Moretti, C.; Bonini, S. In vitro effects of Beta-2 agonists on skeletal muscle differentiation, hypertrophy, and atrophy. World Allergy Organ. J. 2012, 5, 66–72. [Google Scholar] [CrossRef]
- Ito, D.; Ito, H.; Ideta, T.; Kanbe, A.; Ninomiya, S.; Shimizu, M. Systemic and topical administration of spermidine accelerates skin wound healing. Cell Commun. Signal. 2021, 19, 36. [Google Scholar] [CrossRef]
- Wei, Z.X.; Cai, L.; Zhao, X.M.; Jiang, X.R.; Li, X.L. Effects of Spermidine on Cell Proliferation, Migration, and Inflammatory Response in Porcine Enterocytes. Front. Biosci. (Landmark Ed.) 2022, 27, 194. [Google Scholar] [CrossRef]
- Reichhardt, C.C.; Ahmadpour, A.; Christensen, R.G.; Ineck, N.E.; Murdoch, G.K.; Thornton, K.J. Understanding the influence of trenbolone acetate and polyamines on proliferation of bovine satellite cells. Domest. Anim. Endocrinol. 2021, 74, 106479. [Google Scholar] [CrossRef]
- Adhikari, R.; Shah, R.; Reyes-Gordillo, K.; Arellanes-Robledo, J.; Cheng, Y.; Ibrahim, J.; Tuma, P.L. Spermidine Prevents Ethanol and Lipopolysaccharide-Induced Hepatic Injury in Mice. Molecules 2021, 26, 1786. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, J.H.; Hwangbo, H.; Kim, S.Y.; Ji, S.Y.; Kim, M.Y.; Cha, H.J.; Park, C.; Hong, S.H.; Kim, G.Y.; et al. Spermidine Attenuates Oxidative Stress-Induced Apoptosis via Blocking Ca2+ Overload in Retinal Pigment Epithelial Cells Independently of ROS. Int. J. Mol. Sci. 2021, 22, 1361. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, R.; Garg, G.; Singh, A.K.; Verma, A.K.; Bissoyi, A.; Rizvi, S.I. Spermidine, a caloric restriction mimetic, provides neuroprotection against normal and D-galactose-induced oxidative stress and apoptosis through activation of autophagy in male rats during aging. Biogerontology 2021, 22, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Fratini, E.; Amendola, R.; Bianchi, M.; Signori, E.; Ferraro, E.; Lisi, A.; Federico, R.; Marcocci, L.; Mariottini, P. Increased spermine oxidase (SMO) activity as a novel differentiation marker of myogenic C2C12 cells. Int. J. Biochem. Cell Biol. 2009, 41, 934–944. [Google Scholar] [CrossRef]
- Giardino, I.; Fard, A.K.; Hatchell, D.L.; Brownlee, M. Aminoguanidine inhibits reactive oxygen species formation, lipid peroxidation, and oxidant-induced apoptosis. Diabetes 1998, 47, 1114–1120. [Google Scholar] [CrossRef]
- Smirnova, O.A.; Isaguliants, M.G.; Hyvonen, M.T.; Keinanen, T.A.; Tunitskaya, V.L.; Vepsalainen, J.; Alhonen, L.; Kochetkov, S.N.; Ivanov, A.V. Chemically induced oxidative stress increases polyamine levels by activating the transcription of ornithine decarboxylase and spermidine/spermine-N1-acetyltransferase in human hepatoma HUH7 cells. Biochimie 2012, 94, 1876–1883. [Google Scholar] [CrossRef]
- Chopra, S.; Wallace, H.M. Induction of spermidine/spermine N1-acetyltransferase in human cancer cells in response to increased production of reactive oxygen species. Biochem. Pharmacol. 1998, 55, 1119–1123. [Google Scholar] [CrossRef]
- Coker-Gurkan, A.; Celik, M.; Ugur, M.; Arisan, E.D.; Obakan-Yerlikaya, P.; Durdu, Z.B.; Palavan-Unsal, N. Curcumin inhibits autocrine growth hormone-mediated invasion and metastasis by targeting NF-κB signaling and polyamine metabolism in breast cancer cells. Amino Acids 2018, 50, 1045–1069. [Google Scholar] [CrossRef]
- Pietropaoli, S.; Leonetti, A.; Cervetto, C.; Venturini, A.; Mastrantonio, R.; Baroli, G.; Persichini, T.; Colasanti, M.; Maura, G.; Marcoli, M.; et al. Glutamate Excitotoxicity Linked to Spermine Oxidase Overexpression. Mol. Neurobiol. 2018, 55, 7259–7270. [Google Scholar] [CrossRef]
- Leonetti, A.; Baroli, G.; Fratini, E.; Pietropaoli, S.; Marcoli, M.; Mariottini, P.; Cervelli, M. Epileptic seizures and oxidative stress in a mouse model over-expressing spermine oxidase. Amino Acids 2020, 52, 129–139. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, T.W.; Lin, Y.Y.; Ho, W.C.; Tsai, H.C.; Chen, S.P.; Lin, A.M.; Liu, T.Y.; Wang, H.T. Acrolein is involved in ischemic stroke-induced neurotoxicity through spermidine/spermine-N1-acetyltransferase activation. Exp. Neurol. 2020, 323, 113066. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Spermidine/spermine-N(1)-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef]
- Duranti, G.; La Rosa, P.; Dimauro, I.; Wannenes, F.; Bonini, S.; Sabatini, S.; Parisi, P.; Caporossi, D. Effects of salmeterol on skeletal muscle cells: Metabolic and proapoptotic features. Med. Sci. Sports Exerc. 2011, 43, 2259–2273. [Google Scholar] [CrossRef]
- Ceci, R.; Maldini, M.; Olson, M.E.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Duranti, G. Moringa oleifera Leaf Extract Protects C2C12 Myotubes against H2O2-Induced Oxidative Stress. Antioxidants 2022, 11, 1435. [Google Scholar] [CrossRef]
- Di Luigi, L.; Duranti, G.; Antonioni, A.; Sgrò, P.; Ceci, R.; Crescioli, C.; Sabatini, S.; Lenzi, A.; Caporossi, D.; Del Galdo, F.; et al. The Phosphodiesterase Type 5 Inhibitor Sildenafil Improves DNA Stability and Redox Homeostasis in Systemic Sclerosis Fibroblasts Exposed to Reactive Oxygen Species. Antioxidants 2020, 9, 786. [Google Scholar] [CrossRef]
- Duranti, G.; Maldini, M.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Ceci, R. Moringa oleifera leaf extract upregulates Nrf2/HO-1 expression and ameliorates redox status in C2C12 skeletal muscle cells. Molecules 2021, 26, 5041. [Google Scholar] [CrossRef] [PubMed]
- Duranti, G.; Ceci, R.; Di Luigi, L.; Antinozzi, C.; Dimauro, I.; Sabatini, S.; Cervelli, M.; Sgrò, P. Effect of Tadalafil Administration on Redox Homeostasis and Polyamine Levels in Healthy Men with High Level of Physical Activity. Int. J. Environ. Res. Public Health 2021, 18, 9962. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Márquez, J.; García-Caballero, M.; Núñez de Castro, I.; Sánchez-Jiménez, F. Simultaneous fluorometric determination of intracellular polyamines separated by reversed-phase high-performance liquid chromatography. Agents Actions 1992, 36, 17–21. [Google Scholar] [CrossRef]
- Colamartino, M.; Duranti, G.; Ceci, R.; Sabatini, S.; Testa, A.; Cozzi, R. A multi-biomarker analysis of the antioxidant efficacy of Parkinson’s disease therapy. Toxicol. Vitr. 2018, 47, 1–7. [Google Scholar] [CrossRef]
- Duranti, G.; Maldini, M.; Crognale, D.; Sabatini, S.; Corana, F.; Horner, K.; Ceci, R. Moringa Oleifera leaf extract influences oxidative metabolism in C2C12 myotubes through SIRT1-PPARα pathway. Phytomedicine Plus 2021, 1, 100014. [Google Scholar] [CrossRef]
- Ceci, R.; Duranti, G.; Leonetti, A.; Pietropaoli, S.; Spinozzi, F.; Marcocci, L.; Amendola, R.; Cecconi, F.; Sabatini, S.; Mariottini, P.; et al. Adaptive responses of heart and skeletal muscle to spermine oxidase overexpression: Evaluation of a new transgenic mouse model. Free Radic. Biol. Med. 2017, 103, 216–225. [Google Scholar] [CrossRef] [PubMed]





| Gene | Forward 1 | Reverse 1 |
|---|---|---|
| ODC | 5′-GAAGAGATCACCAGTGTAATC-3′ | 5′-CTCATCTTCATCGTCAGAGC-3′ |
| PAOX | 5′-GGGAAGATACATCGCCCTTA-3′ | 5′-GGACCAAAAATCCAATGAGC-3′ |
| SMOX | 5′-ACTCCAAGAATGGCGTGGC-3′ | 5′-CGACGCTGTTCTGACTCTC-3′ |
| SMS | 5′-ACAAGAATGGCAGCTTTGCC-3′ | 5′-GAACTATGGGTGGTAATCGC-3′ |
| SDS | 5′-CGGAAGGTGCTGATCATCG-3′ | 5′-TCGCCCACGTGGAGAGT-3′ |
| SAT1 | 5′-CACTGGACCCCTGAAGGTTA-3′ | 5′-CAGCAACTTGCCAATCCATG-3′ |
| GAPDH | 5′-GGTTGTCTCCTGCGACTTC-3′ | 5′-GGTGGTCCAGGGTTTCTTAC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceci, R.; Duranti, G.; Giuliani, S.; Rossi, M.N.; Dimauro, I.; Sabatini, S.; Mariottini, P.; Cervelli, M. The Impact of Spermidine on C2C12 Myoblasts Proliferation, Redox Status and Polyamines Metabolism under H2O2 Exposure. Int. J. Mol. Sci. 2022, 23, 10986. https://doi.org/10.3390/ijms231910986
Ceci R, Duranti G, Giuliani S, Rossi MN, Dimauro I, Sabatini S, Mariottini P, Cervelli M. The Impact of Spermidine on C2C12 Myoblasts Proliferation, Redox Status and Polyamines Metabolism under H2O2 Exposure. International Journal of Molecular Sciences. 2022; 23(19):10986. https://doi.org/10.3390/ijms231910986
Chicago/Turabian StyleCeci, Roberta, Guglielmo Duranti, Stefano Giuliani, Marianna Nicoletta Rossi, Ivan Dimauro, Stefania Sabatini, Paolo Mariottini, and Manuela Cervelli. 2022. "The Impact of Spermidine on C2C12 Myoblasts Proliferation, Redox Status and Polyamines Metabolism under H2O2 Exposure" International Journal of Molecular Sciences 23, no. 19: 10986. https://doi.org/10.3390/ijms231910986
APA StyleCeci, R., Duranti, G., Giuliani, S., Rossi, M. N., Dimauro, I., Sabatini, S., Mariottini, P., & Cervelli, M. (2022). The Impact of Spermidine on C2C12 Myoblasts Proliferation, Redox Status and Polyamines Metabolism under H2O2 Exposure. International Journal of Molecular Sciences, 23(19), 10986. https://doi.org/10.3390/ijms231910986