Molecular Mechanisms Underlying Ca2+/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction
Abstract
1. Introduction
2. Discovery and Current Members of CaMKK Family
3. Tissue Distribution and Subcellular Localization of CaMKK
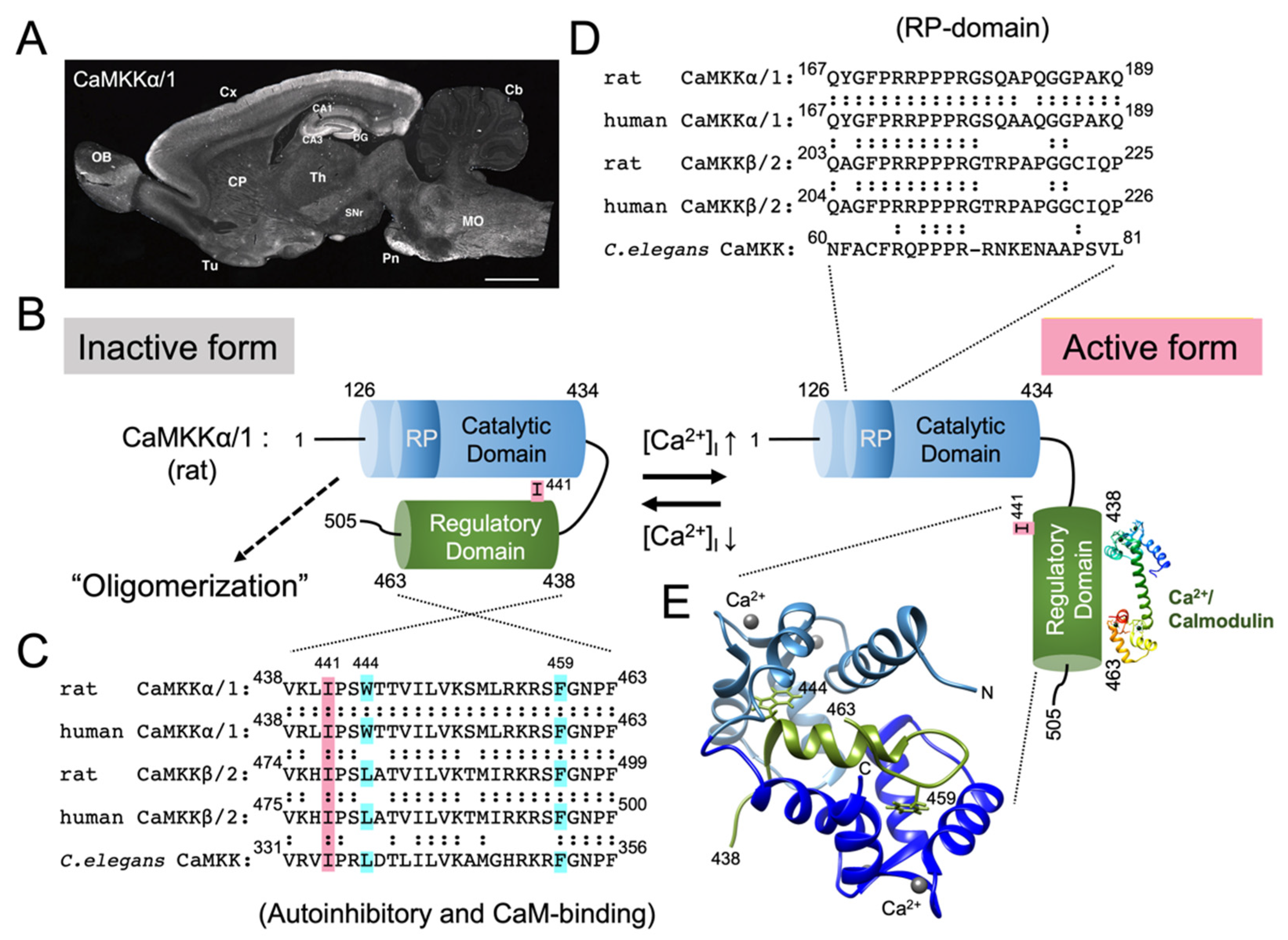
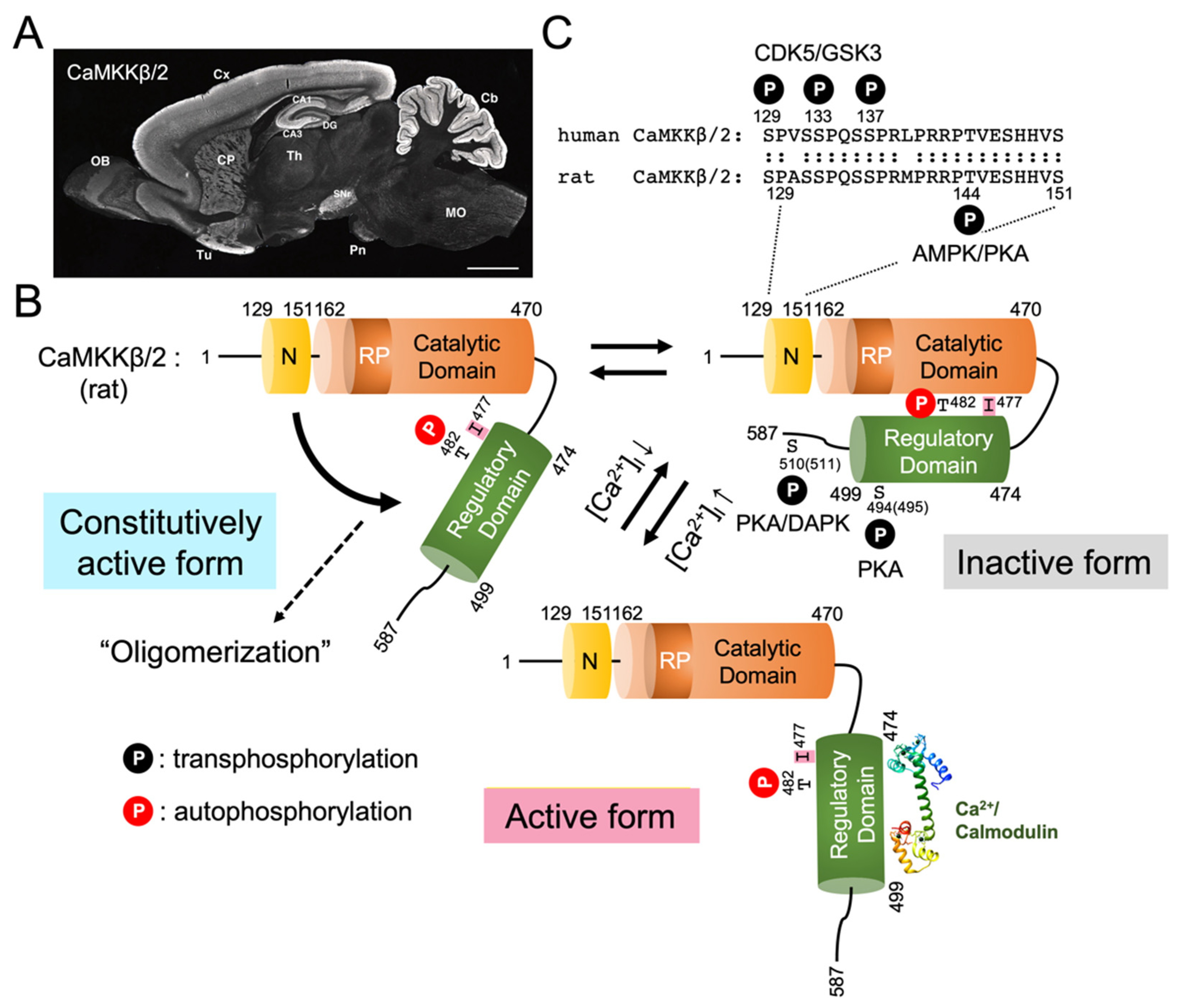
4. Domain Structure and Activation of CaMKK
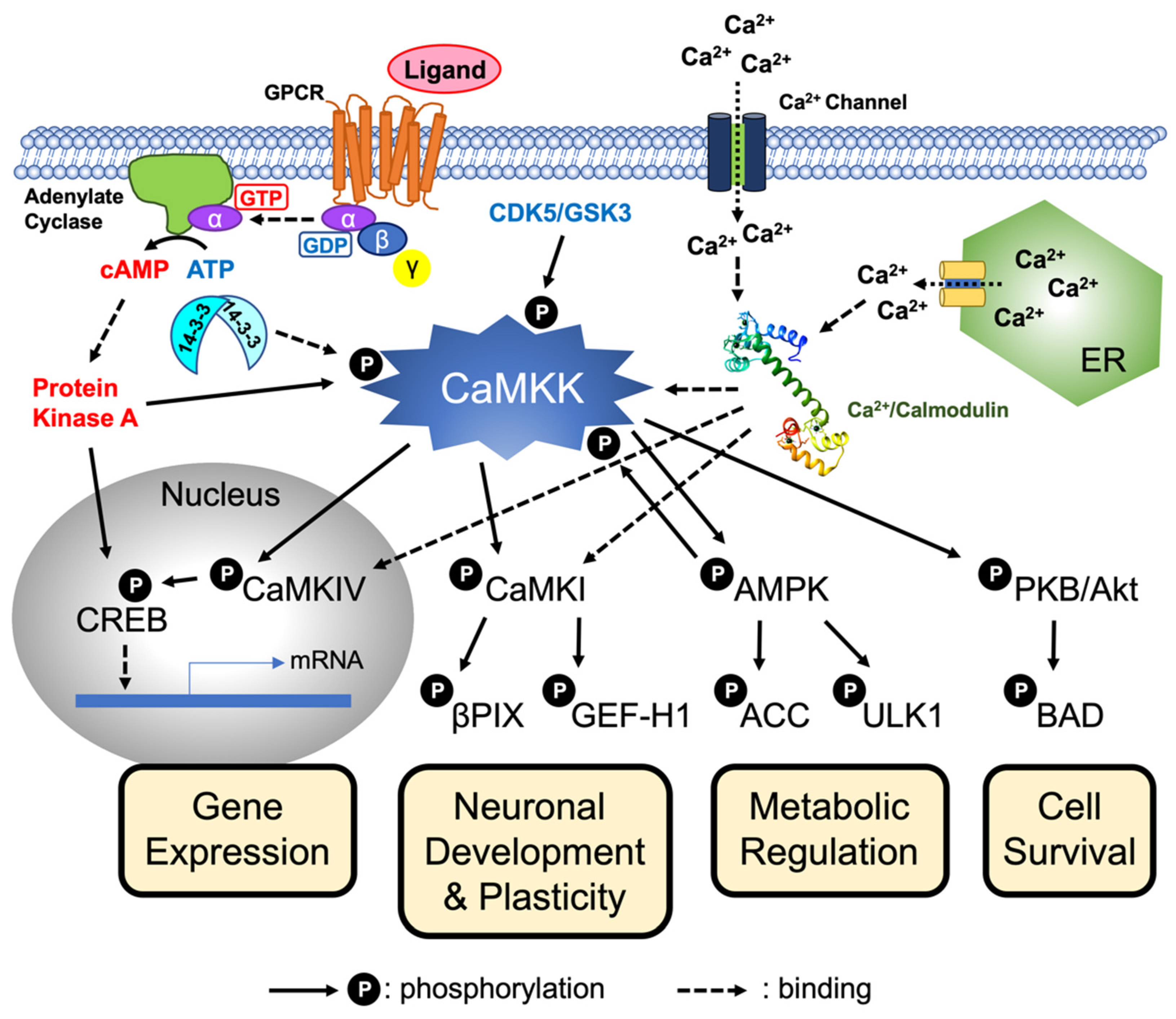
5. CaMKK Signaling Pathway
6. CaMKK Inhibitors and Pharmacological Analyses of Signaling Pathways
| Inhibitor | Structure | IC50 (nM) for | IC50 (µM) | Note | |
|---|---|---|---|---|---|
| CaMKKα/1 | CaMKKβ/2 | Cell-Based Assay | |||
| STO-609 [95] | 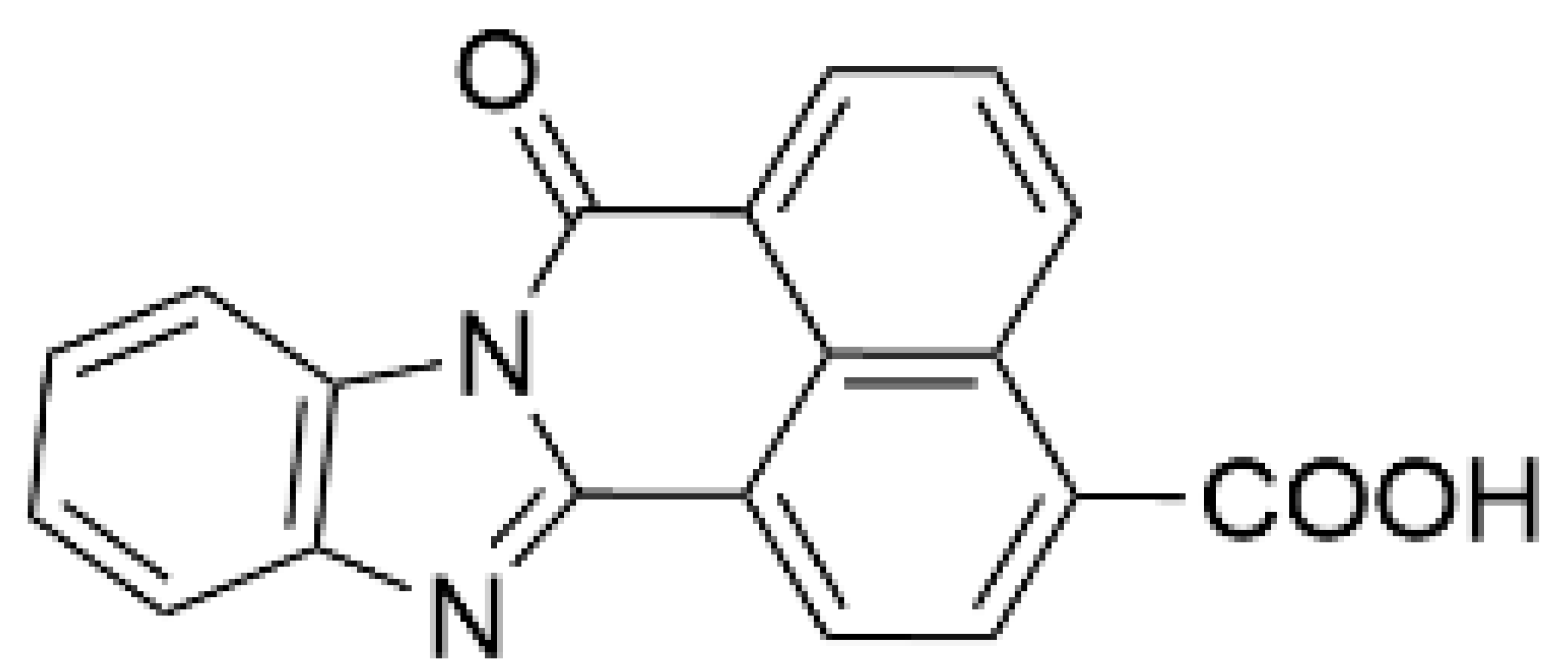 | 120–408 | 10–130 [98,128] | 0.2 | Inhibitor-resistant CaMKK mutants [96,100] |
| TIM-063 [129] | 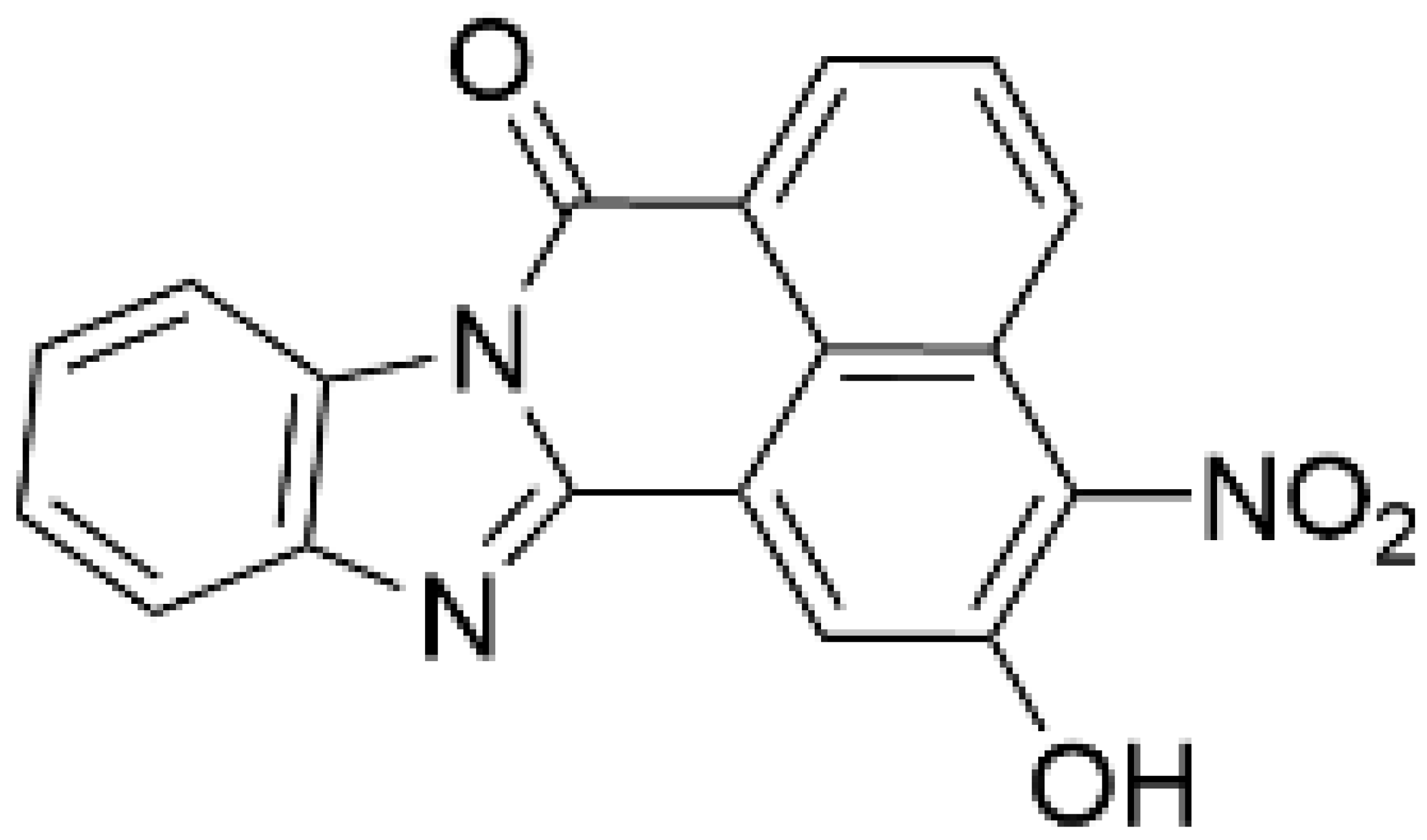 | 630 | 960 | 0.3 | Inactive analogue (TIM-062) [129], Conformation-dependent binding [130] |
| Compound 11 [125] | 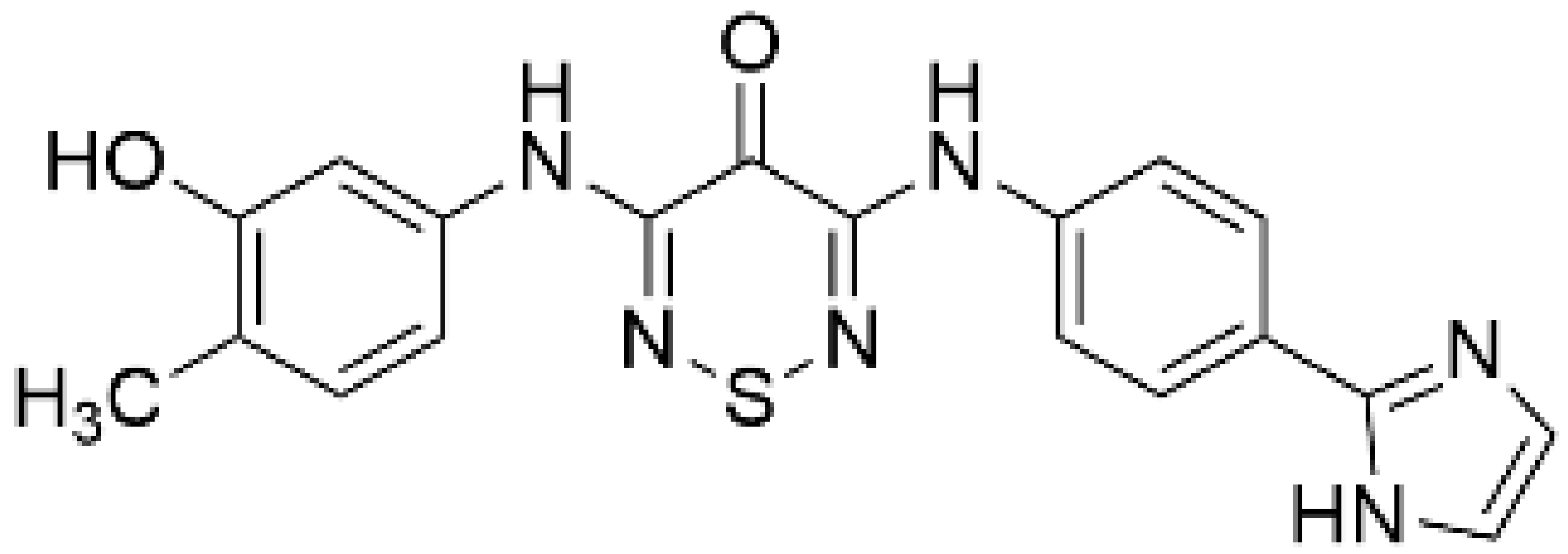 | NA | 6.5 (µM) | NA | – |
| Compound 4t [126] |  | NA | 6 | NA | Orally available |
| GSK650394 [128] | 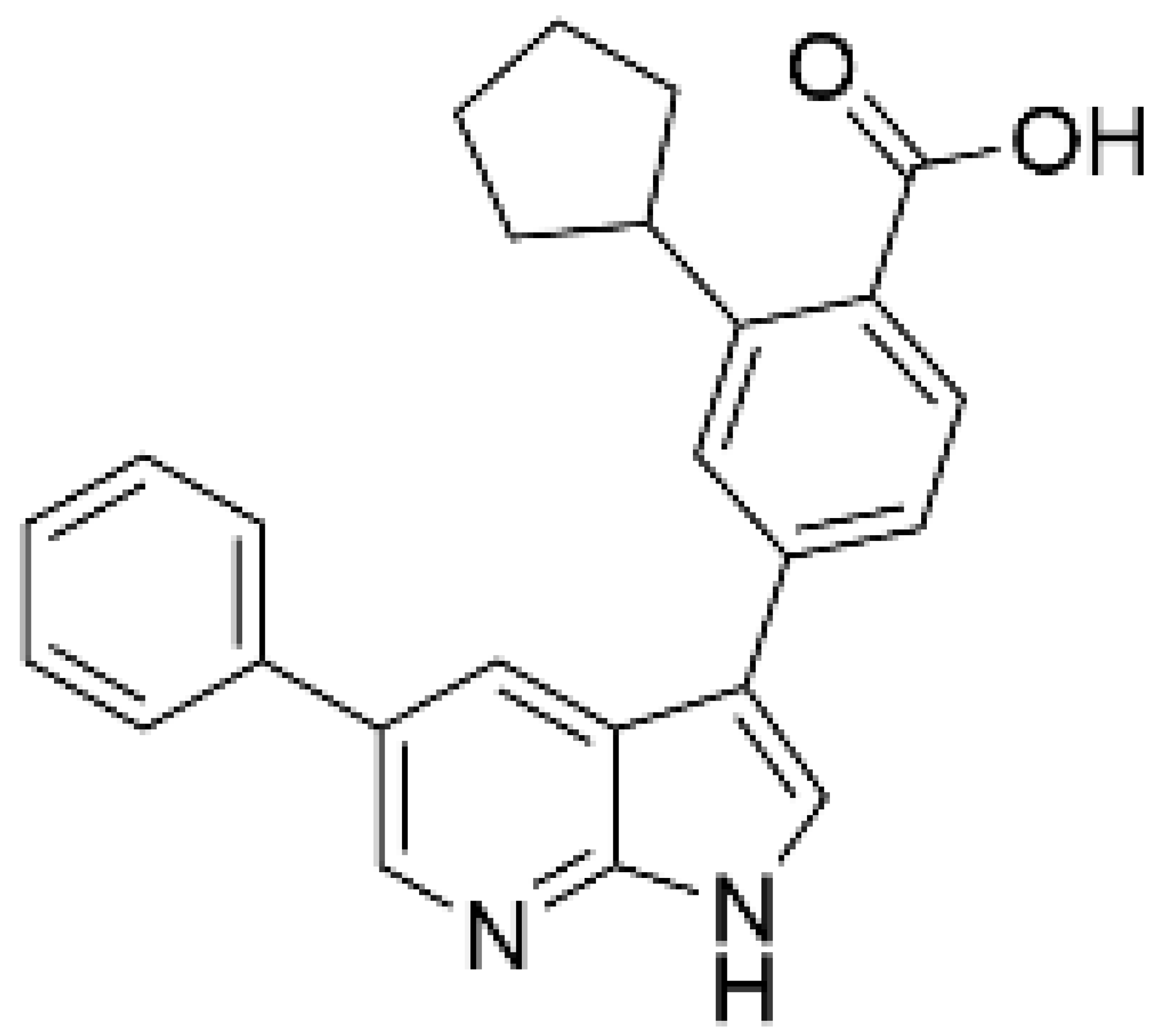 | 33 | 3 | NA | SGK inhibitor [127] |
| SGC-CAMKK2-1 a, [128] | 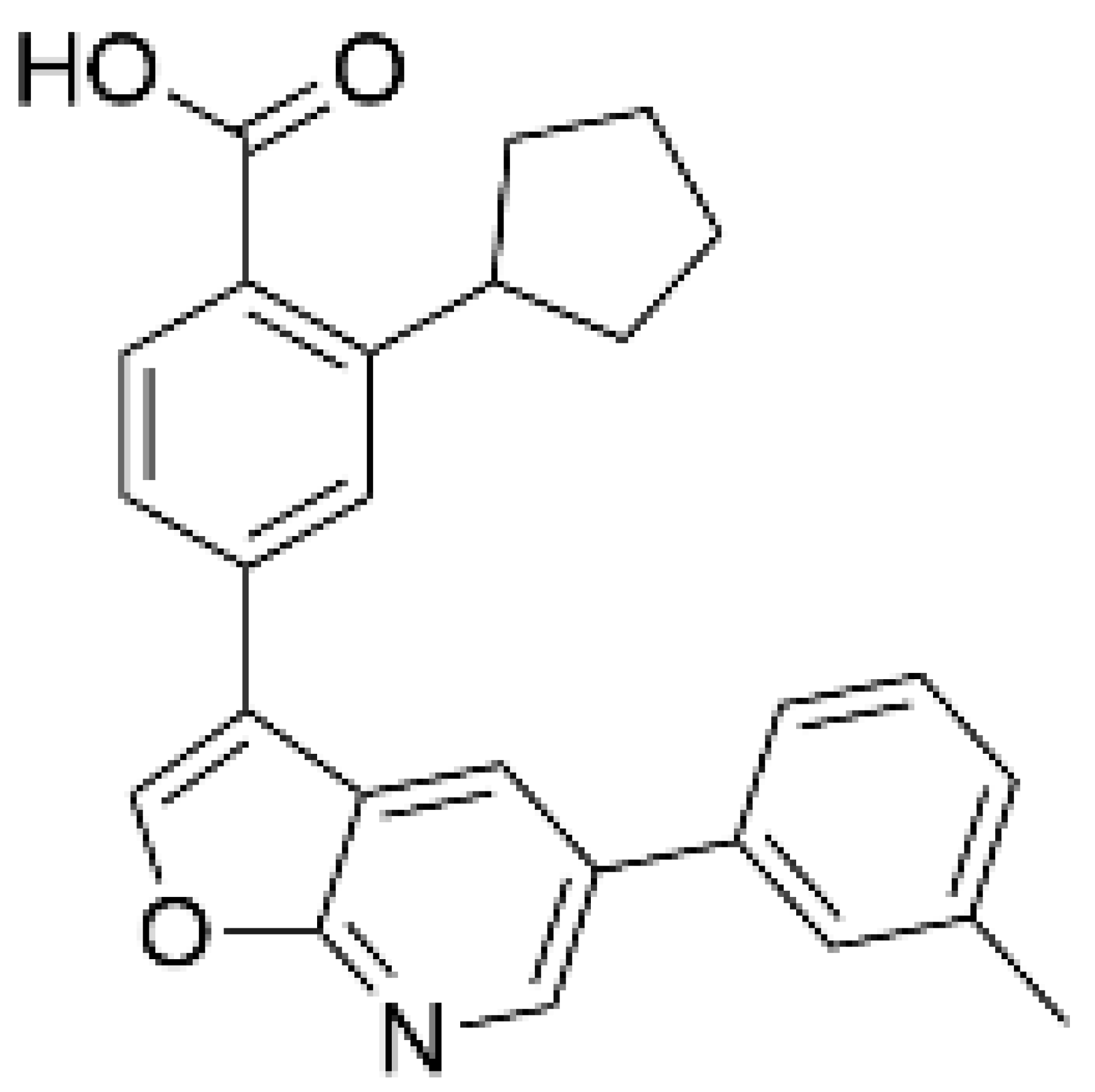 | NA | 30 | 1.6 | Inactive analogue a (SGC-CAMKK2-1N) |
7. Genetic Manipulation and Pathophysiological Role of CaMKK
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soderling, T.R.; Stull, J.T. Structure and regulation of calcium/calmodulin-dependent protein kinases. Chem. Rev. 2001, 101, 2341–2352. [Google Scholar] [CrossRef] [PubMed]
- Hook, S.S.; Means, A.R. Ca2+/CaM-dependent kinases: From activation to function. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 471–505. [Google Scholar] [CrossRef] [PubMed]
- Stull, J.T.; Kamm, K.E.; Vandenboom, R. Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch. Biochem. Biophys. 2011, 510, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kamm, K.E.; Stull, J.T. The Function of Myosin and Myosin Light Chain Kinase Phosphorylation in Smooth Muscle. Annu. Rev. Pharmacol. Toxicol. 1985, 25, 593–620. [Google Scholar] [CrossRef]
- Brushia, R.J.; Walsh, D.A. Phosphorylase kinase: The complexity of its regulation is reflected in the complexity of its structure. Front. Biosci. 1999, 4, D618–D641. [Google Scholar] [CrossRef] [PubMed]
- Takemoto-Kimura, S.; Suzuki, K.; Horigane, S.I.; Kamijo, S.; Inoue, M.; Sakamoto, M.; Fujii, H.; Bito, H. Calmodulin kinases: Essential regulators in health and disease. J. Neurochem. 2017, 141, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Colbran, R.J.; Schworer, C.M.; Hashimoto, Y.; Fong, Y.L.; Rich, D.P.; Smith, M.K.; Soderling, T.R. Calcium/calmodulin-dependent protein kinase II. Biochem. J. 1989, 258, 313–325. [Google Scholar]
- Pearson, R.B.; Wettenhall, R.E.; Means, A.R.; Hartshorne, D.J.; Kemp, B.E. Autoregulation of enzymes by pseudosubstrate prototopes: Myosin light chain kinase. Science 1988, 241, 970–973. [Google Scholar] [CrossRef]
- Yokokura, H.; Picciotto, M.R.; Nairn, A.C.; Hidaka, H. The regulatory region of calcium/calmodulin-dependent protein kinase I contains closely associated autoinhibitory and calmodulin-binding domains. J. Biol. Chem. 1995, 270, 23851–23859. [Google Scholar] [CrossRef]
- Hanson, P.I.; Schulman, H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu. Rev. Biochem. 1992, 61, 559–601. [Google Scholar] [CrossRef]
- Lisman, J.; Schulman, H.; Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002, 3, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Zoli, M.; Bertuzzi, G.; Nairn, A.C. Immunochemical localization of calcium/calmodulin-dependent protein kinase I. Synapse 1995, 20, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ohmstede, C.A.; Jensen, K.F.; Sahyoun, N.E. Ca2+/calmodulin-dependent protein kinase enriched in cerebellar granule cells. Identification of a novel neuronal calmodulin-dependent protein kinase. J. Biol. Chem. 1989, 264, 5866–5875. [Google Scholar] [CrossRef]
- Sakagami, H.; Tsubochi, H.; Kondo, H. Immunohistochemical localization of Ca2+/calmodulin-dependent protein kinase type IV in the peripheral ganglia and paraganglia of developing and mature rats. Brain Res. 1994, 666, 173–181. [Google Scholar] [CrossRef]
- Okuno, S.; Fujisawa, H. Requirement of Brain Extract for the Activity of Brain Calmodulin-Dependent Protein Kinase-IV Expressed in Escherichia coli. J. Biochem. 1993, 114, 167–170. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Brickey, D.A.; Glod, J.; Hidaka, H.; Sikela, J.; Soderling, T.R. Activation mechanisms for Ca2+/calmodulin-dependent protein kinase IV. Identification of a brain CaM-kinase IV kinase. J. Biol. Chem. 1994, 269, 28640–28647. [Google Scholar] [CrossRef]
- Okuno, S.; Kitani, T.; Fujisawa, H. Purification and characterization of Ca2+/calmodulin-dependent protein kinase IV kinase from rat brain. J. Biochem. 1994, 116, 923–930. [Google Scholar] [CrossRef]
- Lee, J.C.; Edelman, A.M. A protein activator of Ca2+-calmodulin-dependent protein kinase Ia. J. Biol. Chem. 1994, 269, 2158–2164. [Google Scholar] [CrossRef]
- Selbert, M.A.; Anderson, K.A.; Huang, Q.H.; Goldstein, E.G.; Means, A.R.; Edelman, A.M. Phosphorylation and activation of Ca2+-calmodulin-dependent protein kinase IV by Ca2+-calmodulin-dependent protein kinase Ia kinase. Phosphorylation of threonine 196 is essential for activation. J. Biol. Chem. 1995, 270, 17616–17621. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Enslen, H.; Soderling, T.R. Characterization of a Ca2+/calmodulin-dependent protein kinase cascade. Molecular cloning and expression of calcium/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 1995, 270, 19320–19324. [Google Scholar] [CrossRef]
- Kitani, T.; Okuno, S.; Fujisawa, H. Molecular cloning of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biochem. 1997, 122, 243–250. [Google Scholar] [CrossRef]
- Anderson, K.A.; Means, R.L.; Huang, Q.H.; Kemp, B.E.; Goldstein, E.G.; Selbert, M.A.; Edelman, A.M.; Fremeau, R.T.; Means, A.R. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biol. Chem. 1998, 273, 31880–31889. [Google Scholar] [CrossRef]
- Hsu, L.S.; Chen, G.D.; Lee, L.S.; Chi, C.W.; Cheng, J.F.; Chen, J.Y. Human Ca2+/calmodulin-dependent protein kinase kinase β gene encodes multiple isoforms that display distinct kinase activity. J. Biol. Chem. 2001, 276, 31113–31123. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Tokumitsu, H.; Inuzuka, H.; Murata-Hori, M.; Hosoya, H.; Kobayashi, R. Identification and characterization of novel components of a Ca2+/calmodulin-dependent protein kinase cascade in HeLa cells. FEBS Lett. 2003, 550, 57–63. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Soderling, T.R. Requirements for calcium and calmodulin in the calmodulin kinase activation cascade. J. Biol. Chem. 1996, 271, 5617–5622. [Google Scholar] [CrossRef]
- Haribabu, B.; Hook, S.S.; Selbert, M.A.; Goldstein, E.G.; Tomhave, E.D.; Edelman, A.M.; Snyderman, R.; Means, A.R. Human calcium-calmodulin dependent protein kinase I: cDNA cloning, domain structure and activation by phosphorylation at threonine-177 by calcium-calmodulin dependent protein kinase I kinase. EMBO J. 1995, 14, 3679–3686. [Google Scholar] [CrossRef]
- Yano, S.; Tokumitsu, H.; Soderling, T.R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 1998, 396, 584–587. [Google Scholar] [CrossRef]
- Fujimoto, T.; Yurimoto, S.; Hatano, N.; Nozaki, N.; Sueyoshi, N.; Kameshita, I.; Mizutani, A.; Mikoshiba, K.; Kobayashi, R.; Tokumitsu, H. Activation of SAD kinase by Ca2+/calmodulin-dependent protein kinase kinase. Biochemistry 2008, 47, 4151–4159. [Google Scholar] [CrossRef]
- Fogarty, S.; Hawley, S.A.; Green, K.A.; Saner, N.; Mustard, K.J.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-β activates AMPK without forming a stable complex: Synergistic effects of Ca2+ and AMP. Biochem. J. 2010, 426, 109–118. [Google Scholar] [CrossRef]
- Fujimoto, T.; Hatano, N.; Nozaki, N.; Yurimoto, S.; Kobayashi, R.; Tokumitsu, H. Identification of a novel CaMKK substrate. Biochem. Biophys. Res. Commun. 2011, 410, 45–51. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Iwabu, M.; Ishikawa, Y.; Kobayashi, R. Differential regulatory mechanism of Ca2+/calmodulin-dependent protein kinase kinase isoforms. Biochemistry 2001, 40, 13925–13932. [Google Scholar] [CrossRef] [PubMed]
- Gocher, A.M.; Azabdaftari, G.; Euscher, L.M.; Dai, S.; Karacosta, L.G.; Franke, T.F.; Edelman, A.M. Akt activation by Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) in ovarian cancer cells. J. Biol. Chem. 2017, 292, 14188–14204. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Dickerson, K.; Heath, R.; Hong, S.P.; Momcilovic, M.; Johnstone, S.R.; Carlson, M.; Carling, D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005, 2, 21–33. [Google Scholar] [CrossRef]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef]
- Hurley, R.L.; Anderson, K.A.; Franzone, J.M.; Kemp, B.E.; Means, A.R.; Witters, L.A. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005, 280, 29060–29066. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Chen, Z.; Zhang, F.; Cui, X.; Sun, W.; Geary, G.G.; Wang, Y.; Johnson, D.A.; Zhu, Y.; Chien, S.; et al. Ca2+/calmodulin-dependent protein kinase kinase β phosphorylation of Sirtuin 1 in endothelium is atheroprotective. Proc. Natl. Acad. Sci. USA 2013, 110, E2420–E2427. [Google Scholar] [CrossRef]
- Stork, B.A.; Dean, A.; Ortiz, A.R.; Saha, P.; Putluri, N.; Planas-Silva, M.D.; Mahmud, I.; Rajapakshe, K.; Coarfa, C.; Knapp, S.; et al. Calcium/calmodulin-dependent protein kinase kinase 2 regulates hepatic fuel metabolism. Mol. Metab. 2022, 62, 101513. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Corcoran, E.E.; Eto, K.; Gengyo-Ando, K.; Muramatsu, M.A.; Kobayashi, R.; Freedman, J.H.; Mitani, S.; Hagiwara, M.; Means, A.R.; et al. A CaMK cascade activates CRE-mediated transcription in neurons of Caenorhabditis elegans. EMBO Rep. 2002, 3, 962–966. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Takahashi, N.; Eto, K.; Yano, S.; Soderling, T.R.; Muramatsu, M. Substrate recognition by Ca2+/Calmodulin-dependent protein kinase kinase. Role of the arg-pro-rich insert domain. J. Biol. Chem. 1999, 274, 15803–15810. [Google Scholar] [CrossRef]
- Eto, K.; Takahashi, N.; Kimura, Y.; Masuho, Y.; Arai, K.; Muramatsu, M.A.; Tokumitsu, H. Ca2+/Calmodulin-dependent protein kinase cascade in Caenorhabditis elegans. Implication in transcriptional activation. J. Biol. Chem. 1999, 274, 22556–22562. [Google Scholar] [CrossRef]
- Joseph, J.D.; Means, A.R. Identification and characterization of two Ca2+/CaM-dependent protein kinases required for normal nuclear division in Aspergillus nidulans. J. Biol. Chem. 2000, 275, 38230–38238. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, Y.; Imai, K.K.; Kawasaki, Y.; Nakamura, T.; Nakaseko, Y.; Nagao, K.; Kokubu, A.; Ebe, M.; Fujisawa, A.; Hayashi, T.; et al. Schizosaccharomyces pombe cell division cycle under limited glucose requires Ssp1 kinase, the putative CaMKK, and Sds23, a PP2A-related phosphatase inhibitor. Genes Cells 2009, 14, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Valbuena, N.; Moreno, S. AMPK phosphorylation by Ssp1 is required for proper sexual differentiation in fission yeast. J. Cell Sci. 2012, 125 Pt 11, 2655–2664. [Google Scholar] [CrossRef]
- Yu, Y.V.; Bell, H.W.; Glauser, D.; Van Hooser, S.D.; Goodman, M.B.; Sengupta, P. CaMKI-dependent regulation of sensory gene expression mediates experience-dependent plasticity in the operating range of a thermosensory neuron. Neuron 2014, 84, 919–926. [Google Scholar] [CrossRef]
- Schild, L.C.; Zbinden, L.; Bell, H.W.; Yu, Y.V.; Sengupta, P.; Goodman, M.B.; Glauser, D.A. The balance between cytoplasmic and nuclear CaM kinase-1 signaling controls the operating range of noxious heat avoidance. Neuron 2014, 84, 983–996. [Google Scholar] [CrossRef]
- Sakagami, H.; Saito, S.; Kitani, T.; Okuno, S.; Fujisawa, H.; Kondo, H. Localization of the mRNAs for two isoforms of Ca2+/calmodulin-dependent protein kinase kinases in the adult rat brain. Brain Res. Mol. Brain Res. 1998, 54, 311–315. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, D.; Zhao, L.; Li, Y.; Yao, X.; Wang, H.; Zhang, S.; Liu, W.; Cao, H.; Yu, S.; et al. CaMKK2 Suppresses Muscle Regeneration through the Inhibition of Myoblast Proliferation and Differentiation. Int. J. Mol. Sci. 2016, 17, 1695. [Google Scholar] [CrossRef]
- Yu, X.; Murao, K.; Sayo, Y.; Imachi, H.; Cao, W.M.; Ohtsuka, S.; Niimi, M.; Tokumitsu, H.; Inuzuka, H.; Wong, N.C.; et al. The role of calcium/calmodulin-dependent protein kinase cascade in glucose upregulation of insulin gene expression. Diabetes 2004, 53, 1475–1481. [Google Scholar] [CrossRef][Green Version]
- Lin, F.; Ribar, T.J.; Means, A.R. The Ca2+/calmodulin-dependent protein kinase kinase, CaMKK2, inhibits preadipocyte differentiation. Endocrinology 2011, 152, 3668–3679. [Google Scholar] [CrossRef]
- Anderson, K.A.; Lin, F.; Ribar, T.J.; Stevens, R.D.; Muehlbauer, M.J.; Newgard, C.B.; Means, A.R. Deletion of CaMKK2 from the liver lowers blood glucose and improves whole-body glucose tolerance in the mouse. Mol. Endocrinol. 2012, 26, 281–291. [Google Scholar] [CrossRef]
- Racioppi, L.; Noeldner, P.K.; Lin, F.; Arvai, S.; Means, A.R. Calcium/calmodulin-dependent protein kinase kinase 2 regulates macrophage-mediated inflammatory responses. J. Biol. Chem. 2012, 287, 11579–11591. [Google Scholar] [CrossRef]
- Cary, R.L.; Waddell, S.; Racioppi, L.; Long, F.; Novack, D.V.; Voor, M.J.; Sankar, U. Inhibition of Ca2+/calmodulin-dependent protein kinase kinase 2 stimulates osteoblast formation and inhibits osteoclast differentiation. J. Bone Miner. Res. 2013, 28, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Racioppi, L.; Lento, W.; Huang, W.; Arvai, S.; Doan, P.L.; Harris, J.R.; Marcon, F.; Nakaya, H.I.; Liu, Y.; Chao, N. Calcium/calmodulin-dependent kinase kinase 2 regulates hematopoietic stem and progenitor cell regeneration. Cell Death Dis. 2017, 8, e3076. [Google Scholar] [CrossRef] [PubMed]
- Teng, E.C.; Racioppi, L.; Means, A.R. A cell-intrinsic role for CaMKK2 in granulocyte lineage commitment and differentiation. J. Leukoc. Biol. 2011, 90, 897–909. [Google Scholar] [CrossRef]
- Marcelo, K.L.; Ribar, T.; Means, C.R.; Tsimelzon, A.; Stevens, R.D.; Ilkayeva, O.; Bain, J.R.; Hilsenbeck, S.G.; Newgard, C.B.; Means, A.R.; et al. Research Resource: Roles for Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CaMKK2) in Systems Metabolism. Mol. Endocrinol. 2016, 30, 557–572. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ozawa, T.; Kurata, T.; Nakajima, N.; Zamponi, G.W.; Giles, W.R.; Imaizumi, Y.; Yamamura, H. A molecular complex of Cav1.2/CaMKK2/CaMK1a in caveolae is responsible for vascular remodeling via excitation-transcription coupling. Proc. Natl. Acad. Sci. USA 2022, 119, e2117435119. [Google Scholar] [CrossRef]
- Stahmann, N.; Woods, A.; Carling, D.; Heller, R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase β. Mol. Cell. Biol. 2006, 26, 5933–5945. [Google Scholar] [CrossRef]
- Nanba, K.; Chen, A.; Nishimoto, K.; Rainey, W.E. Role of Ca2+/calmodulin-dependent protein kinase kinase in adrenal aldosterone production. Endocrinology 2015, 156, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Okuno, S.; Kitani, T.; Otake, K.; Sato, F.; Fujisawa, H. Distribution of Ca2+/calmodulin-dependent protein kinase kinase a in the rat central nervous system: An immunohistochemical study. Neurosci. Lett. 1996, 204, 61–64. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okuno, S.; Kitani, T.; Otake, K.; Sato, F.; Fujisawa, H. Immunohistochemical localization of Ca2+/calmodulin-dependent protein kinase kinase β in the rat central nervous system. Neurosci. Res. 2001, 39, 175–188. [Google Scholar] [CrossRef]
- Sakagami, H.; Umemiya, M.; Saito, S.; Kondo, H. Distinct immunohistochemical localization of two isoforms of Ca2+/calmodulin-dependent protein kinase kinases in the adult rat brain. Eur. J. Neurosci. 2000, 12, 89–99. [Google Scholar] [CrossRef]
- Jensen, K.F.; Ohmstede, C.A.; Fisher, R.S.; Sahyoun, N. Nuclear and axonal localization of Ca2+/calmodulin-dependent protein kinase type Gr in rat cerebellar cortex. Proc. Natl. Acad. Sci. USA 1991, 88, 2850–2853. [Google Scholar] [CrossRef]
- Li, B.; Suutari, B.S.; Sun, S.D.; Luo, Z.; Wei, C.; Chenouard, N.; Mandelberg, N.J.; Zhang, G.; Wamsley, B.; Tian, G.; et al. Neuronal Inactivity Co-opts LTP Machinery to Drive Potassium Channel Splicing and Homeostatic Spike Widening. Cell 2020, 181, 1547–1565.e15. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Muramatsu, M.; Ikura, M.; Kobayashi, R. Regulatory mechanism of Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 2000, 275, 20090–20095. [Google Scholar] [CrossRef]
- Kaneshige, R.; Ohtsuka, S.; Harada, Y.; Kawamata, I.; Magari, M.; Kanayama, N.; Hatano, N.; Sakagami, H.; Tokumitsu, H. Substrate recognition by Arg/Pro-rich insert domain in calcium/calmodulin-dependent protein kinase kinase for target protein kinases. FEBS J. 2022, in press. [Google Scholar] [CrossRef]
- Osawa, M.; Tokumitsu, H.; Swindells, M.B.; Kurihara, H.; Orita, M.; Shibanuma, T.; Furuya, T.; Ikura, M. A novel target recognition revealed by calmodulin in complex with Ca2+-calmodulin-dependent kinase kinase. Nat. Struct. Biol. 1999, 6, 819–824. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Tokumitsu, H. Ca2+/calmodulin-dependent protein kinase kinase: From Ca2+-signal transduction to drug development. J. Jpn. Biochem. Soc. 2018, 90, 452–461. [Google Scholar]
- Meador, W.E.; Means, A.R.; Quiocho, F.A. Modulation of calmodulin plasticity in molecular recognition on the basis of X-ray structures. Science 1993, 262, 1718–1721. [Google Scholar] [CrossRef] [PubMed]
- Ikura, M.; Clore, G.M.; Gronenborn, A.M.; Zhu, G.; Klee, C.B.; Bax, A. Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science 1992, 256, 632–638. [Google Scholar] [CrossRef]
- Meador, W.E.; Means, A.R.; Quiocho, F.A. Target enzyme recognition by calmodulin: 2.4 Å structure of a calmodulin-peptide complex. Science 1992, 257, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.L.; Kim, J.; Truong, K.; Sherman, M.; Yuan, T.; Ikura, M. Calmodulin target database. J. Struct. Funct. Genom. 2000, 1, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Osawa, M.; Kurihara, H.; Katayama, N.; Tokumitsu, H.; Swindells, M.B.; Kainosho, M.; Ikura, M. Target-induced conformational adaptation of calmodulin revealed by the crystal structure of a complex with nematode Ca2+/calmodulin-dependent kinase kinase peptide. J. Mol. Biol. 2001, 312, 59–68. [Google Scholar] [CrossRef]
- Xy Ling, N.; Langendorf, C.G.; Hoque, A.; Galic, S.; Loh, K.; Kemp, B.E.; Gundlach, A.L.; Oakhill, J.S.; Scott, J.W. Functional analysis of an R311C variant of Ca2+-calmodulin-dependent protein kinase kinase-2 (CaMKK2) found as a de novo mutation in a patient with bipolar disorder. Bipolar Disord. 2020, 22, 841–848. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Harada, Y.; Ohtsuka, S.; Kanayama, N.; Magari, M.; Hatano, N.; Sakagami, H.; Tokumitsu, H. Oligomerization of Ca2+/calmodulin-dependent protein kinase kinase. Biochem. Biophys. Res. Commun. 2022, 587, 160–165. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Hatano, N.; Fujimoto, T.; Yurimoto, S.; Kobayashi, R. Generation of autonomous activity of Ca2+/calmodulin-dependent protein kinase kinase β by autophosphorylation. Biochemistry 2011, 50, 8193–8201. [Google Scholar] [CrossRef]
- Green, M.F.; Scott, J.W.; Steel, R.; Oakhill, J.S.; Kemp, B.E.; Means, A.R. Ca2+/Calmodulin-dependent protein kinase kinase β is regulated by multisite phosphorylation. J. Biol. Chem. 2011, 286, 28066–28079. [Google Scholar] [CrossRef]
- Nakanishi, A.; Hatano, N.; Fujiwara, Y.; Sha’ri, A.; Takabatake, S.; Akano, H.; Kanayama, N.; Magari, M.; Nozaki, N.; Tokumitsu, H. AMP-activated protein kinase-mediated feedback phosphorylation controls the Ca2+/calmodulin (CaM) dependence of Ca2+/CaM-dependent protein kinase kinase β. J. Biol. Chem. 2017, 292, 19804–19813. [Google Scholar] [CrossRef]
- Takabatake, S.; Ohtsuka, S.; Sugawara, T.; Hatano, N.; Kanayama, N.; Magari, M.; Sakagami, H.; Tokumitsu, H. Regulation of Ca2+/calmodulin-dependent protein kinase kinase β by cAMP signaling. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 672–680. [Google Scholar] [CrossRef]
- Schmitt, J.M.; Smith, S.; Hart, B.; Fletcher, L. CaM kinase control of AKT and LNCaP cell survival. J. Cell Biochem. 2012, 113, 1514–1526. [Google Scholar] [CrossRef]
- Xin, Y.; Guan, J.; Li, Y.; Duan, C. Regulation of cell quiescence-proliferation balance by Ca2+-CaMKK-Akt signaling. J. Cell Sci. 2021, 134, jcs253807. [Google Scholar] [CrossRef]
- Marcelo, K.L.; Means, A.R.; York, B. The Ca2+/Calmodulin/CaMKK2 Axis: Nature’s Metabolic CaMshaft. Trends Endocrinol. Metab. 2016, 27, 706–718. [Google Scholar] [CrossRef]
- Bright, N.J.; Carling, D.; Thornton, C. Investigating the regulation of brain-specific kinases 1 and 2 by phosphorylation. J. Biol. Chem. 2008, 283, 14946–14954. [Google Scholar] [CrossRef]
- Okuno, S.; Kitani, T.; Fujisawa, H. Studies on the substrate specificity of Ca2+/calmodulin-dependent protein kinase kinase α. J. Biochem. 1997, 122, 337–343. [Google Scholar] [CrossRef]
- Wayman, G.A.; Tokumitsu, H.; Soderling, T.R. Inhibitory cross-talk by cAMP kinase on the calmodulin-dependent protein kinase cascade. J. Biol. Chem. 1997, 272, 16073–16076. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Nairn, A.C. Inhibition of the Ca2+/calmodulin-dependent protein kinase I cascade by cAMP-dependent protein kinase. J. Biol. Chem. 1999, 274, 10086–10093. [Google Scholar] [CrossRef] [PubMed]
- Davare, M.A.; Saneyoshi, T.; Guire, E.S.; Nygaard, S.C.; Soderling, T.R. Inhibition of calcium/calmodulin-dependent protein kinase kinase by protein 14-3-3. J. Biol. Chem. 2004, 279, 52191–52199. [Google Scholar] [CrossRef]
- Ichimura, T.; Taoka, M.; Hozumi, Y.; Goto, K.; Tokumitsu, H. 14-3-3 Proteins directly regulate Ca2+/calmodulin-dependent protein kinase kinase α through phosphorylation-dependent multisite binding. FEBS Lett. 2008, 582, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Langendorf, C.G.; O’Brien, M.T.; Ngoei, K.R.W.; McAloon, L.M.; Dhagat, U.; Hoque, A.; Ling, N.X.Y.; Dite, T.A.; Galic, S.; Loh, K.; et al. CaMKK2 is inactivated by cAMP-PKA signaling and 14-3-3 adaptor proteins. J. Biol. Chem. 2020, 295, 16239–16250. [Google Scholar] [CrossRef] [PubMed]
- Psenakova, K.; Petrvalska, O.; Kylarova, S.; Lentini Santo, D.; Kalabova, D.; Herman, P.; Obsilova, V.; Obsil, T. 14-3-3 protein directly interacts with the kinase domain of calcium/calmodulin-dependent protein kinase kinase (CaMKK2). Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1612–1625. [Google Scholar] [CrossRef] [PubMed]
- Takabatake, S.; Fukumoto, Y.; Ohtsuka, S.; Kanayama, N.; Magari, M.; Sakagami, H.; Hatano, N.; Tokumitsu, H. Phosphorylation and dephosphorylation of Ca2+/calmodulin-dependent protein kinase kinase β at Thr144 in HeLa cells. Biochem. Biophys. Res. Commun. 2020, 525, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.M.; Schavocky, J.P.; Velentza, A.V.; Mirzoeva, S.; Watterson, D.M. A calmodulin-regulated protein kinase linked to neuron survival is a substrate for the calmodulin-regulated death-associated protein kinase. Biochemistry 2004, 43, 8116–8124. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.W.; Park, E.; Rodriguiz, R.M.; Oakhill, J.S.; Issa, S.M.; O’Brien, M.T.; Dite, T.A.; Langendorf, C.G.; Wetsel, W.C.; Means, A.R.; et al. Autophosphorylation of CaMKK2 generates autonomous activity that is disrupted by a T85S mutation linked to anxiety and bipolar disorder. Sci. Rep. 2015, 5, 14436. [Google Scholar] [CrossRef]
- Erhardt, A.; Lucae, S.; Unschuld, P.G.; Ising, M.; Kern, N.; Salyakina, D.; Lieb, R.; Uhr, M.; Binder, E.B.; Keck, M.E.; et al. Association of polymorphisms in P2RX7 and CaMKKb with anxiety disorders. J. Affect. Disord. 2007, 101, 159–168. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Inuzuka, H.; Ishikawa, Y.; Ikeda, M.; Saji, I.; Kobayashi, R. STO-609, a specific inhibitor of the Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 2002, 277, 15813–15818. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Inuzuka, H.; Ishikawa, Y.; Kobayashi, R. A single amino acid difference between α and β Ca2+/calmodulin-dependent protein kinase kinase dictates sensitivity to the specific inhibitor, STO-609. J. Biol. Chem. 2003, 278, 10908–10913. [Google Scholar] [CrossRef]
- Kukimoto-Niino, M.; Yoshikawa, S.; Takagi, T.; Ohsawa, N.; Tomabechi, Y.; Terada, T.; Shirouzu, M.; Suzuki, A.; Lee, S.; Yamauchi, T.; et al. Crystal structure of the Ca2+/calmodulin-dependent protein kinase kinase in complex with the inhibitor STO-609. J. Biol. Chem. 2011, 286, 22570–22579. [Google Scholar] [CrossRef]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; McLauchlan, H.; Klevernic, I.; Arthur, J.S.; Alessi, D.R.; Cohen, P. The selectivity of protein kinase inhibitors: A further update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef]
- Monteiro, P.; Gilot, D.; Langouet, S.; Fardel, O. Activation of the aryl hydrocarbon receptor by the calcium/calmodulin-dependent protein kinase kinase inhibitor 7-oxo-7H-benzimidazo [2,1-a]benz[de]isoquinoline-3-carboxylic acid (STO-609). Drug Metab. Dispos. 2008, 36, 2556–2563. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Hiraoka, Y.; Fujimoto, T.; Kanayama, N.; Magari, M.; Tokumitsu, H. Analysis of Distinct Roles of CaMKK Isoforms Using STO-609-Resistant Mutants in Living Cells. Biochemistry 2015, 54, 3969–3977. [Google Scholar] [CrossRef]
- Wayman, G.A.; Kaech, S.; Grant, W.F.; Davare, M.; Impey, S.; Tokumitsu, H.; Nozaki, N.; Banker, G.; Soderling, T.R. Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I. J. Neurosci. 2004, 24, 3786–3794. [Google Scholar] [CrossRef] [PubMed]
- Wayman, G.A.; Impey, S.; Marks, D.; Saneyoshi, T.; Grant, W.F.; Derkach, V.; Soderling, T.R. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 2006, 50, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Saneyoshi, T.; Wayman, G.; Fortin, D.; Davare, M.; Hoshi, N.; Nozaki, N.; Natsume, T.; Soderling, T.R. Activity-dependent synaptogenesis: Regulation by a CaM-kinase kinase/CaM-kinase I/βPIX signaling complex. Neuron 2008, 57, 94–107. [Google Scholar] [CrossRef]
- Schmitt, J.M.; Guire, E.S.; Saneyoshi, T.; Soderling, T.R. Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation. J. Neurosci. 2005, 25, 1281–1290. [Google Scholar] [CrossRef]
- Massie, C.E.; Lynch, A.; Ramos-Montoya, A.; Boren, J.; Stark, R.; Fazli, L.; Warren, A.; Scott, H.; Madhu, B.; Sharma, N.; et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011, 30, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Marcelo, K.L.; Rajapakshe, K.; Coarfa, C.; Dean, A.; Wilganowski, N.; Robinson, H.; Sevick, E.; Bissig, K.D.; Goldie, L.C.; et al. The camKK2/camKIV relay is an essential regulator of hepatic cancer. Hepatology 2015, 62, 505–520. [Google Scholar] [CrossRef] [PubMed]
- York, B.; Li, F.; Lin, F.; Marcelo, K.L.; Mao, J.; Dean, A.; Gonzales, N.; Gooden, D.; Maity, S.; Coarfa, C.; et al. Pharmacological inhibition of CaMKK2 with the selective antagonist STO-609 regresses NAFLD. Sci. Rep. 2017, 7, 11793. [Google Scholar] [CrossRef]
- Horigane, S.; Ageta-Ishihara, N.; Kamijo, S.; Fujii, H.; Okamura, M.; Kinoshita, M.; Takemoto-Kimura, S.; Bito, H. Facilitation of axon outgrowth via a Wnt5a-CaMKK-CaMKIα pathway during neuronal polarization. Mol. Brain 2016, 9, 8. [Google Scholar] [CrossRef]
- Fortin, D.A.; Davare, M.A.; Srivastava, T.; Brady, J.D.; Nygaard, S.; Derkach, V.A.; Soderling, T.R. Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J. Neurosci. 2010, 30, 11565–11575. [Google Scholar] [CrossRef]
- Srivastava, T.; Fortin, D.A.; Nygaard, S.; Kaech, S.; Sonenberg, N.; Edelman, A.M.; Soderling, T.R. Regulation of neuronal mRNA translation by CaM-kinase I phosphorylation of eIF4GII. J. Neurosci. 2012, 32, 5620–5630. [Google Scholar] [CrossRef]
- Ageta-Ishihara, N.; Takemoto-Kimura, S.; Nonaka, M.; Adachi-Morishima, A.; Suzuki, K.; Kamijo, S.; Fujii, H.; Mano, T.; Blaeser, F.; Chatila, T.A.; et al. Control of cortical axon elongation by a GABA-driven Ca2+/calmodulin-dependent protein kinase cascade. J. Neurosci. 2009, 29, 13720–13729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, L.; Collage, R.D.; Stripay, J.L.; Tsung, A.; Lee, J.S.; Rosengart, M.R. Calcium/calmodulin-dependent protein kinase (CaMK) Iα mediates the macrophage inflammatory response to sepsis. J. Leukoc. Biol. 2011, 90, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Murao, K.; Li, J.; Imachi, H.; Muraoka, T.; Masugata, H.; Zhang, G.X.; Kobayashi, R.; Ishida, T.; Tokumitsu, H. Exendin-4 regulates glucokinase expression by CaMKK/CaMKIV pathway in pancreatic β-cell line. Diabetes Obes. Metab. 2009, 11, 939–946. [Google Scholar] [CrossRef]
- Li, J.; Murao, K.; Imachi, H.; Masugata, H.; Iwama, H.; Tada, S.; Zhang, G.X.; Kobayashi, R.; Ishida, T.; Tokumitsu, H. Exendin-4 regulates pancreatic ABCA1 transcription via CaMKK/CaMKIV pathway. J. Cell. Mol. Med. 2010, 14, 1083–1087. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, K.; Yu, X.; Murao, K.; Imachi, H.; Li, J.; Muraoka, T.; Masugata, H.; Zhang, G.X.; Kobayashi, R.; Ishida, T.; et al. Exendin-4 regulates GLUT2 expression via the CaMKK/CaMKIV pathway in a pancreatic β-cell line. Metabolism 2011, 60, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M.; Mishima, T.; Wang, Y.; Kasahara, J.; Fukunaga, K.; Ohashi, K.; Mizuno, K. Ca2+/calmodulin-dependent protein kinase IV-mediated LIM kinase activation is critical for calcium signal-induced neurite outgrowth. J. Biol. Chem. 2009, 284, 28554–28562. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.A.; Ribar, T.J.; Lin, F.; Noeldner, P.K.; Green, M.F.; Muehlbauer, M.J.; Witters, L.A.; Kemp, B.E.; Means, A.R. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008, 7, 377–388. [Google Scholar] [CrossRef]
- Jensen, T.E.; Rose, A.J.; Jorgensen, S.B.; Brandt, N.; Schjerling, P.; Wojtaszewski, J.F.; Richter, E.A. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1308–E1317. [Google Scholar] [CrossRef]
- Merlin, J.; Evans, B.A.; Csikasz, R.I.; Bengtsson, T.; Summers, R.J.; Hutchinson, D.S. The M3-muscarinic acetylcholine receptor stimulates glucose uptake in L6 skeletal muscle cells by a CaMKK-AMPK-dependent mechanism. Cell. Signal. 2010, 22, 1104–1113. [Google Scholar] [CrossRef]
- Tamás, P.; Hawley, S.A.; Clarke, R.G.; Mustard, K.J.; Green, K.; Hardie, D.G.; Cantrell, D.A. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med. 2006, 203, 1665–1670. [Google Scholar] [CrossRef]
- Hoyer-Hansen, M.; Bastholm, L.; Szyniarowski, P.; Campanella, M.; Szabadkai, G.; Farkas, T.; Bianchi, K.; Fehrenbacher, N.; Elling, F.; Rizzuto, R.; et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-β, and Bcl-2. Mol. Cell 2007, 25, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Ghislat, G.; Patron, M.; Rizzuto, R.; Knecht, E. Withdrawal of essential amino acids increases autophagy by a pathway involving Ca2+/calmodulin-dependent kinase kinase-β (CaMKK-β). J. Biol. Chem. 2012, 287, 38625–38636. [Google Scholar] [CrossRef]
- Krasner, N.M.; Ido, Y.; Ruderman, N.B.; Cacicedo, J.M. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE 2014, 9, e97554. [Google Scholar] [CrossRef]
- Li, T.; Xu, W.; Ouyang, J.; Lu, X.; Sherchan, P.; Lenahan, C.; Irio, G.; Zhang, J.H.; Zhao, J.; Zhang, Y.; et al. Orexin A alleviates neuroinflammation via OXR2/CaMKKβ/AMPK signaling pathway after ICH in mice. J. Neuroinflamm. 2020, 17, 187. [Google Scholar] [CrossRef] [PubMed]
- Asquith, C.R.M.; Godoi, P.H.; Counago, R.M.; Laitinen, T.; Scott, J.W.; Langendorf, C.G.; Oakhill, J.S.; Drewry, D.H.; Zuercher, W.J.; Koutentis, P.A.; et al. 1,2,6-Thiadiazinones as Novel Narrow Spectrum Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CaMKK2) Inhibitors. Molecules 2018, 23, 1221. [Google Scholar] [CrossRef] [PubMed]
- Price, D.J.; Drewry, D.H.; Schaller, L.T.; Thompson, B.D.; Reid, P.R.; Maloney, P.R.; Liang, X.; Banker, P.; Buckholz, R.G.; Selley, P.K.; et al. An orally available, brain-penetrant CAMKK2 inhibitor reduces food intake in rodent model. Bioorg. Med. Chem. Lett. 2018, 28, 1958–1963. [Google Scholar] [CrossRef]
- Sherk, A.B.; Frigo, D.E.; Schnackenberg, C.G.; Bray, J.D.; Laping, N.J.; Trizna, W.; Hammond, M.; Patterson, J.R.; Thompson, S.K.; Kazmin, D.; et al. Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 2008, 68, 7475–7483. [Google Scholar] [CrossRef] [PubMed]
- Eduful, B.J.; O’Byrne, S.N.; Temme, L.; Asquith, C.R.M.; Liang, Y.; Picado, A.; Pilotte, J.R.; Hossain, M.A.; Wells, C.I.; Zuercher, W.J.; et al. Hinge Binder Scaffold Hopping Identifies Potent Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CAMKK2) Inhibitor Chemotypes. J. Med. Chem. 2021, 64, 10849–10877. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Ozeki, Y.; Fujiwara, M.; Miyagawa, T.; Kanayama, N.; Magari, M.; Hatano, N.; Suizu, F.; Ishikawa, T.; Tokumitsu, H. Development and Characterization of Novel Molecular Probes for Ca2+/Calmodulin-Dependent Protein Kinase Kinase, Derived from STO-609. Biochemistry 2020, 59, 1701–1710. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Okumura, T.; Tauabuchi, Y.; Miyagawa, T.; Kanayama, N.; Magari, M.; Hatano, N.; Sakagami, H.; Suizu, F.; Ishikawa, T.; et al. Conformation-Dependent Reversible Interaction of Ca2+/Calmodulin-Dependent Protein Kinase Kinase with an Inhibitor, TIM-063. Biochemistry 2022, 61, 545–553. [Google Scholar] [CrossRef]
- Kitazawa, T.; Matsui, T.; Katsuki, S.; Goto, A.; Akagi, K.; Hatano, N.; Tokumitsu, H.; Takeya, K.; Eto, M. A temporal Ca2+ desensitization of myosin light chain kinase in phasic smooth muscles induced by CaMKKβ/PP2A pathways. Am. J. Physiol. Cell Physiol. 2021, 321, C549–C558. [Google Scholar] [CrossRef]
- Mizuno, K.; Ris, L.; Sanchez-Capelo, A.; Godaux, E.; Giese, K.P. Ca2+/calmodulin kinase kinase α is dispensable for brain development but is required for distinct memories in male, though not in female, mice. Mol. Cell. Biol. 2006, 26, 9094–9104. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Mizuno, K.; Ris, L.; Angelo, M.; Godaux, E.; Giese, K.P. Loss of Ca2+/calmodulin kinase kinase β affects the formation of some, but not all, types of hippocampus-dependent long-term memory. J. Neurosci. 2003, 23, 9752–9760. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Antunes-Martins, A.; Ris, L.; Peters, M.; Godaux, E.; Giese, K.P. Calcium/calmodulin kinase kinase β has a male-specific role in memory formation. Neuroscience 2007, 145, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Blaeser, F.; Sanders, M.J.; Truong, N.; Ko, S.; Wu, L.J.; Wozniak, D.F.; Fanselow, M.S.; Zhuo, M.; Chatila, T.A. Long-term memory deficits in Pavlovian fear conditioning in Ca2+/calmodulin kinase kinase α-deficient mice. Mol. Cell. Biol. 2006, 26, 9105–9115. [Google Scholar] [CrossRef]
- Wei, F.; Qiu, C.S.; Liauw, J.; Robinson, D.A.; Ho, N.; Chatila, T.; Zhuo, M. Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat. Neurosci. 2002, 5, 573–579. [Google Scholar] [CrossRef]
- Matsushita, M.; Nairn, A.C. Characterization of the mechanism of regulation of Ca2+/calmodulin-dependent protein kinase I by calmodulin and by Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 1998, 273, 21473–21481. [Google Scholar] [CrossRef]
- Kaitsuka, T.; Li, S.T.; Nakamura, K.; Takao, K.; Miyakawa, T.; Matsushita, M. Forebrain-specific constitutively active CaMKKα transgenic mice show deficits in hippocampus-dependent long-term memory. Neurobiol. Learn Mem. 2011, 96, 238–247. [Google Scholar] [CrossRef]
- Frigo, D.E.; Howe, M.K.; Wittmann, B.M.; Brunner, A.M.; Cushman, I.; Wang, Q.; Brown, M.; Means, A.R.; McDonnell, D.P. CaM kinase kinase β-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 2011, 71, 528–537. [Google Scholar] [CrossRef]
- Luo, X.J.; Li, M.; Huang, L.; Steinberg, S.; Mattheisen, M.; Liang, G.; Donohoe, G.; Shi, Y.; Chen, C.; Yue, W.; et al. Convergent lines of evidence support CAMKK2 as a schizophrenia susceptibility gene. Mol. Psychiatry 2014, 19, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Matoba, N.; Sawada, T.; Kazuno, A.A.; Ishiwata, M.; Fujii, K.; Matsuo, K.; Takata, A.; Kato, T. Exome sequencing for bipolar disorder points to roles of de novo loss-of-function and protein-altering mutations. Mol. Psychiatry 2016, 21, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhong, F.; Chen, Y. Association of calcium/calmodulin-dependent protein kinase kinase1 rs7214723 polymorphism with lung cancer risk in a Chinese population. Biosci. Rep. 2017, 37, BSR20170762. [Google Scholar] [CrossRef] [PubMed]
- Beghi, S.; Cavaliere, F.; Manfredini, M.; Ferrarese, S.; Corazzari, C.; Beghi, C.; Buschini, A. Polymorphism rs7214723 in CAMKK1: A new genetic variant associated with cardiovascular diseases. Biosci. Rep. 2021, 41, BSR20210326. [Google Scholar] [CrossRef]
- Jin, L.; Chun, J.; Pan, C.; Kumar, A.; Zhang, G.; Ha, Y.; Li, D.; Alesi, G.N.; Kang, Y.; Zhou, L.; et al. The PLAG1-GDH1 Axis Promotes Anoikis Resistance and Tumor Metastasis through CamKK2-AMPK Signaling in LKB1-Deficient Lung Cancer. Mol. Cell 2018, 69, 87–99.e7. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Bu, F.; Min, J.W.; Munshi, Y.; Howe, M.D.; Liu, L.; Koellhoffer, E.C.; Qi, L.; McCullough, L.D.; Li, J. Inhibition of calcium/calmodulin-dependent protein kinase kinase (CaMKK) exacerbates impairment of endothelial cell and blood-brain barrier after stroke. Eur. J. Neurosci. 2019, 49, 27–39. [Google Scholar] [CrossRef]
- Liu, L.; McCullough, L.; Li, J. Genetic deletion of calcium/calmodulin-dependent protein kinase kinase β (CaMKK β) or CaMK IV exacerbates stroke outcomes in ovariectomized (OVXed) female mice. BMC Neurosci. 2014, 15, 118. [Google Scholar] [CrossRef]
| CaMKK | Species | UniProtKB | M.M. (Da) | Ca2+/CaM | Substrates | |
|---|---|---|---|---|---|---|
| (a.a. Residues) | -Dependency | (Phosphorylation Site) | ||||
| CaMKKα/1 | rat | P97756 | 55,908 (505) [20] | YES [25] | CaMKI (α: Thr177) [20,26] | |
| mouse | Q8VBY2 | 55,838 (505) | CaMKIV (Thr196) [20,25] | |||
| human | Q8N5S9 | 55,735 (505) | PKB/Akt (Thr308) [27] | |||
| BRSK1 (Thr189) [28,29] | ||||||
| Syndapin I (Thr355) [30] | ||||||
| CaMKKβ/2 | rat | O88831 | 64,446 (587) [21,22] | YES [22] | CaMKI (α: Thr177) [22] | |
| mouse | Q8C078 | 64,618 (588) | (autonomous activity) [22,31] | CaMKIV (Thr196) [21,22] | ||
| –1 | human | Q96RR4-1 | 64.746 (588) [22] | PKB/Akt (Thr308) [32] | ||
| –2 | human | Q96RR4-2 | 58,899 (533) [23] | AMPK (α: Thr172) [33,34,35] | ||
| –3 | human | Q96RR4-3 | 59,602 (541) [24] | SIRT1 (Ser27, Ser47) [36] | ||
| GAPDH, Pex3 [37] | ||||||
| CKK-1 [38] | –a | C. elegans | Q3Y416-2 | 48,940 (432) | YES [39] | CMK1 (Thr179) [40] |
| –b | C. elegans | Q3Y416-1 | 60,804 (541) | |||
| CMKC [41] | A. nidulans | Q9Y898 | 59,153 (518) | YES | CMKB (Thr179) [41] | |
| Ssp1 [42] | S. pombe | P50526 | 73,992 (652) | ND | Ssp2 (Thr189) [42,43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokumitsu, H.; Sakagami, H. Molecular Mechanisms Underlying Ca2+/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction. Int. J. Mol. Sci. 2022, 23, 11025. https://doi.org/10.3390/ijms231911025
Tokumitsu H, Sakagami H. Molecular Mechanisms Underlying Ca2+/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction. International Journal of Molecular Sciences. 2022; 23(19):11025. https://doi.org/10.3390/ijms231911025
Chicago/Turabian StyleTokumitsu, Hiroshi, and Hiroyuki Sakagami. 2022. "Molecular Mechanisms Underlying Ca2+/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction" International Journal of Molecular Sciences 23, no. 19: 11025. https://doi.org/10.3390/ijms231911025
APA StyleTokumitsu, H., & Sakagami, H. (2022). Molecular Mechanisms Underlying Ca2+/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction. International Journal of Molecular Sciences, 23(19), 11025. https://doi.org/10.3390/ijms231911025





