Hyperbaric Oxygen Therapy Alleviates Social Behavior Dysfunction and Neuroinflammation in a Mouse Model for Autism Spectrum Disorders
Abstract
1. Introduction
2. Results
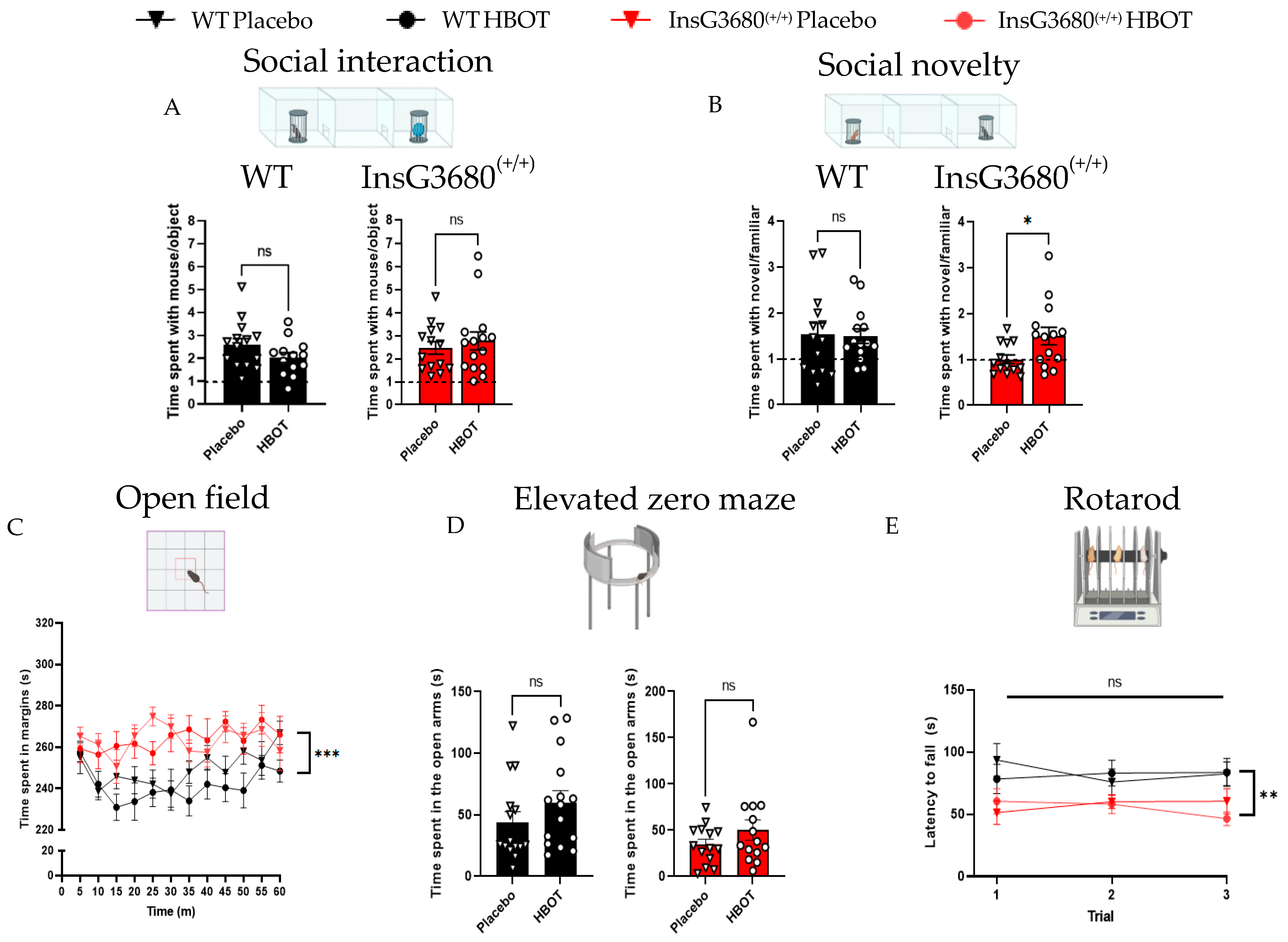
2.1. HBOT Improves Social Novelty Preference but Not Anxiety-like Behavior and Motor Coordination in InsG3680 Mouse Model for ASD
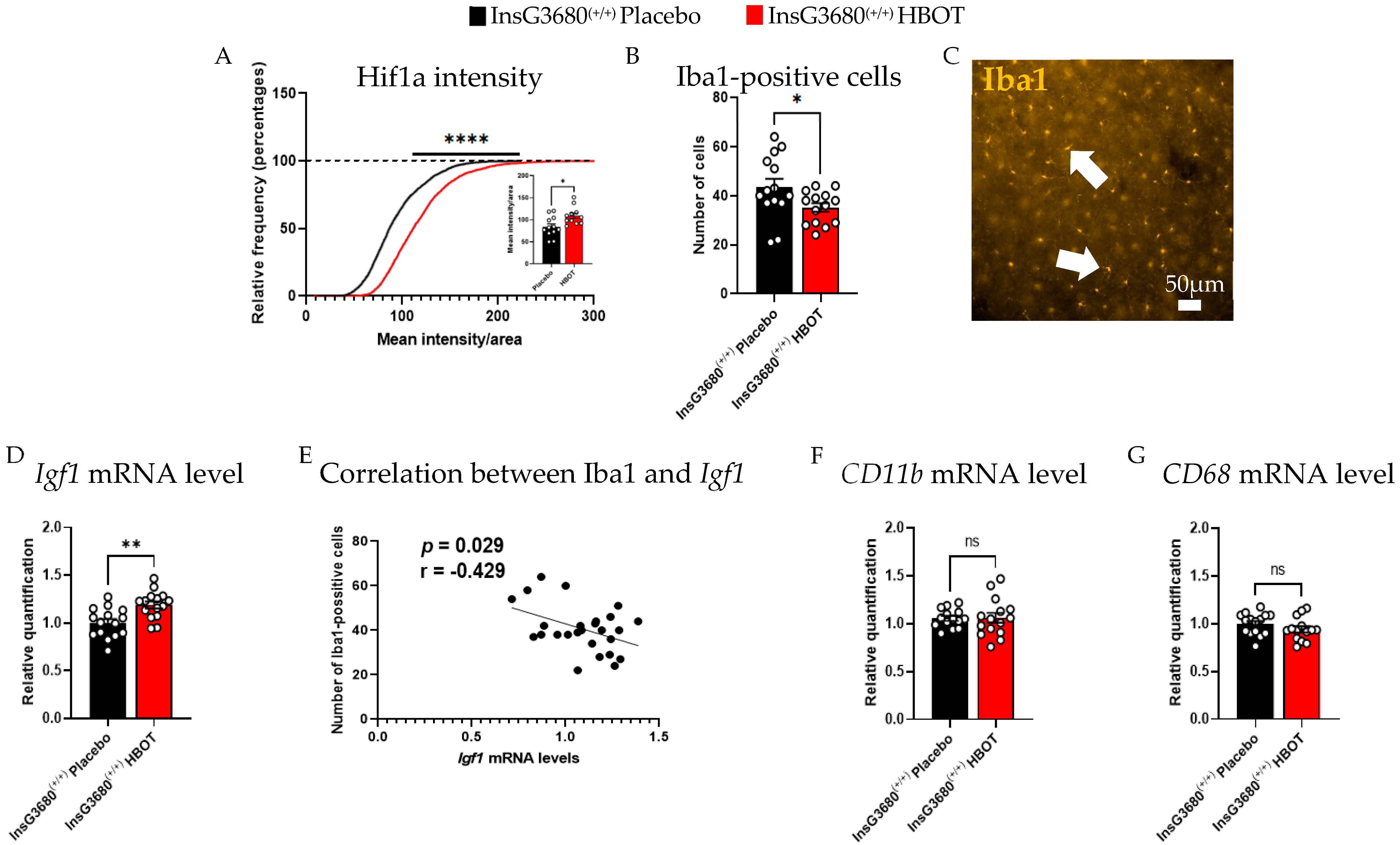
2.2. HBOT Increases the Expression Level of Insulin-like Growth Factor 1 (Igf1) and Hif1a and Decreases Neuroinflammation in the Brain of InsG3680 Mouse Model for ASD
3. Discussion
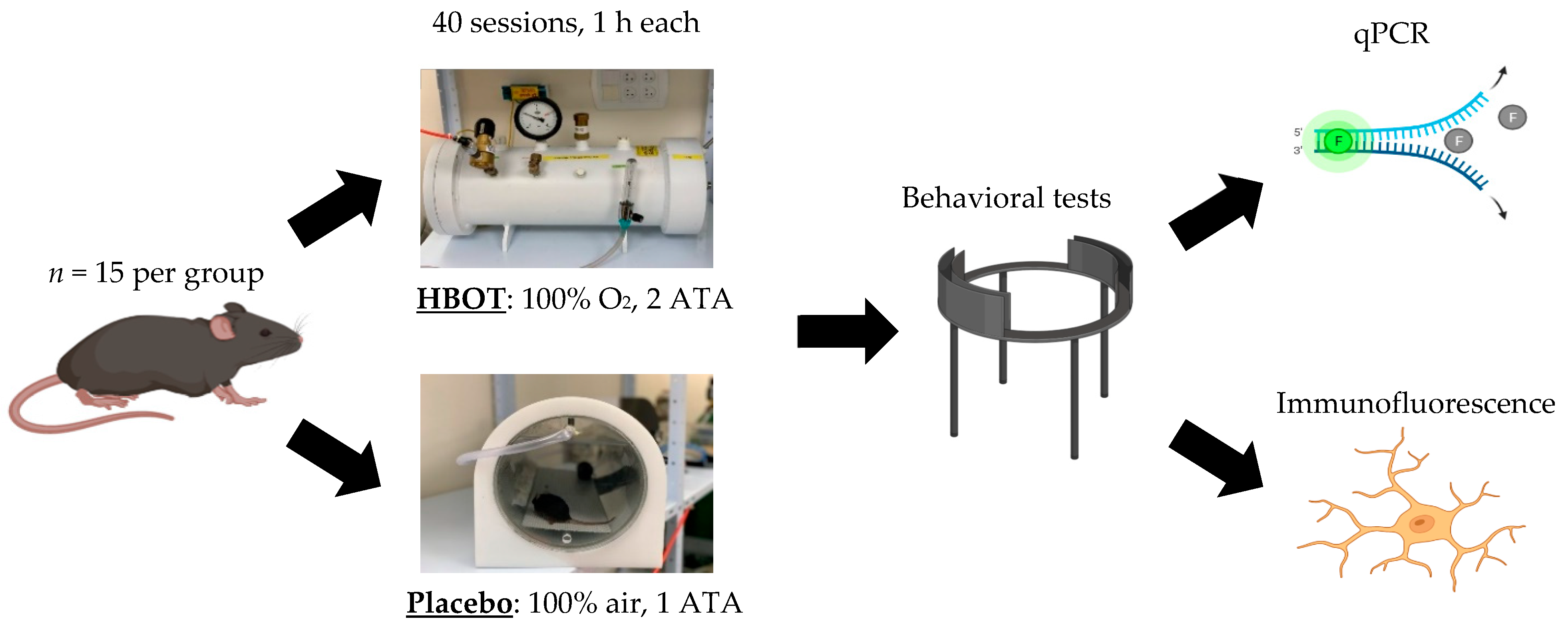
4. Materials and Methods
4.1. Animal Work Statement
4.2. HBOT
4.3. Behavioral Studies
4.3.1. Social Preference Test
4.3.2. Open Field Exploration Test
4.3.3. Elevated Zero Maze
4.3.4. Rotarod
4.4. Brain Tissue Extraction
4.5. RNA Isolation and Quantitative PCR (qPCR)
4.5.1. RNA Extraction
4.5.2. Complementary Deoxyribonucleic Acid (cDNA) Preparation
4.5.3. Real-Time PCR
4.6. Immunofluorescence Staining
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Barak, B.; Feng, G. Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat. Neurosci. 2016, 19, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and Characteristics of Autism Spectrum Disorder among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill Summ. 2021, 70, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Huguet, G.; Ey, E.; Bourgeron, T. The Genetic Landscapes of Autism Spectrum Disorders. Annu. Rev. Genom. Hum. Genet. 2013, 14, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.; Feng, G. SHANK proteins: Roles at the synapse and in autism spectrum disorder. Nat. Rev. Neurosci. 2017, 18, 147–157. [Google Scholar] [CrossRef]
- Mitz, A.R.; Philyaw, T.J.; Boccuto, L.; Shcheglovitov, A.; Sarasua, S.M.; Kaufmann, W.E.; Thurm, A. Identification of 22q13 genes most likely to contribute to Phelan McDermid syndrome. Eur. J. Hum. Genet. 2018, 26, 293–302. [Google Scholar] [CrossRef]
- Durand, C.M.; Betancur, C.; Boeckers, T.M.; Bockmann, J.; Chaste, P.; Fauchereau, F.; Nygren, G.; Rastam, M.; Gillberg, I.C.; Anckarsäter, H.; et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 2006, 39, 25–27. [Google Scholar] [CrossRef]
- Zhou, Y.; Kaiser, T.; Monteiro, P.; Zhang, X.; Van de Goes, A.S.; Wang, D.; Barak, B.; Zenf, M.; Li, C.; Lu, C.; et al. Mice with Shank3 Mutations Associated with ASD and Schizophrenia Display Both Shared and Distinct Defects. Neuron 2016, 89, 147–162. [Google Scholar] [CrossRef]
- Amal, H.; Barak, B.; Bhat, V.; Gong, G.; Joughin, B.A.; Wang, X.; Wishnok, J.S.; Feng, G.; Tannenbaum, S.R. Shank3 mutation in a mouse model of autism leads to changes in the S-nitroso-proteome and affects key proteins involved in vesicle release and synaptic function. Mol. Psychiatry 2020, 25, 1835–1848. [Google Scholar] [CrossRef]
- Ivashko-Pachima, Y.; Ganaiem, M.; Ben-Horin-Hazak, I.; Lobyntseva, A.; Bellaiche, N.; Fischer, I.; Levy, G.; Sragovich, S.; Karmon, G.; Giladi, E.; et al. SH3- and actin-binding domains connect ADNP and SHANK3, revealing a fundamental shared mechanism underlying autism. Mol. Psychiatry 2022. [Google Scholar] [CrossRef]
- Poleg, S.; Kourieh, E.; Ruban, A.; Shapira, G.; Shomron, N.; Barak, B.; Offen, D. Behavioral aspects and neurobiological properties underlying medical cannabis treatment in Shank3 mouse model of autism spectrum disorder. Transl. Psychiatry 2021, 11, 524. [Google Scholar] [CrossRef]
- Voineagu, I.; Eapen, V. Converging Pathways in Autism Spectrum Disorders: Interplay between Synaptic Dysfunction and Immune Responses. Front. Hum. Neurosci. 2013, 7, 738. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Caldeira, G.L.; Peça, J.; Carvalho, A.L. New insights on synaptic dysfunction in neuropsychiatric disorders. Curr. Opin. Neurobiol. 2019, 57, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kirk, S.; Karlik, S. VEGF and vascular changes in chronic neuroinflammation. J. Autoimmun. 2003, 21, 353–363. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Rossignol, L.W. Hyperbaric oxygen therapy may improve symptoms in autistic children. Med. Hypotheses 2006, 67, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef]
- Bar, E.; Barak, B. Microglia roles in synaptic plasticity and myelination in homeostatic conditions and neurodevelopmental disorders. Glia 2019, 67, 2125–2141. [Google Scholar] [CrossRef]
- Lecavalier, L. Phenotypic Variability in Autism Spectrum Disorder: Clinical Considerations. In Handbook of Autism and Anxiety Davis, T.E., III; White, S.W., Ollendick, T.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 15–29. [Google Scholar]
- Grzadzinski, R.; Huerta, M.; Lord, C. DSM-5 and autism spectrum disorders (ASDs): An opportunity for identifying ASD subtypes. Mol. Autism 2013, 4, 12. [Google Scholar] [CrossRef]
- Boccuto, L.; Lauri, M.; Sarasua, S.M.; Skinner, C.D.; Buccella, D.; Dwivedi, A.; Orteschi, D.; Collins, J.S.; Zollino, M.; Visconti, P.; et al. Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders. Eur. J. Hum. Genet. 2012, 21, 310–316. [Google Scholar] [CrossRef]
- Eissa, N.; Al-Houqani, M.; Sadeq, A.; Ojha, S.K.; Sasse, A.; Sadek, B. Current Enlightenment About Etiology and Pharmacological Treatment of Autism Spectrum Disorder. Front. Neurosci. 2018, 12, 304. [Google Scholar] [CrossRef]
- Boddaert, N.; Zilbovicius, M. Functional neuroimaging and childhood autism. Pediatr. Radiol. 2001, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A. Hyperbaric oxygen therapy might improve certain pathophysiological findings in autism. Med. Hypotheses 2007, 68, 1208–1227. [Google Scholar] [CrossRef] [PubMed]
- Efrati, S.; Ben-Jacob, E. Reflections on the neurotherapeutic effects of hyperbaric oxygen. Expert Rev. Neurother. 2014, 14, 233–236. [Google Scholar] [CrossRef]
- Hadanny, A.; Golan, H.; Fishlev, G.; Bechor, Y.; Volkov, O.; Suzin, G.; Ben-Jacob, E.; Efrati, S. Hyperbaric oxygen can induce neuroplasticity and improve cognitive functions of patients suffering from anoxic brain damage. Restor. Neurol. Neurosci. 2015, 33, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Tal, S.; Hadanny, A.; Sasson, E.; Suzin, G.; Efrati, S. Hyperbaric Oxygen Therapy Can Induce Angiogenesis and Regeneration of Nerve Fibers in Traumatic Brain Injury Patients. Front. Hum. Neurosci. 2017, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.; Barak, B. Molecular and Therapeutic Aspects of Hyperbaric Oxygen Therapy in Neurological Conditions. Biomolecules 2020, 10, 1247. [Google Scholar] [CrossRef]
- Hadanny, A.; Efrati, S. The Hyperoxic-Hypoxic Paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef]
- Günther, A.; Küppers-Tiedt, L.; Schneider, P.-M.; Kunert, I.; Berrouschot, J.; Schneider, D.; Rossner, S. Reduced infarct volume and differential effects on glial cell activation after hyperbaric oxygen treatment in rat permanent focal cerebral ischaemia. Eur. J. Neurosci. 2005, 21, 3189–3194. [Google Scholar] [CrossRef]
- Rossignol, D.; Rossignol, L.W.; Smith, S.; Schneider, C.; Logerquist, S.; Usman, A.; Neubrander, J.; Madren, E.M.; Hintz, G.; Grushkin, B.; et al. Hyperbaric treatment for children with autism: A multicenter, randomized, double-blind, controlled trial. BMC Pediatr. 2009, 9, 21. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Rossignol, L.W.; James, S.J.; Melnyk, S.; Mumper, E. The effects of hyperbaric oxygen therapy on oxidative stress, inflammation, and symptoms in children with autism: An open-label pilot study. BMC Pediatr. 2007, 7, 36. [Google Scholar] [CrossRef]
- Granpeesheh, D.; Tarbox, J.; Dixon, D.R.; Wilke, A.E.; Allen, M.S.; Bradstreet, J.J. Randomized trial of hyperbaric oxygen therapy for children with autism. Res. Autism Spectr. Disord. 2010, 4, 268–275. [Google Scholar] [CrossRef]
- Jepson, B.; Granpeesheh, D.; Tarbox, J.; Olive, M.L.; Stott, C.; Braud, S.; Yoo, J.H.; Wakefield, A.; Allen, M.S. Controlled Evaluation of the Effects of Hyperbaric Oxygen Therapy on the Behavior of 16 Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2011, 41, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Sampanthavivat, M.; Singkhwa, W.; Chaiyakul, T.; Karoonyawanich, S.; Ajpru, H. Hyperbaric oxygen in the treatment of childhood autism: A randomised controlled trial. Diving Hyperb. Med. J. 2012, 42, 128–133. [Google Scholar]
- El-Baz, F.; Elhossiny, R.M.; Azeem, Y.A.; Girgis, M. Study the effect of hyperbaric oxygen therapy in Egyptian autistic children: A clinical trial. Egypt. J. Med. Hum. Genet. 2014, 15, 155–162. [Google Scholar] [CrossRef]
- Chungpaibulpatana, J.; Sumpatanarax, T.; Thadakul, N.; Chantharatreerat, C.; Konkaew, M.; Aroonlimsawas, M. Hyperbaric oxygen therapy in Thai autistic children. Med. J. Med. Assoc. Thail. 2008, 91, 1232. [Google Scholar]
- Bent, S.; Bertoglio, K.; Ashwood, P.; Nemeth, E.; Hendren, R.L. Brief Report: Hyperbaric Oxygen Therapy (HBOT) in Children with Autism Spectrum Disorder: A Clinical Trial. J. Autism Dev. Disord. 2012, 42, 1127–1132. [Google Scholar] [CrossRef]
- Kolevzon, A.; Bush, L.; Wang, A.T.; Halpern, D.; Frank, Y.; Grodberg, D.; Rapaport, R.; Tavassoli, T.; Chaplin, W.; Soorya, L.; et al. A pilot controlled trial of insulin-like growth factor-1 in children with Phelan-McDermid syndrome. Mol. Autism 2014, 5, 54. [Google Scholar] [CrossRef]
- Xie, R.J.; Li, T.X.; Sun, C.; Cheng, C.; Zhao, J.; Xu, H.; Liu, Y. A case report of Phelan-McDermid syndrome: Preliminary results of the treatment with growth hormone therapy. Ital. J. Pediatrics 2021, 47, 49. [Google Scholar] [CrossRef]
- Manning, E.E.; van den Buuse, M. Altered social cognition in male BDNF heterozygous mice and following chronic methamphetamine exposure. Behav. Brain Res. 2016, 305, 181–185. [Google Scholar] [CrossRef]
- Schottlender, N.; Gottfried, I.; Ashery, U. Hyperbaric Oxygen Treatment: Effects on Mitochondrial Function and Oxidative Stress. Biomolecules 2021, 11, 1827. [Google Scholar] [CrossRef]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.-M.; Ji, L.; Brown, T.; Malik, M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2004, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.J.; Behringer, R.R.; Brinster, R.L.; McMorris, F. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron 1993, 10, 729–740. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Torres-Alemán, I. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 2012, 13, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Costales, J.; Kolevzon, A. The therapeutic potential of insulin-like growth factor-1 in central nervous system disorders. Neurosci. Biobehav. Rev. 2016, 63, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Corvin, A.P.; Molinos, I.; Little, G.; Donohoe, G.; Gill, M.; Morris, D.W.; Tropea, D. Insulin-like growth factor 1 (IGF1) and its active peptide (1–3)IGF1 enhance the expression of synaptic markers in neuronal circuits through different cellular mechanisms. Neurosci. Lett. 2012, 520, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Riikonen, R.; Makkonen, I.; Vanhala, R.; Turpeinen, U.; Kuikka, J.; Kokki, H. Cerebrospinal fluid insulin-like growth factors IGF-1 and IGF-2 in infantile autism. Dev. Med. Child Neurol. 2006, 48, 751–755. [Google Scholar] [CrossRef]
- Vanhala, R.; Turpeinen, U.; Riikonen, R. Low levels of insulin-like growth factor-I in cerebrospinal fluid in children with autism. Dev. Med. Child Neurol. 2001, 43, 614–616. [Google Scholar] [CrossRef]
- Shcheglovitov, A.; Shcheglovitova, O.; Yazawa, M.; Portmann, T.; Shu, R.; Sebastiano, V.; Krawisz, A.; Froehlich, W.; Bernstein, J.A.; Hallmayer, J.F.; et al. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature 2013, 503, 267–271. [Google Scholar] [CrossRef]
- Bozdagi, O.; Tavassoli, T.; Buxbaum, J.D. Insulin-like growth factor-1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay. Mol. Autism 2013, 4, 9. [Google Scholar] [CrossRef]
- Sztainberg, Y.; Zoghbi, H. Lessons learned from studying syndromic autism spectrum disorders. Nat. Neurosci. 2016, 19, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Vahdatpour, C.; Dyer, A.H.; Tropea, D. Insulin-Like Growth Factor 1 and Related Compounds in the Treatment of Childhood-Onset Neurodevelopmental Disorders. Front. Neurosci. 2016, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Riikonen, R. Treatment of autistic spectrum disorder with insulin-like growth factors. Eur. J. Paediatr. Neurol. 2016, 20, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Bake, S.; Selvamani, A.; Cherry, J.; Sohrabji, F. Blood Brain Barrier and Neuroinflammation Are Critical Targets of IGF-1-Mediated Neuroprotection in Stroke for Middle-Aged Female Rats. PLoS ONE 2014, 9, e91427. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Gao, W.; Zhou, K.; Liu, X.; Jiang, W.; Xue, R.; Wu, W. Role of IGF-1 in neuroinflammation and cognition deficits induced by sleep deprivation. Neurosci. Lett. 2022, 776, 136575. [Google Scholar] [CrossRef]
- Shandilya, A.; Mehan, S. Dysregulation of IGF-1/GLP-1 signaling in the progression of ALS: Potential target activators and influences on neurological dysfunctions. Neurol. Sci. 2021, 42, 3145–3166. [Google Scholar] [CrossRef]
- Herrera, M.L.; Bandín, S.; Champarini, L.G.; Hereñú, C.B.; Bellini, M.J. Intramuscular insulin-like growth factor-1 gene therapy modulates reactive microglia after traumatic brain injury. Brain Res. Bull. 2021, 175, 196–204. [Google Scholar] [CrossRef]
- Tien, L.-T.; Lee, Y.-J.; Pang, Y.; Lu, S.; Lee, J.W.; Tseng, C.-H.; Bhatt, A.J.; Savich, R.D.; Fan, L.-W. Neuroprotective Effects of Intranasal IGF-1 against Neonatal Lipopolysaccharide-Induced Neurobehavioral Deficits and Neuronal Inflammation in the Substantia Nigra and Locus Coeruleus of Juvenile Rats. Dev. Neurosci. 2017, 39, 443–459. [Google Scholar] [CrossRef]
- Serhan, A.; Aerts, J.L.; Boddeke, E.W.; Kooijman, R. Neuroprotection by Insulin-like Growth Factor-1 in Rats with Ischemic Stroke is Associated with Microglial Changes and a Reduction in Neuroinflammation. Neuroscience 2020, 426, 101–114. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
| Origin | Forward Sequence | Reverse Sequence |
|---|---|---|
| Gapdh | GCCTTCCGTGTTCCTACC | CCTCAGTGTAGCCCAAGATG |
| Cd11b | CTCTTGGCTCTCATCACTGCTG | GCAGCTTCATTCATCATGTCCT |
| Igf1 | CAGCAGCCTTCCAACTCAATTAT | CGCCAGGTAGAAGAGGTGTGAA |
| Cd68 | GGACAGCTTACCTTTGGATTCA | AAGGACACATTGTATTCCACCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, I.; Shohat, S.; Levy, G.; Bar, E.; Trangle, S.S.; Efrati, S.; Barak, B. Hyperbaric Oxygen Therapy Alleviates Social Behavior Dysfunction and Neuroinflammation in a Mouse Model for Autism Spectrum Disorders. Int. J. Mol. Sci. 2022, 23, 11077. https://doi.org/10.3390/ijms231911077
Fischer I, Shohat S, Levy G, Bar E, Trangle SS, Efrati S, Barak B. Hyperbaric Oxygen Therapy Alleviates Social Behavior Dysfunction and Neuroinflammation in a Mouse Model for Autism Spectrum Disorders. International Journal of Molecular Sciences. 2022; 23(19):11077. https://doi.org/10.3390/ijms231911077
Chicago/Turabian StyleFischer, Inbar, Sophie Shohat, Gilad Levy, Ela Bar, Sari Schokoroy Trangle, Shai Efrati, and Boaz Barak. 2022. "Hyperbaric Oxygen Therapy Alleviates Social Behavior Dysfunction and Neuroinflammation in a Mouse Model for Autism Spectrum Disorders" International Journal of Molecular Sciences 23, no. 19: 11077. https://doi.org/10.3390/ijms231911077
APA StyleFischer, I., Shohat, S., Levy, G., Bar, E., Trangle, S. S., Efrati, S., & Barak, B. (2022). Hyperbaric Oxygen Therapy Alleviates Social Behavior Dysfunction and Neuroinflammation in a Mouse Model for Autism Spectrum Disorders. International Journal of Molecular Sciences, 23(19), 11077. https://doi.org/10.3390/ijms231911077






