Low Dose and Non-Targeted Radiation Effects in Environmental Protection and Medicine—A New Model Focusing on Electromagnetic Signaling
Abstract
:1. Introduction to Low Dose and Non-Targeted Radiobiology
1.1. General Background
1.2. The Importance of Signalling
1.3. Non-Targeted Effects
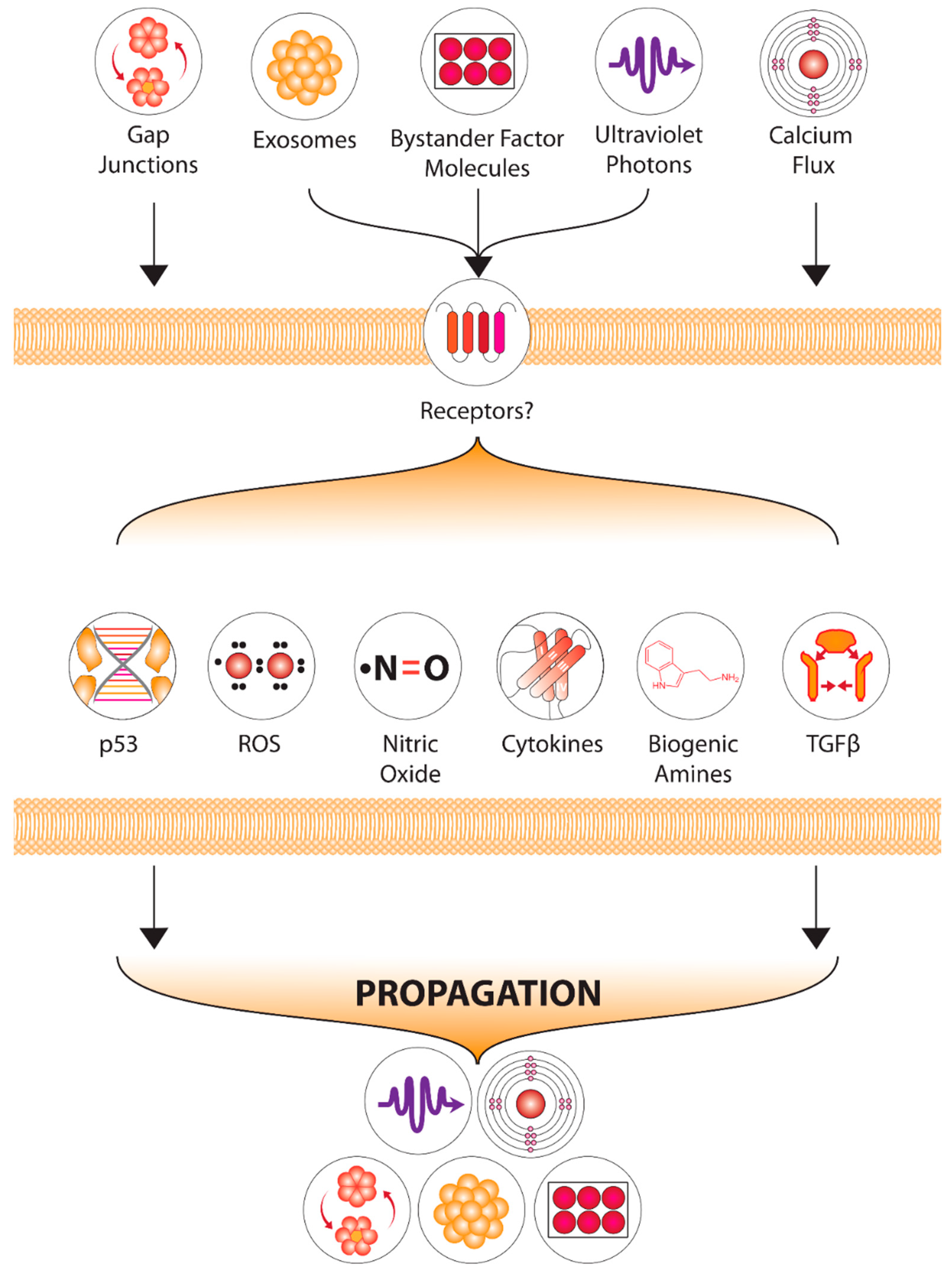
1.4. Candidate Signals-Chemical and Physical
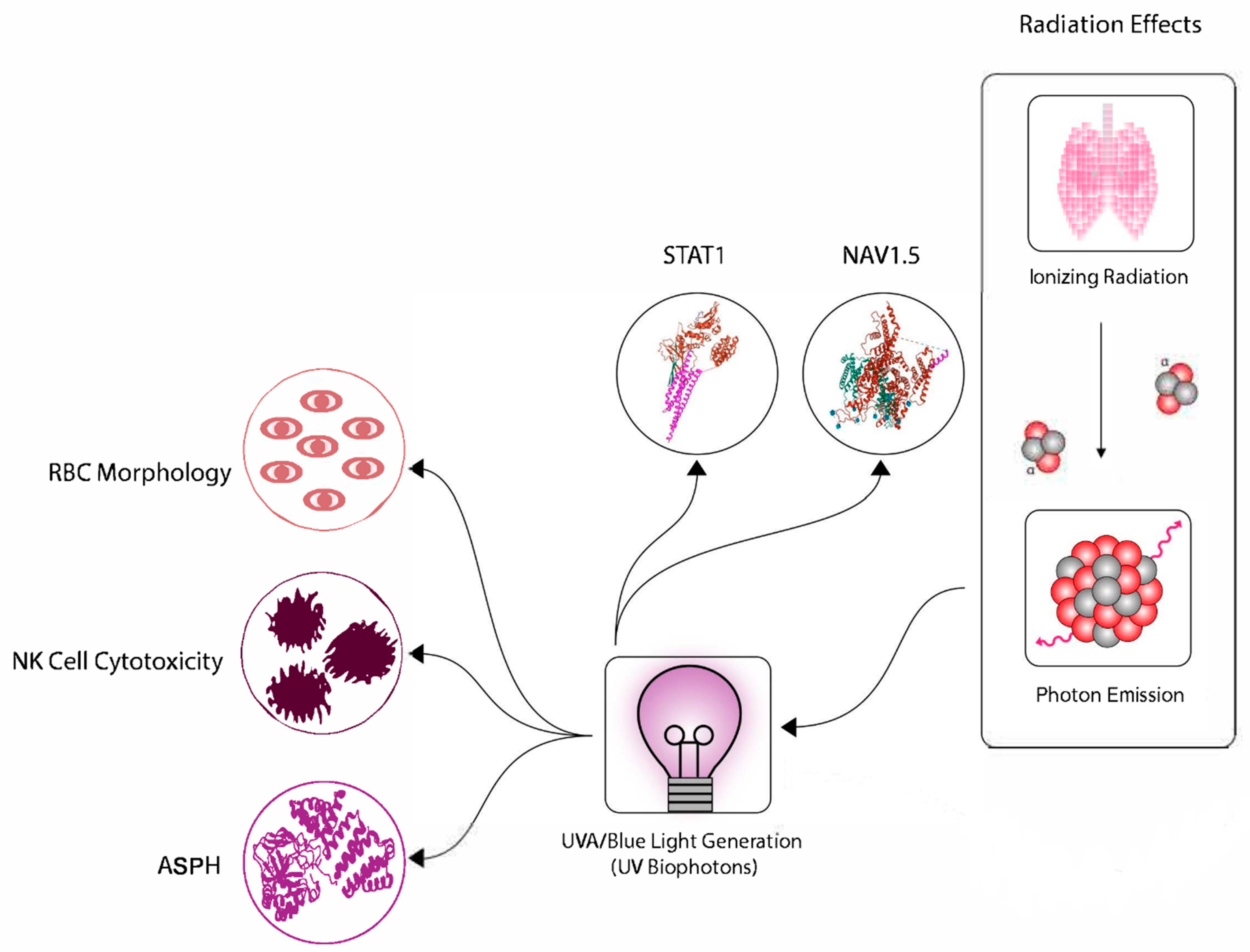
1.5. History of Physical Signal Discoveries
1.6. Physical Signals Can Trigger the Bystander Effect
1.7. Acoustic Signals
2. Downstream Processing of Electromagnetic Signals
3. Chemical Ecology, Mychorrysal and Microbiome Evidence
4. Downstream Events—The Role of RIBE and RIGI
Beneficial Outcomes and Adaptive Responses
5. Proposal of Common Mechanism
6. Relevance and Impacts in Radiation Protection and Medicine
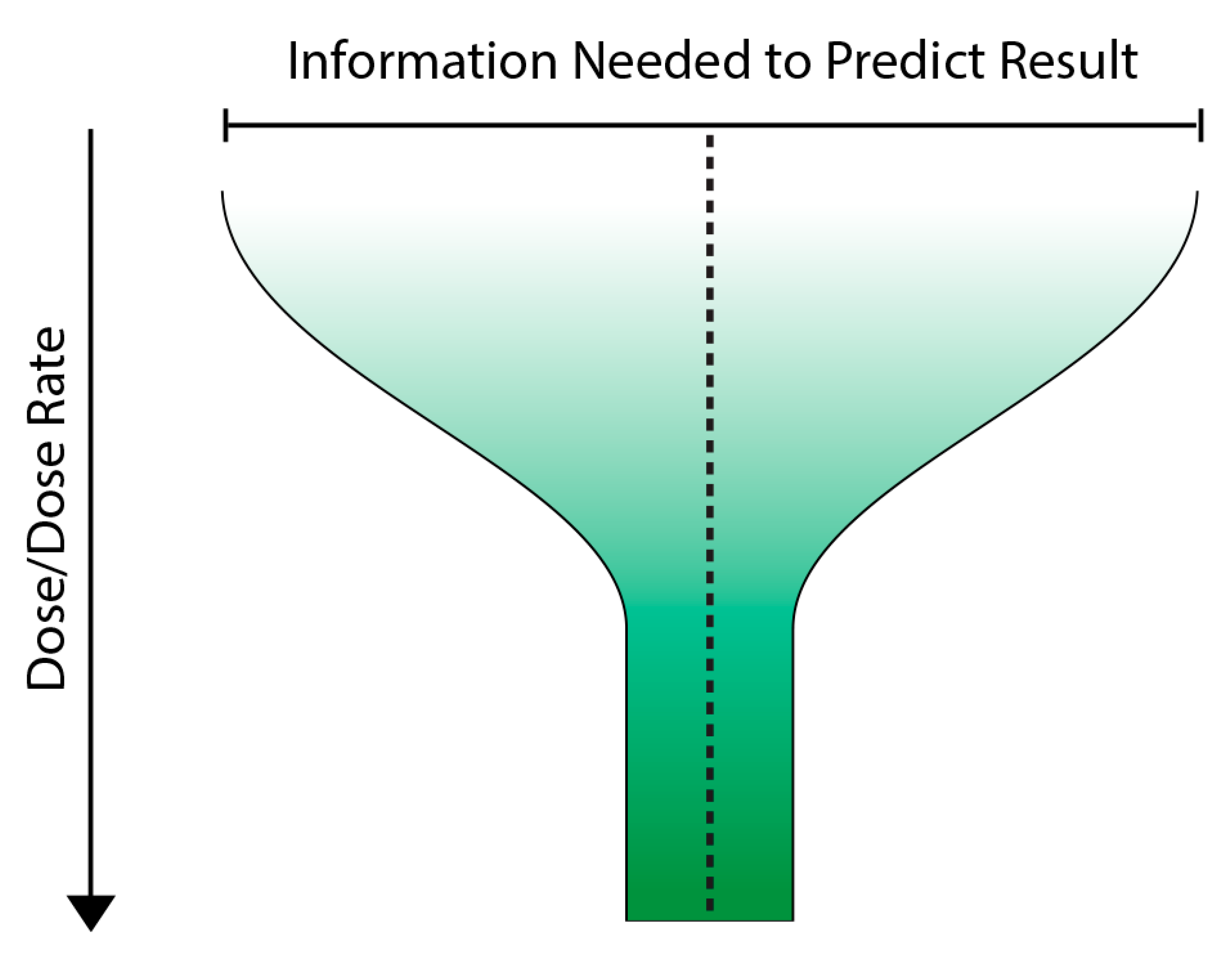
7. A Way Forward? The Variable Response Model
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nation Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation. Report to General Assembly, with Scientific Annexes; United Nations: New York, NY, USA, 2000.
- Mothersill, C.; Seymour, C. Old Data-New Concepts: Integrating “Indirect Effects” Into Radiation Protection. Health Phys. 2018, 115, 170–178. [Google Scholar] [CrossRef]
- Owusu, S.B.; Dupré-Crochet, S.; Bizouarn, T.; Houée-Levin, C.; Baciou, L. Accumulation of Cytochrome b558 at the Plasma Membrane: Hallmark of Oxidative Stress in Phagocytic Cells. Int. J. Mol. Sci. 2022, 23, 767. [Google Scholar] [CrossRef]
- Kabilan, U.; Graber, T.E.; Alain, T.; Klokov, D. Ionizing Radiation and Translation Control: A Link to Radiation Hormesis? Int. J. Mol. Sci. 2020, 21, 6650. [Google Scholar] [CrossRef]
- Tang, F.R.; Loke, W.K.; Khoo, B.C. Low-dose or low-dose-rate ionizing radiation-induced bioeffects in animal models. J. Radiat. Res. 2017, 58, 165–182. [Google Scholar] [CrossRef]
- Argacha, J.F.; Mizukami, T.; Bourdrel, T.; Bind, M.A. Ecology of the cardiovascular system: Part II—A focus on non-air related pollutants. Trends Cardiovasc. Med. 2019, 29, 274–282. [Google Scholar] [CrossRef]
- Lumniczky, K.; Impens, N.; Armengol, G.; Candéias, S.; Georgakilas, A.G.; Hornhardt, S.; Martin, O.A.; Rödel, F.; Schaue, D. Low dose ionizing radiation effects on the immune system. Environ. Int. 2021, 149, 106212. [Google Scholar] [CrossRef]
- Murad, H.Y.; Chandra, P.K.; Kelly, C.A.; Khurana, N.; Yu, H.; Bortz, E.P.; Hong, S.N.; Mondal, D.; Khismatullin, D.B. Pre-Exposure to Stress-Inducing Agents Increase the Anticancer Efficacy of Focused Ultrasound against Aggressive Prostate Cancer Cells. Antioxidants 2022, 11, 341. [Google Scholar] [CrossRef]
- Wen, S.; Li, C.; Zhan, X. Muti-omics integration analysis revealed molecular network alterations in human nonfunctional pituitary neuroendocrine tumors in the framework of 3P medicine. EPMA J. 2022, 13, 9–37. [Google Scholar] [CrossRef]
- Sun, P.Y.; Wang, A.S.; Zhang, Z.F.; Zhang, Y.L.; Zheng, X. Network pharmacology-based strategy to investigate the active ingredients and molecular mechanisms of Scutellaria Barbata D. Don against radiation pneumonitis. Medicine 2021, 100, e27957. [Google Scholar]
- Xu, P.; Wang, M.; Song, W.M.; Wang, Q.; Yuan, G.C.; Sudmant, P.H.; Zare, H.; Tu, Z.; Orr, M.E.; Zhang, B. The landscape of human tissue and cell type specific expression and co-regulation of senescence genes. Mol. Neurodegener. 2022, 17, 5. [Google Scholar] [CrossRef]
- Powathil, G.G.; Munro, A.J.; Chaplain, M.A.; Swat, M. Bystander effects and their implications for clinical radiation therapy: Insights from multiscale in silico experiments. J. Theor. Biol. 2016, 401, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Naumann, R.B.; Sabounchi, N.S.; Kuhlberg, J.; Singichetti, B.; Marshall, S.W.; Hassmiller Lich, K. Simulating congestion pricing policy impacts on pedestrian safety using a system dynamics approach. Accid. Anal. Prev. 2022, 171, 106662. [Google Scholar] [CrossRef]
- Social Messes: Robert E. Horn. Available online: https://nautilus.org/gps/solving/social-messes-robert-e-horn/ (accessed on 6 June 2022).
- Bell, I.R.; Ives, J.A.; Jonas, W.B. Nonlinear effects of nanoparticles: Biological variability from hormetic doses, small particle sizes, and dynamic adaptive interactions. Dose-Response A Publ. Int. Hormesis Soc. 2013, 12, 202–232. [Google Scholar] [CrossRef]
- Averbeck, D.; Rodriguez-Lafrasse, C. Role of Mitochondria in Radiation Responses: Epigenetic, Metabolic, and Signaling Impacts. Int. J. Mol. Sci. 2021, 22, 11047. [Google Scholar] [CrossRef]
- Bell, I.R.; Koithan, M. A model for homeopathic remedy effects: Low dose nanoparticles, allostatic cross-adaptation, and time-dependent sensitization in a complex adaptive system. BMC Complementary Altern. Med. 2012, 12, 191. [Google Scholar] [CrossRef]
- Murray, D.; Mirzayans, R. Nonlinearities in the cellular response to ionizing radiation and the role of p53 therein. Int. J. Radiat. Biol. 2021, 97, 1088–1098. [Google Scholar] [CrossRef]
- McCallum, E.S.; Dey, C.J.; Cerveny, D.; Bose, A.; Brodin, T. Social status modulates the behavioral and physiological consequences of a chemical pollutant in animal groups. Ecol. Appl. A Publ. Ecol. Soc. Am. 2021, 31, e02454. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C. Low dose radiation mechanisms: The certainty of uncertainty. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2022, 876–877, 503451. [Google Scholar] [CrossRef]
- Sacks, B.; Meyerson, G. Linear No-threshold (LNT) vs. Hormesis: Paradigms, Assumptions, and Mathematical Conventions that Bias the Conclusions in Favor of LNT and Against hormesis. Health Phys. 2019, 116, 807–816. [Google Scholar] [CrossRef]
- Oughton, D. Protection of the environment from ionising radiation: Ethical issues. J. Environ. Radioact. 2003, 66, 3–18. [Google Scholar] [CrossRef]
- Britel, M.; Bourguignon, M.; Preau, M. Radiation protection in mammography for breast cancer screening: Not covered by the French press. Public Health 2020, 183, 119–121. [Google Scholar] [CrossRef]
- Lierman, S.; Veuchelen, L. The optimisation approach of ALARA in nuclear practice: An early application of the precautionary principle. Scientific uncertainty versus legal uncertainty. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2005, 52, 81–86. [Google Scholar]
- Devic, C.; Ferlazzo, M.L.; Berthel, E.; Foray, N. Influence of Individual Radiosensitivity on the Hormesis Phenomenon: Toward a Mechanistic Explanation Based on the Nucleoshuttling of ATM Protein. Dose-Response A Publ. Int. Hormesis Soc. 2020, 18, 1559325820913784. [Google Scholar] [CrossRef]
- Tanaka, Y.; Furuta, M. Biological effects of low-dose γ-ray irradiation on chromosomes and DNA of Drosophila melanogaster. J. Radiat. Res. 2021, 62, 1–11. [Google Scholar] [CrossRef]
- Das, S.; Singh, R.; George, D.; Vijaykumar, T.S.; John, S. Radiobiological Response of Cervical Cancer Cell Line in Low Dose Region: Evidence of Low Dose Hypersensitivity (HRS) and Induced Radioresistance (IRR). J. Clin. Diagn. Res. JCDR 2015, 9, XC05–XC08. [Google Scholar] [CrossRef]
- Morgan, W.F. Communicating non-targeted effects of ionizing radiation to achieve adaptive homeostasis in tissues. Curr. Mol. Pharmacol. 2011, 4, 135–140. [Google Scholar] [CrossRef]
- Dawood, A.; Mothersill, C.; Seymour, C. Low dose ionizing radiation and the immune response: What is the role of non-targeted effects? Int. J. Radiat. Biol. 2021, 97, 1368–1382. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dutta, A.; Chakraborty, A. External modulators and redox homeostasis: Scenario in radiation-induced bystander cells. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108368. [Google Scholar] [CrossRef]
- Wang, X.; Undi, R.B.; Ali, N.; Huycke, M.M. It takes a village: Microbiota, parainflammation, paligenosis and bystander effects in colorectal cancer initiation. Dis. Models Mech. 2021, 14, dmm048793. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, H.; Lv, C.; Lan, F.; Wang, Y.; Deng, Y. Exosomes and exosomal microRNA in non-targeted radiation bystander and abscopal effects in the central nervous system. Cancer Lett. 2021, 499, 73–84. [Google Scholar] [CrossRef]
- Chauhan, V.; Beaton, D.; Hamada, N.; Wilkins, R.; Burtt, J.; Leblanc, J.; Cool, D.; Garnier-Laplace, J.; Laurier, D.; Le, Y.; et al. Adverse outcome pathway: A path toward better data consolidation and global co-ordination of radiation research. Int. J. Radiat. Biol. 2022, 1–10, Advance online publication. [Google Scholar] [CrossRef]
- Chauhan, V.; Wilkins, R.C.; Beaton, D.; Sachana, M.; Delrue, N.; Yauk, C.; O’Brien, J.; Marchetti, F.; Halappanavar, S.; Boyd, M.; et al. Bringing together scientific disciplines for collaborative undertakings: A vision for advancing the adverse outcome pathway framework. Int. J. Radiat. Biol. 2021, 97, 431–441. [Google Scholar] [CrossRef]
- Preston, R.J.; Rühm, W.; Azzam, E.I.; Boice, J.D.; Bouffler, S.; Held, K.D.; Little, M.P.; Shore, R.E.; Shuryak, I.; Weil, M.M. Adverse outcome pathways, key events, and radiation risk assessment. Int. J. Radiat. Biol. 2021, 97, 804–814. [Google Scholar] [CrossRef]
- Heeran, A.B.; Berrigan, H.P.; O’Sullivan, J. The Radiation-Induced Bystander Effect (RIBE) and its Connections with the Hallmarks of Cancer. Radiat. Res. 2019, 192, 668–679. [Google Scholar] [CrossRef]
- Burdak-Rothkamm, S.; Rothkamm, K. Radiation-induced bystander and systemic effects serve as a unifying model system for genotoxic stress responses. Mutat. Res. Rev. Mutat. Res. 2018, 778, 13–22. [Google Scholar] [CrossRef]
- Mothersill, C.; Rusin, A.; Fernandez-Palomo, C.; Seymour, C. History of bystander effects research 1905-present; what is in a name? Int. J. Radiat. Biol. 2018, 94, 696–707. [Google Scholar] [CrossRef]
- Du, Y.; Du, S.; Liu, L.; Gan, F.; Jiang, X.; Wangrao, K.; Lyu, P.; Gong, P.; Yao, Y. Radiation-Induced Bystander Effect can be Transmitted Through Exosomes Using miRNAs as Effector Molecules. Radiat. Res. 2020, 194, 89–100. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C.B. Radiation-induced bystander effects--implications for cancer. Nat. Rev. Cancer 2004, 4, 158–164. [Google Scholar]
- Gurwitsch, A.A. A historical review of the problem of mitogenetic radiation. Experientia 1988, 44, 545–550. [Google Scholar] [CrossRef]
- Popp, F.A.; Nagl, W.; Li, K.H.; Scholz, W.; Weingrtner, O.; Wolf, R. Biophoton emission. New evidence for coherence and DNA as source. Cell Biophys. 1984, 6, 33–52. [Google Scholar] [CrossRef]
- Gurwitsch, A. Les mitoses de croissance embryonnaire exigent-elles une stimulation extracellulaire? Compt. Rend. Soc. Biol. 1920, 83, 1552–1553. [Google Scholar]
- Baron, M.A. Uber mitogenetische Strahlung bei Protisten. Arch. Entw. Organ. 1926, 108, 619–628. [Google Scholar] [CrossRef]
- Bajpai, R.P.; Bajpai, P.K.; Roy, D. Ultraweak photon emission in germinating seeds: A signal of biological order. J. Biolumin. Chemilumin. 1991, 6, 227–230. [Google Scholar] [CrossRef]
- Devaraj, B.; Scott, R.Q.; Roschger, P.; Inaba, H. Ultraweak light emission from rat liver nuclei. Photochem. Photobiol. 1991, 54, 289–293. [Google Scholar] [CrossRef]
- Evelson, P.; Ordez, C.P.; Llesuy, S.; Boveris, A. Oxidative stress and in vivo chemiluminescence in mouse skin exposed to UVA radiation. J. Photochem. Photobiol. B Biol. 1997, 38, 215–219. [Google Scholar] [CrossRef]
- Van Wijk, R.; Van Wijk EP, A.; van Wietmarschen, H.A.; van der Greef, J. Towards whole-body ultra-weak photon counting and imaging with a focus on human beings: A review. J. Photochem. Photobiol. B Biol. 2014, 139, 39–46. [Google Scholar] [CrossRef]
- Niggli, H.J. The cell nucleus of cultured melanoma cells as a source of ultraweak photon emission. Naturwissenschaften 1996, 83, 41–44. [Google Scholar] [CrossRef]
- Niggli, H.J.; Tudisco, S.; Lanzan, L.; Applegate, L.A.; Scordino, A.; Musumeci, F. 2008 Laser-ultraviolet-A induced ultra weak photon emission in human skin cells: A biophotonic comparison between keratinocytes and fibroblasts. Indian J. Exp. Biol. 2008, 46, 358–363. [Google Scholar]
- Van Wijk, E.; Kobayashi, M.; van Wijk, R.; van der Greef, J. Imaging of ultra-weak photon emission in a rheumatoid arthritis mouse model. PLoS ONE 2013, 8, e84579. [Google Scholar] [CrossRef]
- Bajpai, R.P.; Van Wijk EP, A.; Van Wijk, R.; van der Greef, J. Attributes characterizing spontaneous ultra-weak photon signals of human subjects. J. Photochem. Photobiol. B Biol. 2013, 129, 6–16. [Google Scholar] [CrossRef]
- Borodin, D.N. Energy emanation during cell division processes (M-rays). Plant Physiol. 1930, 5, 119–129. [Google Scholar] [CrossRef]
- Rahn, O. Invisible Radiations Of Organisms; Gebreuder Borntrarger: Berlin, Germany, 1936. [Google Scholar]
- Slawinski, J.; Ezzahir, A.; Godlewski, M.; Kwiecinska, T.; Rajfur, Z.; Sitko, D.; Wierzuchowska, D. Stress-induced photon emission from perturbed organisms. Experientia 1992, 48, 1041–1058. [Google Scholar] [CrossRef]
- Ahmad, S.B.; McNeill, F.E.; Byun, S.H.; Prestwich, W.V.; Mothersill, C.; Seymour, C.; Armstrong, A.; Fernandez, C. Ultra-violet light emission from HPV-G cells irradiated with low let radiation from (90)Y; consequences for radiation induced bystander effects. Dose-Response 2013, 11, 498–516. [Google Scholar] [CrossRef]
- Le, M.; McNeill, F.E.; Seymour, C.; Rainbow, A.J.; Mothersill, C.E. An observed effect of ultraviolet radiation emitted from beta-irradiated HaCaT cells upon non-beta-irradiated bystander cells. Radiat. Res. 2015, 183, 279–290. [Google Scholar] [CrossRef]
- Ruth, B.; Popp, F.A. Experimentelle Untersuchungen zur ultraschwachen Photonenemission biologischer Systeme [Experimental investigations on ultraweak photonemission form biological systems (author’s transl)]. Z. Fur Naturforschung. Sect. C Biosci. 1976, 31, 741–745. [Google Scholar] [CrossRef]
- Van Wijk, R.; Van Wijk, E.P.; Wiegant, F.A.; Ives, J. Free radicals and low-level photon emission in human pathogenesis: State of the art. Indian J. Exp. Biol. 2008, 46, 273–309. [Google Scholar]
- Le, M.; Mothersill, C.E.; Seymour, C.B.; Rainbow, A.J.; McNeill, F.E. An Observed Effect of p53 Status on the Bystander Response to Radiation-Induced Cellular Photon Emission. Radiat. Res. 2017, 187, 169–185. [Google Scholar] [CrossRef]
- Ahmad, S.B.; McNeill, F.E.; Prestwich, W.V.; Byun, S.H.; Seymour, C.; Mothersill, C.E. Quantification of ultraviolet photon emission from interaction of charged particles in materials of interest in radiation biology research. Nucl. Instrum. Methods Phys. Res. 2014, 319, 48–54. [Google Scholar] [CrossRef]
- Cohen, J.; Vo, N.; Chettle, D.R.; McNeill, F.E.; Seymour, C.B.; Mothersill, C.E. Quantifying Biophoton Emissions From Human Cells Directly Exposed to Low-Dose Gamma Radiation. Dose-Response A Publ. Int. Hormesis Soc. 2020, 18, 1559325820926763. [Google Scholar] [CrossRef]
- Matarèse, B.; Lad, J.; Seymour, C.; Schofield, P.N.; Mothersill, C. Bio-acoustic signaling; exploring the potential of sound as a mediator of low-dose radiation and stress responses in the environment. Int. J. Radiat. Biol. 2022, 98, 1083–1097. [Google Scholar] [CrossRef]
- Iyer, R.; Lehnert, B.E.; Svensson, R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000, 60, 1290–1298. [Google Scholar]
- Lyng, F.M.; Seymour, C.B.; Mothersill, C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: A possible mechanism for bystander-induced genomic instability? Radiat. Res. 2002, 157, 365–370. [Google Scholar] [CrossRef]
- Lyng, F.M.; Howe, O.L.; McClean, B. Reactive oxygen species-induced release of signalling factors in irradiated cells triggers membrane signalling and calcium influx in bystander cells. Int. J. Radiat. Biol. 2011, 87, 683–695. [Google Scholar] [CrossRef]
- Carmichael, A.J.; Mossoba, M.M.; Riesz, P.; Christman, C.L. Free radical production in aqueous solutions exposed to simulated ultrasonic diagnostic conditions. IEEE Trans. Ultrason. Ferroelectr. Freq. Cont. 1986, 33, 148–155. [Google Scholar] [CrossRef]
- Christman, C.L.; Carmichael, A.J.; Mossoba, M.M.; Riesz, P. Evidence for free radicals produced in aqueous solutions by diagnostic ultrasound. Ultrasonics 1987, 25, 31–34. [Google Scholar] [CrossRef]
- Riesz, P.; Berdahl, D.; Christman, C.L. Free radical generation by ultrasound in aqueous and nonaqueous solutions. Environ. Health Perspect. 1985, 64, 233–252. [Google Scholar]
- Xia, C.; Zeng, H.; Zheng, Y. Low-intensity ultrasound enhances the antitumor effects of doxorubicin on hepatocellular carcinoma cells through the ROS-miR-21-PTEN axis. Mol. Med. Rep. 2020, 21, 989–998. [Google Scholar] [CrossRef]
- Ivaskovic, P.; Ainseba, B.; Nicolas, Y.; Toupance, T.; Tardy, P.; Thiéry, D. Sensing of Airborne Infochemicals for Green Pest Management: What Is the Challenge? ACS Sens. 2021, 6, 3824–3840. [Google Scholar] [CrossRef]
- Mori, N.; Noge, K. Recent advances in chemical ecology: Complex interactions mediated by molecules. Biosci. Biotechnol. Biochem. 2021, 85, 33–41. [Google Scholar] [CrossRef]
- Koprivova, A.; Kopriva, S. Plant secondary metabolites altering root microbiome composition and function. Curr. Opin. Plant Biol. 2022, 67, 102227, Advance online publication. [Google Scholar] [CrossRef]
- Allievi, S.; Arru, L.; Forti, L. A tuning point in plant acoustics investigation. Plant Signal. Behav. 2021, 16, 1919836. [Google Scholar] [CrossRef]
- Cook, T.M.; Mansuy-Aubert, V. Communication between the gut microbiota and peripheral nervous system in health and chronic disease. Gut Microbes 2022, 14, 2068365. [Google Scholar] [CrossRef]
- Jovanovic, P.; Riera, C.E. Olfactory system and energy metabolism: A two-way street. Trends Endocrinol. Metab. TEM 2022, 33, 281–291. [Google Scholar] [CrossRef]
- Clark-Cotton, M.R.; Jacobs, K.C.; Lew, D.J. Chemotropism and Cell-Cell Fusion in Fungi. Microbiol. Mol. Biol. Rev. MMBR 2022, 86, e0016521. [Google Scholar] [CrossRef]
- Čepulytė, R.; Būda, V. Toward Chemical Ecology of Plant-Parasitic Nematodes: Kairomones, Pheromones, and Other Behaviorally Active Chemical Compounds. J. Agric. Food Chem. 2022, 70, 1367–1390. [Google Scholar] [CrossRef]
- Müller, C.; Caspers, B.A.; Gadau, J.; Kaiser, S. The Power of Infochemicals in Mediating Individualized Niches. Trends Ecol. Evol. 2020, 35, 981–989. [Google Scholar] [CrossRef]
- Fox, E.; Adams, R. On the Biological Diversity of Ant Alkaloids. Annu. Rev. Entomol. 2022, 67, 367–385. [Google Scholar] [CrossRef]
- Budzałek, G.; Śliwińska-Wilczewska, S.; Wiśniewska, K.; Wochna, A.; Bubak, I.; Latała, A.; Wiktor, J.M. Macroalgal Defense against Competitors and Herbivores. Int. J. Mol. Sci. 2021, 22, 7865. [Google Scholar] [CrossRef]
- McNab, J.M.; Rodríguez, J.; Karuso, P.; Williamson, J.E. Natural Products in Polyclad Flatworms. Mar. Drugs 2021, 19, 47. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Qasim, M.; Haider, I.; Lou, Y. Plant-insect-microbe interaction: A love triangle between enemies in ecosystem. Sci. Total Environ. 2020, 699, 134181. [Google Scholar] [CrossRef]
- Weiss, L.C. Sensory Ecology of Predator-Induced Phenotypic Plasticity. Front. Behav. Neurosci. 2019, 12, 330. [Google Scholar] [CrossRef]
- Zhang, P.; He, E.; Romero-Freire, A.; Xia, B.; Ying, R.; Liu, Y.; Qiu, H. Plant intelligence in a rapidly changing world: Implementation of plant-plant communications in managed plant systems. Sustain. Horiz. 2022, 2, 100008. [Google Scholar] [CrossRef]
- Carreon-Ortiz, H.; Valdez, F. A new mycorrhized tree optimization nature-inspired algorithm. Soft Comput. 2022, 26, 4797–4817. [Google Scholar] [CrossRef]
- Lovett, L.; Hay, D.; Hudson-Smith, A.; de Jode, M. Mobile Communications Technologies in Tree Time: The Listening Wood. Leonardo 2021, 54, 220–221. [Google Scholar] [CrossRef]
- Steidinger, B.S.; Mukul, S.; Crowther, T.W.; Liang, J.; Van, D.T.; Werner, G.; Reich, P.B.; Nabuurs, G.J.; de Miguel, S.; Zhou, M.; et al. Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 2019, 569, 404–408. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Wolters Kluwer Health: Philadelphia, PA, USA, 2018. [Google Scholar]
- Fournier, B.; Mahlaoui, N.; Moshous, D.; de Villartay, J.P. Inborn errors of immunity caused by defects in the DNA damage response pathways: Importance of minimizing treatment-related genotoxicity. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2022, 33, e13820. [Google Scholar] [CrossRef]
- Rusin, A.; Seymour, C.; Cocchetto, A.; Mothersill, C. Commonalities in the Features of Cancer and Chronic Fatigue Syndrome (CFS): Evidence for Stress-Induced Phenotype Instability? Int. J. Mol. Sci. 2022, 23, 691. [Google Scholar] [CrossRef]
- Shibata, A.; Jeggo, P.A. ATM’s Role in the Repair of DNA Double-Strand Breaks. Genes 2021, 12, 1370. [Google Scholar] [CrossRef]
- Dahal, S.; Raghavan, S.C. Mitochondrial genome stability in human: Understanding the role of DNA repair pathways. Biochem. J. 2021, 478, 1179–1197. [Google Scholar] [CrossRef]
- Jit, B.P.; Pradhan, B.; Dash, R.; Bhuyan, P.P.; Behera, C.; Behera, R.K.; Sharma, A.; Alcaraz, M.; Jena, M. Phytochemicals: Potential Therapeutic Modulators of Radiation Induced Signaling Pathways. Antioxidants 2021, 11, 49. [Google Scholar] [CrossRef]
- Balajee, A.S.; Livingston, G.K.; Escalona, M.B.; Ryan, T.L.; Goans, R.E.; Iddins, C.J. Cytogenetic follow-up studies on humans with internal and external exposure to ionizing radiation. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2021, 41, S578–S601. [Google Scholar] [CrossRef] [PubMed]
- Nagane, M.; Yasui, H.; Kuppusamy, P.; Yamashita, T.; Inanami, O. DNA damage response in vascular endothelial senescence: Implication for radiation-induced cardiovascular diseases. J. Radiat. Res. 2021, 62, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Correia, M.; Dias, A.G.; Pestana, A.; Soares, P.; Nunes, J.; Lima, J.; Máximo, V.; Boaventura, P. Evaluation of the role of mitochondria in the non-targeted effects of ionizing radiation using cybrid cellular models. Sci. Rep. 2020, 10, 6131. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, K.; Miura, T.; Kasai, K.; Fujishima, Y.; Nakata, A.; Yoshida, M. Radiation-Induced Bystander Effect is Mediated by Mitochondrial DNA in Exosome-Like Vesicles. Sci. Rep. 2019, 9, 9103. [Google Scholar] [CrossRef]
- Rusin, A.; Li, M.; Cocchetto, A.; Seymour, C.; Mothersill, C. Radiation exposure and mitochondrial insufficiency in chronic fatigue and immune dysfunction syndrome. Med. Hypotheses 2021, 154, 110647. [Google Scholar] [CrossRef]
- Schirrmacher, V. Less Can Be More: The Hormesis Theory of Stress Adaptation in the Global Biosphere and Its Implications. Biomedicines 2021, 9, 293. [Google Scholar] [CrossRef]
- Wang, J.; Duan, Z.; Nugent, Z.; Zou, J.X.; Borowsky, A.D.; Zhang, Y.; Tepper, C.G.; Li, J.J.; Fiehn, O.; Xu, J.; et al. Reprogramming metabolism by histone methyltransferase NSD2 drives endocrine resistance via coordinated activation of pentose phosphate pathway enzymes. Cancer Lett. 2016, 378, 69–79. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Z.; Xu, S.; Liao, C.; Chen, X.; Li, B.; Peng, J.; Li, D.; Yang, L. Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Cancer Lett. 2020, 478, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Dadar, M.; Pivina, L.; Doşa, M.D.; Semenova, Y.; Maes, M. Environmental, Neuro-immune, and Neuro-oxidative Stress Interactions in Chronic Fatigue Syndrome. Mol. Neurobiol. 2020, 57, 4598–4607. [Google Scholar] [CrossRef]
- Olivieri, G.; Bodycote, J.; Wolff, S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science 1984, 223, 594–597. [Google Scholar] [CrossRef]
- Wolff, S.; Afzal, V.; Wiencke, J.K.; Olivieri, G.; Michaeli, A. Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double-strand breaks in DNA. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1988, 53, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Van Hoeck, A.; Horemans, N.; Nauts, R.; Van Hees, M.; Vandenhove, H.; Blust, R. Lemna minor plants chronically exposed to ionising radiation: RNA-seq analysis indicates a dose rate dependent shift from acclimation to survival strategies. Plant Sci. Int. J. Exp. Plant Biol. 2017, 257, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Cherednichenko, O.; Pilyugina, A.; Nuraliev, S. Chronic human exposure to ionizing radiation: Individual variability of chromosomal aberration frequencies and G0 radiosensitivities. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2022, 873, 503434. [Google Scholar] [CrossRef]
- Audette-Stuart, M.; Kim, S.B.; McMullin, D.; Festarini, A.; Yankovich, T.L.; Carr, J.; Mulpuru, S. Adaptive response in frogs chronically exposed to low doses of ionizing radiation in the environment. J. Environ. Radioact. 2011, 102, 566–573. [Google Scholar] [CrossRef]
- Barescut, J.; Lariviere, D.; Stocki, T.; Audette-Stuart, M.; Yankovich, T. Bystander effects in bullfrog tadpoles. Radioprotection 2011, 46, S497. [Google Scholar]
- Vo, N.; Singh, H.; Stuart, M.; Seymour, C.B.; Mothersill, C.E. A pilot study of radiation-induced bystander effect in radio-adapting frogs at a radiologically contaminated site located on the chalk river laboratories property. Int. J. Radiat. Biol. 2022, 98, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.E.; Oughton, D.H.; Schofield, P.N.; Abend, M.; Adam-Guillermin, C.; Ariyoshi, K.; Beresford, N.A.; Bonisoli-Alquati, A.; Cohen, J.; Dubrova, Y.; et al. From tangled banks to toxic bunnies; a reflection on the issues involved in developing an ecosystem approach for environmental radiation protection. Int. J. Radiat. Biol. 2022, 98, 1185–1200. [Google Scholar] [CrossRef]
- Joiner, M.C.; Lambin, P.; Malaise, E.P.; Robson, T.; Arrand, J.E.; Skov, K.A.; Marples, B. Hypersensitivity to very-low single radiation doses: Its relationship to the adaptive response and induced radioresistance. Mutat. Res. 1996, 358, 171–183. [Google Scholar] [CrossRef]
- Marples, B.; Adomat, H.; Koch, C.J.; Skov, K.A. Response of V79 cells to low doses of X-rays and negative pi-mesons: Clonogenic survival and DNA strand breaks. Int. J. Radiat. Biol. 1996, 70, 429–436. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis: A General Biological Principle. Chem. Res. Toxicol. 2022, 35, 547–549. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Hormesis: Transforming disciplines that rely on the dose response. IUBMB Life 2022, 74, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Key historical study findings questioned in debate over threshold versus linear non-threshold for cancer risk assessment. Chem.-Biol. Interact. 2022, 359, 109917. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, K.; Nair, J.; Shankar, B.S. Differential radio-adaptive responses in BALB/c and C57BL/6 mice: Pivotal role of calcium and nitric oxide signalling. Int. J. Radiat. Biol. 2019, 95, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Li, X.K.; Sakai, K.; Cai, L. Low-dose radiation and its clinical implications: Diabetes. Hum. Exp. Toxicol. 2008, 27, 135–142. [Google Scholar] [CrossRef]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Karar, J.; Cerniglia, G.J.; Lindsten, T.; Koumenis, C.; Maity, A. Dual PI3K/mTOR inhibitor NVP-BEZ235 suppresses hypoxia-inducible factor (HIF)-1α expression by blocking protein translation and increases cell death under hypoxia. Cancer Biol. Ther. 2012, 13, 1102–1111. [Google Scholar] [CrossRef]
- Boice, J.D., Jr.; Cohen, S.S.; Mumma, M.T.; Ellis, E.D. The Million Person Study, whence it came and why. Int. J. Radiat. Biol. 2022, 98, 537–550. [Google Scholar] [CrossRef]
- Hiller, M.; Woda, C.; Degteva, M.; Bugrov, N.; Shishkina, E.; Pryakhin, E.; Ivanov, O. External dose reconstruction at the shore of the Metlinsky Pond in the former village of Metlino (Techa River, Russia) based on environmental surveys, luminescence measurements and radiation transport modelling. Radiat. Environ. Biophys. 2022, 61, 87–109. [Google Scholar] [CrossRef]
- Brooks, A.L.; Hoel, D.; Glines, W.M. Radiobiology of Select Radionuclides in Hanford Site Tank Waste. Health Phys. 2022, 123, 99–115. [Google Scholar] [CrossRef]
- Little, M.P.; Brenner, A.V.; Grant, E.J.; Sugiyama, H.; Preston, D.L.; Sakata, R.; Cologne, J.; Velazquez-Kronen, R.; Utada, M.; Mabuchi, K.; et al. Age effects on radiation response: Summary of a recent symposium and future perspectives. Int. J. Radiat. Biol. 2022, 1–11, Advance online publication. [Google Scholar] [CrossRef]
- Lyon, M.F.; Phillips, R.J.; Fisher, G. Dose-response curves for radiation-induced gene mutations in mouse oocytes and their interpretation. Mutat. Res. 1979, 63, 161–173. [Google Scholar] [CrossRef]
- Fisher, D.R.; Weller, R.E. Carcinogenesis from inhaled (239)PuO(2) in beagles: Evidence for radiation homeostasis at low doses? Health Phys. 2010, 99, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Schofield, P.N.; Kulka, U.; Tapio, S.; Grosche, B. Big data in radiation biology and epidemiology; an overview of the historical and contemporary landscape of data and biomaterial archives. Int. J. Radiat. Biol. 2019, 95, 861–878. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, A. Reflections on effects of low doses and risk inference based on the UNSCEAR 2021 report on ‘biological mechanisms relevant for the inference of cancer risks from low-dose and low-dose-rate radiation’. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2022, 42, 023501. [Google Scholar] [CrossRef] [PubMed]
- Busby, C. Ionizing radiation and cancer: The failure of the risk model. Cancer Treat. Res. Commun. 2022, 31, 100565. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Selby, P.B.; Giordano, J. Ethical challenges of the linear non-threshold (LNT) cancer risk assessment revolution: History, insights, and lessons to be learned. Sci. Total Environ. 2022, 832, 155054. [Google Scholar] [CrossRef]
- Beyea, J. Lessons to be learned from a contentious challenge to mainstream radiobiological science (the linear no-threshold theory of genetic mutations). Environ. Res. 2017, 154, 362–379. [Google Scholar] [CrossRef]
- Kugathasan, T.; Mothersill, C. Radiobiological and social considerations following a radiological terrorist attack; mechanisms, detection and mitigation: Review of new research developments. Int. J. Radiat. Biol. 2022, 98, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Charrasse, B.; Mora, J.C.; Anderson, T.; Bonchuk, Y.; Telleria, D. Bounding uncertainties around the conceptual representation of species in radiological assessment in the context of routine atmospheric release. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2022, 42, 020506. [Google Scholar] [CrossRef]
- Rühm, W.; Laurier, D.; Wakeford, R. Cancer risk following low doses of ionising radiation—Current epidemiological evidence and implications for radiological protection. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2022, 873, 503436. [Google Scholar] [CrossRef]
- Beaumelle, L.; Della Vedova, C.; Beaugelin-Seiller, K.; Garnier-Laplace, J.; Gilbin, R. Ecological risk assessment of mixtures of radiological and chemical stressors: Methodology to implement an msPAF approach. Environ. Pollut. (Barking 2017 Essex 1987) 2017, 231 Pt 2, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Garnier-Laplace, J.; Geras’kin, S.; Della-Vedova, C.; Beaugelin-Seiller, K.; Hinton, T.G.; Real, A.; Oudalova, A. Are radiosensitivity data derived from natural field conditions consistent with data from controlled exposures? A case study of Chernobyl wildlife chronically exposed to low dose rates. J. Environ. Radioact. 2013, 121, 12–21. [Google Scholar] [PubMed]
- Shakhtarin, V.V.; Tsyb, A.F.; Stepanenko, V.F.; Orlov, M.Y.; Kopecky, K.J.; Davis, S. Iodine deficiency, radiation dose, and the risk of thyroid cancer among children and adolescents in the Bryansk region of Russia following the Chernobyl power station accident. Int. J. Epidemiol. 2003, 32, 584–591. [Google Scholar] [CrossRef]
- Cardis, E.; Kesminiene, A.; Ivanov, V.; Malakhova, I.; Shibata, Y.; Khrouch, V.; Drozdovitch, V.; Maceika, E.; Zvonova, I.; Vlassov, O.; et al. Risk of thyroid cancer after exposure to 131I in childhood. J. Natl. Cancer Inst. 2005, 97, 724–732. [Google Scholar] [CrossRef]
- Linkov, I.; Morel, B.; Schell, W.R. Remedial policies in radiologically-contaminated forests: Environmental consequences and risk assessment. Risk Anal. Off. Publ. Soc. Risk Anal. 1997, 17, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, E.K. Commentary: Breast cancer risk among women exposed to fallout after the Chernobyl accident. Int. J. Epidemiol. 2020, 49, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Zupunski, L.; Yaumenenka, A.; Ryzhov, A.; Veyalkin, I.; Drozdovitch, V.; Masiuk, S.; Ivanova, O.; Kesminiene, A.; Pukkala, E.; Moiseev, P.; et al. Breast cancer incidence in the regions of Belarus and Ukraine most contaminated by the Chernobyl accident: 1978 to 2016. Int. J. Cancer 2021, 148, 1839–1849. [Google Scholar] [CrossRef]
- Ilienko, I.M.; Bazyka, D.A.; Golyarnyk, N.A.; Zvarych, L.M.; Shvayko, L.I.; Bazyka, K.D. Changes in gene expression associated with non-cancer effects of the chornobyl clean-up workers in the remote period after exposure. Zminy gennoï ekspresiï, asotsiĭovani z nepukhlynnymy efektamy viddalenogo periodu pislia oprominennia v uchasnykiv ikvidatsiï naslidkiv avariï na chaes. Probl. Radiatsiinoi Medytsyny Ta Radiobiolohii 2020, 25, 456–477. [Google Scholar]
- Boice, J.D., Jr. The linear nonthreshold (LNT) model as used in radiation protection: An NCRP update. Int. J. Radiat. Biol. 2017, 93, 1079–1092. [Google Scholar] [CrossRef]
- Møller, A.P.; Mousseau, T.A. Strong effects of ionizing radiation from Chernobyl on mutation rates. Sci. Rep. 2015, 5, 8363. [Google Scholar] [CrossRef] [Green Version]
- Møller, A.P.; Mousseau, T.A. Biological consequences of Chernobyl: 20 years on. Trends Ecol. Evol. 2006, 21, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Taira, W.; Hiyama, A.; Nohara, C.; Sakauchi, K.; Otaki, J.M. Ingestional and transgenerational effects of the Fukushima nuclear accident on the pale grass blue butterfly. J. Radiat. Res. 2015, 56 (Suppl. 1), i2–i18. [Google Scholar] [CrossRef]
- Bréchignac, F.; Oughton, D.; Mays, C.; Barnthouse, L.; Beasley, J.C.; Bonisoli-Alquati, A.; Bradshaw, C.; Brown, J.; Dray, S.; Geras’Kin, S.; et al. Addressing ecological effects of radiation on populations and ecosystems to improve protection of the environment against radiation: Agreed statements from a Consensus Symposium. J. Environ. Radioact. 2016, 158–159, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Cardis, E.; Vrijheid, M.; Blettner, M.; Gilbert, E.; Hakama, M.; Hill, C.; Howe, G.; Kaldor, J.; Muirhead, C.R.; Schubauer-Berigan, M.; et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: Estimates of radiation-related cancer risks. Radiat. Res. 2007, 167, 396–416. [Google Scholar] [CrossRef]
- Ashmore, J.P.; Gentner, N.E.; Osborne, R.V. Incomplete data on the Canadian cohort may have affected the results of the study by the International Agency for Research on Cancer on the radiogenic cancer risk among nuclear industry workers in 15 countries. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2010, 30, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Boice, J.D.; Cohen, S.S.; Mumma, M.T.; Chen, H.; Golden, A.P.; Beck, H.L.; Till, J.E. Mortality among U.S. military participants at eight aboveground nuclear weapons test series. Int. J. Radiat. Biol. 2022, 98, 679–700. [Google Scholar] [CrossRef]
- Roff, S.R. Establishing the possible radiogenicity of morbidity and mortality from participation in UK nuclear weapons development. Med. Confl. Surviv. 2004, 20, 218–241. [Google Scholar] [CrossRef]
- Shi, H.M.; Sun, Z.C.; Ju, F.H. Understanding the harm of low-dose computed tomography radiation to the body (Review). Exp. Ther. Med. 2022, 24, 534. [Google Scholar] [CrossRef]
- Francone, M.; Gimelli, A.; Budde, R.P.J.; Caro-Dominguez, P.; Einstein, A.J.; Gutberlet, M.; Maurovich-Horvat, P.; Miller, O.; Nagy, E.; Natale, L.; et al. Radiation safety for cardiovascular computed tomography imaging in paediatric cardiology: A joint expert consensus document of the EACVI, ESCR, AEPC, and ESPR. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e279–e289. [Google Scholar] [CrossRef]
- Frush, D.P.; Donnelly, L.F.; Rosen, N.S. Computed tomography and radiation risks: What pediatric health care providers should know. Pediatrics 2003, 112, 951–957. [Google Scholar] [CrossRef]

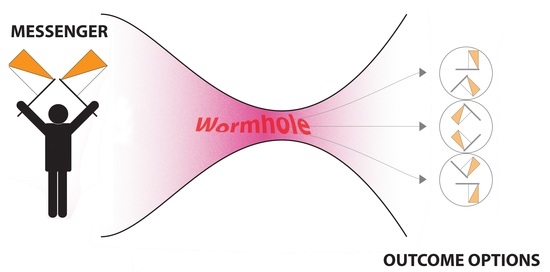
| Direct Effects |
|---|
| Adaptive response: A low “priming” dose of radiation induces protection against a later higher dose. |
| HRS/IRR: Hyper-radiosensitivity after low dose exposure is lost as the dose increases and a region of induced radioresistence is seen at higher doses |
| Hormesis: A beneficial effect of low dose exposure is seen compared to unirradiated controls. |
| Non-Targeted Effects |
| Bystander effect: An effect detected in non-exposed cells which received signals from irradiated cells. Can also apply to tissues and organisms. |
| Genomic instability: Detection of chromosomal or other DNA damage in progeny of irradiated cells which was not present in the first post-irradiation mitosis. |
| Lethal Mutations: A form of genomic instability leading to a permanently reduced plating efficiency in progeny cell lineages which survived irradiation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mothersill, C.; Cocchetto, A.; Seymour, C. Low Dose and Non-Targeted Radiation Effects in Environmental Protection and Medicine—A New Model Focusing on Electromagnetic Signaling. Int. J. Mol. Sci. 2022, 23, 11118. https://doi.org/10.3390/ijms231911118
Mothersill C, Cocchetto A, Seymour C. Low Dose and Non-Targeted Radiation Effects in Environmental Protection and Medicine—A New Model Focusing on Electromagnetic Signaling. International Journal of Molecular Sciences. 2022; 23(19):11118. https://doi.org/10.3390/ijms231911118
Chicago/Turabian StyleMothersill, Carmel, Alan Cocchetto, and Colin Seymour. 2022. "Low Dose and Non-Targeted Radiation Effects in Environmental Protection and Medicine—A New Model Focusing on Electromagnetic Signaling" International Journal of Molecular Sciences 23, no. 19: 11118. https://doi.org/10.3390/ijms231911118