Molecular Identification of Pro-Excitogenic Receptor and Channel Phenotypes of the Deafferented Lumbar Motoneurons in the Early Phase after SCT in Rats
Abstract
:1. Introduction
2. Results
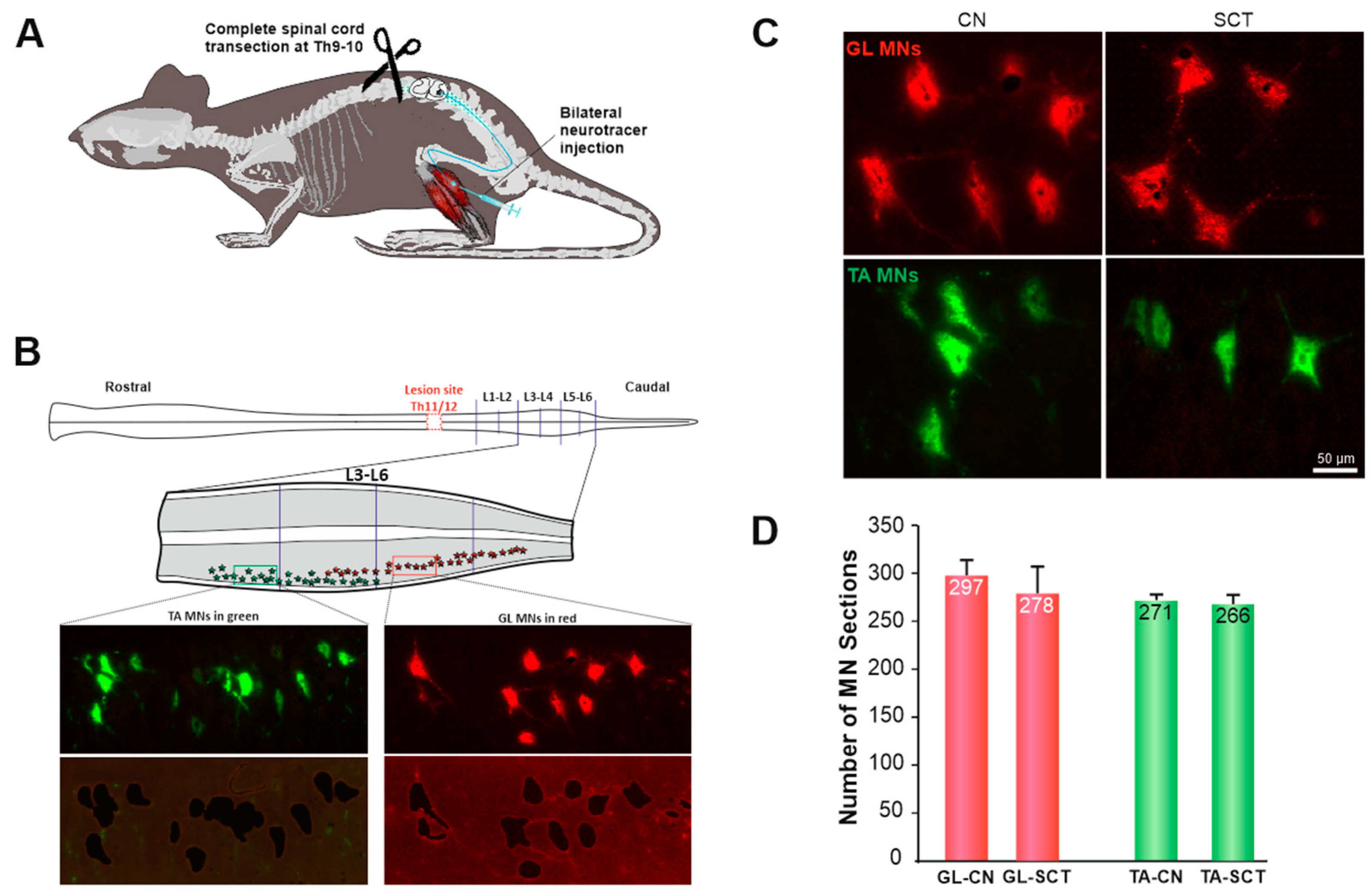
2.1. MNs at Lumbar 3–6 Segments Show Normal Morphology after SCT at Low Thoracic Level
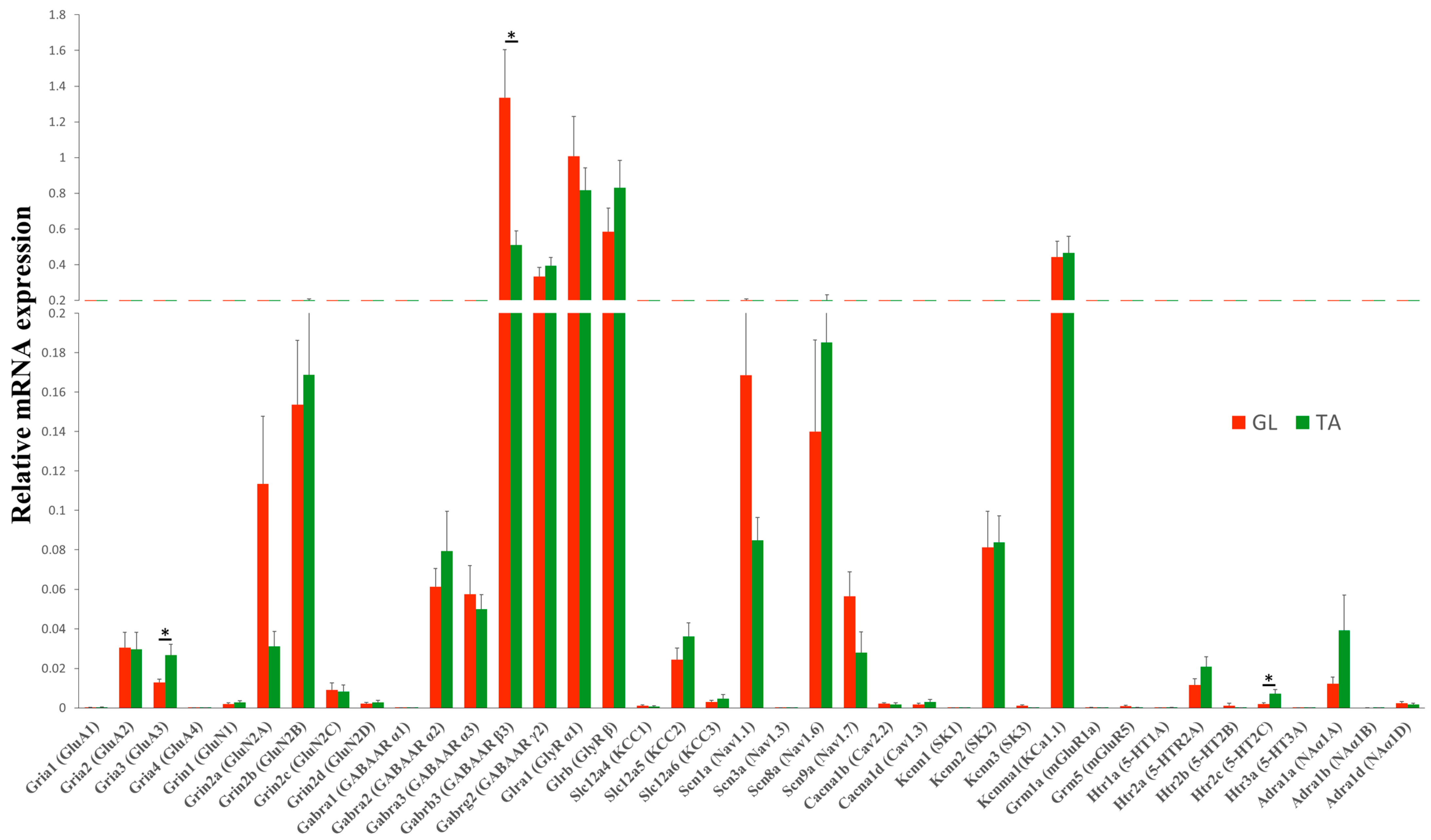
2.2. Steady-State mRNA Abundance of Receptor and Ion Channel Subunits in GL and TA MNs of the Intact Rats
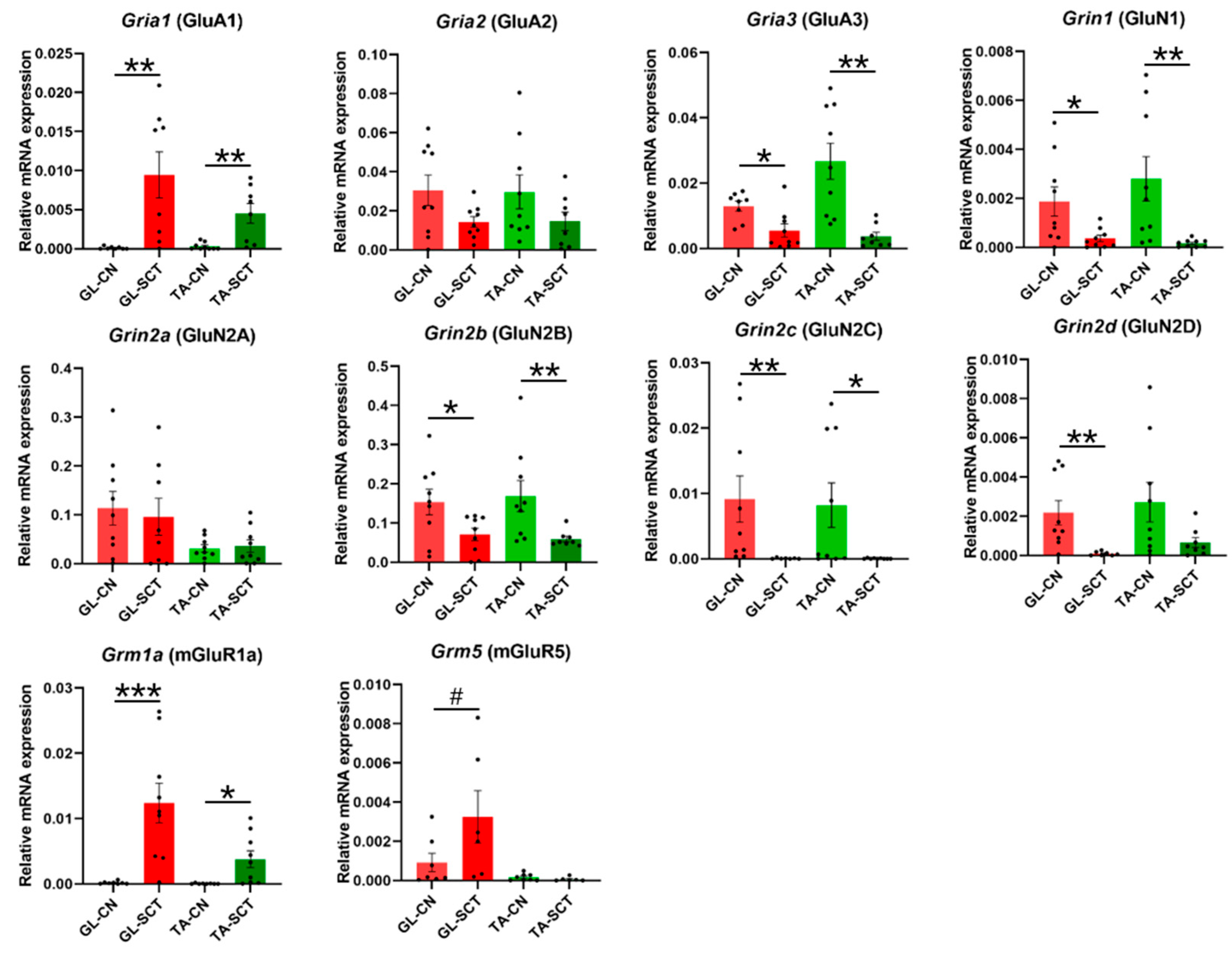
2.3. SCT Differentially Alters Transcript Level of Subunits of Glutamatergic AMPA, NMDA, and mGlu Receptors
2.4. SCT Downregulates Transcript Level of GABAergic and Glycinergic Receptor Subunits and KCC Transporters Similar to in GL and TA MNs
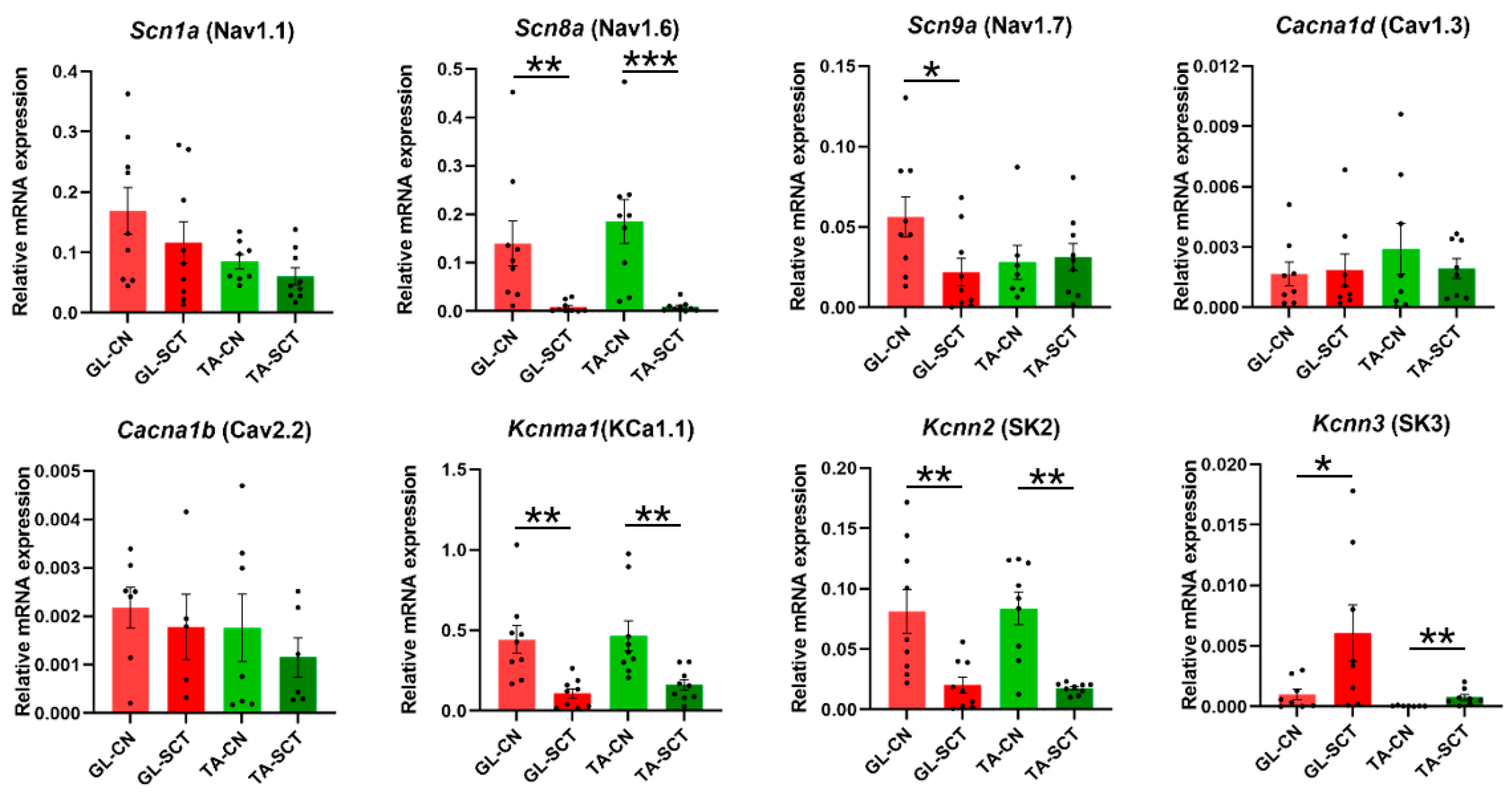
2.5. SCT Downregulates Transcript Level of Nav1.6, KCa1.1(BK), and SK2 Channels Similar to in GL and TA MNs
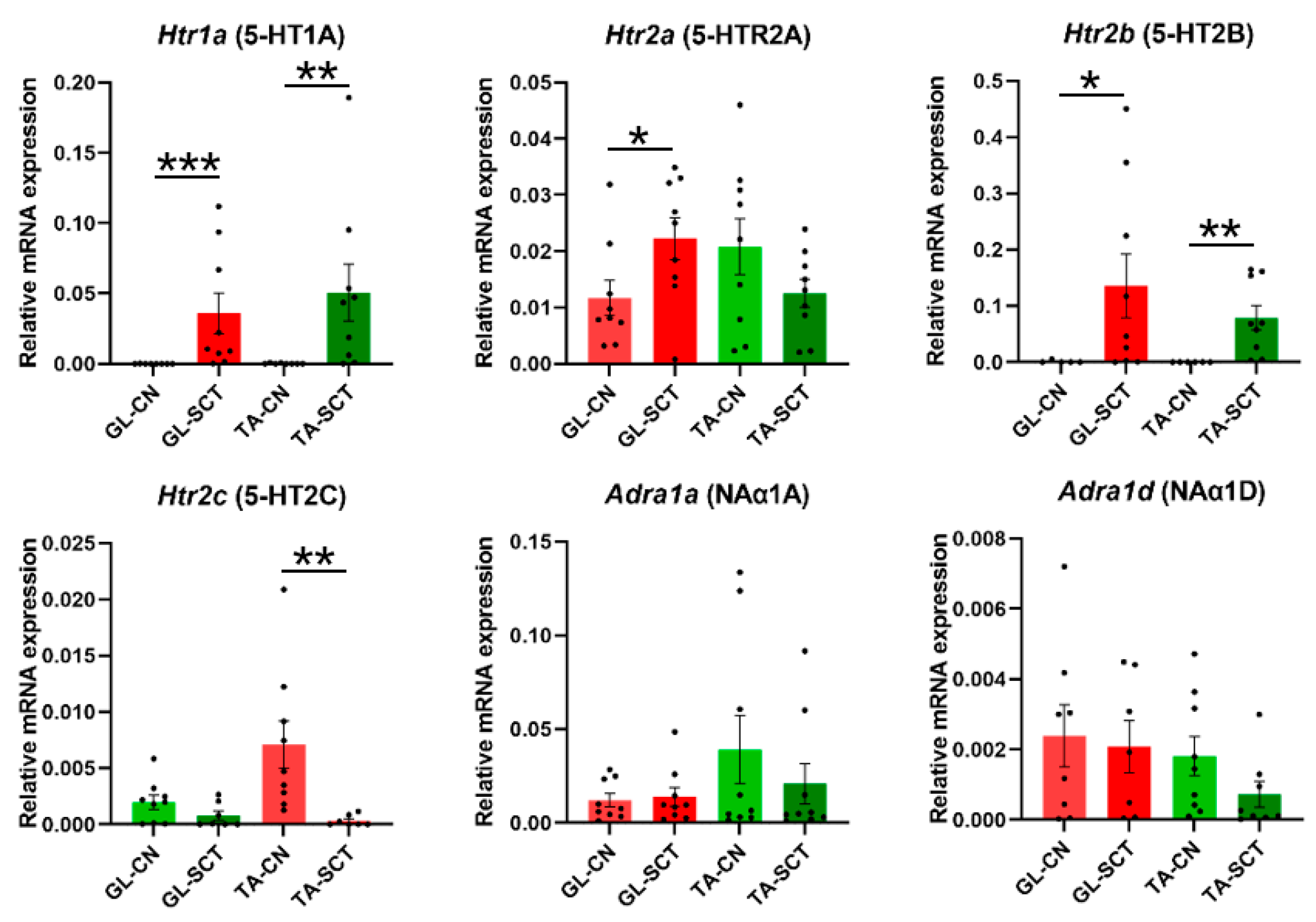
2.6. SCT Alters Transcript Levels of 5-HT Receptors but Not NA Receptors
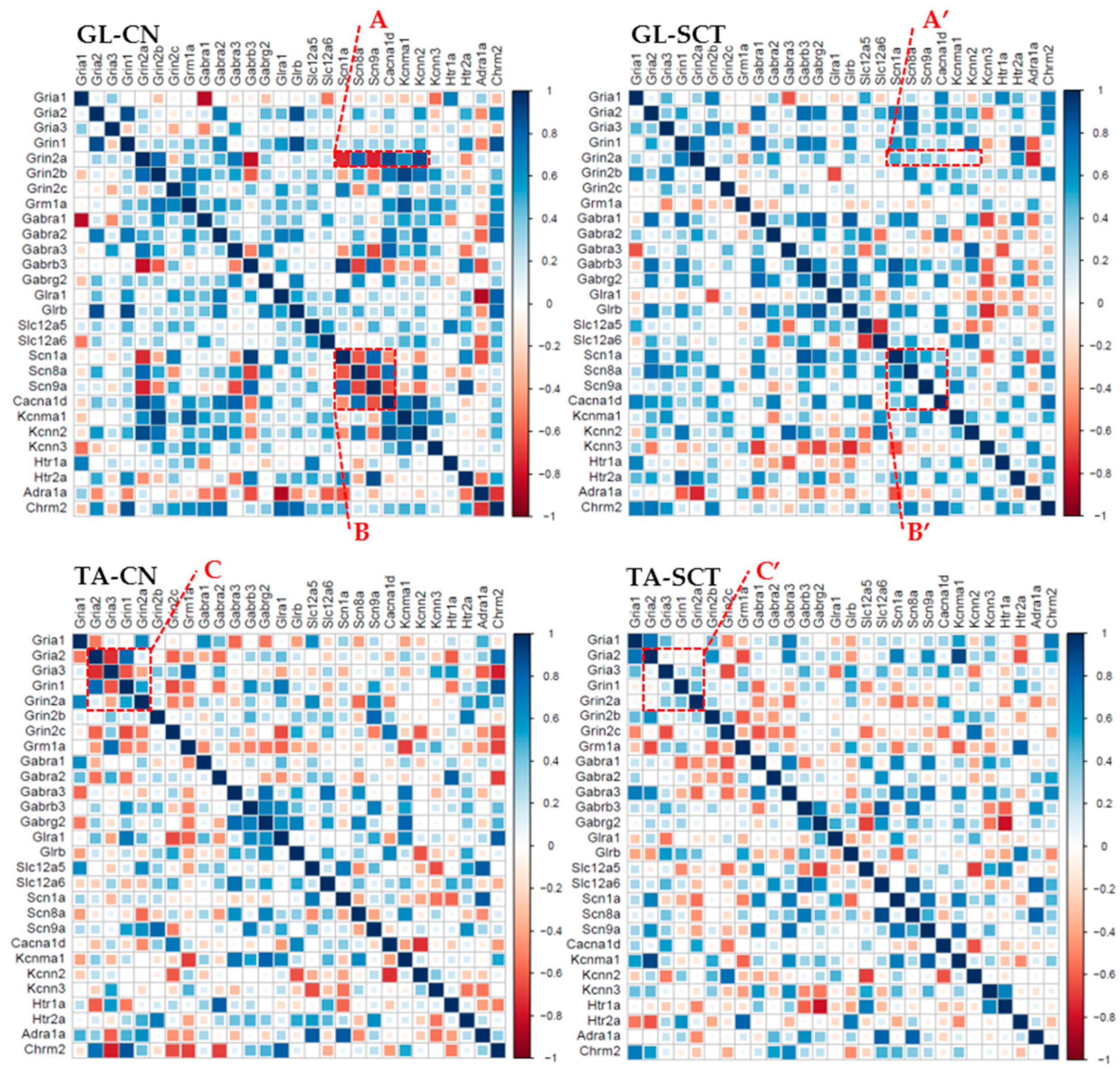
2.7. Correlation Analyses of Gene Expression in GL and TA MNs after SCT
3. Discussion
3.1. Functional Implications of Early Changes in Gene Expression of Glutamatergic Receptors after SCT
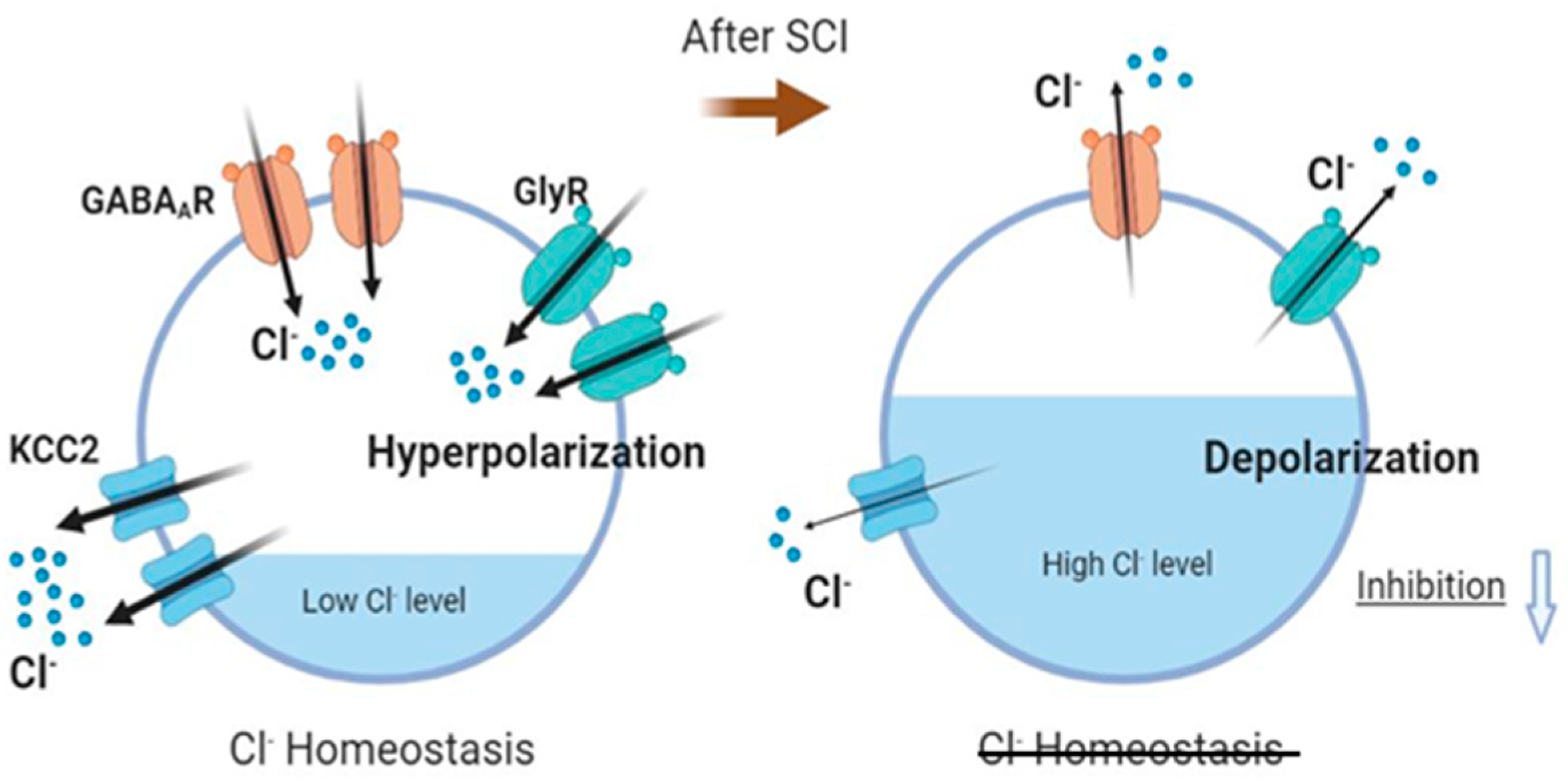
3.2. Functional Implications of Downregulation of Gene Expression of GABAergic and Glycinergic Receptors, KCC2 and ion Channels after SCT
3.3. SCT Affects Gene Expression of Ion Channels and Modulatory Receptors
4. Materials and Methods
4.1. Animals
4.2. Retrograde labeling of MNs
4.3. Complete Spinal Cord Transection
4.4. Tissue Preparation and Laser Microdissection (LMD)
4.5. RNA Isolation and qPCR
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Levasseur, A.; Mac-Thiong, J.M.; Richard-Denis, A. Are early clinical manifestations of spasticity associated with long-term functional outcome following spinal cord injury? A retrospective study. Spinal Cord 2021, 59, 910–916. [Google Scholar] [CrossRef]
- Richard-Denis, A.; Nguyen, B.H.; Mac-Thiong, J.M. The impact of early spasticity on the intensive functional rehabilitation phase and community reintegration following traumatic spinal cord injury. J. Spinal Cord Med. 2020, 43, 435–443. [Google Scholar] [CrossRef]
- Mayer, N.H. Clinicophysiologic concepts of spasticity and motor dysfunction in adults with an upper motoneuron lesion. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 1997, 6, S1–S13. [Google Scholar] [CrossRef]
- Wienecke, J.; Westerdahl, A.C.; Hultborn, H.; Kiehn, O.; Ryge, J. Global gene expression analysis of rodent motor neurons following spinal cord injury associates molecular mechanisms with development of postinjury spasticity. J. Neurophysiol. 2010, 103, 761–778. [Google Scholar] [CrossRef]
- Benson, C.A.; Olson, K.-L.; Patwa, S.; Reimer, M.L.; Bangalore, L.; Hill, M.; Waxman, S.G.; Tan, A.M. Conditional RAC1 knockout in motor neurons restores H-reflex rate-dependent depression after spinal cord injury. Sci. Rep. 2021, 11, 7838. [Google Scholar] [CrossRef]
- Sharif-Alhoseini, M.; Khormali, M.; Rezaei, M.; Safdarian, M.; Hajighadery, A.; Khalatbari, M.M.; Safdarian, M.; Meknatkhah, S.; Rezvan, M.; Chalangari, M.; et al. Animal models of spinal cord injury: A systematic review. Spinal Cord 2017, 55, 714–721. [Google Scholar] [CrossRef]
- Sadlaoud, K.; Khalki, L.; Brocard, F.; Vinay, L.; Boulenguez, P.; Bras, H. Alteration of glycinergic receptor expression in lumbar spinal motoneurons is involved in the mechanisms underlying spasticity after spinal cord injury. J. Chem. Neuroanat. 2020, 106, 101787. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Tsai, J.Y.; Wu, Y.N.; Hwang, I.S.; Chen, T.I.; Chen, J.J. Time course quantification of spastic hypertonia following spinal hemisection in rats. Neuroscience 2010, 167, 185–198. [Google Scholar] [CrossRef]
- Loy, K.; Bareyre, F.M. Rehabilitation following spinal cord injury: How animal models can help our understanding of exercise-induced neuroplasticity. Neural Regen. Res. 2019, 14, 405–412. [Google Scholar]
- Filipp, M.E.; Travis, B.J.; Henry, S.S.; Idzikowski, E.C.; Magnuson, S.A.; Loh, M.Y.; Hellenbrand, D.J.; Hanna, A.S. Differences in neuroplasticity after spinal cord injury in varying animal models and humans. Neural Regen. Res. 2019, 14, 7–19. [Google Scholar] [PubMed]
- D’Amico, J.M.; Condliffe, E.G.; Martins, K.J.B.; Bennett, D.J.; Gorassini, M.A. Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity. Front. Integr. Neurosci. 2014, 8, 36. [Google Scholar] [CrossRef]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. Management of spasticity after spinal cord injury: Current techniques and future directions. Neurorehabil. Neural Repair. 2010, 2, 23–33. [Google Scholar] [CrossRef]
- Bandaru, S.P.; Liu, S.; Waxman, S.G.; Tan, A.M. Dendritic spine dysgenesis contributes to hyperreflexia after spinal cord injury. J. Neurophysiol. 2015, 113, 1598–1615. [Google Scholar] [CrossRef]
- Rekling, J.C.; Funk, G.D.; Bayliss, D.A.; Dong, X.W.; Feldman, J.L. Synaptic control of motoneuronal excitability. Physiol. Rev. 2000, 80, 767–852. [Google Scholar] [CrossRef]
- Leis, A.A.; Kronenberg, M.F.; Stĕtkárová, I.; Paske, W.C.; Stokić, D.S. Spinal motoneuron excitability after acute spinal cord injury in humans. Neurology 1996, 47, 231–237. [Google Scholar] [CrossRef]
- Hiersemenzel, L.P.; Curt, A.; Dietz, V. From spinal shock to spasticity: Neuronal adaptations to a spinal cord injury. Neurology 2000, 54, 1574–1582. [Google Scholar] [CrossRef]
- Li, Y.; Gorassini, M.A.; Bennett, D.J. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J. Neurophysiol. 2004, 91, 767–783. [Google Scholar] [CrossRef]
- Ryge, J.; Winther, O.; Wienecke, J.; Sandelin, A.; Westerdahl, A.C.; Hultborn, H.; Kiehn, O. Transcriptional regulation of gene expression clusters in motor neurons following spinal cord injury. BMC Genom. 2010, 11, 365. [Google Scholar] [CrossRef]
- Garcia, V.B.; Abbinanti, M.D.; Harris-Warrick, R.M.; Schulz, D.J. Effects of Chronic Spinal Cord Injury on Relationships among Ion Channel and Receptor mRNAs in Mouse Lumbar Spinal Cord. Neuroscience 2018, 393, 42–60. [Google Scholar] [CrossRef]
- Murray, K.C.; Nakae, A.; Stephens, M.J.; Rank, M.; D’Amico, J.; Harvey, P.J.; Li, X.; Harris, R.L.; Ballou, E.W.; Anelli, R.; et al. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat. Med. 2010, 16, 694–700. [Google Scholar] [CrossRef] [Green Version]
- Murray, K.C.; Stephens, M.J.; Ballou, E.W.; Heckman, C.J.; Bennett, D.J. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J. Neurophysiol. 2011, 105, 731–748. [Google Scholar] [CrossRef]
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010, 16, 302–307. [Google Scholar] [CrossRef]
- Gazula, V.R.; Roberts, M.; Luzzio, C.; Jawad, A.F.; Kalb, R.G. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J. Comp. Neurol. 2004, 476, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Kalb, R.G. Regulation of motor neuron dendrite growth by NMDA receptor activation. Development 1994, 120, 3063–3071. [Google Scholar] [CrossRef]
- Inglis, F.M.; Crockett, R.; Korada, S.; Abraham, W.C.; Hollmann, M.; Kalb, R.G. The AMPA receptor subunit GluR1 regulates dendritic architecture of motor neurons. J. Neurosci. 2002, 22, 8042–8051. [Google Scholar] [CrossRef] [PubMed]
- Grycz, K.; Glowacka, A.; Ji, B.; Czarkowska-Bauch, J.; Gajewska-Wozniak, O.; Skup, M. Early pre- and postsynaptic decrease in glutamatergic and cholinergic signaling after spinalization is not modified when stimulating proprioceptive input to the ankle extensor alpha-motoneurons: Anatomical and neurochemical study. PLoS ONE 2019, 14, e0222849. [Google Scholar]
- Giroux, N.; Rossignol, S.; Reader, T.A. Autoradiographic study of α1- and α2-noradrenergic and serotonin1A receptors in the spinal cord of normal and chronically transected cats. J. Comp. Neurol. 1999, 406, 402–414. [Google Scholar] [CrossRef]
- Lee, J.K.; Johnson, C.S.; Wrathall, J.R. Up-regulation of 5-HT2 receptors is involved in the increased H-reflex amplitude after contusive spinal cord injury. Exp. Neurol. 2007, 203, 502–511. [Google Scholar] [CrossRef]
- Heckmann, C.J.; Gorassini, M.A.; Bennett, D.J. Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle Nerve 2005, 31, 135–156. [Google Scholar] [CrossRef]
- Kong, X.Y.; Wienecke, J.; Hultborn, H.; Zhang, M. Robust upregulation of serotonin 2A receptors after chronic spinal transection of rats: An immunohistochemical study. Brain Res. 2010, 1320, 60–68. [Google Scholar] [CrossRef]
- Rank, M.M.; Murray, K.C.; Stephens, M.J.; D’Amico, J.; Gorassini, M.A.; Bennett, D.J. Adrenergic receptors modulate motoneuron excitability, sensory synaptic transmission and muscle spasms after chronic spinal cord injury. J. Neurophysiol. 2011, 105, 410–422. [Google Scholar] [CrossRef] [Green Version]
- Hultborn, H.; Brownstone, R.B.; Toth, T.I.; Gossard, J.-P. Key mechanisms for setting the input–output gain across the motoneuron pool. Prog Brain Res. 2004, 143, 75–95. [Google Scholar]
- Chopek, J.W.; Sheppard, P.C.; Gardiner, K.; Gardiner, P.F. Serotonin receptor and KCC2 gene expression in lumbar flexor and extensor motoneurons posttransection with and without passive cycling. J. Neurophysiol. 2015, 113, 1369–1376. [Google Scholar] [CrossRef]
- Bras, H.; Liabeuf, S. Differential effects of spinal cord transection on glycinergic and GABAergic synaptic signaling in sub-lesional lumbar motoneurons. J. Chem. Neuroanat. 2021, 113, 101847. [Google Scholar] [CrossRef]
- Liabeuf, S.; Stuhl-Gourmand, L.; Gackiere, F.; Mancuso, R.; Sanchez Brualla, I.; Marino, P.; Brocard, F.; Vinay, L. Prochlorperazine Increases KCC2 Function and Reduces Spasticity after Spinal Cord Injury. J. Neurotrauma 2017, 34, 3397–3406. [Google Scholar] [CrossRef]
- Skup, M.; Gajewska-Wozniak, O.; Grygielewicz, P.; Mankovskaya, T.; Czarkowska-Bauch, J. Different effects of spinalization and locomotor training of spinal animals on cholinergic innervation of the soleus and tibialis anterior motoneurons. Eur. J. Neurosci. 2012, 36, 2679–2688. [Google Scholar] [CrossRef]
- Macias, M.; Nowicka, D.; Czupryn, A.; Sulejczak, D.; Skup, M.; Skangiel-Kramska, J.; Czarkowska-Bauch, J. Exercise-induced motor improvement after complete spinal cord transection and its relation to expression of brain-derived neurotrophic factor and presynaptic markers. BMC Neurosci. 2009, 10, 144. [Google Scholar] [CrossRef]
- Gajewska-Wozniak, O. Reduction in cholinergic and glutamatergic innervation of ankle extensor but not flexor motoneurons after spinalization calls for selective therapies. Acta Neurobiol. Exp. 2017, 77, 26. [Google Scholar]
- Mohan, R.; Tosolini, A.P.; Morris, R. Segmental distribution of the motor neuron columns that supply the rat hindlimb: A muscle/motor neuron tract-tracing analysis targeting the motor end plates. Neuroscience 2015, 307, 98–108. [Google Scholar] [CrossRef]
- Nicolopoulos-Stournaras, S.; Iles, J.F. Motor neuron columns in the lumbar spinal cord of the rat. J. Comp. Neurol. 1983, 217, 75–85. [Google Scholar] [CrossRef]
- Jacob, J.M. Lumbar motor neuron size and number is affected by age in male F344 rats. Mech. Ageing Dev. 1998, 106, 205–216. [Google Scholar] [CrossRef]
- Ling, X.; Bao, F.; Qian, H.; Liu, D. The temporal and spatial profiles of cell loss following experimental spinal cord injury: Effect of antioxidant therapy on cell death and functional recovery. BMC Neurosci. 2013, 14, 146. [Google Scholar] [CrossRef]
- Hassannejad, Z.; Zadegan, S.A.; Shakouri-Motlagh, A.; Mokhatab, M.; Rezvan, M.; Sharif-Alhoseini, M.; Shokraneh, F.; Moshayedi, P.; Rahimi-Movaghar, V. The fate of neurons after traumatic spinal cord injury in rats: A systematic review. Iran. J. Basic Med. Sci. 2018, 21, 546–557. [Google Scholar]
- Byrnes, K.R.; Stoica, B.A.; Fricke, S.; Di Giovanni, S.; Faden, A.I. Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain 2007, 130, 2977–2992. [Google Scholar] [CrossRef] [Green Version]
- Li, G.L.; Brodin, G.; Farooque, M.; Funa, K.; Holtz, A.; Wang, W.L.; Olsson, Y. Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J. Neuropathol. Exp. Neurol. 1996, 55, 280–289. [Google Scholar] [CrossRef]
- Diering, G.H.; Huganir, R.L. The AMPA Receptor Code of Synaptic Plasticity. Neuron 2018, 100, 314–329. [Google Scholar] [CrossRef]
- Man, H.Y. GluA2-lacking, calcium-permeable AMPA receptors—Inducers of plasticity? Curr. Opin. Neurobiol. 2011, 21, 291–298. [Google Scholar] [CrossRef]
- Kohr, G. NMDA receptor function: Subunit composition versus spatial distribution. Cell Tissue Res. 2006, 326, 439–446. [Google Scholar] [CrossRef]
- Berthele, A.; Boxall, S.J.; Urban, A.; Anneser, J.M.; Zieglgänsberger, W.; Urban, L.; Tölle, T.R. Distribution and developmental changes in metabotropic glutamate receptor messenger RNA expression in the rat lumbar spinal cord. Brain Res. Dev. Brain Res. 1999, 112, 39–53. [Google Scholar] [CrossRef]
- Stocker, M. Ca(2+)-activated K+ channels: Molecular determinants and function of the SK family. Nat. Rev. Neurosci. 2004, 5, 758–770. [Google Scholar] [CrossRef]
- Fukuoka, T.; Kobayashi, K.; Noguchi, K. Laminae-specific distribution of alpha-subunits of voltage-gated sodium channels in the adult rat spinal cord. Neuroscience 2010, 169, 994–1006. [Google Scholar] [CrossRef]
- Deardorff, A.S.; Romer, S.H.; Deng, Z.; Bullinger, K.L.; Nardelli, P.; Cope, T.C.; Fyffe, R.E.W. Expression of postsynaptic Ca2+-activated K+(SK) channels at C-bouton synapses in mammalian lumbar α-motoneurons. J. Physiol. 2013, 591, 875–897. [Google Scholar] [CrossRef] [PubMed]
- Heckman, C.J.; Mottram, C.; Quinlan, K.; Theiss, R.; Schuster, J. Motoneuron excitability: The importance of neuromodulatory inputs. Clin. Neurophysiol. 2009, 120, 2040–2054. [Google Scholar] [CrossRef]
- Ziemlińska, E.; Kügler, S.; Schachner, M.; Wewiór, I.; Czarkowska-Bauch, J.; Skup, M. Overexpression of BDNF Increases Excitability of the Lumbar Spinal Network and Leads to Robust Early Locomotor Recovery in Completely Spinalized Rats. PLoS ONE 2014, 9, e88833. [Google Scholar] [CrossRef] [Green Version]
- Cotel, F.; Antri, M.; Barthe, J.Y.; Orsal, D. Identified ankle extensor and flexor motoneurons display different firing profiles in the neonatal rat. J. Neurosci. 2009, 29, 2748–2753. [Google Scholar] [CrossRef]
- Kim, E.H.; Wilson, J.M.; Thompson, C.K.; Heckman, C.J. Differences in estimated persistent inward currents between ankle flexors and extensors in humans. J. Neurophysiol. 2020, 124, 525–535. [Google Scholar] [CrossRef]
- Hollmann, M.; Heinemann, S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994, 17, 31–108. [Google Scholar] [CrossRef]
- Shi, S.; Hayashi, Y.; Esteban, J.A.; Malinow, R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 2001, 105, 331–343. [Google Scholar] [CrossRef]
- Malinow, R. AMPA receptor trafficking and long-term potentiation. Philos. Trans. R Soc. Lond. B Biol. Sci. 2003, 358, 707–714. [Google Scholar] [CrossRef]
- Barry, M.F.; Ziff, E.B. Receptor trafficking and the plasticity of excitatory synapses. Curr. Opin. Neurobiol. 2002, 12, 279–286. [Google Scholar] [CrossRef]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar]
- Ozawa, S.; Kamiya, H.; Tsuzuki, K. Glutamate receptors in the mammalian central nervous system. Prog. Neurobiol. 1998, 54, 581–618. [Google Scholar] [CrossRef]
- Sommer, B.; Kohler, M.; Sprengel, R.; Seeburg, P.H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 1991, 67, 11–19. [Google Scholar] [CrossRef]
- Seeburg, P.H. The role of RNA editing in controlling glutamate receptor channel properties. J. Neurochem. 1996, 66, 1–5. [Google Scholar] [CrossRef]
- Cull-Candy, S.G.; Farrant, M. Ca(2+) -permeable AMPA receptors and their auxiliary subunits in synaptic plasticity and disease. J. Physiol. 2021, 599, 2655–2671. [Google Scholar] [CrossRef]
- Li, D.P.; Byan, H.S.; Pan, H.L. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J. Neurosci. 2012, 32, 372–380. [Google Scholar] [CrossRef]
- Konen, L.M.; Wright, A.L.; Royle, G.A.; Morris, G.P.; Lau, B.K.; Seow, P.W.; Zinn, R.; Milham, L.T.; Vaughan, C.W.; Vissel, B. A new mouse line with reduced GluA2 Q/R site RNA editing exhibits loss of dendritic spines, hippocampal CA1-neuron loss, learning and memory impairments and NMDA receptor-independent seizure vulnerability. Mol. Brain 2020, 13, 27. [Google Scholar] [CrossRef]
- Vikman, K.S.; Rycroft, B.K.; Christie, M.J. Switch to Ca2+-permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J. Physiol. 2008, 586, 515–527. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.R.; Zhou, M.H.; Wang, L.; Li, D.P.; Chen, H.; Lee, G.; Jayaraman, V.; Pan, H.L. alpha2delta-1 switches the phenotype of synaptic AMPA receptors by physically disrupting heteromeric subunit assembly. Cell Rep. 2021, 36, 109396. [Google Scholar] [CrossRef]
- Huang, X.; Roet, K.C.D.; Zhang, L.; Brault, A.; Berg, A.P.; Jefferson, A.B.; Klug-McLeod, J.; Leach, K.L.; Vincent, F.; Yang, H.; et al. Human amyotrophic lateral sclerosis excitability phenotype screen: Target discovery and validation. Cell Rep. 2021, 35, 109224. [Google Scholar] [CrossRef]
- Higuchi, M.; Maas, S.; Single, F.N.; Hartner, J.; Rozov, A.; Burnashev, N.; Feldmeyer, D.; Sprengel, R.; Seeburg, P.H. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature 2000, 406, 78–81. [Google Scholar] [CrossRef]
- Yang, J.H.; Sklar, P.; Axel, R.; Maniatis, T. Purification and characterization of a human RNA adenosine deaminase for glutamate receptor B pre-mRNA editing. Proc. Natl. Acad. Sci. USA 1997, 94, 4354–4359. [Google Scholar] [CrossRef]
- Hideyama, T.; Yamashita, T.; Aizawa, H.; Tsuji, S.; Kakita, A.; Takahashi, H.; Kwak, S. Profound downregulation of the RNA editing enzyme ADAR2 in ALS spinal motor neurons. Neurobiol. Dis. 2012, 45, 1121–1128. [Google Scholar] [CrossRef]
- Peng, P.L.; Zhong, X.; Tu, W.; Soundarapandian, M.M.; Molner, P.; Zhu, D.; Lau, L.; Liu, S.; Liu, F.; Lu, Y. ADAR2-dependent RNA editing of AMPA receptor subunit GluR2 determines vulnerability of neurons in forebrain ischemia. Neuron 2006, 49, 719–733. [Google Scholar] [CrossRef]
- Wright, A.; Vissel, B. The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front. Mol. Neurosci. 2012, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Goncalves, J.; Bartol, T.M.; Camus, C.; Levet, F.; Menegolla, A.P.; Sejnowski, T.J.; Sibarita, J.B.; Vivaudou, M.; Choquet, D.; Hosy, E. Nanoscale co-organization and coactivation of AMPAR, NMDAR, and mGluR at excitatory synapses. Proc. Natl. Acad. Sci. USA 2020, 117, 14503–14511. [Google Scholar] [CrossRef]
- Amakhin, D.V.; Soboleva, E.B.; Chizhov, A.V.; Zaitsev, A.V. Insertion of Calcium-Permeable AMPA Receptors during Epileptiform Activity In Vitro Modulates Excitability of Principal Neurons in the Rat Entorhinal Cortex. Int. J. Mol. Sci. 2021, 22, 12174. [Google Scholar] [CrossRef]
- Fukushima, F.; Nakao, K.; Shinoe, T.; Fukaya, M.; Muramatsu, S.; Sakimura, K.; Kataoka, H.; Mori, H.; Watanabe, M.; Manabe, T.; et al. Ablation of NMDA receptors enhances the excitability of hippocampal CA3 neurons. PLoS ONE 2009, 4, e3993. [Google Scholar] [CrossRef]
- Gold, M.S.; Flake, N.M. Inflammation-mediated hyperexcitability of sensory neurons. Neurosignals 2005, 14, 147–157. [Google Scholar] [CrossRef]
- Twomey, E.C.; Yelshanskaya, M.V.; Vassilevski, A.A.; Sobolevsky, A.I. Mechanisms of Channel Block in Calcium-Permeable AMPA Receptors. Neuron 2018, 99, 956–968.e4. [Google Scholar] [CrossRef]
- Chamma, I.; Chevy, Q.; Poncer, J.C.; Levi, S. Role of the neuronal K-Cl co-transporter KCC2 in inhibitory and excitatory neurotransmission. Front. Cell. Neurosci. 2012, 6, 5. [Google Scholar] [CrossRef]
- Côté, M.-P. Role of chloride cotransporters in the development of spasticity and neuropathic pain after spinal cord injury. In Neuronal Chloride Transporters in Health and Disease; Academic Press: Cambridge, MA, USA, 2020; pp. 463–516. [Google Scholar]
- Hebert, S.C.; Mount, D.B.; Gamba, G. Molecular physiology of cation-coupled Cl- cotransport: The SLC12 family. Pflugers Arch. 2004, 447, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Garzon-Muvdi, T.; Di Fulvio, M.; Chen, Y.; Delpire, E.; Alvarez, F.J.; Alvarez-Leefmans, F.J. Molecular and functional expression of cation-chloride cotransporters in dorsal root ganglion neurons during postnatal maturation. J. Neurophysiol. 2012, 108, 834–852. [Google Scholar] [CrossRef] [PubMed]
- Gillen, C.M.; Brill, S.; Payne, J.A.; Forbush, B., 3rd. Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human. A new member of the cation-chloride cotransporter family. J. Biol. Chem. 1996, 271, 16237–16244. [Google Scholar] [CrossRef] [Green Version]
- Crable, S.C.; Hammond, S.M.; Papes, R.; Rettig, R.K.; Zhou, G.P.; Gallagher, P.G.; Joiner, C.H.; Anderson, K.P. Multiple isoforms of the KC1 cotransporter are expressed in sickle and normal erythroid cells. Exp. Hematol. 2005, 33, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Giuliodori, M.J.; Zuccolilli, G. Postsynaptic potential summation and action potential initiation: Function following form. Adv. Physiol. Educ. 2004, 28, 79–80. [Google Scholar] [CrossRef]
- Westenbroek, R.E.; Hoskins, L.; Catterall, W.A. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J. Neurosci. 1998, 18, 6319–6330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.C.; Birch, D.V.; Heckman, C.J.; Tysseling, V.M. The Involvement of CaV1.3 Channels in Prolonged Root Reflexes and Its Potential as a Therapeutic Target in Spinal Cord Injury. Front. Neural Circuits 2021, 15, 642111. [Google Scholar] [CrossRef] [PubMed]
- Marcantoni, M.; Fuchs, A.; Löw, P.; Bartsch, D.; Kiehn, O.; Bellardita, C. Early delivery and prolonged treatment with nimodipine prevents the development of spasticity after spinal cord injury in mice. Sci. Transl. Med. 2020, 12, eaay0167. [Google Scholar] [CrossRef] [PubMed]
- Perrier, J.F.; Hounsgaard, J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J. Neurophysiol. 2003, 89, 954–959. [Google Scholar] [CrossRef]
- Sukiasyan, N.; Hultborn, H.; Zhang, M. Distribution of calcium channel Ca(V)1.3 immunoreactivity in the rat spinal cord and brain stem. Neuroscience 2009, 159, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Bodzeta, A.; Scheefhals, N.; MacGillavry, H.D. Membrane trafficking and positioning of mGluRs at presynaptic and postsynaptic sites of excitatory synapses. Neuropharmacology 2021, 200, 108799. [Google Scholar] [CrossRef] [PubMed]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Scheefhals, N.; MacGillavry, H.D. Functional organization of postsynaptic glutamate receptors. Mol. Cell. Neurosci. 2018, 91, 82–94. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Villalba, R.M.; Carr, P.A.; Grandes, P.; Somohano, P.M. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. J. Comp. Neurol. 2000, 422, 464–487. [Google Scholar] [CrossRef]
- Hovelsø, N.; Sotty, F.; Montezinho, L.P.; Pinheiro, P.S.; Herrik, K.F.; Mørk, A. Therapeutic potential of metabotropic glutamate receptor modulators. Curr. Neuropharmacol. 2012, 10, 12–48. [Google Scholar] [CrossRef]
- Vidnyánszky, Z.; Hámori, J.; Négyessy, L.; Rüegg, D.; Knöpfel, T.; Kuhn, R.; Görcs, T.J. Cellular and subcellular localization of the mGluR5a metabotropic glutamate receptor in rat spinal cord. Neuroreport. 1994, 6, 209–213. [Google Scholar] [CrossRef]
- Ma, L.; Ostrovsky, H.; Miles, G.; Lipski, J.; Funk, G.D.; Nicholson, L.F. Differential expression of group I metabotropic glutamate receptors in human motoneurons at low and high risk of degeneration in amyotrophic lateral sclerosis. Neuroscience 2006, 143, 95–104. [Google Scholar] [CrossRef]
- Anneser, J.M.; Ince, P.G.; Shaw, P.J.; Borasio, G.D. Differential expression of mGluR5 in human lumbosacral motoneurons. Neuroreport 2004, 15, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Iwagaki, N.; Miles, G.B. Activation of group I metabotropic glutamate receptors modulates locomotor-related motoneuron output in mice. J. Neurophysiol. 2011, 105, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Nistri, A.; Ostroumov, K.; Sharifullina, E.; Taccola, G. Tuning and playing a motor rhythm: How metabotropic glutamate receptors orchestrate generation of motor patterns in the mammalian central nervous system. J. Physiol. 2006, 572, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.R.; Abraham, W.C. Group I mGluRs increase excitability of hippocampal CA1 pyramidal neurons by a PLC-independent mechanism. J. Neurophysiol. 2002, 88, 107–116. [Google Scholar] [CrossRef]
- Li, D.P.; Zhu, L.H.; Pachuau, J.; Lee, H.A.; Pan, H.L. mGluR5 Upregulation Increases Excitability of Hypothalamic Presympathetic Neurons through NMDA Receptor Trafficking in Spontaneously Hypertensive Rats. J. Neurosci. 2014, 34, 4309–4317. [Google Scholar] [CrossRef]
- Yu, W.; Sohn, J.-W.; Kwon, J.; Lee, S.-H.; Kim, S.; Ho, W.-K. Enhancement of dendritic persistent Na+ currents by mGluR5 leads to an advancement of spike timing with an increase in temporal precision. Mol. Brain 2018, 11, 67. [Google Scholar] [CrossRef] [Green Version]
- Fagni, L.; Chavis, P.; Ango, F.; Bockaert, J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000, 23, 80–88. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Hulsebosch, C.E. Upregulation of Group I metabotropic glutamate receptors in neurons and astrocytes in the dorsal horn following spinal cord injury. Exp. Neurol. 2005, 195, 236–243. [Google Scholar] [CrossRef]
- Heinke, B.; Sandkuhler, J. Group I metabotropic glutamate receptor-induced Ca(2+)-gradients in rat superficial spinal dorsal horn neurons. Neuropharmacology 2007, 52, 1015–1023. [Google Scholar] [CrossRef]
- Harvey, P.J.; Li, X.; Li, Y.; Bennett, D.J. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J. Neurophysiol. 2006, 96, 1158–1170. [Google Scholar] [CrossRef]
- Heckman, C.J.; Johnson, M.; Mottram, C.; Schuster, J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist. 2008, 14, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bennett, D.J. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J. Neurophysiol. 2003, 90, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Carlin, K.P.; Jones, K.E.; Jiang, Z.; Jordan, L.M.; Brownstone, R.M. Dendritic L-type calcium currents in mouse spinal motoneurons: Implications for bistability. Eur. J. Neurosci. 2000, 12, 1635–1646. [Google Scholar] [CrossRef]
- D’Amico, J.M.; Murray, K.C.; Li, Y.; Chan, K.M.; Finlay, M.G.; Bennett, D.J.; Gorassini, M.A. Constitutively active 5-HT2/alpha1 receptors facilitate muscle spasms after human spinal cord injury. J. Neurophysiol. 2013, 109, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Wikström, M.; Hill, R.; Hellgren, J.; Grillner, S. The action of 5-HT on calcium-dependent potassium channels and on the spinal locomotor network in lamprey is mediated by 5-HT1A-like receptors. Brain Res. 1995, 678, 191–199. [Google Scholar] [CrossRef]
- Grunnet, M.; Jespersen, T.; Perrier, J.F. 5-HT1A receptors modulate small-conductance Ca2+-activated K+ channels. J. Neurosci. Res. 2004, 78, 845–854. [Google Scholar] [CrossRef]


 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, B.; Wojtaś, B.; Skup, M. Molecular Identification of Pro-Excitogenic Receptor and Channel Phenotypes of the Deafferented Lumbar Motoneurons in the Early Phase after SCT in Rats. Int. J. Mol. Sci. 2022, 23, 11133. https://doi.org/10.3390/ijms231911133
Ji B, Wojtaś B, Skup M. Molecular Identification of Pro-Excitogenic Receptor and Channel Phenotypes of the Deafferented Lumbar Motoneurons in the Early Phase after SCT in Rats. International Journal of Molecular Sciences. 2022; 23(19):11133. https://doi.org/10.3390/ijms231911133
Chicago/Turabian StyleJi, Benjun, Bartosz Wojtaś, and Małgorzata Skup. 2022. "Molecular Identification of Pro-Excitogenic Receptor and Channel Phenotypes of the Deafferented Lumbar Motoneurons in the Early Phase after SCT in Rats" International Journal of Molecular Sciences 23, no. 19: 11133. https://doi.org/10.3390/ijms231911133





