Assessment of Efficacy and Safety Using PPAR-γ Agonist-Loaded Nanocarriers for Inflammatory Eye Diseases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of PZ-NCs
2.2. In Vitro HET-CAM Test
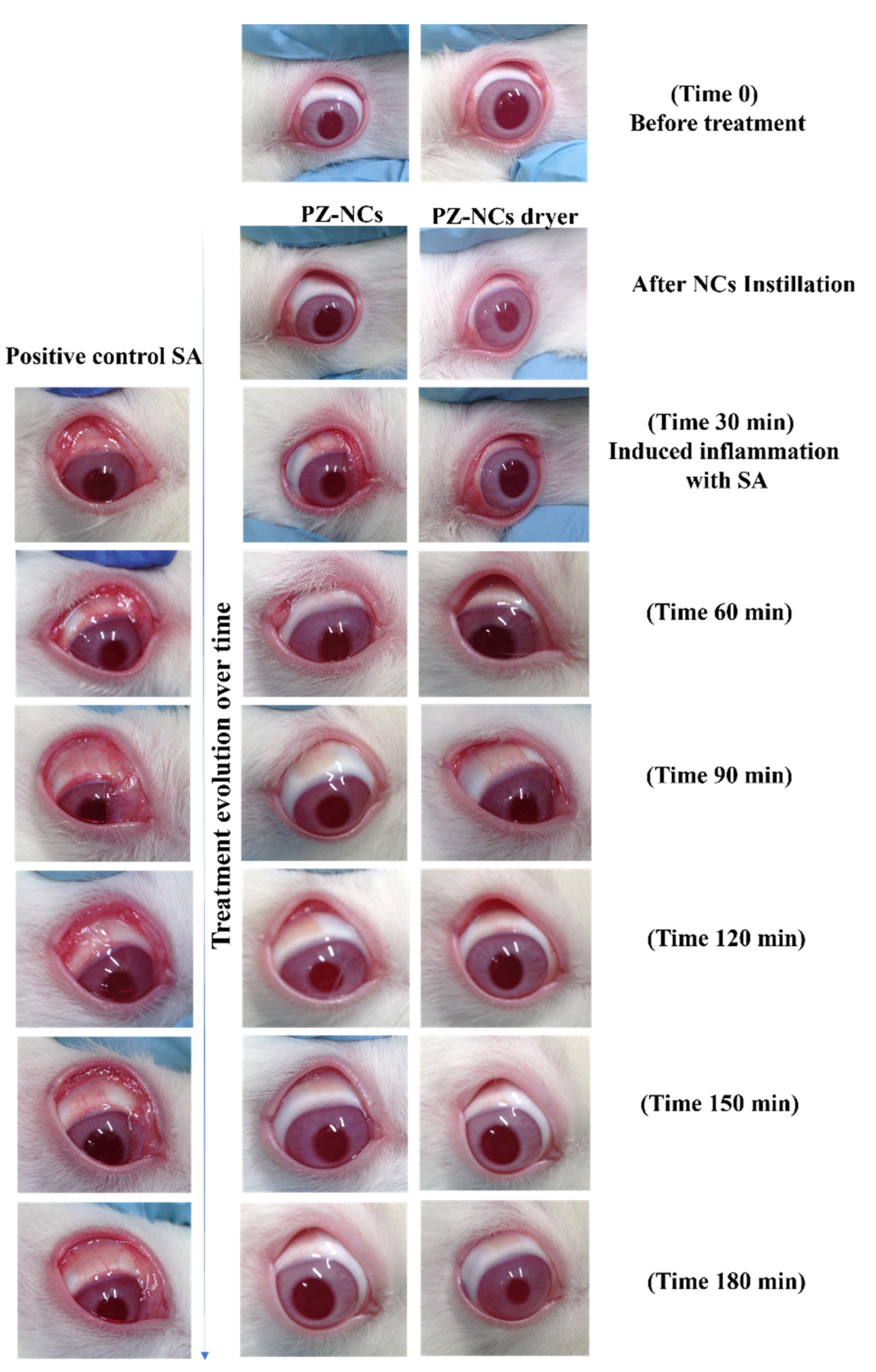
2.3. In Vivo Ocular Tolerance (Draize Test)
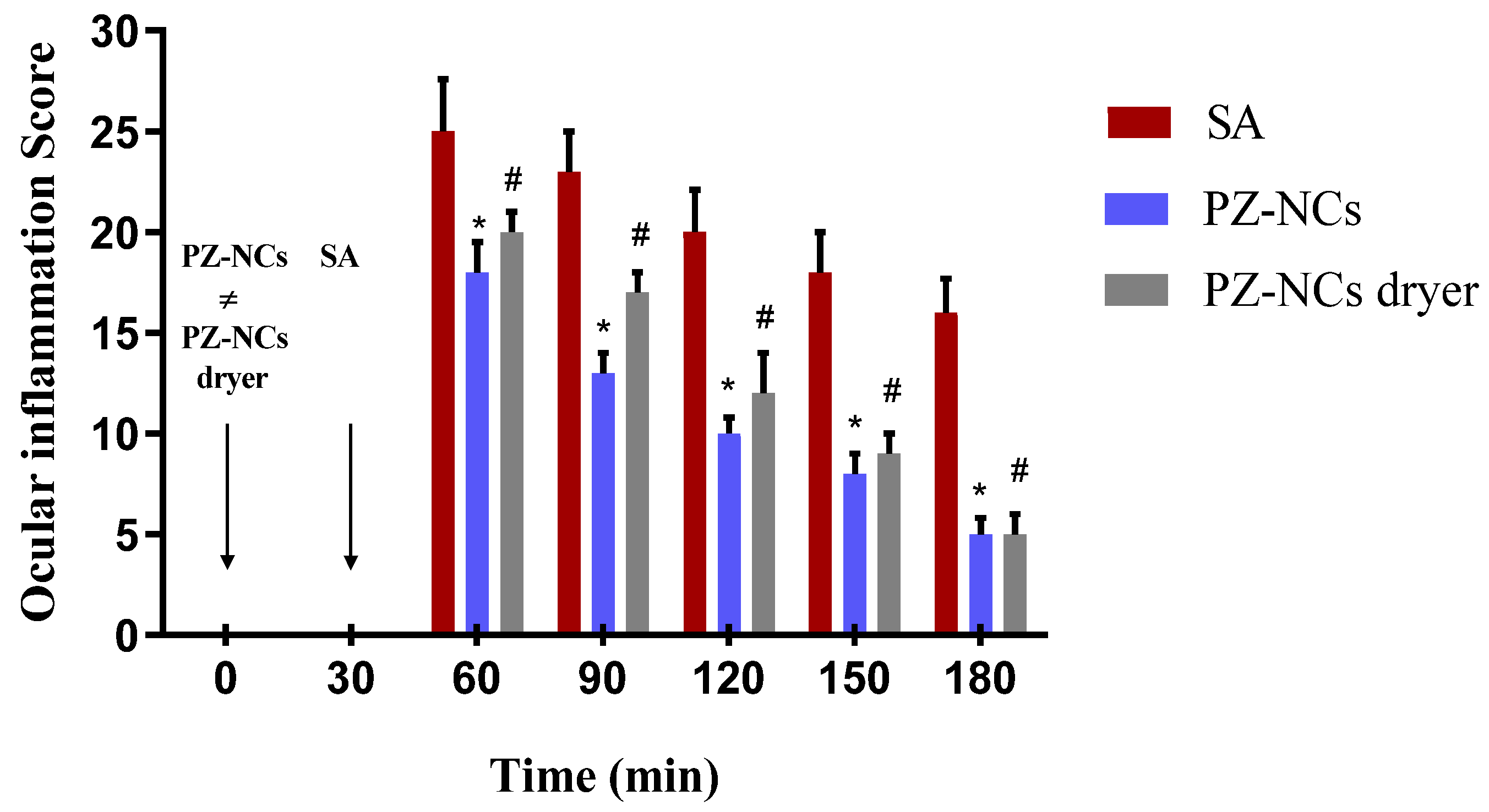
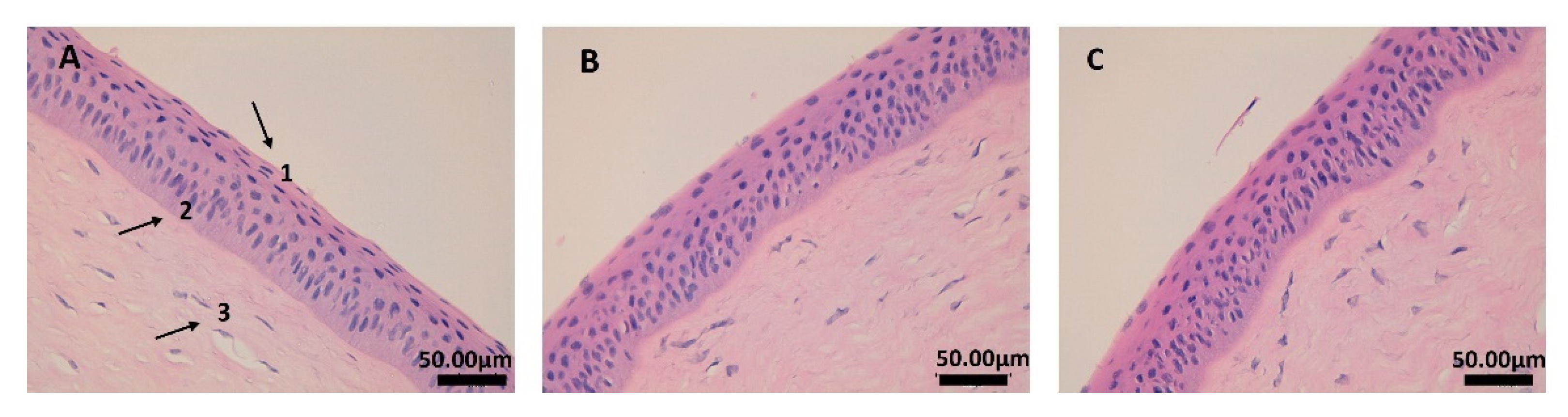
2.4. In Vivo Efficacy Assay: Anti-Inflammatory and Histological Studies
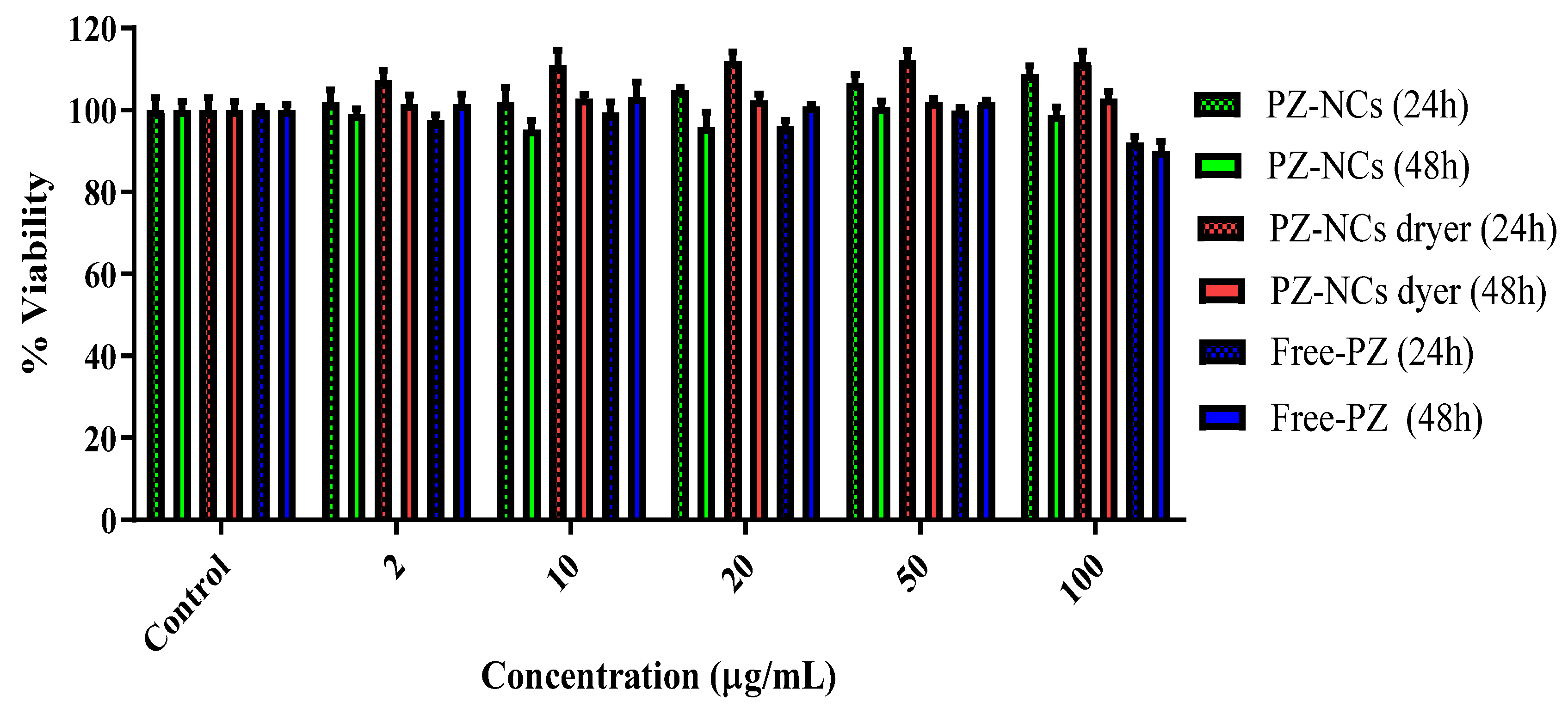
2.5. Toxicity Assay (Cell Culture Cell Line Y-79)
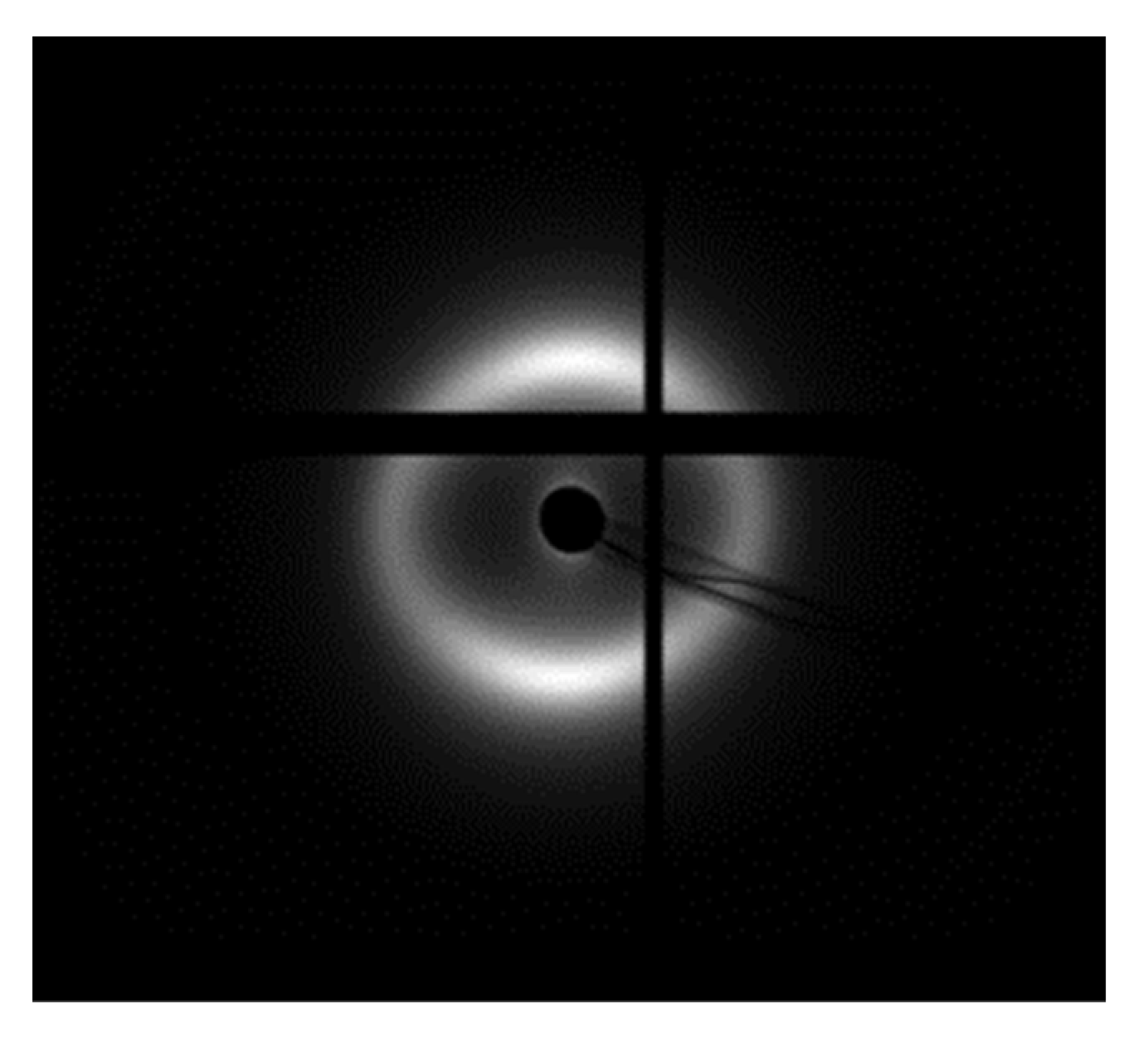
2.6. SAXS Analysis
2.7. Corneal Transparency
3. Materials and Methods
3.1. Materials
3.2. Nanocarrier Synthesis and Stabilization Process
3.3. In Vitro Ocular Irritation Test (HET-CAM)
3.4. In Vivo Draize Test
3.5. In Vivo Efficacy Assay: Anti-Inflammatory and Histological Studies
3.6. Assessment of Cytotoxicity
3.7. Synchrotron Small Angle X-ray Diffraction (SAXS)
3.7.1. Sample Preparation
3.7.2. Data Collection
3.8. Corneal Transparency
3.9. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dua, H.S.; Faraj, L.A.; Said, D.G.; Gray, T.; Lowe, J. Human corneal anatomy redefined: A novel pre-descemet’s layer (Dua’s Layer). Ophthalmology 2013, 120, 1778–1785. [Google Scholar] [CrossRef]
- Dua, H.S.; Faraj, L.A.; Said, D.G. Duas layer: Discovery, characteristics, clinical applications, controversy and potential relevance to glaucoma. Expert Rev. Ophthalmol. 2015, 10, 531–547. [Google Scholar] [CrossRef]
- Hayes, S.; Boote, C.; Tuft, S.J.; Quantock, A.J.; Meek, K.M. A study of corneal thickness, shape and collagen organisation in keratoconus using videokeratography and X-ray scattering techniques. Exp. Eye Res. 2007, 84, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Dooley, E.P.; Albon, J.; Boulton, M.E.; Meek, K.M.; Kamma-Lorger, C.S. Adhesion of laser in situ keratomileusis-like flaps in the cornea: Effects of crosslinking, stromal fibroblasts, and cytokine treatment. J. Cataract Refract. Surg. 2011, 37, 166–172. [Google Scholar] [CrossRef]
- Meek, K.M.; Fullwood, N.J.; Cooke, P.H.; Elliott, G.F.; Maurice, D.M.; Quantock, A.J.; Wall, R.S.; Worthington, C.R. Synchrotron x-ray diffraction studies of the cornea, with implications for stromal hydration. Biophys. J. 1991, 60, 467–474. [Google Scholar] [CrossRef]
- Kamma-Lorger, C.S.; Boote, C.; Hayes, S.; Albon, J.; Boulton, M.E.; Meek, K.M. Collagen ultrastructural changes during stromal wound healing in organ cultured bovine corneas. Exp. Eye Res. 2009, 88, 953–959. [Google Scholar] [CrossRef]
- Boote, C.; Hayes, S.; Young, R.D.; Kamma-Lorger, C.S.; Hocking, P.M.; Elsheikh, A.; Inglehearn, C.F.; Ali, M.; Meek, K.M. Ultrastructural changes in the retinopathy, globe enlarged (rge) chick cornea. J. Struct. Biol. 2009, 166, 195–204. [Google Scholar] [CrossRef]
- Kamma-Lorger, C.S.; Hayes, S.; Boote, C.; Burghammer, M.; Boulton, M.E.; Meek, K.M. Effects on collagen orientation in the cornea after trephine injury. Mol. Vis. 2009, 15, 378–385. [Google Scholar]
- Kamma-Lorger, C.S.; Boote, C.; Hayes, S.; Moger, J.; Burghammer, M.; Knupp, C.; Quantock, A.J.; Sorensen, T.; Di Cola, E.; White, N.; et al. Collagen and mature elastic fibre organisation as a function of depth in the human cornea and limbus. J. Struct. Biol. 2010, 169, 424–430. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Mahankali, A.; Matsuda, M.; Glass, L.; Mahankali, S.; Ferrannini, E.; Cusi, K.; Mandarino, L.J.; DeFronzo, R.A. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care 2001, 24, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Lazar, M.A. The many faces of PPARγ. Cell 2005, 123, 993–999. [Google Scholar] [CrossRef] [Green Version]
- Tokutome, M.; Matoba, T.; Nakano, Y.; Okahara, A.; Fujiwara, M.; Koga, J.I.; Nakano, K.; Tsutsui, H.; Egashira, K. Peroxisome proliferator-activated receptor-gamma targeting nanomedicine promotes cardiac healing after acute myocardial infarction by skewing monocyte/macrophage polarization in preclinical animal models. Cardiovasc. Res. 2019, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Egashira, K.; Hiasa, K.I.; Inoue, S.; Ni, W.; Zhao, Q.; Usui, M.; Kitamoto, S.; Ichiki, T.; Takeshita, A. Antiinflammatory and antiarteriosclerotic effects of pioglitazone. Hypertension 2002, 40, 687–693. [Google Scholar] [CrossRef]
- Nagahama, R.; Matoba, T.; Nakano, K.; Kim-Mitsuyama, S.; Sunagawa, K.; Egashira, K. Nanoparticle-mediated delivery of pioglitazone enhances therapeutic neovascularization in a murine model of hindlimb ischemia. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Kakuta, H.; Miyachi, H.; Sugimoto, Y. Involvement of the retinoid X receptor ligand in the anti-inflammatory effect induced by peroxisome proliferator-activated receptor agonist in vivo. PPAR Res. 2011, 2011, 840194. [Google Scholar] [CrossRef]
- Aoki, Y.; Maeno, T.; Aoyagi, K.; Ueno, M.; Aoki, F.; Aoki, N.; Nakagawa, J.; Sando, Y.; Shimizu, Y.; Suga, T.; et al. Pioglitazone, a peroxisome proliferator-activated receptor gamma ligand, suppresses bleomycin-induced acute lung injury and fibrosis. Respiration 2009, 77, 311–319. [Google Scholar] [CrossRef]
- Mehta, M. Biopharmaceutics Classification System (BCS): Development, Implementation, and Growth; Wiley: Hoboken, NJ, USA, 2016; ISBN 978-1-118-47661-1. [Google Scholar]
- Silva-Abreu, M.; Calpena, A.C.; Espina, M.; Silva, A.M.; Gimeno, A.; Egea, M.A.; García, M.L. Optimization, Biopharmaceutical Profile and Therapeutic Efficacy of Pioglitazone-loaded PLGA-PEG Nanospheres as a Novel Strategy for Ocular Inflammatory Disorders. Pharm. Res. 2018, 35, 11. [Google Scholar] [CrossRef]
- Laddha, U.D.; Kshirsagar, S.J. Formulation of nanoparticles loaded in situ gel for treatment of dry eye disease: In vitro, ex vivo and in vivo evidences. J. Drug Deliv. Sci. Technol. 2021, 61, 102112. [Google Scholar] [CrossRef]
- Wong, L.R.; Wong, P.; Ho, P.C.L. Metabolic profiling of female tg2576 mouse brains provides novel evidence supporting intranasal low-dose pioglitazone for long-term treatment at an early stage of alzheimer’s disease. Biomedicines 2020, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Silva-Abreu, M.; Gonzalez-Pizarro, R.; Espinoza, L.C.; Rodríguez-Lagunas, M.J.; Espina, M.; García, M.L.; Calpena, A.C. Thiazolidinedione as an alternative to facilitate oral administration in geriatric patients with Alzheimer’s disease. Eur. J. Pharm. Sci. 2019, 129, 173–180. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Espinoza, L.C.; Rodríguez-Lagunas, M.J.; Fábrega, M.J.; Espina, M.; García, M.L.; Calpena, A.C. Human skin permeation studies with PPARγ agonist to improve its permeability and efficacy in inflammatory processes. Int. J. Mol. Sci. 2017, 18, 2548. [Google Scholar] [CrossRef] [Green Version]
- Hirenkumar, M.; Steven, S. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2012, 3, 1377–1397. [Google Scholar] [CrossRef]
- Locatelli, E.; Franchini, M.C. Biodegradable PLGA-b-PEG polymeric nanoparticles: Synthesis, properties, and nanomedical applications as drug delivery system. J. Nanoparticle Res. 2012, 14, 1316. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Fowler, R.; Stolnik, S. PEGylated nanomedicines: Recent progress and remaining concerns. Expert Opin. Drug Deliv. 2014, 11, 139–154. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Salama, A.H. Spray drying as an advantageous strategy for enhancing pharmaceuticals bioavailability. Drug Deliv. Transl. Res. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ghafoorianfar, S.; Ghorani-Azam, A.; Mohajeri, S.A.; Farzin, D. Efficiency of nanoparticles for treatment of ocular infections: Systematic literature review. J. Drug Deliv. Sci. Technol. 2020, 57, 101765. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L. Ocular drug delivery: Role of degradable polymeric nanocarriers for ophthalmic application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef]
- Guo, D.; Li, Q.; Sun, Y.; Guo, J.; Zhao, Q.; Yin, X.; Wei, H.; Wu, S.; Bi, H. Evaluation of controlled-release triamcinolone acetonide-loaded mPEG-PLGA nanoparticles in treating experimental autoimmune uveitis. Nanotechnology 2019, 30, 165702. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Carvajal-Vidal, P.; Halbault Bellowa, L.; Calpena, A.C.; Espina, M.; García, M.L. In-situ forming gels containing fluorometholone-loaded polymeric nanoparticles for ocular inflammatory conditions. Colloids Surfaces B Biointerfaces 2019, 175, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Katara, R.; Sachdeva, S.; Majumdar, D.K. Enhancement of ocular efficacy of aceclofenac using biodegradable PLGA nanoparticles: Formulation and characterization. Drug Deliv. Transl. Res. 2017, 7, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Mallandrich, M.; Clares, B.; Egea, M.A.; Espina, M.; García, M.L.; Calpena, A.C. Colloids and Surfaces B: Biointerfaces Design and elaboration of freeze-dried PLGA nanoparticles for the transcorneal permeation of carprofen: Ocular anti-inflammatory applications. Colloids Surf. B Biointerfaces 2015, 136, 935–943. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular drug delivery barriers—role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Miralles, E.; Kamma-Lorger, C.S.; Espina, M.; García, M.L.; Calpena, A.C. Stabilization by Nano Spray Dryer of Pioglitazone Polymeric Nanosystems: Development, In Vivo, Ex Vivo and Synchrotron Analysis. Pharmaceutics 2021, 13, 1751. [Google Scholar] [CrossRef] [PubMed]
- Miralles-Cardiel, E.; Silva-Abreu, M.; Calpena, A.C.; Casals, I. Development and validation of an hplc–ms/ms method for pioglitazone from nanocarriers quantitation in ex vivo and in vivo ocular tissues. Pharmaceutics 2021, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A.; Alshamsan, A. Poly (D,L-lactide-co-glycolide) nanoparticles for sustained release of tacrolimus in rabbit eyes. Biomed. Pharmacother. 2017, 94, 402–411. [Google Scholar] [CrossRef]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomed.Nanotechnol. Biol. Med. 2010, 6, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N.P. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Parra, A.; Calpena, A.; Garcia, M.L. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. Eur. J. Pharm. Biopharm. 2015, 95, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, H.L.; Abrego, G.; Garduño-Ramirez, M.L.; Clares, B.; Calpena, A.C.; García, M.L. Design and optimization of oleanolic/ursolic acid-loaded nanoplatforms for ocular anti-inflammatory applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 521–530. [Google Scholar] [CrossRef]
- Andreani, T.; Miziara, L.; Lorenzón, E.N.; De Souza, A.L.R.; Kiill, C.P.; Fangueiro, J.F.; Garcia, M.L.; Gremião, P.D.; Silva, A.M.; Souto, E.B. Effect of mucoadhesive polymers on the in vitro performance of insulin-loaded silica nanoparticles: Interactions with mucin and biomembrane models. Eur. J. Pharm. Biopharm. 2015, 93, 118–126. [Google Scholar] [CrossRef]
- Huckaby, J.T.; Lai, S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018, 124, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Lema, M.I.; Otero-Espinar, F.J. Mucoadhesive PLGA Nanospheres and Nanocapsules for Lactoferrin Controlled Ocular Delivery. Pharmaceutics 2022, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Kaldybekov, D.B.; Filippov, S.K.; Radulescu, A.; Khutoryanskiy, V.V. Maleimide-functionalised PLGA-PEG nanoparticles as mucoadhesive carriers for intravesical drug delivery. Eur. J. Pharm. Biopharm. 2019, 143, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schön, A.; Gong, X.; Yang, J.C.; Lamb, N.W.; Cai, S.; Yu, T.; Freire, E.; et al. Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Esteruelas, G.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Cano, A.; Calpena, A.C.; Ettcheto, M.; Camins, A.; et al. Article dexibuprofen biodegradable nanoparticles: One step closer towards a better ocular interaction study. Nanomaterials 2020, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pizarro, R.; Parrotta, G.; Vera, R.; Sánchez-López, E.; Galindo, R.; Kjeldsen, F.; Badia, J.; Baldoma, L.; Espina, M.; García, M.L. Ocular penetration of fluorometholone-loaded PEG-PLGA nanoparticles functionalized with cell-penetrating peptides. Nanomedicine 2019, 14, 3089–3104. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, C.; Alvarado, H.; Calpena, A.C.; Silva, A.M.; Souto, E.B.; García, M.L.; Abrego, G. In vitro, ex vivo and in vivo characterization of PLGA nanoparticles loading pranoprofen for ocular administration. Int. J. Pharm. 2016, 511, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Giannavola, C.; Bucolo, C.; Maltese, A.; Paolino, D.; Vandelli, M.A.; Puglisi, G.; Lee, V.H.L.; Fresta, M. Influence of preparation conditions on acyclovir-loaded poly-d,l-lactic acid nanospheres and effect of PEG coating on ocular drug bioavailability. Pharm. Res. 2003, 20, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Ramos Yacasi, G.R.; Calpena Campmany, A.C.; Egea Gras, M.A.; Espina García, M.; García López, M.L. Freeze drying optimization of polymeric nanoparticles for ocular flurbiprofen delivery: Effect of protectant agents and critical process parameters on long-term stability. Drug Dev. Ind. Pharm. 2017, 43, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Luis de Redín, I.; Boiero, C.; Martínez-Ohárriz, M.C.; Agüeros, M.; Ramos, R.; Peñuelas, I.; Allemandi, D.; Llabot, J.M.; Irache, J.M. Human serum albumin nanoparticles for ocular delivery of bevacizumab. Int. J. Pharm. 2018, 541, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Feczkó, T.; Piiper, A.; Pleli, T.; Schmithals, C.; Denk, D.; Hehlgans, S.; Rödel, F.; Vogl, T.J.; Wacker, M.G. Theranostic sorafenib-loaded polymeric nanocarriers manufactured by enhanced gadolinium conjugation techniques. Pharmaceutics 2019, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; García, M.L. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen-in vitro, ex vivo and in vivo characterization. Colloids Surfaces B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Ruberti, J.W.; Klyce, S.D.; Smolek, M.K.; Karon, M.D. Anomalous Acute Inflammatory Response in Rabbit Corneal Stroma. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2523–2530. [Google Scholar]
- Connon, C.J.; Meek, K.M. Organization of corneal collagen fibrils during the healing of trephined wounds in rabbits. Wound Repair Regen. 2003, 11, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Quantock, A.J.; Padroni, S.; Connon, C.J.; Milne, G.; Schanzlin, D.J. Proteoglycan alterations in the rabbit corneal stroma after a lamellar incision. J. Cataract Refract. Surg. 2003, 29, 821–824. [Google Scholar] [CrossRef]
- Crespo-Moral, M.; García-Posadas, L.; López-García, A.; Diebold, Y. Histological and immunohistochemical characterization of the porcine ocular surface. PLoS ONE 2020, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Quantock, A.J.; Boote, C.; Young, R.D.; Hayes, S.; Tanioka, H.; Kawasaki, S.; Ohta, N.; Iida, T.; Yagi, N.; Kinoshita, S.; et al. Small-angle fibre diffraction studies of corneal matrix structure: A depth-profiled investigation of the human eye-bank cornea. J. Appl. Crystallogr. 2007, 40, 335–340. [Google Scholar] [CrossRef]
- Boote, C.; Dennis, S.; Newton, R.H.; Puri, H.; Meek, K.M. Collagen fibrils appear more closely packed in the prepupillary cornea: Optical and biomechanical implications. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Reis, S.; Sarmento, B. Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. J. Control. Release 2016, 225, 75–86. [Google Scholar] [CrossRef]
- Franks, F.; Auffret, T. Freeze-Drying of Pharmaceuticals and Biopharmaceuticals; Chemistry, R.S., Ed.; Royal Society of Chemistry: London, UK, 2008; ISBN 0854041516. [Google Scholar]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Arpagaus, C.; Belleteix, F.; Vandamme, T.F. Nanoparticles by spray drying using innovative new technology: The Büchi Nano Spray Dryer B-90. J. Control. Release 2010, 147, 304–310. [Google Scholar] [CrossRef]
- Heng, D.; Lee, S.H.; Ng, W.K.; Tan, R.B. The nano spray dryer B-90. Expert Opin. Drug Deliv. 2011, 8, 965–972. [Google Scholar] [CrossRef]
- INVITTOX 1992 Protocol No. 47: HET-CAM Test. Available online: https://www.researchgate.net/profile/Horst-Spielmann/publication/15647765_HET-CAM_test/links/00b7d523a17404a3c4000000/HET-CAM-test.pdf (accessed on 13 March 2022).
- Kay, J.; Calandra, J. Interpretation of eye irritation tests. J. Soc. Cosmet. Chem. 1962, 13, 281–289. [Google Scholar]
- Wilhelmus, K.R. The Draize eye test. Surv. Ophthalmol. 2001, 45, 493–515. [Google Scholar] [CrossRef]
- Spampinato, S.; Marino, A.; Bucolo, C.; Canossa, M.; Bachetti, T.M.S. Effects of sodium naproxen eye drops on rabbit ocular inflammation induced by sodium arachidonate. J. Ocul. Pharmacol. Ther. 1991, 7, 125–133. [Google Scholar] [CrossRef]
- Andreani, T.; Kiill, C.P.; Souza, A.L.R.d.; Fangueiro, J.F.; Fernandes, L.; Doktorovová, S.; Santos, D.L.; Garcia, M.L.; Gremião, M.P.D.; Souto, E.B.; et al. Surface engineering of silica nanoparticles for oral insulin delivery: Characterization and cell toxicity studies. Colloids Surfaces B Biointerfaces 2014, 123, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Abass, A.; Bell, J.S.; Spang, M.T.; Hayes, S.; Meek, K.M.; Boote, C. SAXS4COLL: An integrated software tool for analysing fibrous collagen-based tissues. J. Appl. Crystallogr. 2017, 50, 1235–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAXS4COLL Software. Available online: https://bio.tools/saxs4coll (accessed on 16 October 2021).



| Position * | No Treatment | Positive Control | PZ-NCs Treatment | PZ-NCs Dryer Treatment |
|---|---|---|---|---|
| 1 | 34.3 ± 0.5 | 35.3 ± 2.1 | 35.5 ± 1.8 | 33.5 ± 0.3 |
| 2 | 35.3 ± 0.3 | 35.0 ± 1.9 | 35.0 ± 1.3 | 33.4 ± 0.3 |
| 3 | 35.3 ± 0.3 | 35.2 ± 1.6 | 34.6 ± 0.8 | 33.6 ± 0.3 |
| 4 | 34.9 ± 0.1 | 34.7 ± 0.8 | 34.0 ± 0.3 | 33.6 ± 0.3 |
| 5 | 34.5 ± 0.3 | 34.2 ± 0.7 | 33.9 ± 0.7 | 33.8 ± 0.6 |
| 6 | 34.4 ± 1.2 | 32.7 ± 0.6 | 33.7 ± 1.0 | 33.5 ± 0.4 |
| Position * | No Treatment | Positive Control | PZ-NCs Treatment | PZ-NCs Dryer Treatment |
|---|---|---|---|---|
| 1 | 59.8 ± 0.8 | 45.6 ± 9.9 | 53.5 ± 4.0 | 40.9 ± 9.8 |
| 2 | 52.3 ± 4.7 | 44.4 ± 8.1 | 55.6 ± 3.4 | 45.6 ± 10.4 |
| 3 | 51.0 ± 3.3 | 44.0 ± 7.9 | 54.6 ± 4.2 | 42.8 ± 10.9 |
| 4 | 49.4 ± 3.2 | 42.9 ± 6.1 | 51.5 ± 3.0 | 47.4 ± 11.1 |
| 5 | 50.0 ± 1.9 | 41.9 ± 5.5 | 51.0 ± 3.6 | 51.2 ± 19.1 |
| 6 | 51.3 ± 1.8 | 42.2 ± 5.7 | 48.5 ± 4.0 | 51.5 ± 16.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miralles, E.; Kamma-Lorger, C.S.; Domènech, Ò.; Sosa, L.; Casals, I.; Calpena, A.C.; Silva-Abreu, M. Assessment of Efficacy and Safety Using PPAR-γ Agonist-Loaded Nanocarriers for Inflammatory Eye Diseases. Int. J. Mol. Sci. 2022, 23, 11184. https://doi.org/10.3390/ijms231911184
Miralles E, Kamma-Lorger CS, Domènech Ò, Sosa L, Casals I, Calpena AC, Silva-Abreu M. Assessment of Efficacy and Safety Using PPAR-γ Agonist-Loaded Nanocarriers for Inflammatory Eye Diseases. International Journal of Molecular Sciences. 2022; 23(19):11184. https://doi.org/10.3390/ijms231911184
Chicago/Turabian StyleMiralles, Esther, Christina S. Kamma-Lorger, Òscar Domènech, Lilian Sosa, Isidre Casals, Ana Cristina Calpena, and Marcelle Silva-Abreu. 2022. "Assessment of Efficacy and Safety Using PPAR-γ Agonist-Loaded Nanocarriers for Inflammatory Eye Diseases" International Journal of Molecular Sciences 23, no. 19: 11184. https://doi.org/10.3390/ijms231911184







