Methamphetamine Induces Systemic Inflammation and Anxiety: The Role of the Gut–Immune–Brain Axis
Abstract
1. Introduction
2. Results and Discussion
2.1. METH Alters Intestinal Permeability
2.2. METH Increases Fatty Acid Binding Protein 1 in Blood
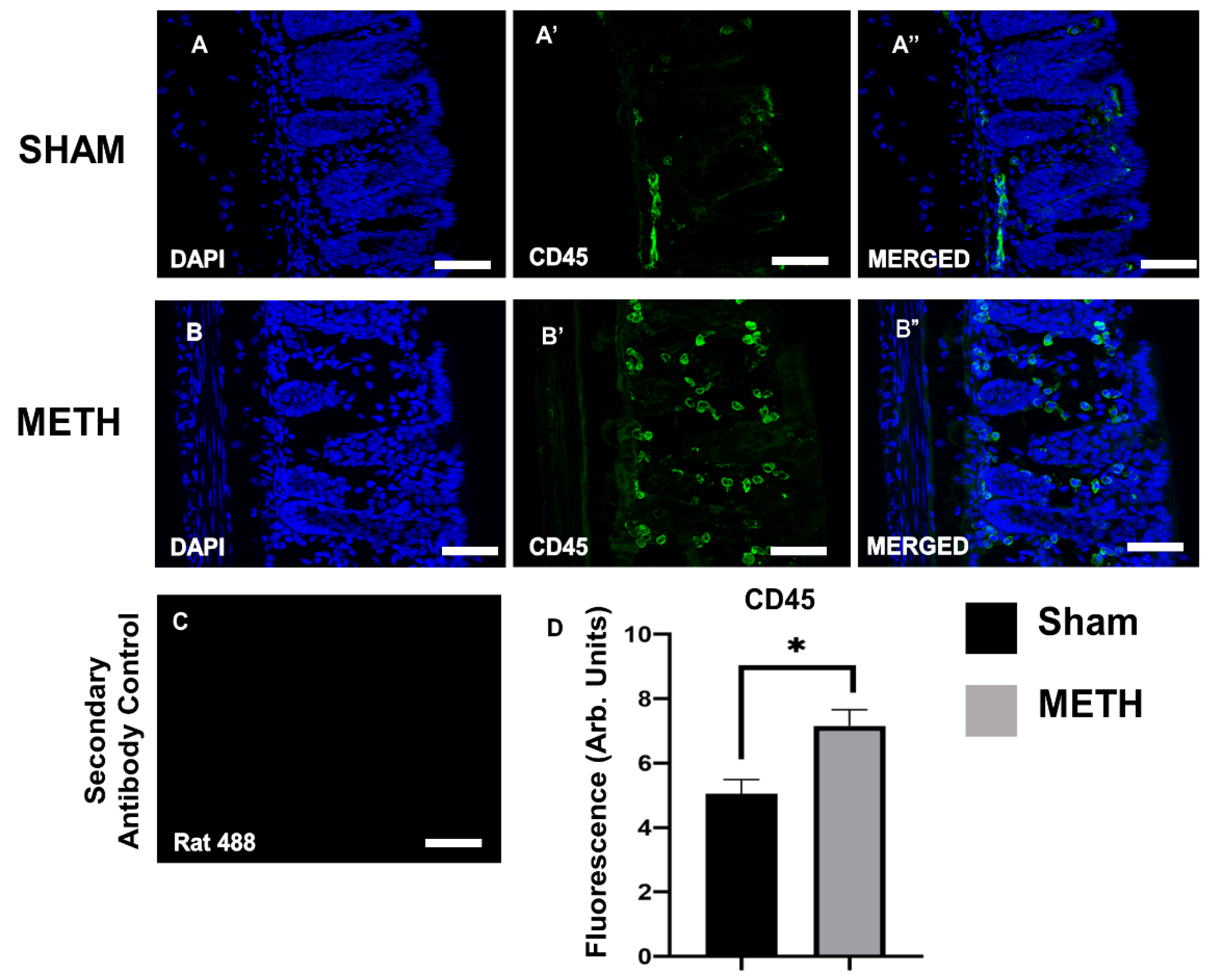
2.3. METH Alters Cellular Immune Responses in the Colon
2.4. METH Increases BBB Permeability
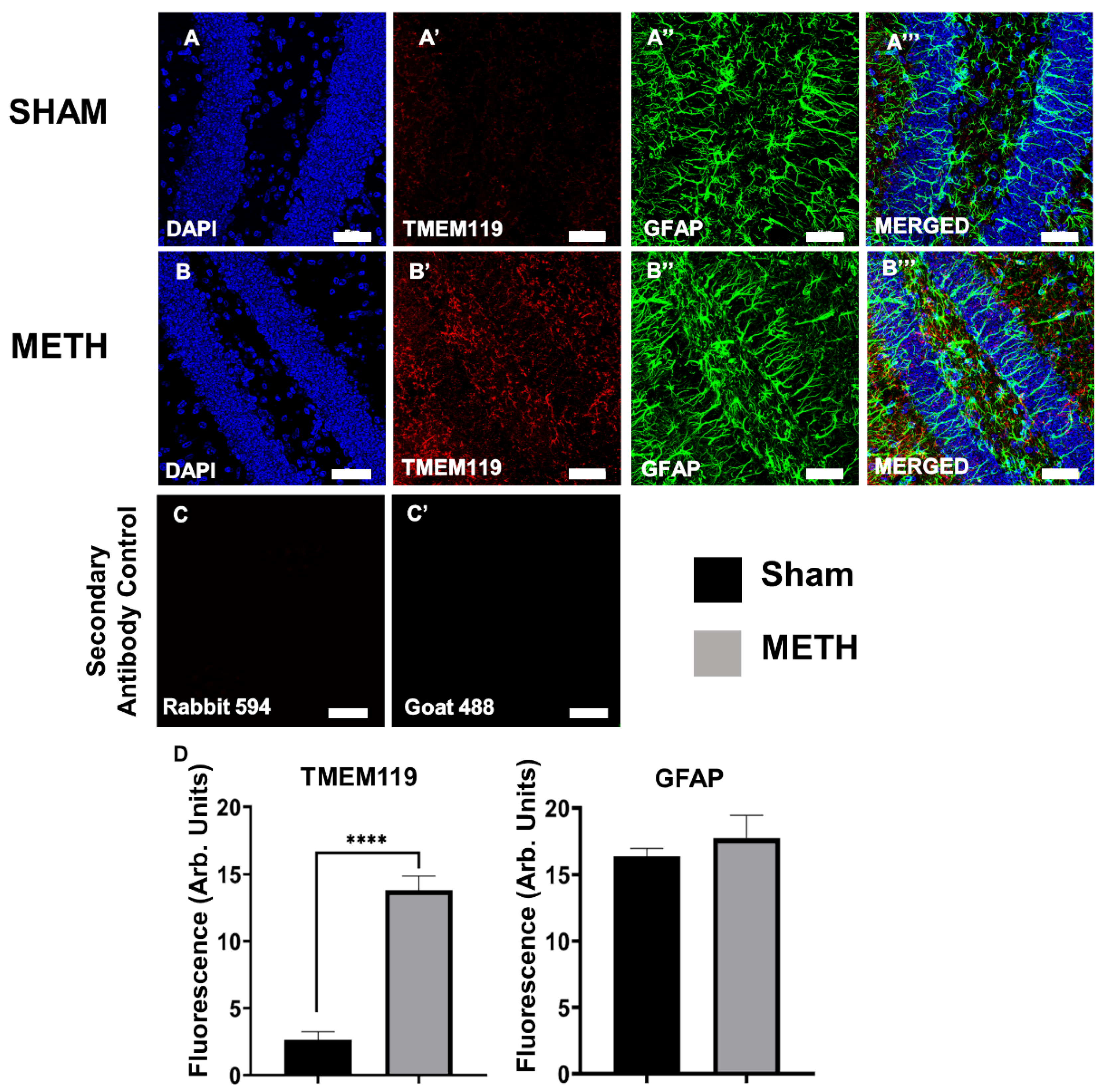
2.5. METH Causes Gliosis
2.6. METH Administration Changes Gene Expression in the Mid-Brain
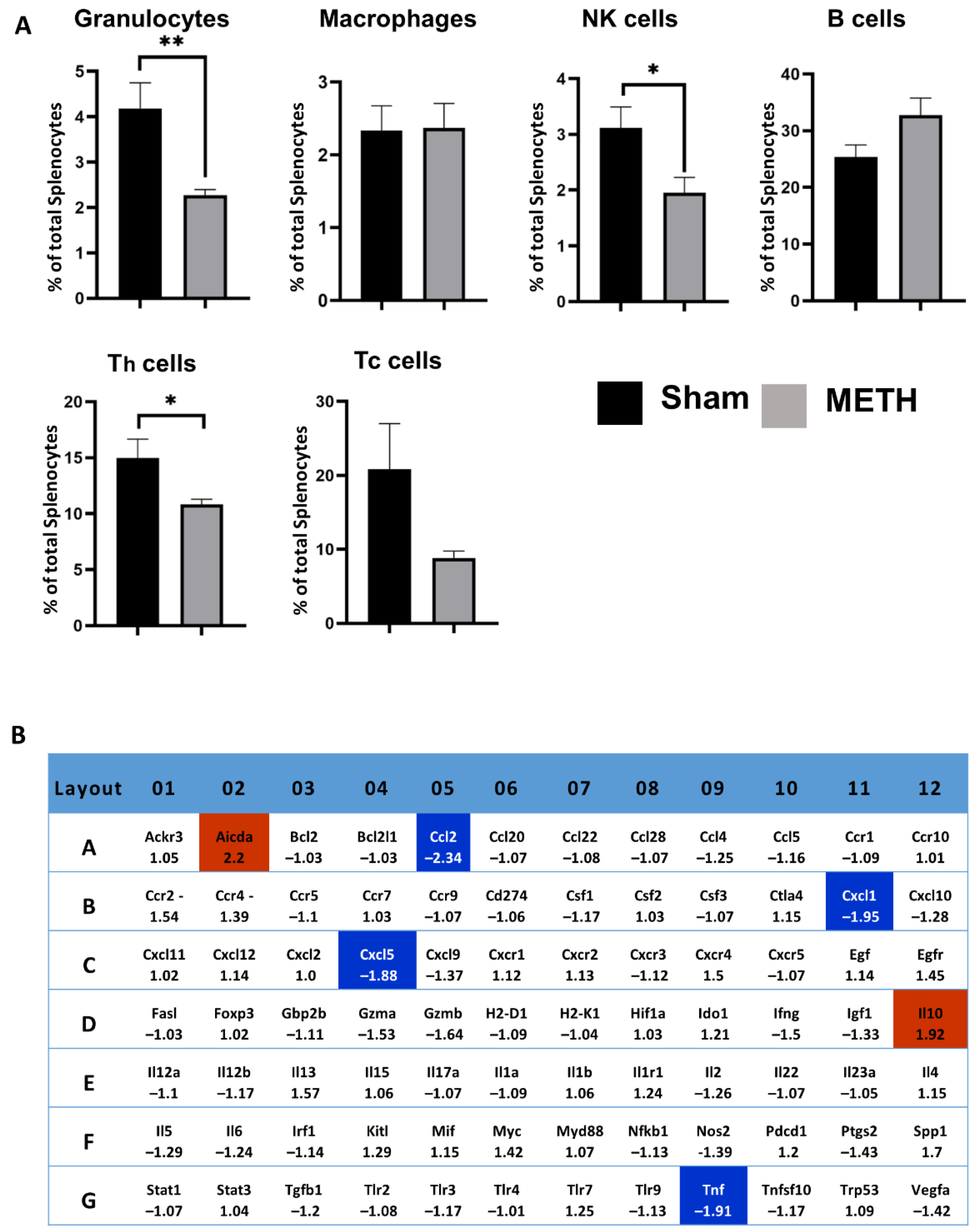
2.7. METH Alters Cellular Immune Responses and Genes in the Spleen
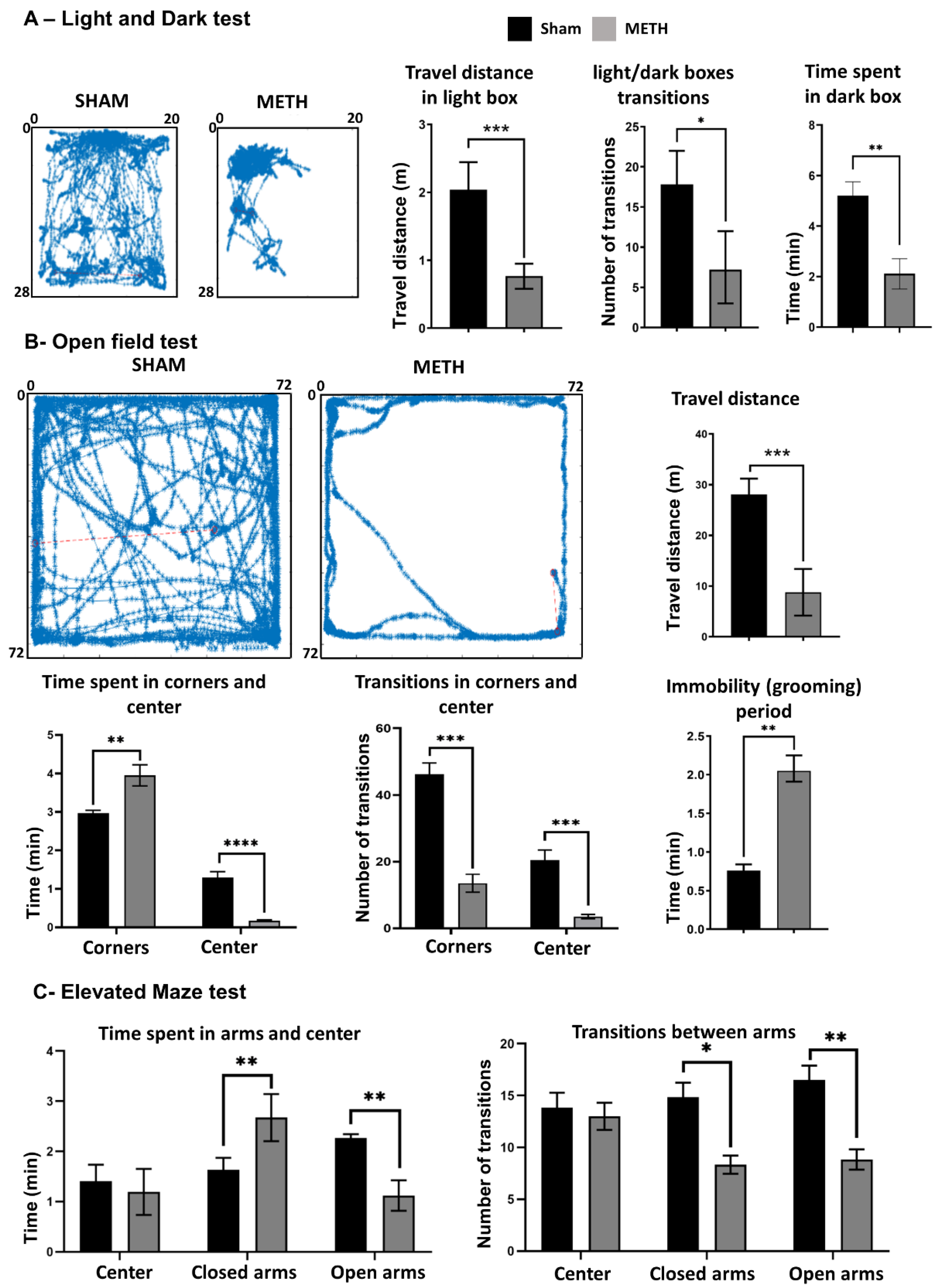
2.8. METH Causes Behavioral Changes
3. Materials and Methods
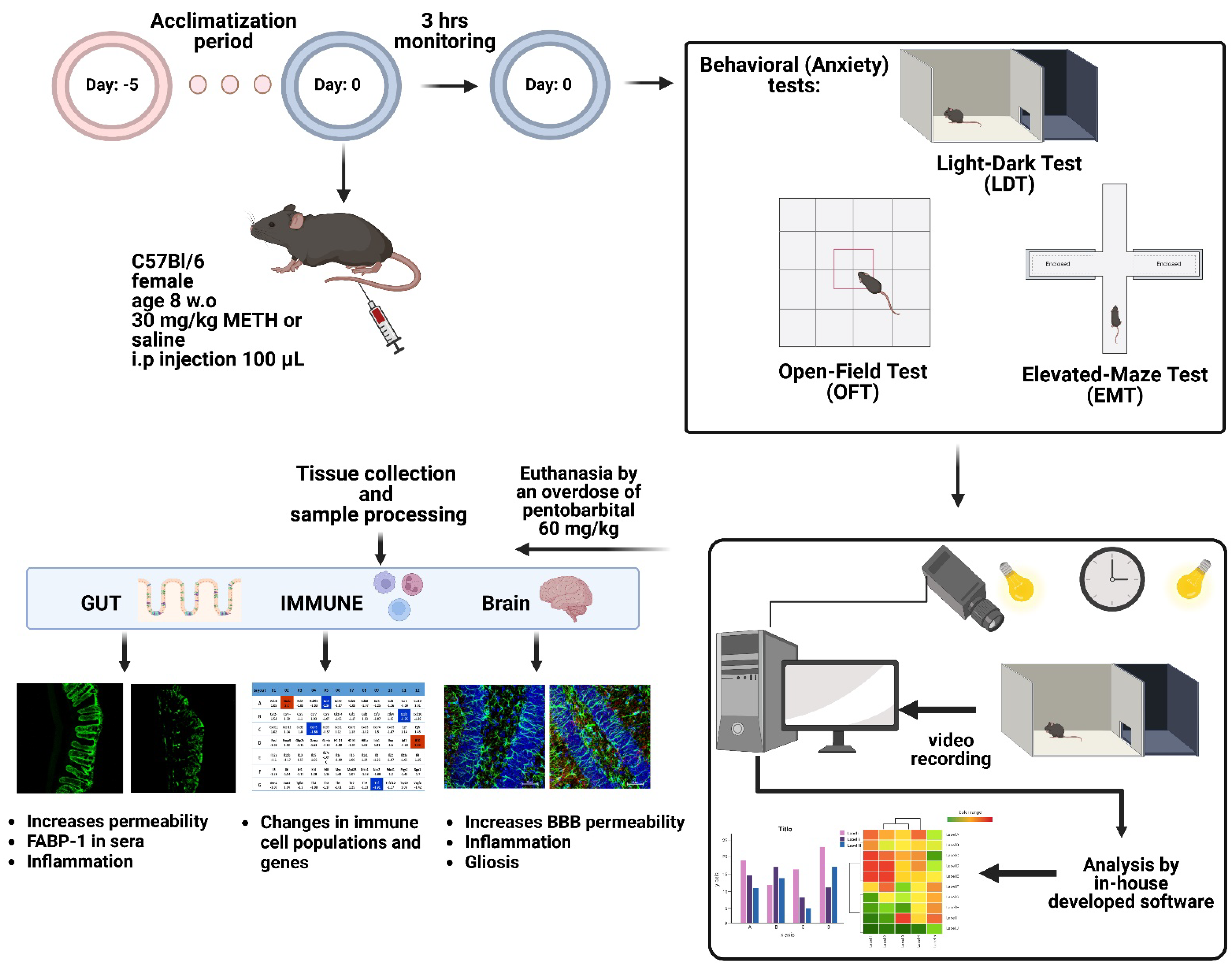
3.1. Animals
3.2. Treatment Protocols
3.3. Behavioral Assessment
3.3.1. Light and Dark Test (LDT)
3.3.2. Open-Field Test (OFT)
3.3.3. Elevated-Maze Test (EMT)
3.4. Blood and Tissue Collection
3.5. Immunohistocemistry
3.5.1. Gut Tissue
3.5.2. Mid-Brain Tissue
3.6. Fatty Acid Binding Protein-1 Assay
3.7. RNA Isolation
3.8. RT2 Profiler PCR Gene Arrays
3.9. RT-PCR
3.10. Statistical Analysis
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papageorgiou, M.; Raza, A.; Fraser, S.; Nurgali, K.; Apostolopoulos, V. Methamphetamine and its immune-modulating effects. Maturitas 2019, 121, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Hassanzadeganroudsari, M.; Kypreos, E.; Feehan, J.; Apostolopoulos, V. Immune to addiction: How immunotherapies can be used to combat methamphetamine addiction. Expert Rev. Vaccines 2021, 20, 707–715. [Google Scholar] [PubMed]
- Kevil, C.G.; Goeders, N.E.; Woolard, M.D.; Bhuiyan, M.S.; Dominic, P.; Kolluru, G.K.; Arnold, C.L.; Traylor, J.G.; Orr, A.W. Methamphetamine Use and Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2019, 39, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.; Lombardi, D.; Du, J.; Makrum, U.; Sitthichai, R.; Harrington, A.; Shukair, N.; Zhao, M.; Fan, X. Methamphetamine-associated psychosis: Clinical presentation, biological basis, and treatment options. Hum. Psychopharmacol. Clin. Exp. 2019, 34, e2710. [Google Scholar] [CrossRef] [PubMed]
- Glasner-Edwards, S.; Mooney, L.J. Methamphetamine psychosis: Epidemiology and management. CNS Drugs 2014, 28, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.S.; Boxenbaum, H.; Everhart, E.T.; Sequeira, G.; Mendelson, J.E.; Jones, R.T. The bioavailability of intranasal and smoked methamphetamine. Clin. Pharmacol. Ther. 2003, 74, 475–486. [Google Scholar] [CrossRef]
- Spalding, F. Methamphetamine: The Dangers of Crystal Meth; The Rosen Publishing Group, Inc.: New York, NY, USA, 2006. [Google Scholar]
- May, A.C.; Aupperle, R.L.; Stewart, J.L. Dark Times: The Role of Negative Reinforcement in Methamphetamine Addiction. Front. Psychiatry 2020, 11, 114. [Google Scholar] [CrossRef]
- Kirkpatrick, M.G.; Haney, M.; Vosburg, S.K.; Comer, S.D.; Foltin, R.W.; Hart, C.L. Methamphetamine self-administration by humans subjected to abrupt shift and sleep schedule changes. Psychopharmacology 2009, 203, 771–780. [Google Scholar] [CrossRef]
- Mendelson, J.; Rawson, R.; Newton, T.; Galloway, G.; de Wit, H.; Dewey, S.L.; Hart, C.L.; Epstein, D.H. Treatment of methamphetamine dependence. Mayo Clin. Proc. 2008, 83, 369–370. [Google Scholar] [CrossRef]
- Perez, A.Y.; Kirkpatrick, M.G.; Gunderson, E.W.; Marrone, G.; Silver, R.; Foltin, R.W.; Hart, C.L. Residual effects of intranasal methamphetamine on sleep, mood, and performance. Drug Alcohol Depend. 2008, 94, 258–262. [Google Scholar] [CrossRef][Green Version]
- Shaner, J.W.; Kimmes, N.; Saini, T.; Edwards, P. “Meth mouth”: Rampant caries in methamphetamine abusers. AIDS Patient Care STDs 2006, 20, 146–150. [Google Scholar] [CrossRef]
- Williams, N.; Covington, J.S., 3rd. Methamphetamine and meth mouth: An overview. J. Tenn. Dent. Assoc. 2006, 86, 32–35. [Google Scholar]
- Herr, R.D.; Caravati, E.M. Acute transient ischemic colitis after oral methamphetamine ingestion. Am. J. Emerg. Med. 1991, 9, 406–409. [Google Scholar] [CrossRef]
- Prakash, M.D.; Tangalakis, K.; Antonipillai, J.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. Methamphetamine: Effects on the brain, gut and immune system. Pharmacol. Res. 2017, 120, 60–67. [Google Scholar] [CrossRef]
- Persons, A.L.; Bradaric, B.D.; Dodiya, H.B.; Ohene-Nyako, M.; Forsyth, C.B.; Keshavarzian, A.; Shaikh, M.; Napier, T.C. Colon dysregulation in methamphetamine self-administering HIV-1 transgenic rats. PLoS ONE 2018, 13, e0190078. [Google Scholar] [CrossRef]
- Amini, A.; Nagalli, S. Bowel Ischemia. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2020. [Google Scholar]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. CMLS 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Northrop, N.A.; Yamamoto, B.K. Methamphetamine effects on blood-brain barrier structure and function. Front. Osci. 2015, 9, 69. [Google Scholar] [CrossRef]
- Rusyniak, D.E. Neurologic manifestations of chronic methamphetamine abuse. Neurol. Clin. 2011, 29, 641–655. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Zaheer, S.; Ahmed, M.E.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Natteru, P.A.; Iyer, S.; et al. Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front. Cell. Neurosci. 2017, 11, 216. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Berridge, C.W. Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2006, 31, 2332–2340. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef]
- Sharma, H.S.; Sjöquist, P.O.; Ali, S.F. Drugs of abuse-induced hyperthermia, blood-brain barrier dysfunction and neurotoxicity: Neuroprotective effects of a new antioxidant compound H-290/51. Curr. Pharm. Des. 2007, 13, 1903–1923. [Google Scholar] [CrossRef]
- Martins, T.; Burgoyne, T.; Kenny, B.A.; Hudson, N.; Futter, C.E.; Ambrosio, A.F.; Silva, A.P.; Greenwood, J.; Turowski, P. Methamphetamine-induced nitric oxide promotes vesicular transport in blood-brain barrier endothelial cells. Neuropharmacology 2013, 65, 74–82. [Google Scholar] [CrossRef]
- Sajja, R.K.; Rahman, S.; Cucullo, L. Drugs of abuse and blood-brain barrier endothelial dysfunction: A focus on the role of oxidative stress. J. Cereb. Blood Flow Metab. 2016, 36, 539–554. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Glasner-Edwards, S.; Marinelli-Casey, P.; Hillhouse, M.; Ang, A.; Mooney, L.J.; Rawson, R. Depression among methamphetamine users: Association with outcomes from the Methamphetamine Treatment Project at 3-year follow-up. J. Nerv. Ment. Dis. 2009, 197, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Kaye, S.; Duflou, J.; Lappin, J. Completed suicide among methamphetamine users: A national study. Suicide Life-Threat. Behav. 2019, 49, 328–337. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hassanzadeganroudsari, M.; Nurgali, K.; Apostolopoulos, V. Vaccine development against methamphetamine drug addiction. Expert Rev. Vaccines 2020, 19, 1105–1114. [Google Scholar] [CrossRef]
- Kamal Hossain, M.; Davidson, M.; Feehan, J.; Deraos, G.; Nurgali, K.; Matsoukas, J.; Apostolopoulos, V. Development and characterization of a novel conjugated methamphetamine vaccine. Vaccine 2022, 40, 5882–5891. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, S.; Dai, Y.; Chen, F.; Wang, K. Methamphetamine Induces Intestinal Inflammatory Injury via Nod-Like Receptor 3 Protein (NLRP3) Inflammasome Overexpression In Vitro and In Vivo. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 8515–8526. [Google Scholar] [CrossRef]
- Attaran, H. Fatal Small Intestinal Ischemia Due to Methamphetamine Intoxication: Report of a Case with Autopsy Results. Acta Med. Iran. 2017, 55, 344–347. [Google Scholar]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef]
- Mahajan, S.D.; Aalinkeel, R.; Sykes, D.E.; Reynolds, J.L.; Bindukumar, B.; Adal, A.; Qi, M.; Toh, J.; Xu, G.; Prasad, P.N.; et al. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: Implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res. 2008, 1203, 133–148. [Google Scholar] [CrossRef]
- Kozan, P.A.; McGeough, M.D.; Peña, C.A.; Mueller, J.L.; Barrett, K.E.; Marchelletta, R.R.; Sivagnanam, M. Mutation of EpCAM leads to intestinal barrier and ion transport dysfunction. J. Mol. Med. 2015, 93, 535–545. [Google Scholar] [CrossRef]
- Trzpis, M.; McLaughlin, P.M.J.; de Leij, L.M.F.H.; Harmsen, M.C. Epithelial cell adhesion molecule: More than a carcinoma marker and adhesion molecule. Am. J. Pathol. 2007, 171, 386–395. [Google Scholar] [CrossRef]
- Straub, R.H.; Schradin, C. Chronic inflammatory systemic diseases: An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol. Med. Public Health 2016, 2016, 37–51. [Google Scholar] [CrossRef]
- Pelsers, M.M.A.L.; Namiot, Z.; Kisielewski, W.; Namiot, A.; Januszkiewicz, M.; Hermens, W.T.; Glatz, J.F.C. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin. Biochem. 2003, 36, 529–535. [Google Scholar] [CrossRef]
- Lau, E.; Marques, C.; Pestana, D.; Santoalha, M.; Carvalho, D.; Freitas, P.; Calhau, C. The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr. Metab. 2016, 13, 31. [Google Scholar] [CrossRef]
- Celi, P.; Verlhac, V.; Pérez Calvo, E.; Schmeisser, J.; Kluenter, A.-M. Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim. Feed. Sci. Technol. 2019, 250, 9–31. [Google Scholar] [CrossRef]
- Sulaiman, S.; Marciani, L. MRI of the Colon in the Pharmaceutical Field: The Future before us. Pharmaceutics 2019, 11, 416. [Google Scholar] [CrossRef]
- Precup, G.; Vodnar, D.C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: A comprehensive literature review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef]
- Azzouz, L.L.; Sharma, S. Physiology, large intestine. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2019. [Google Scholar]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.R.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.-F.; Gasche, C.; Geboes, K.; et al. Toward an Integrated Clinical, Molecular and Serological Classification of Inflammatory Bowel Disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19, 269076. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Park, M.; Kim, H.-J.; Lim, B.; Wylegala, A.; Toborek, M. Methamphetamine-induced Occludin Endocytosis Is Mediated by the Arp2/3 Complex-regulated Actin Rearrangement*♦. J. Biol. Chem. 2013, 288, 33324–33334. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Potula, R.; Fan, S.; Eidem, T.; Papugani, A.; Reichenbach, N.; Dykstra, H.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; et al. Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J. Cereb. Blood Flow Metab. 2009, 29, 1933–1945. [Google Scholar] [CrossRef]
- Martins, T.; Baptista, S.; Gonçalves, J.; Leal, E.; Milhazes, N.; Borges, F.; Ribeiro, C.F.; Quintela, O.; Lendoiro, E.; López-Rivadulla, M.; et al. Methamphetamine transiently increases the blood-brain barrier permeability in the hippocampus: Role of tight junction proteins and matrix metalloproteinase-9. Brain Res. 2011, 1411, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Turowski, P.; Kenny, B.A. The blood-brain barrier and methamphetamine: Open sesame? Front. Neurosci. 2015, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, J.F.; Robinson, B.; Ali, S.; Schmued, L.C. Neurotoxic-related changes in tyrosine hydroxylase, microglia, myelin, and the blood-brain barrier in the caudate-putamen from acute methamphetamine exposure. Synapse 2008, 62, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D. Markers for blood-brain barrier integrity: How appropriate is Evans blue in the twenty-first century and what are the alternatives? Front. Neurosci. 2015, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, J.F.; Ali, S. High doses of methamphetamine that cause disruption of the blood-brain barrier in limbic regions produce extensive neuronal degeneration in mouse hippocampus. Synapse 2006, 60, 521–532. [Google Scholar] [CrossRef]
- Frausto, R.F.; Chung, D.D.; Boere, P.M.; Swamy, V.S.; Duong, H.N.V.; Kao, L.; Azimov, R.; Zhang, W.; Carrigan, L.; Wong, D. ZEB1 insufficiency causes corneal endothelial cell state transition and altered cellular processing. PLoS ONE 2019, 14, e0218279. [Google Scholar] [CrossRef]
- Sheng, P.; Cerruti, C.; Cadet, J.L. Methamphetamine (METH) causes reactive gliosis in vitro: Attenuation by the ADP-ribosylation (ADPR) inhibitor, benzamide. Life Sci. 1994, 55, Pl51–Pl54. [Google Scholar] [CrossRef]
- Kays, J.S.; Yamamoto, B.K. Evaluation of Microglia/Macrophage Cells from Rat Striatum and Prefrontal Cortex Reveals Differential Expression of Inflammatory-Related mRNA after Methamphetamine. Brain Sci. 2019, 9, 340. [Google Scholar] [CrossRef]
- Kitamura, O.; Takeichi, T.; Wang, E.L.; Tokunaga, I.; Ishigami, A.; Kubo, S. Microglial and astrocytic changes in the striatum of methamphetamine abusers. Leg. Med. 2010, 12, 57–62. [Google Scholar] [CrossRef]
- Gonçalves, J.; Martins, T.; Ferreira, R.; Milhazes, N.; Borges, F.; Ribeiro, C.F.; Malva, J.O.; Macedo, T.R.; Silva, A.P. Methamphetamine-Induced Early Increase of IL-6 and TNF-α mRNA Expression in the Mouse Brain. Ann. N. Y. Acad. Sci. 2008, 1139, 103–111. [Google Scholar] [CrossRef]
- Moreira da Silva Santos, A.; Kelly, J.P.; Doyle, K.M. Dose-Dependent Effects of Binge-Like Methamphetamine Dosing on Dopamine and Neurotrophin Levels in Rat Brain. Neuropsychobiology 2017, 75, 63–71. [Google Scholar] [CrossRef]
- Dang, J.; Tiwari, S.K.; Agrawal, K.; Hui, H.; Qin, Y.; Rana, T.M. Glial cell diversity and methamphetamine-induced neuroinflammation in human cerebral organoids. Mol. Psychiatry 2021, 26, 1194–1207. [Google Scholar] [CrossRef]
- Castellano, P.; Nwagbo, C.; Martinez, L.R.; Eugenin, E.A. Methamphetamine compromises gap junctional communication in astrocytes and neurons. J. Neurochem. 2016, 137, 561–575. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, L.; Shen, Q.; Bai, X.; Di, X. Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behav. Neurol. 2015, 2015, 103969. [Google Scholar] [CrossRef]
- Wang, Z.; He, D.; Zeng, Y.Y.; Zhu, L.; Yang, C.; Lu, Y.J.; Huang, J.Q.; Cheng, X.Y.; Huang, X.H.; Tan, X.J. The spleen may be an important target of stem cell therapy for stroke. J. Neuroinflamm. 2019, 16, 20. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin. J. Traumatol. 2018, 21, 137–151. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Kim, E.; Yang, J.; Beltran, C.D.; Cho, S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J. Cereb. Blood Flow Metab. 2014, 34, 1411–1419. [Google Scholar] [CrossRef]
- Jickling, G.C.; Liu, D.; Ander, B.P.; Stamova, B.; Zhan, X.; Sharp, F.R. Targeting neutrophils in ischemic stroke: Translational insights from experimental studies. J. Cereb. Blood Flow Metab. 2015, 35, 888–901. [Google Scholar] [CrossRef]
- Harms, R.; Morsey, B.; Boyer, C.W.; Fox, H.S.; Sarvetnick, N. Methamphetamine administration targets multiple immune subsets and induces phenotypic alterations suggestive of immunosuppression. PLoS ONE 2012, 7, e49897. [Google Scholar] [CrossRef]
- Gires, O.; Kieu, C.; Fix, P.; Schmitt, B.; Munz, M.; Wollenberg, B.; Zeidler, R. Tumor necrosis factor alpha negatively regulates the expression of the carcinoma-associated antigen epithelial cell adhesion molecule. Cancer 2001, 92, 620–628. [Google Scholar] [CrossRef]
- Nakajima, A.; Yamada, K.; Nagai, T.; Uchiyama, T.; Miyamoto, Y.; Mamiya, T.; He, J.; Nitta, A.; Mizuno, M.; Tran, M.H.; et al. Role of Tumor Necrosis Factor-α in Methamphetamine-Induced Drug Dependence and Neurotoxicity. J. Neurosci. 2004, 24, 2212–2225. [Google Scholar] [CrossRef]
- Parikh, N.U.; Aalinkeel, R.; Reynolds, J.L.; Nair, B.B.; Sykes, D.E.; Mammen, M.J.; Schwartz, S.A.; Mahajan, S.D. Galectin-1 suppresses methamphetamine induced neuroinflammation in human brain microvascular endothelial cells: Neuroprotective role in maintaining blood brain barrier integrity. Brain Res. 2015, 1624, 175–187. [Google Scholar] [CrossRef]
- Kara, N.; Stukalin, Y.; Einat, H. Revisiting the validity of the mouse forced swim test: Systematic review and meta-analysis of the effects of prototypic antidepressants. Neurosci. Biobehav. Rev. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Tanaka, S.; Young, J.W.; Halberstadt, A.L.; Masten, V.L.; Geyer, M.A. Four factors underlying mouse behavior in an open field. Behav. Brain Res. 2012, 233, 55–61. [Google Scholar] [CrossRef]
- Molendijk, M.L.; de Kloet, E.R. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 2015, 62, 389–391. [Google Scholar] [CrossRef]
- Burghardt, G.M. Insights found in century-old writings on animal behaviour and some cautions for today. Anim. Behav. 2020, 164, 241–249. [Google Scholar] [CrossRef]
- Santos, N.; Beck, A.; Fontbonne, A. A review of maternal behaviour in dogs and potential areas for further research. J. Small Anim. Pract. 2020, 61, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Belovicova, K.; Bogi, E.; Csatlosova, K.; Dubovicky, M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017, 10, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Arrant, A.E.; Schramm-Sapyta, N.L.; Kuhn, C.M. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav. Brain Res. 2013, 256, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.R.; Crawley, J.N. Anxiety-Related Behaviors in Mice. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; Buccafusco, J.J., Ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated plus maze for mice. JoVE J. Vis. Exp. 2008, 22, e1088. [Google Scholar] [CrossRef]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. JoVE J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Fukumura, M.; Cappon, G.D.; Pu, C.; Broening, H.W.; Vorhees, C.V. A single dose model of methamphetamine-induced neurotoxicity in rats: Effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Res. 1998, 806, 1–7. [Google Scholar] [CrossRef]
- Hendrickson, H.P.; Hardwick, W.C.; McMillan, D.E.; Owens, S.M. Bioavailability of (+)-methamphetamine in the pigeon following an intramuscular dose. Pharm. Biochem. Behav. 2008, 90, 382–386. [Google Scholar] [CrossRef]
- Misslin, R.; Belzung, C.; Vogel, E. Behavioural validation of a light/dark choice procedure for testing anti-anxiety agents. Behav. Processes 1989, 18, 119–132. [Google Scholar] [CrossRef]
- Nku, C.; Oghale, G.; Ajiwhen, I. Locomotor Behaviour and Anxiety in the Open Field and Light/Dark Box in CD1 Mice Treated with Aspirin, Cataflam and Ethanolic Extract of Cannabis sativa. Br. J. Med. Med. Res. 2015, 6, 563–572. [Google Scholar] [CrossRef]
- Brown, R.E.; Corey, S.C.; Moore, A.K. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behav. Genet. 1999, 29, 263–271. [Google Scholar] [CrossRef]
- Jayathilake, A.G.; Hassanzadeganroudsari, M.; Jovanovska, V.; Luwor, R.B.; Nurgali, K.; Su, X.Q. The comparative anti-cancer effects of krill oil and oxaliplatin in an orthotopic mouse model of colorectal cancer. Nutr. Metab. 2022, 19, 12. [Google Scholar] [CrossRef]
- Vassileva, G.; Huwyler, L.; Poirier, K.; Agellon, L.B.; Toth, M.J. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. FASEB J. 2000, 14, 2040–2046. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schep, L.J.; Slaughter, R.J.; Beasley, D.M.G. The clinical toxicology of metamfetamine. Clin. Toxicol. 2010, 48, 675–694. [Google Scholar] [CrossRef]
- Rommel, N.; Rohleder, N.H.; Wagenpfeil, S.; Haertel-Petri, R.; Kesting, M.R. Evaluation of methamphetamine-associated socioeconomic status and addictive behaviors, and their impact on oral health. Addict. Behav. 2015, 50, 182–187. [Google Scholar] [CrossRef]
- Salamanca, S.A.; Sorrentino, E.E.; Nosanchuk, J.D.; Martinez, L.R. Impact of methamphetamine on infection and immunity. Front. Neurosci. 2014, 8, 445. [Google Scholar] [CrossRef]
- Ellis, M.S.; Kasper, Z.A.; Cicero, T.J. Twin epidemics: The surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend. 2018, 193, 14–20. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hassanzadeganroudsari, M.; Apostolopoulos, V. Why METH users are at high risk of fatality due to COVID-19 infection? Expert Rev. Vaccines 2020, 19, 1101–1103. [Google Scholar] [CrossRef]
- Brannan, T.A.; Soundararajan, S.; Houghton, B.L. Methamphetamine-associated shock with intestinal infarction. Medscape Gen. Med. 2004, 6, 6. [Google Scholar]
- Carlson, T.L.; Plackett, T.P.; Gagliano, R.A., Jr.; Smith, R.R. Methamphetamine-induced paralytic ileus. Hawai’i J. Med. Public Health 2012, 71, 44. [Google Scholar]
- Saito, M.; Terada, M.; Kawata, T.; Ito, H.; Shigematsu, N.; Kromkhun, P.; Yokosuka, M.; Saito, T.R. Effects of single or repeated administrations of methamphetamine on immune response in mice. Exp. Anim. 2008, 57, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Mihu, M.R.; Gacser, A.; Santambrogio, L.; Nosanchuk, J.D. Methamphetamine enhances histoplasmosis by immunosuppression of the host. J. Infect. Dis. 2009, 200, 131–141. [Google Scholar] [CrossRef]
- Potula, R.; Hawkins, B.J.; Cenna, J.M.; Fan, S.; Dykstra, H.; Ramirez, S.H.; Morsey, B.; Brodie, M.R.; Persidsky, Y. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J. Immunol. 2010, 185, 2867–2876. [Google Scholar] [CrossRef]
- Tallóczy, Z.; Martinez, J.; Joset, D.; Ray, Y.; Gácser, A.; Toussi, S.; Mizushima, N.; Nosanchuk, J.D.; Goldstein, H.; Loike, J.; et al. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008, 4, e28. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidson, M.; Mayer, M.; Habib, A.; Rashidi, N.; Filippone, R.T.; Fraser, S.; Prakash, M.D.; Sinnayah, P.; Tangalakis, K.; Mathai, M.L.; et al. Methamphetamine Induces Systemic Inflammation and Anxiety: The Role of the Gut–Immune–Brain Axis. Int. J. Mol. Sci. 2022, 23, 11224. https://doi.org/10.3390/ijms231911224
Davidson M, Mayer M, Habib A, Rashidi N, Filippone RT, Fraser S, Prakash MD, Sinnayah P, Tangalakis K, Mathai ML, et al. Methamphetamine Induces Systemic Inflammation and Anxiety: The Role of the Gut–Immune–Brain Axis. International Journal of Molecular Sciences. 2022; 23(19):11224. https://doi.org/10.3390/ijms231911224
Chicago/Turabian StyleDavidson, Majid, Marina Mayer, Amanda Habib, Niloufar Rashidi, Rhiannon Talia Filippone, Sarah Fraser, Monica D. Prakash, Puspha Sinnayah, Kathy Tangalakis, Michael L. Mathai, and et al. 2022. "Methamphetamine Induces Systemic Inflammation and Anxiety: The Role of the Gut–Immune–Brain Axis" International Journal of Molecular Sciences 23, no. 19: 11224. https://doi.org/10.3390/ijms231911224
APA StyleDavidson, M., Mayer, M., Habib, A., Rashidi, N., Filippone, R. T., Fraser, S., Prakash, M. D., Sinnayah, P., Tangalakis, K., Mathai, M. L., Nurgali, K., & Apostolopoulos, V. (2022). Methamphetamine Induces Systemic Inflammation and Anxiety: The Role of the Gut–Immune–Brain Axis. International Journal of Molecular Sciences, 23(19), 11224. https://doi.org/10.3390/ijms231911224








