Characterization of a Novel Myrosinase with High Activity from Marine Bacterium Shewanella baltica Myr-37
Abstract
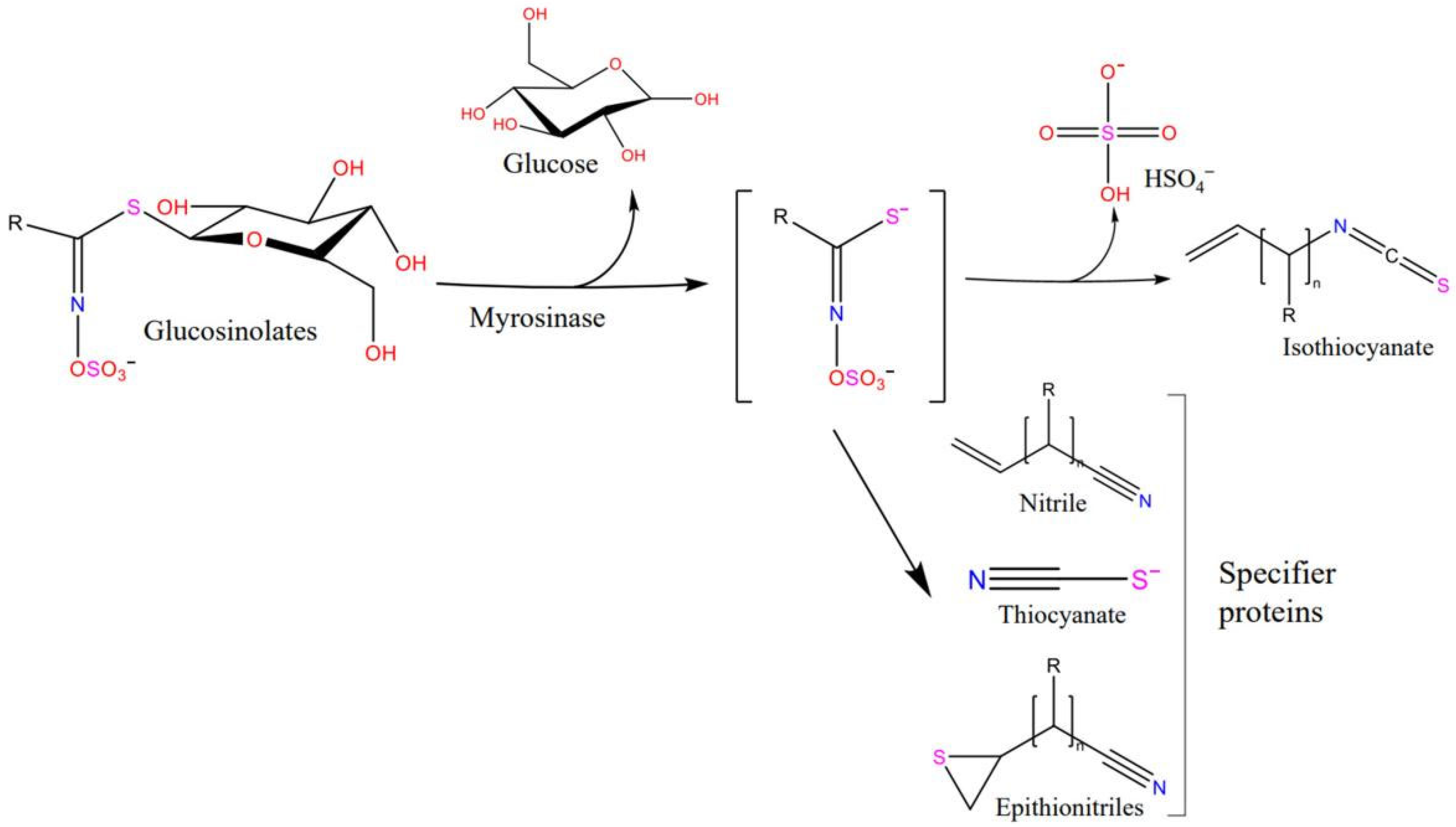
:1. Introduction
2. Results
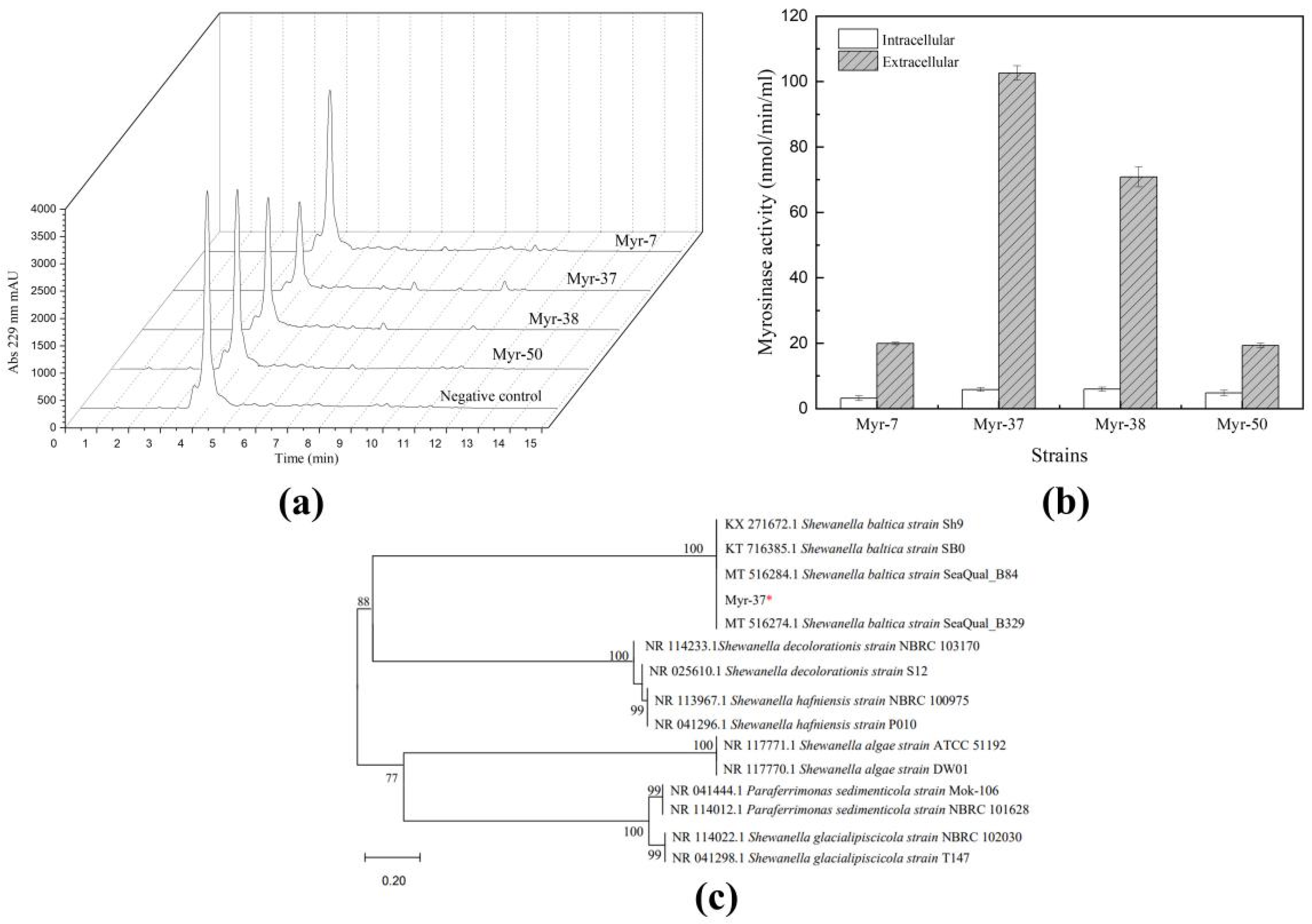
2.1. Isolation and Identification of Myrosinase-Producing Bacteria
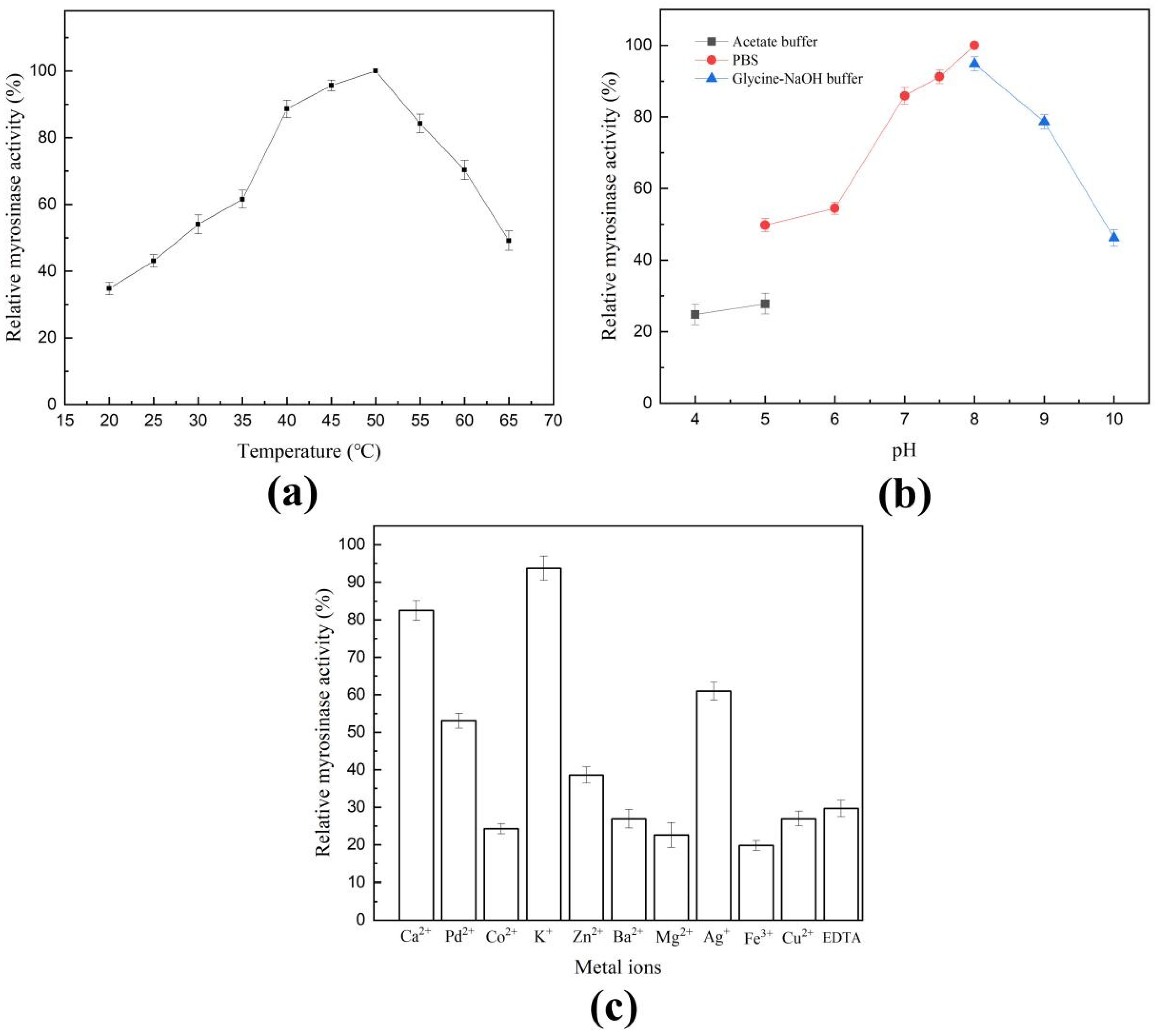
2.2. Characterization of Shewanella baltica Myr-37 Myrosinase
2.3. Purification of Shewanella baltica Myr-37 Myrosinase
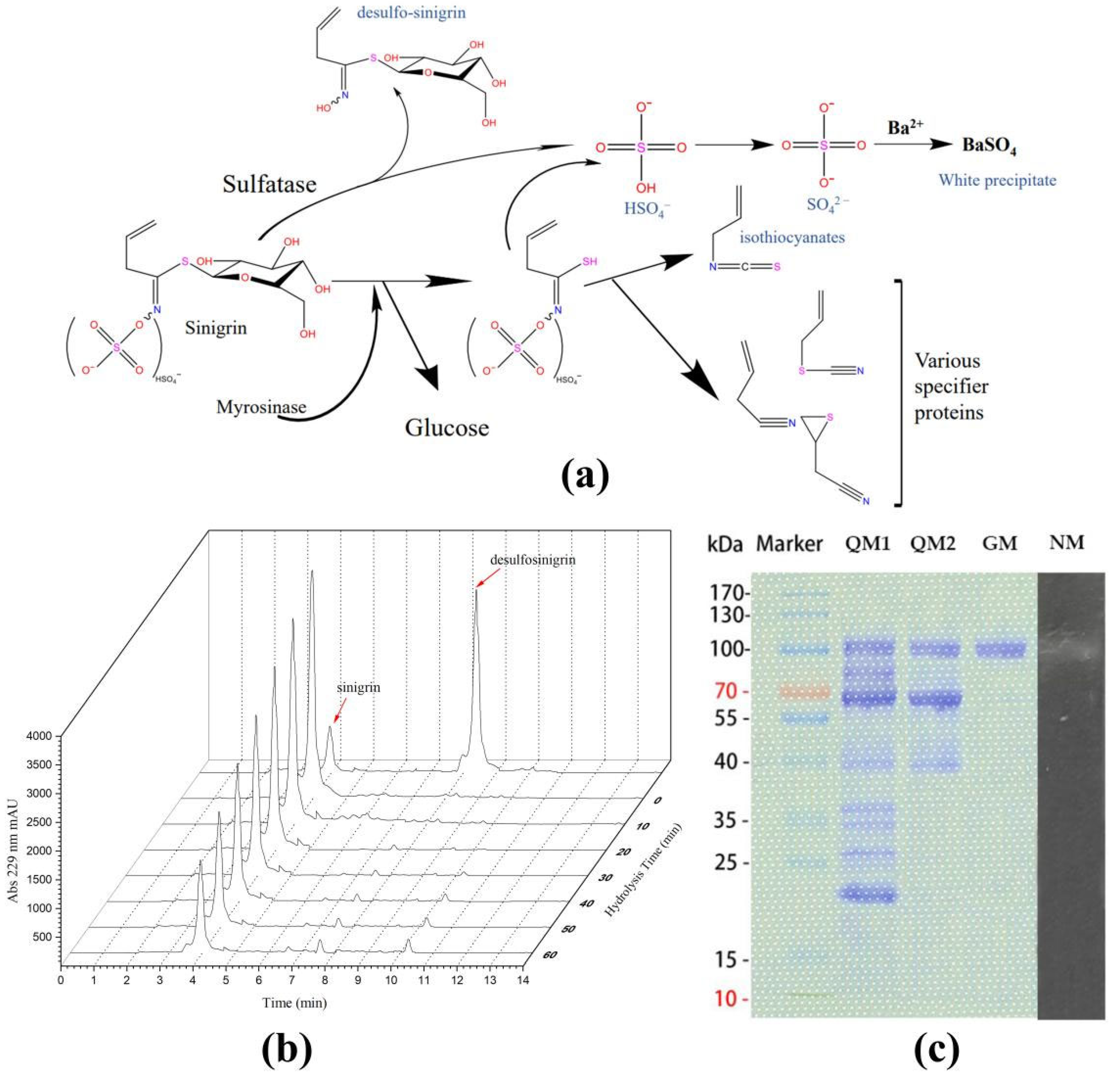
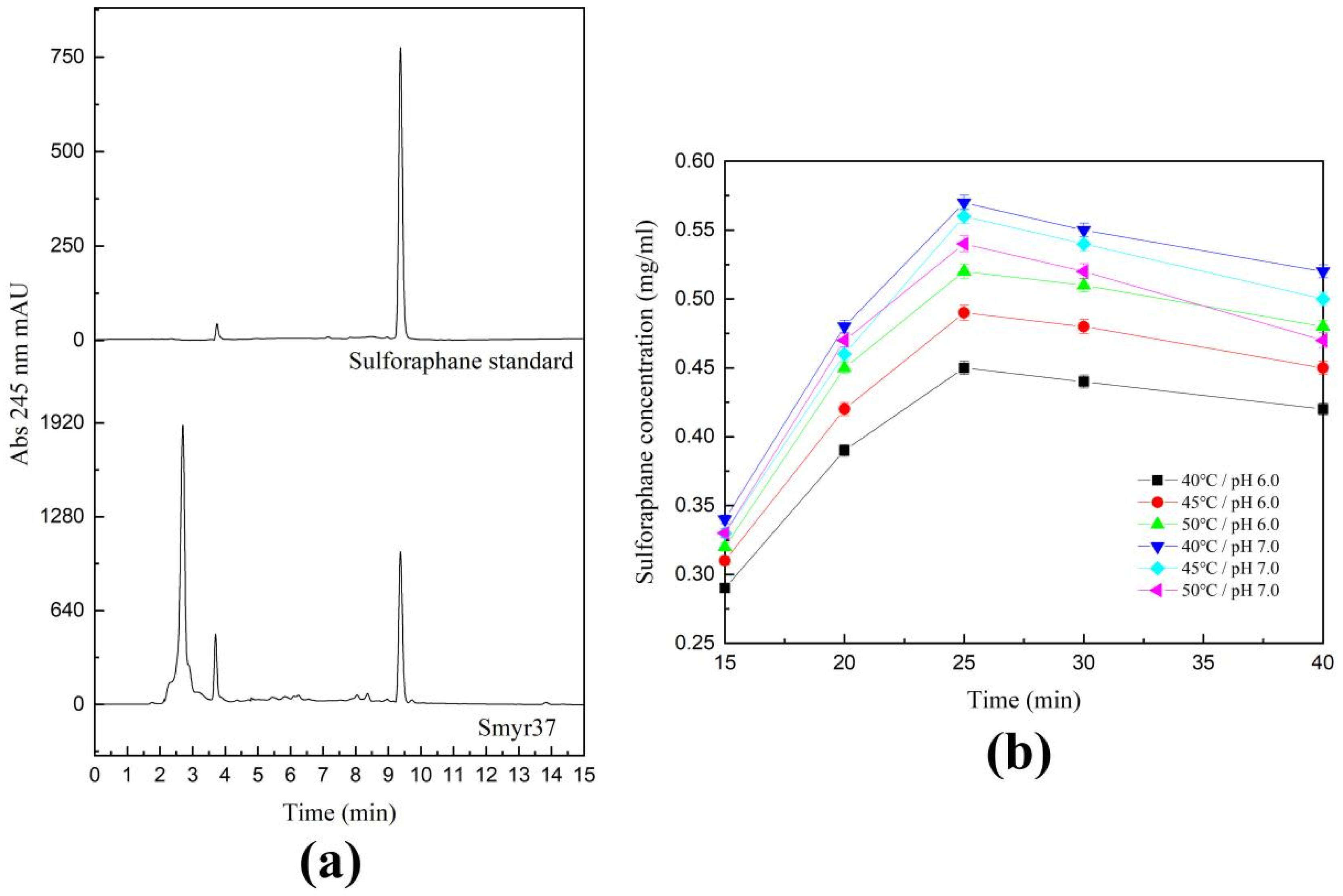
2.4. Catalytic Activity of Shewanella baltica Myr-37 Myrosinase against Glucoraphanin
2.5. Exogenous Hydrolysis of Glucoraphanin by Shewanella baltica Myr-37 Myrosinase
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Screening of the Sinigrin-Degrading Strains from the Marine Mud
4.3. Myrosinase Activity Assay
4.4. Identification of the Myrosinase-Producing Strain
4.5. Characterization of Myrosinase Smyr37 Produced by Shewanella baltica Myr-37
4.6. Native-PAGE
4.7. Myrosinase Purification
4.8. HPLC Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kissen, R.; Rossiter, J.T.; Bones, A.M. The ‘mustard oil bomb’: Not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 2008, 8, 69–86. [Google Scholar] [CrossRef]
- Lv, Q.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. The Cellular and subcellular organization of the glucosinolate-myrosinase system against herbivores and pathogens. Int. J. Mol. Sci. 2022, 23, 1577. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Zhang, J.; Yan, Q.; Miao, C.L.; Han, W.K.; Hou, W.; Yang, K.; Hansson, B.S.; Peng, Y.C.; Guo, J.M. The molecular basis of host selection in a crucifer-specialized moth. Curr. Biol. 2020, 30, 4476–4482.e5. [Google Scholar] [CrossRef] [PubMed]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Xu, L.; Wei, Y.; Tang, Y.; Hu, Q.; Liu, X.; Li, X.; Davis, J.; Smith, R.; et al. Decreasing risk of psychosis by sulforaphane study protocol for a randomized, double-blind, placebo-controlled, clinical multi-centre trial. Early Interv. Psychiatry 2021, 15, 585–594. [Google Scholar] [CrossRef]
- Zhang, C.; Su, Z.Y.; Khor, T.O.; Shu, L.; Kong, A.N. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochem. Pharmacol. 2013, 85, 1398–1404. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Deregowska, A.; Wnuk, M. Sulforaphane-induced cell cycle arrest and senescence are accompanied by DNA hypomethylation and changes in microRNA profile in breast cancer cells. Theranostics 2017, 7, 3461–3477. [Google Scholar] [CrossRef]
- Plaszko, T.; Szucs, Z.; Vasas, G.; Gonda, S. Effects of glucosinolate-derived isothiocyanates on Fungi: A comprehensive review on direct effects, mechanisms, structure-activity relationship data and possible agricultural applications. J. Fungi 2021, 7, 539. [Google Scholar] [CrossRef]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A broccoli bioactive phytocompound with cancer preventive potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef]
- Agerbirk, N.; Vos, M.; Kim, J.H.; Jander, G. Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 2009, 8, 101–120. [Google Scholar] [CrossRef]
- Liou, C.S.; Sirk, S.J.; Diaz, C.; Klein, A.P.; Sattely, E.S. A metabolic pathway for activation of dietary glucosinolates by a human gut symbiont. Cell 2020, 180, 717–728.e19. [Google Scholar] [CrossRef] [PubMed]
- Plaszko, T.; Szucs, Z.; Vasas, G.; Gonda, S. Interactions of fungi with non-isothiocyanate products of the plant glucosinolate pathway: A review on product formation, antifungal activity, mode of action and biotransformation. Phytochemistry 2022, 200, 113245–113274. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, H.; Qiu, Y.; Dong, Y.; Hamouda, H.I.; Balah, M.A.; Mao, X. Biochemical characterization of a novel myrosinase Rmyr from Rahnella inusitata for high-level preparation of sulforaphene and sulforaphane. J. Agric. Food Chem. 2022, 70, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Schepici, G.; Bramanti, P.; Mazzon, E. Efficacy of sulforaphane in neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 8637. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, B.; Domenica, M.; Di, M.; Malfa, G.A.; Renis, M. Rapha Myr, a blend of sulforaphane and myrosinase, exerts antitumor and Anoikis-Sensitizing effects on human astrocytoma cells modulating sirtuins and DNA methylation. Int. J. Mol. Sci. 2020, 21, 5328. [Google Scholar] [CrossRef] [PubMed]
- Eisenschmidt-Bonn, D.; Schneegans, N.; Backenkohler, A.; Wittstock, U.; Brandt, W. Structural diversification during glucosinolate breakdown: Mechanisms of thiocyanate, epithionitrile and simple nitrile formation. Plant J. 2019, 99, 329–343. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, C.; Tan, X.; Hagedoorn, P.L.; Gu, C.; Xu, H.; Zhou, X. Effect of aliphatic diamine spacer length on enzymatic performance of myrosinase immobilized on chitosan microsphere and its application for sulforaphene production. J. Biotechnol. 2019, 299, 79–85. [Google Scholar] [CrossRef]
- Sangkret, S.; Pongmalai, P.; Devahastin, S.; Chiewchan, N. Enhanced production of sulforaphane by exogenous glucoraphanin hydrolysis catalyzed by myrosinase extracted from Chinese flowering cabbage (Brassica rapa var. parachinensis). Sci. Rep. 2019, 9, 9882–9889. [Google Scholar] [CrossRef]
- Bhat, R.; Vyas, D. Myrosinase: Insights on structural, catalytic, regulatory, and environmental interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef]
- Li, X.; Kushad, M.M. Purification and characterization of myrosinase from horseradish (Armoracia rusticana) roots. Plant Physiol. Biochem. 2005, 43, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.; Lognay, G.; Wathelet, J.P. Characterisation of aphid myrosinase and degradation studies of glucosinolates. Arch. Insect Biochem. 2002, 50, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Albaser, A.; Kazana, E.; Bennett, M.H.; Cebeci, F.; Luang-In, V.; Spanu, P.D.; Rossiter, J.T. Discovery of a bacterial glycoside hydrolase family 3 (GH3) beta-gglucosidase with myrosinase activity from a Citrobacter strain isolated from soil. J. Agric. Food Chem. 2016, 64, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhu, W.; Zhang, C.; Yin, L.; Zhang, Y.; Liu, L.; Yuan, H. Identification of two myrosinases from a Leclercia adecarboxylata Strain and investigation of its tolerance mechanism to glucosinolate hhydrolysate. J. Agric. Food Chem. 2021, 69, 14151–14164. [Google Scholar] [CrossRef]
- Luang-In, V.; Narbad, A.; Nueno-Palop, C.; Mithen, R.; Bennett, M.; Rossiter, J.T. The metabolism of methylsulfinylalkyl- and methylthioalkyl-glucosinolates by a selection of human gut bacteria. Mol. Nutr. Food Res. 2014, 58, 875–883. [Google Scholar] [CrossRef]
- Cordeiro, R.P.; Doria, J.H.; Zhanel, G.G.; Sparling, R.; Holley, R.A. Role of glycoside hydrolase genes in sinigrin degradation by E. coli O157:H7. Int. J. Food Mic. 2015, 205, 105–111. [Google Scholar] [CrossRef]
- Zhou, C.; Tokuhisa, J.G.; Bevan, D.R.; Esen, A. Properties of beta-thioglucoside hydrolases (TGG1 and TGG2) from leaves of Arabidopsis thaliana. Plant Sci. 2012, 191–192, 82–92. [Google Scholar] [CrossRef]
- Loebers, A.; Müller-Uri, F.; Kreis, W. A young root-specific gene (ArMY2) from horseradish encoding a MYR II myrosinase with kinetic preference for the root-specific glucosinolate gluconasturtiin. Phytochemistry 2014, 99, 26–35. [Google Scholar] [CrossRef]
- Andersson, D.; Chakrabarty, R.; Bejai, S.; Zhang, J.; Rask, L.; Meijer, J. Myrosinases from root and leaves of Arabidopsis thaliana have different catalytic properties. Phytochemistry 2009, 70, 1345–1354. [Google Scholar] [CrossRef]
- Galletti, S.; Sala, E.; Leoni, O.; Cinti, S.; Cerato, C. Aspergillus flavus transformation of glucosinolates to nitriles by an arylsulfatase and a β-thio-glucosidase. Soil Boil. Biochem. 2008, 40, 2170–2173. [Google Scholar] [CrossRef]
- Bhat, R.; Kaur, T.; Khajuria, M.; Vyas, R.; Vyas, D. Purification and characterization of a novel rredox-regulated isoform of myrosinase (beta-thioglucoside glucohydrolase) from Lepidium latifolium L. J. Agric. Food Chem. 2015, 63, 10218–10226. [Google Scholar] [CrossRef] [PubMed]
- Rakariyatham, K.; Yang, X.; Gao, Z.; Song, M.; Han, Y.; Chen, X.; Xiao, H. Synergistic chemopreventive effect of allyl isothiocyanate and sulforaphane on non-small cell lung carcinoma cells. Food Funct. 2019, 10, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lv, C.; Zou, L.; Sun, J.; Song, X.; Zhang, Y.; Mao, J. Approaches for enhancing the stability and formation of sulforaphane. Food Chem. 2021, 345, 128771. [Google Scholar] [CrossRef]
- Shen, L.; Su, G.; Wang, X.; Du, Q.; Wang, K. Endogenous and exogenous enzymolysis of vegetable-sourced glucosinolates and influencing factors. Food Chem. 2010, 119, 987–994. [Google Scholar] [CrossRef]

| Purification Step | Volume [mL] | Total Protein [mg] | Specific Activity [nmol/min/mg] | Yield [%] |
|---|---|---|---|---|
| Crude enzyme | 10 | 107 | 662 | 100 |
| Ion exchange first (HiTrap Capto Q column) | 3 | 2.95 | 1245 | 5.18 |
| Ion exchange second (HiTrap Capto Q column) | 1.5 | 0.131 | 2297 | 0.42 |
| Gelfiltration (Superdex200 Increase 10/300 column) | 0.5 | 0.0159 | 6951 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Fang, Y.; Li, M.; Mi, H.; Liu, S.; Yang, G.; Lu, J.; Zhao, Y.; Liu, Q.; Zhang, W.; et al. Characterization of a Novel Myrosinase with High Activity from Marine Bacterium Shewanella baltica Myr-37. Int. J. Mol. Sci. 2022, 23, 11258. https://doi.org/10.3390/ijms231911258
Ye Q, Fang Y, Li M, Mi H, Liu S, Yang G, Lu J, Zhao Y, Liu Q, Zhang W, et al. Characterization of a Novel Myrosinase with High Activity from Marine Bacterium Shewanella baltica Myr-37. International Journal of Molecular Sciences. 2022; 23(19):11258. https://doi.org/10.3390/ijms231911258
Chicago/Turabian StyleYe, Qinwen, Yaowei Fang, Mengjiao Li, Haoyu Mi, Shu Liu, Guang Yang, Jing Lu, Yaling Zhao, Qitong Liu, Wei Zhang, and et al. 2022. "Characterization of a Novel Myrosinase with High Activity from Marine Bacterium Shewanella baltica Myr-37" International Journal of Molecular Sciences 23, no. 19: 11258. https://doi.org/10.3390/ijms231911258
APA StyleYe, Q., Fang, Y., Li, M., Mi, H., Liu, S., Yang, G., Lu, J., Zhao, Y., Liu, Q., Zhang, W., & Hou, X. (2022). Characterization of a Novel Myrosinase with High Activity from Marine Bacterium Shewanella baltica Myr-37. International Journal of Molecular Sciences, 23(19), 11258. https://doi.org/10.3390/ijms231911258






