Downregulation of miR-30b-5p Facilitates Chondrocyte Hypertrophy and Apoptosis via Targeting Runx2 in Steroid-Induced Osteonecrosis of the Femoral Head
Abstract
:1. Introduction
2. Results
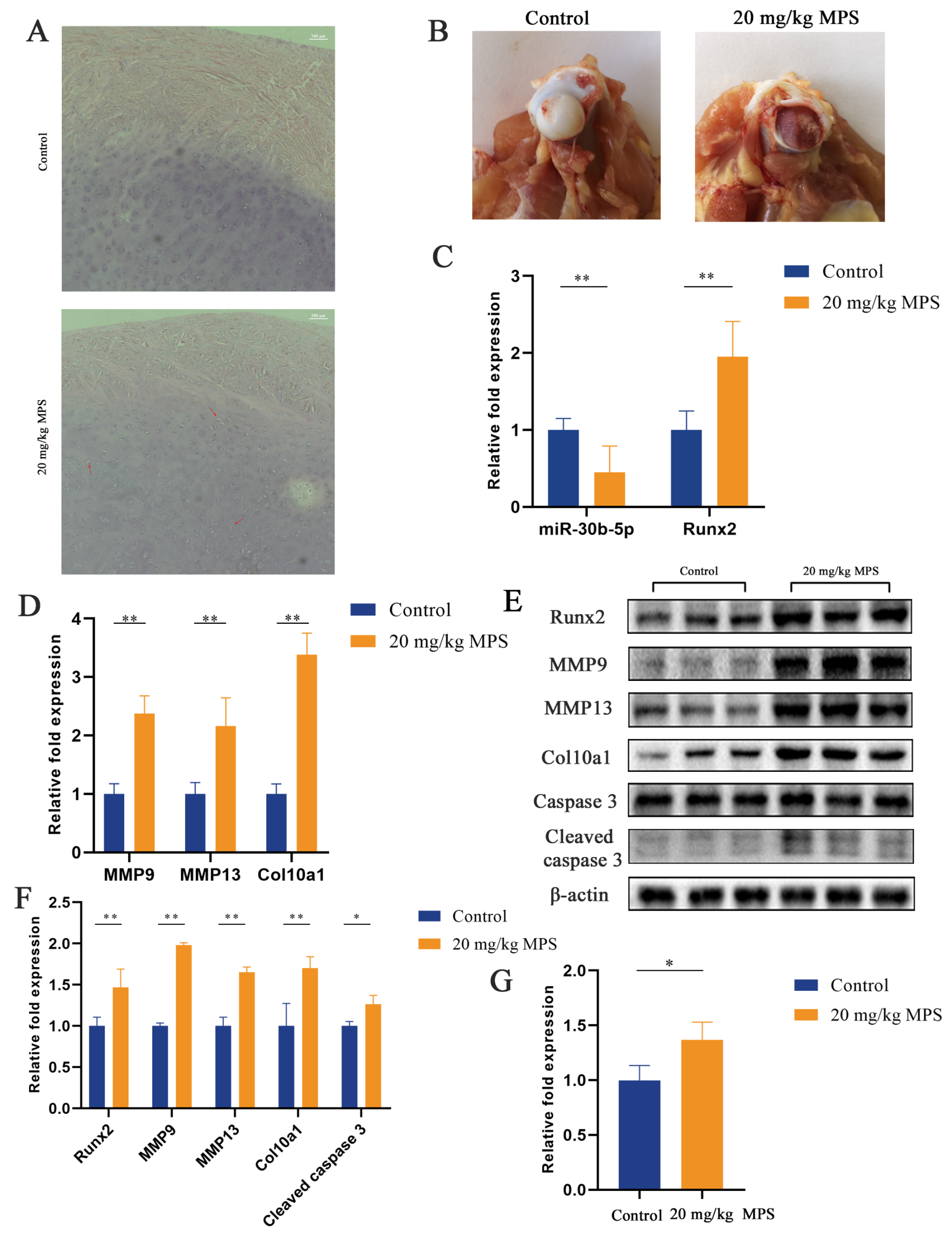
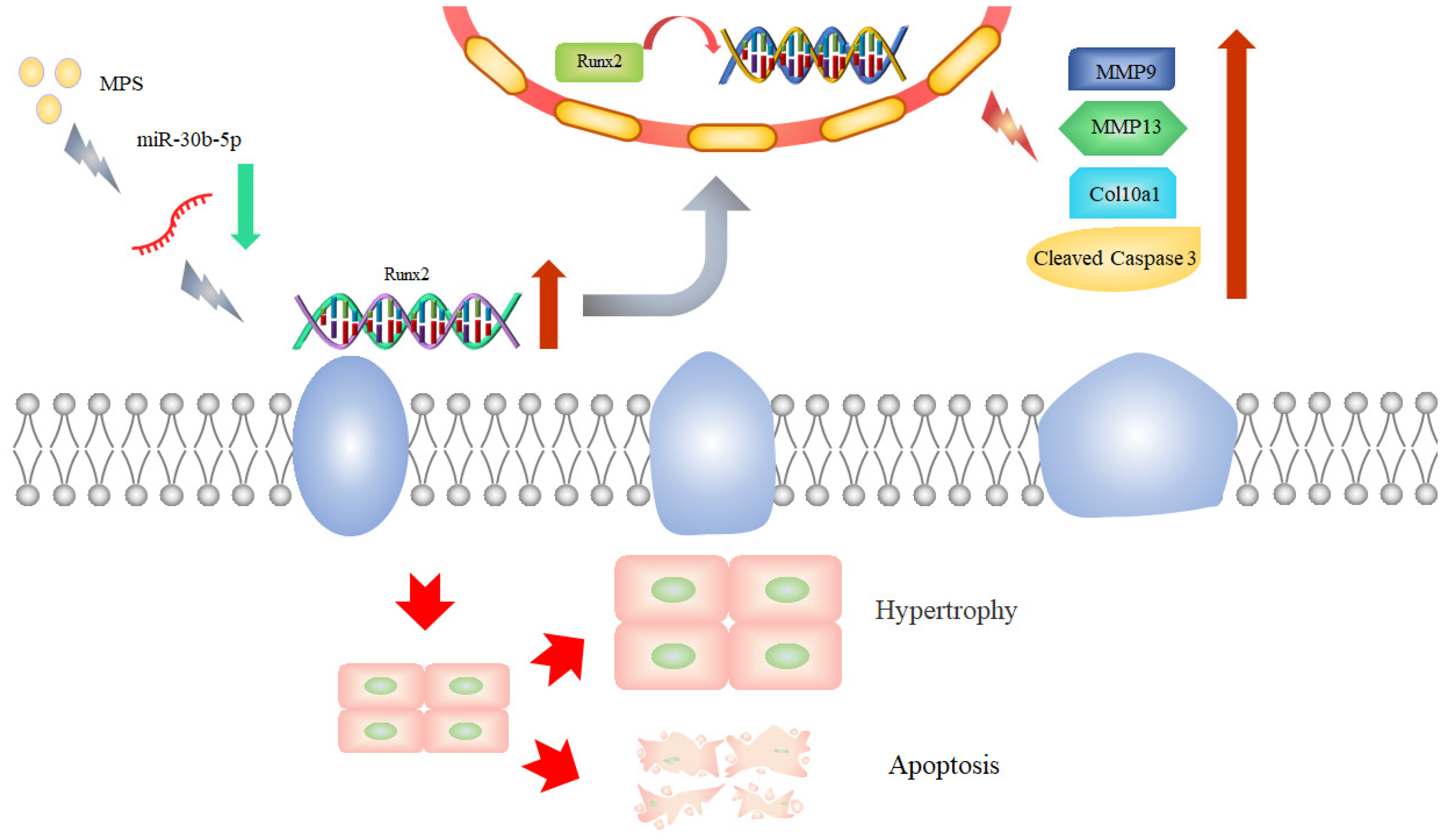
2.1. Methylprednisolone (MPS) Exposure Led to Chondrocyte Hypertrophy and Apoptosis in the SONFH Animal Model
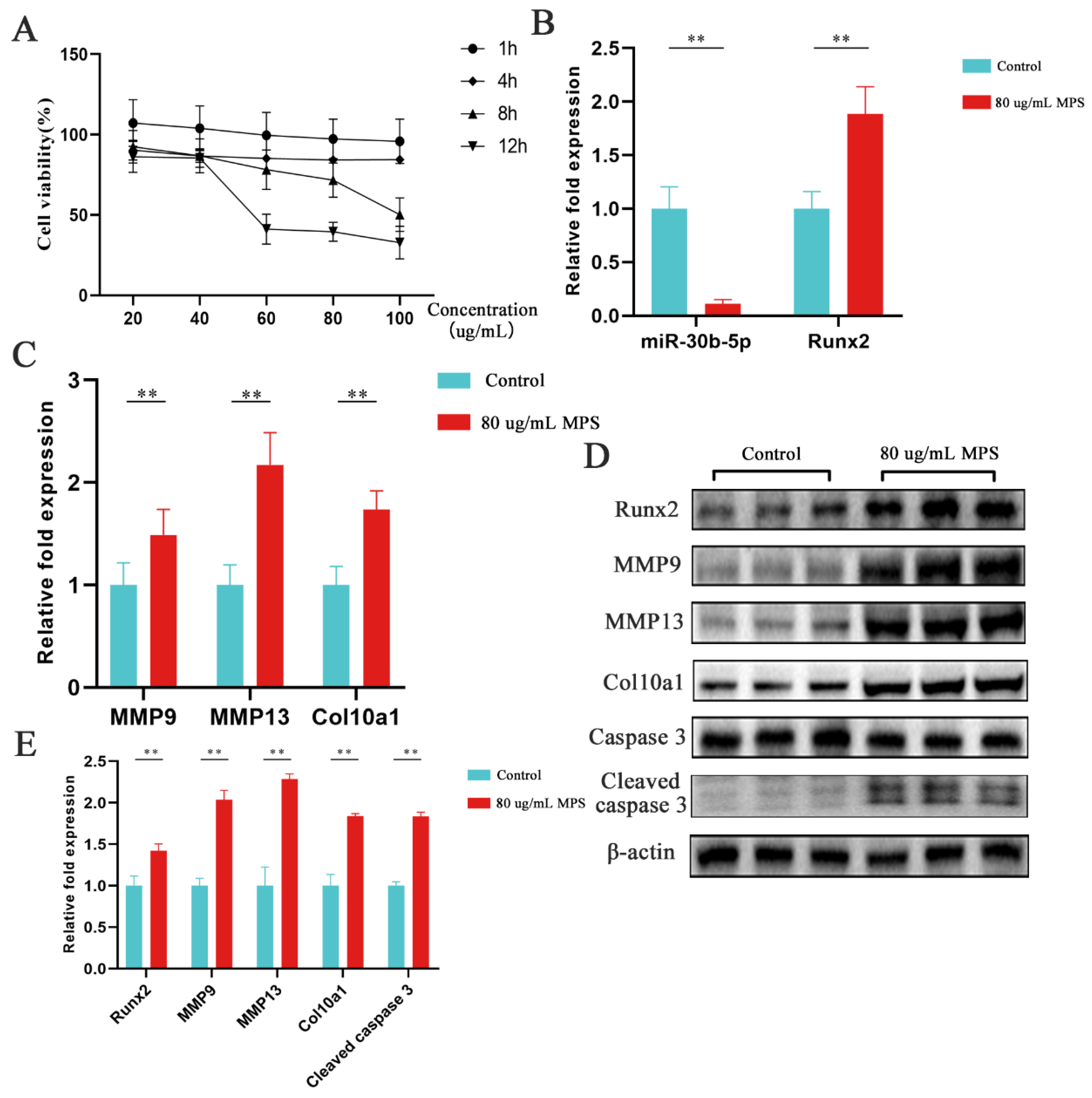
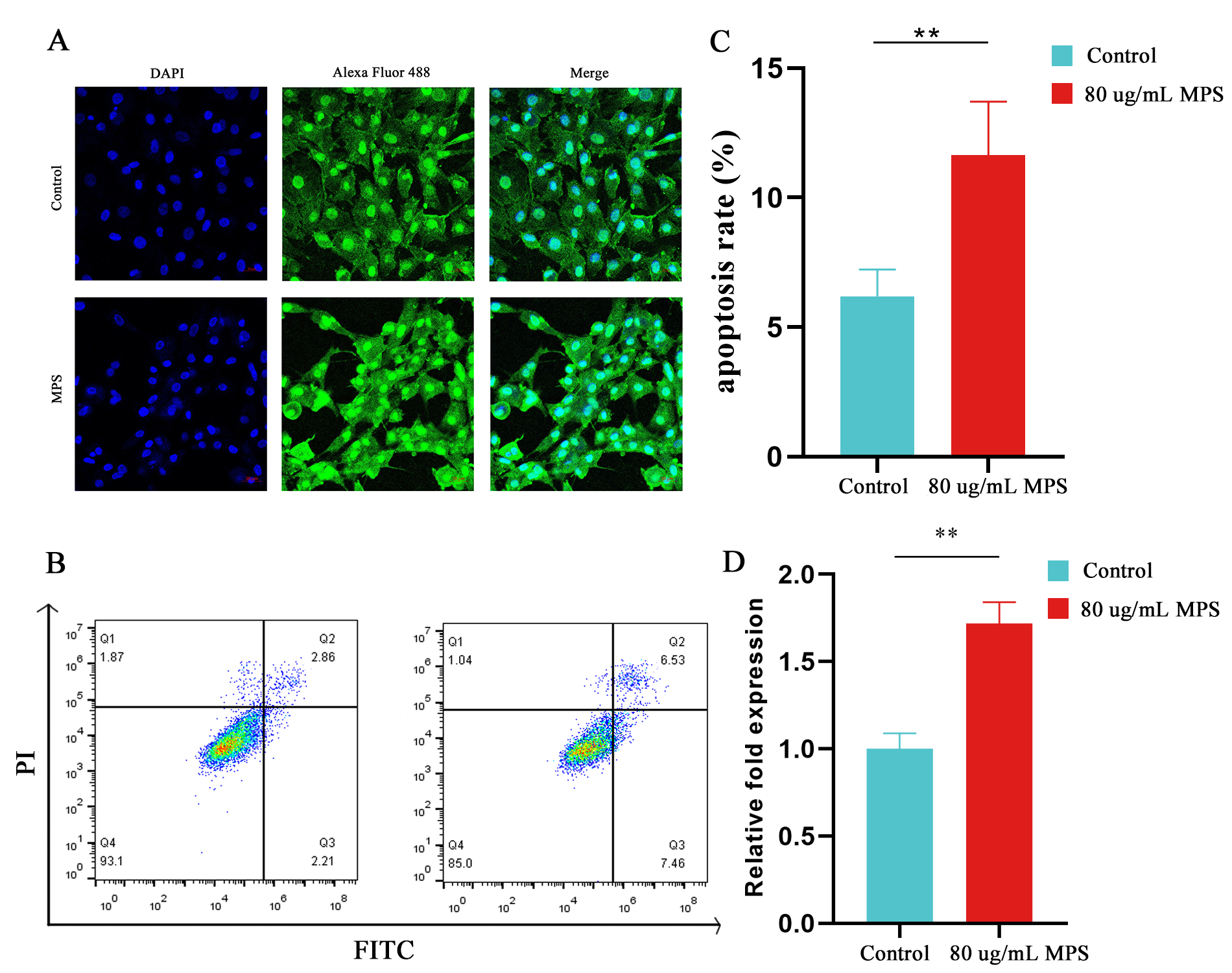
2.2. Effects of MPS Exposure on Hypertrophic Differentiation and Apoptosis in Chondrocytes
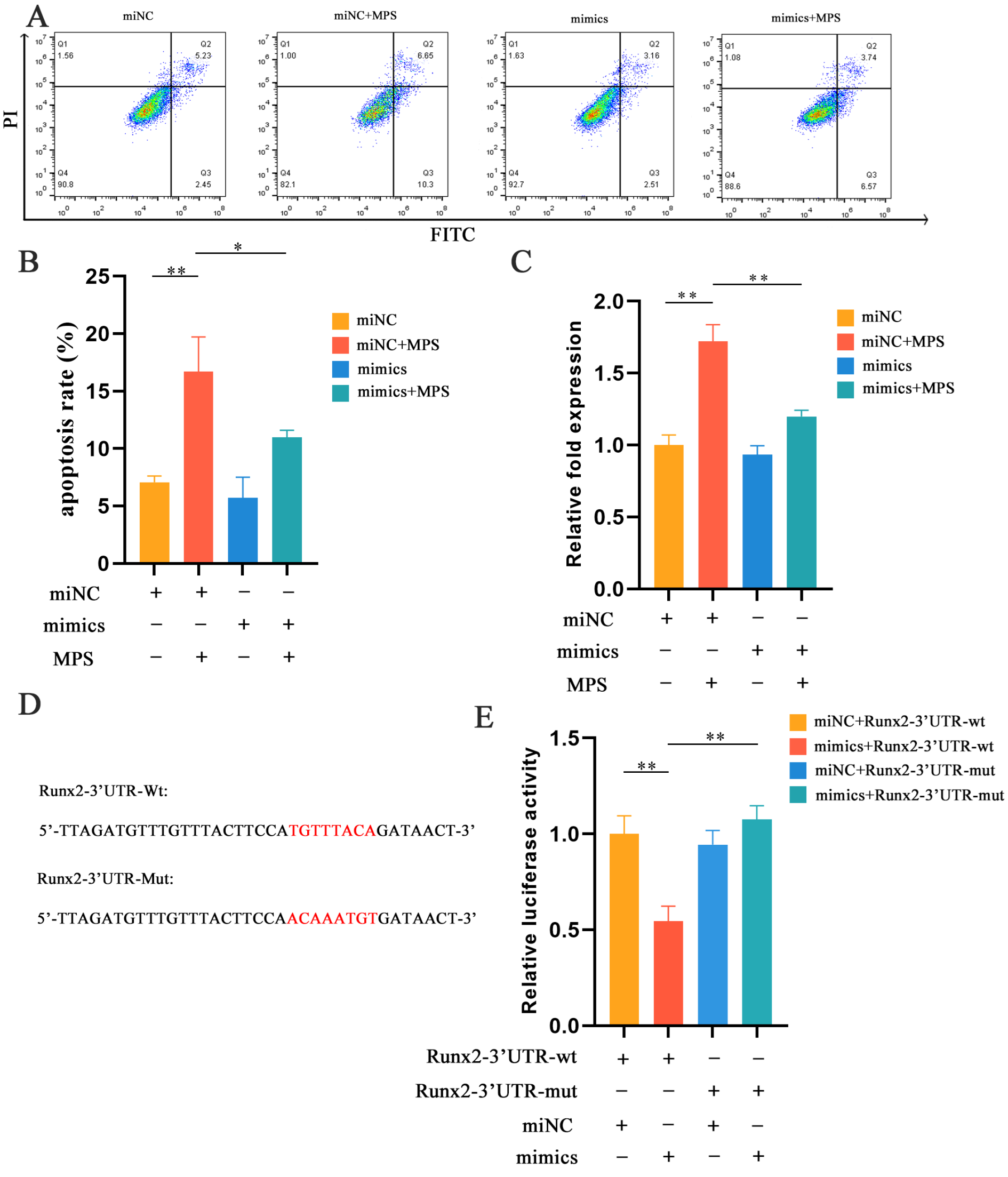
2.3. MiR-30b-5p Regulated Chondrocyte Hypertrophy with MPS Treatment in Chondrocytes
2.4. MPS-Induced Apoptosis in Chondrocytes Was Reduced by miR-30b-5p
3. Discussion
4. Materials and Methods
4.1. Femoral Head Necrosis Model Animals
4.2. Cell Culture, Drug Stimulation, Cell Viability Assay
4.3. RNA Isolation and Quantitative Real-Time PCR
4.4. Western Blotting
4.5. Immunofluorescent Staining
4.6. Flow Cytometry
4.7. Measurement of Caspase-3 Activity
4.8. Cell Transfection
4.9. Dual Fluorescence Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef]
- Pietrogrande, V.; Marino, V. Study of the circulation of the femoral head in some patients with arthritis deformans of the hip. Reumatismo 1953, 5, 219–222. [Google Scholar] [PubMed]
- Wang, A.; Ren, M.; Wang, J. The pathogenesis of steroid-induced osteonecrosis of the femoral head: A systematic review of the literature. Gene 2018, 671, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Beermann, J.; Piccoli, M.-T.; Viereck, J.; Thum, T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, C.; Li, X.; Wu, W.K.K.; Chen, X.; Zhu, S.; Ye, C.; Chan, M.T.V.; Qian, W. Circulating microRNA signature of steroid-induced osteonecrosis of the femoral head. Cell Prolif. 2018, 51, e12418. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Wan, F.; Zhang, Q.; Wen, P.; Cheng, L.; Li, P.; Guo, W. Effect of glucocorticoids on miRNA expression spectrum of rat femoral head microcirculation endothelial cells. Gene 2018, 651, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Qi, J.; Huang, P.; Jiang, M.; Zhou, Q.; Zhou, H.; Kang, H.; Qian, N.; Yang, Q.; Guo, L.; et al. MicroRNA-17/20a inhibits glucocorticoid-induced osteoclast differentiation and function through targeting RANKL expression in osteoblast cells. Bone 2014, 68, 67–75. [Google Scholar] [CrossRef]
- Shi, C.; Huang, P.; Kang, H.; Hu, B.; Qi, J.; Jiang, M.; Zhou, H.; Guo, L.; Deng, L. Glucocorticoid inhibits cell proliferation in differentiating osteoblasts by microRNA-199a targeting of WNT signaling. J. Mol. Endocrinol. 2015, 54, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. Molecular Mechanism of Runx2-Dependent Bone Development. Mol. Cells 2020, 43, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cells Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Enomoto-Iwamoto, M.; Iwamoto, M.; Nomura, S.; Himeno, M.; Kitamura, Y.; Kishimoto, T.; Komori, T. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J. Biol. Chem. 2000, 275, 8695–8702. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.P.; Cummings, C.E.; Chapman, J.A.; Grant, M.E. Macromolecular organization of chicken type X collagen in vitro. J. Cell Biol. 1991, 114, 597–604. [Google Scholar] [CrossRef]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef]
- Wong, M.; Siegrist, M.; Goodwin, K. Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone 2003, 33, 685–693. [Google Scholar] [CrossRef]
- Qiu, H.; Shen, X.; Chen, B.; Chen, T.; Feng, G.; Chen, S.; Feng, D.; Xu, Q. miR-30b-5p inhibits cancer progression and enhances cisplatin sensitivity in lung cancer through targeting LRP8. Apoptosis 2021, 26, 261–276. [Google Scholar] [CrossRef]
- Ji, L.; Wang, Z.-H.; Zhang, Y.-H.; Zhou, Y.; Tang, D.-S.; Yan, C.-S.; Ma, J.-M.; Fang, K.; Gao, L.; Ren, N.-S.; et al. ATG7-enhanced impaired autophagy exacerbates acute pancreatitis by promoting regulated necrosis via the miR-30b-5p/CAMKII pathway. Cell Death Dis. 2022, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, H.; Wang, Y.; Zhao, X.; Guo, Y.; Jin, J.; Chi, R. MiR-30b-5p functions as a tumor suppressor in cell proliferation, metastasis and epithelial-to-mesenchymal transition by targeting G-protein subunit α-13 in renal cell carcinoma. Gene 2017, 626, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Nurzati, Y.; Zhu, Z.; Xu, H.; Zhang, Y. Identification and Validation of Novel Diagnostic Biomarkers for Keloid Based on GEO Database. Int. J. Gen. Med. 2022, 15, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, H.; Zhang, B.; Xu, F.; Zhu, H.; Chen, B.; Zhu, C.; Shen, J. miR-30b-5p acts as a tumor suppressor microRNA in esophageal squamous cell carcinoma. J. Thorac. Dis. 2019, 11, 3015–3029. [Google Scholar] [CrossRef]
- Cardozo, J.B.; Andrade, D.M.S.; Santiago, M.B. The use of bisphosphonate in the treatment of avascular necrosis: A systematic review. Clin. Rheumatol. 2008, 27, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J. Nontraumatic necrosis of bone (osteonecrosis). N. Engl. J. Med. 1992, 326, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, S.; Pang, K.; Zhou, Z. Endoplasmic reticulum stress affected chondrocyte apoptosis in femoral head necrosis induced by glucocorticoid in broilers. Poult. Sci. 2019, 98, 1111–1120. [Google Scholar] [CrossRef]
- Liao, W.; Ning, Y.; Xu, H.-J.; Zou, W.-Z.; Hu, J.; Liu, X.-Z.; Yang, Y.; Li, Z.-H. BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin. Sci. 2019, 133, 1955–1975. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, M.; Yang, W.; Tang, M.; Chen, T.; Gan, D.; Zhang, D.; Ding, X.; Zhao, A.; Zhao, P.; et al. CD41-deficient exosomes from non-traumatic femoral head necrosis tissues impair osteogenic differentiation and migration of mesenchymal stem cells. Cell Death Dis. 2020, 11, 293. [Google Scholar] [CrossRef]
- Xu, J.; Gong, H.; Lu, S.; Deasey, M.J.; Cui, Q. Animal models of steroid-induced osteonecrosis of the femoral head—A comprehensive research review up to 2018. Int. Orthop. 2018, 42, 1729–1737. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, G.J.; Su, C.C.; Balian, G. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin. Orthop. Relat. Res. 1997, 344, 8–19. [Google Scholar] [CrossRef]
- Erken, H.Y.; Ofluoglu, O.; Aktas, M.; Topal, C.; Yildiz, M. Effect of pentoxifylline on histopathological changes in steroid-induced osteonecrosis of femoral head: Experimental study in chicken. Int. Orthop. 2012, 36, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Pu, M.; Tai, Y.; Chen, Y.; Lu, H.; Qiao, J.; Wang, G.; Chen, J.; Qi, X.; Huang, R.; et al. Nuclear miR-30b-5p suppresses TFEB-mediated lysosomal biogenesis and autophagy. Cell Death Differ. 2021, 28, 320–336. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Q.; Liu, X.; Wang, F.; Yang, Y.; Tian, X. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. Int. J. Biol. Sci. 2022, 18, 1220–1237. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010, 339, 189–195. [Google Scholar] [CrossRef]
- Wang, X.; Manner, P.A.; Horner, A.; Shum, L.; Tuan, R.S.; Nuckolls, G.H. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthr. Cartil. 2004, 12, 963–973. [Google Scholar] [CrossRef]
- Lu, Y.; Ding, M.; Li, N.; Wang, Q.; Li, J.; Li, X.; Gu, J.; Im, H.-J.; Lei, G.; Zheng, Q. Col10a1-Runx2 transgenic mice with delayed chondrocyte maturation are less susceptible to developing osteoarthritis. Am. J. Transl. Res. 2014, 6, 736–745. [Google Scholar]
- Vu, T.H.; Shipley, J.M.; Bergers, G.; Berger, J.E.; Helms, J.A.; Hanahan, D.; Shapiro, S.D.; Senior, R.M.; Werb, Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93, 411–422. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, S.; Gu, J.; Takarada, T.; Yoneda, Y.; Huang, J.; Zhao, L.; Oh, C.-D.; Li, J.; Wang, B.; et al. Deletion of Runx2 in Articular Chondrocytes Decelerates the Progression of DMM-Induced Osteoarthritis in Adult Mice. Sci. Rep. 2017, 7, 2371. [Google Scholar] [CrossRef]
- Rashid, H.; Chen, H.; Javed, A. Runx2 is required for hypertrophic chondrocyte mediated degradation of cartilage matrix during endochondral ossification. Matrix Biol. Plus 2021, 12, 100088. [Google Scholar] [CrossRef]
- Zhao, X.; Chang, G.; Cheng, Y.; Zhou, Z. GABAA Receptor/STEP61 Signaling Pathway May Be Involved in Emulsified Isoflurane Anesthesia in Rats. Int. J. Mol. Sci. 2020, 21, 4078. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Yu, Y.; Liu, K.; Jiang, Y.; Zhou, Z. Downregulation of miR-30b-5p Facilitates Chondrocyte Hypertrophy and Apoptosis via Targeting Runx2 in Steroid-Induced Osteonecrosis of the Femoral Head. Int. J. Mol. Sci. 2022, 23, 11275. https://doi.org/10.3390/ijms231911275
Lin L, Yu Y, Liu K, Jiang Y, Zhou Z. Downregulation of miR-30b-5p Facilitates Chondrocyte Hypertrophy and Apoptosis via Targeting Runx2 in Steroid-Induced Osteonecrosis of the Femoral Head. International Journal of Molecular Sciences. 2022; 23(19):11275. https://doi.org/10.3390/ijms231911275
Chicago/Turabian StyleLin, Lishan, Yaling Yu, Kangping Liu, Yixin Jiang, and Zhenlei Zhou. 2022. "Downregulation of miR-30b-5p Facilitates Chondrocyte Hypertrophy and Apoptosis via Targeting Runx2 in Steroid-Induced Osteonecrosis of the Femoral Head" International Journal of Molecular Sciences 23, no. 19: 11275. https://doi.org/10.3390/ijms231911275



