Association of Hypomagnesemia and Liver Injury, Role of Gut-Barrier Dysfunction and Inflammation: Efficacy of Abstinence, and 2-Week Medical Management in Alcohol Use Disorder Patients
Abstract
1. Introduction
2. Results
2.1. Demographics, Drinking, and Nutritional Status
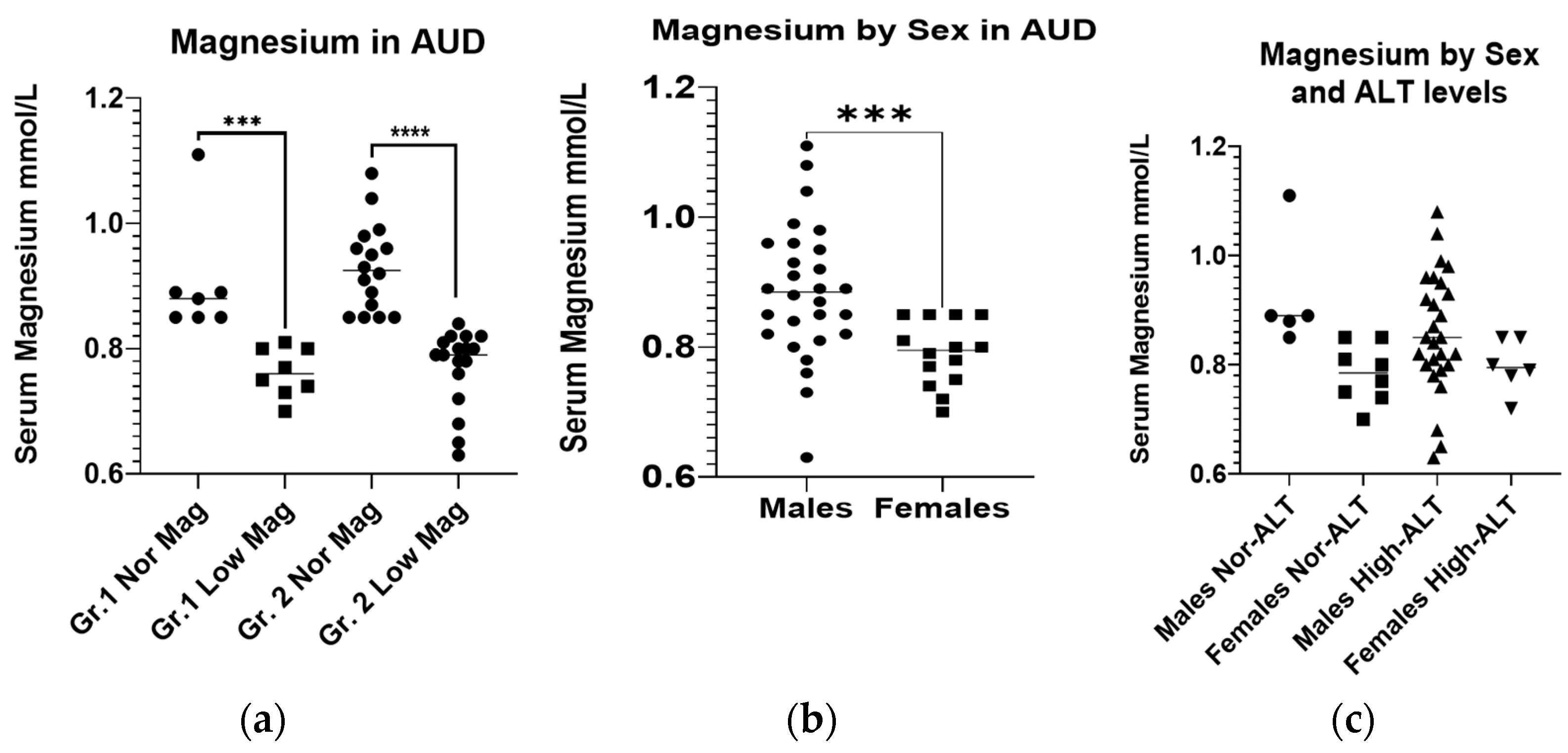
2.2. Baseline Magnesium Level and Nutritional Status
2.3. Baseline Gut-Dysfunction and Pro-Inflammatory Response
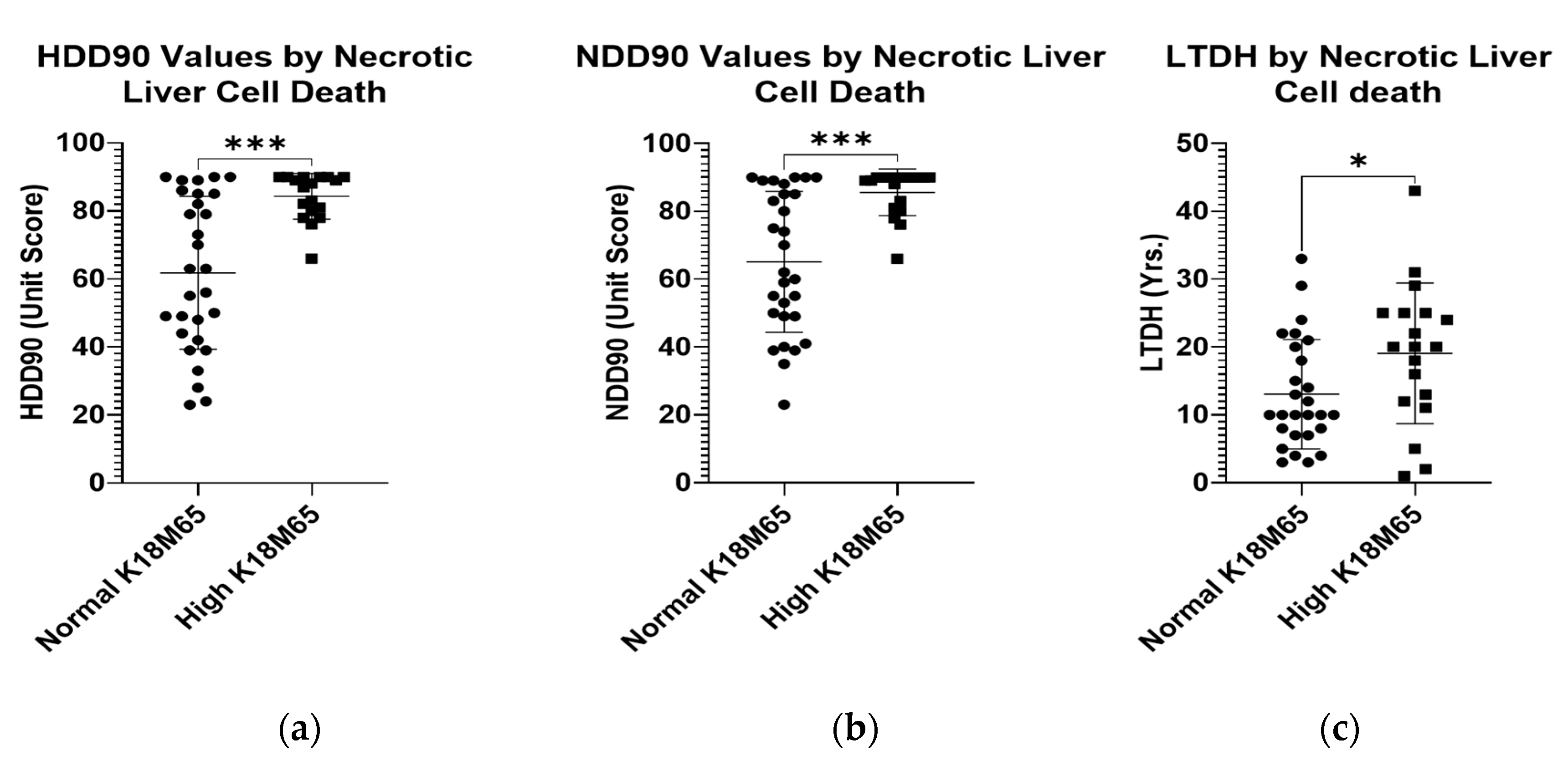
2.4. Baseline Liver Injury, and Liver Cell Death
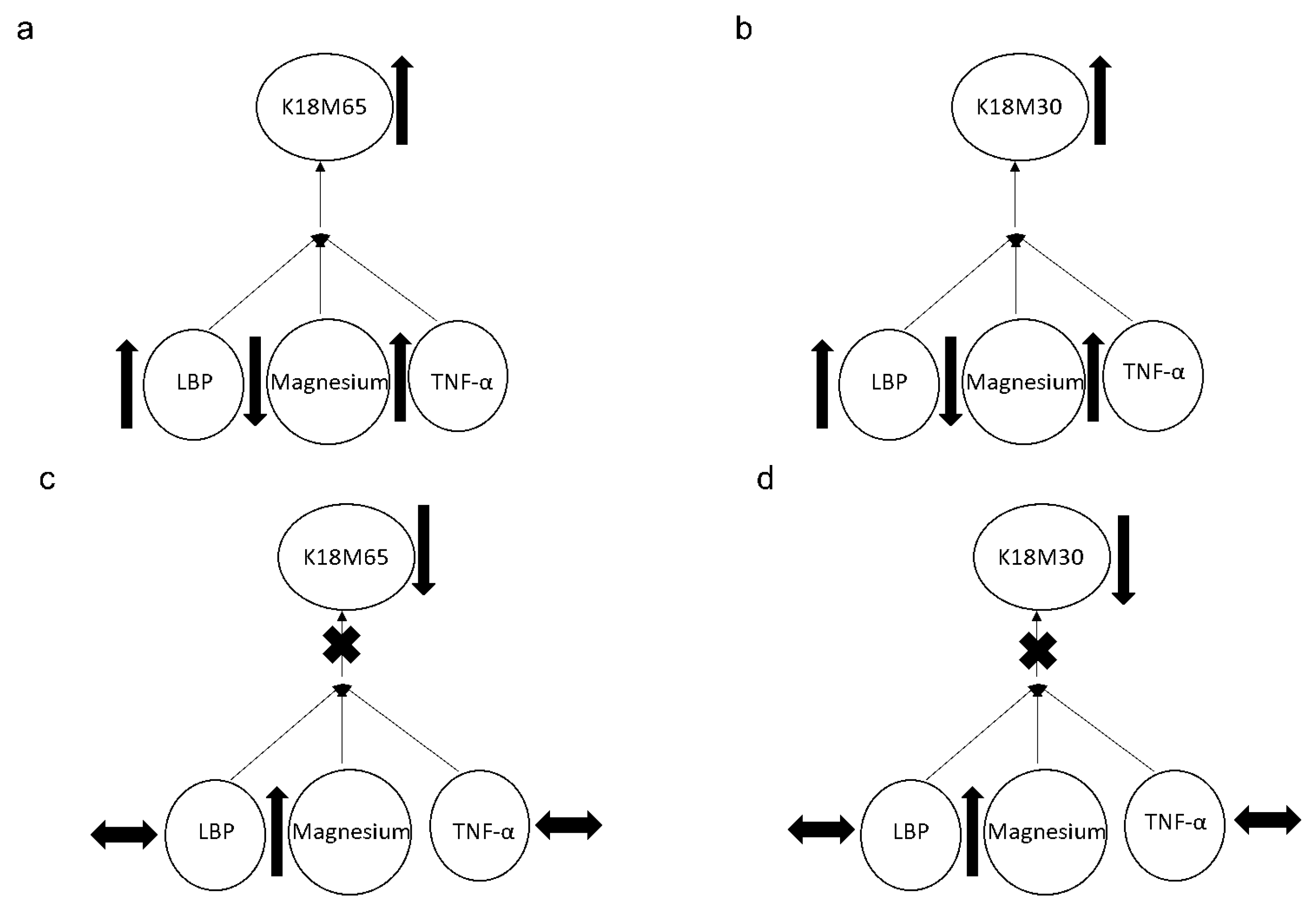
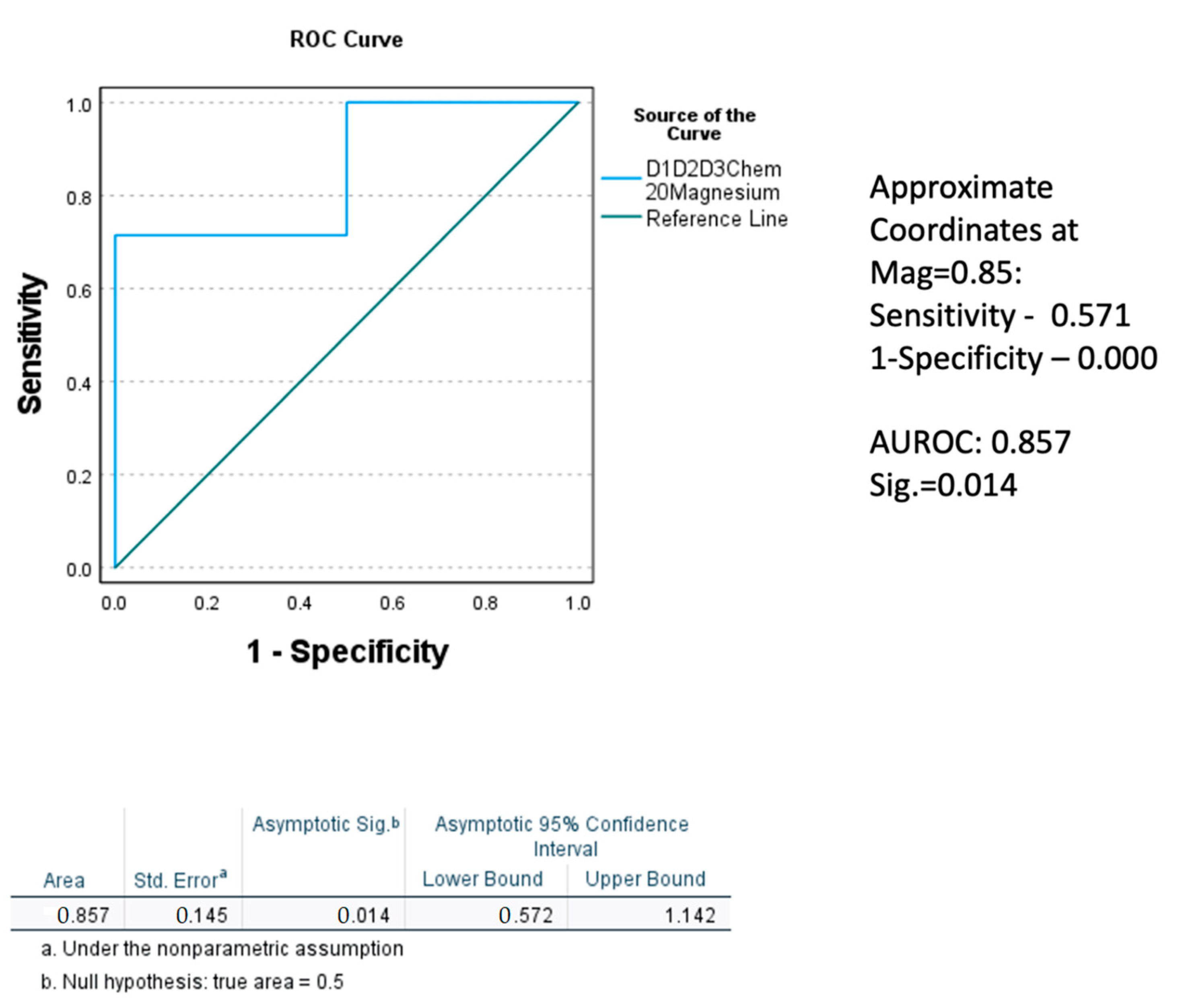
2.5. Association of Magnesium with Gut-Dysfunction, Inflammation, and Liver Cell Death Markers
2.6. Post-SOC Evaluation
3. Discussion
4. Materials and Methods
4.1. Demographics, Drinking, and Laboratory Evaluations
4.2. Laboratory Assays
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic liver disease: Pathogenesis and current management. Alcohol Res. Curr. Rev. 2017, 38, 147. [Google Scholar]
- Shah, N.D.; Ventura-Cots, M.; Abraldes, J.G.; Alboraie, M.; Alfadhli, A.; Argemi, J.; Badia-Aranda, E.; Arús-Soler, E.; Barritt, A.S., IV; Bessone, F. Alcohol-related liver disease is rarely detected at early stages compared with liver diseases of other etiologies worldwide. Clin. Gastroenterol. Hepatol. 2019, 17, 2320–2329.e12. [Google Scholar] [CrossRef]
- Sagaram, M.; Parthasarathy, R.; Condon, S.L.; Closson, C.F.; Kong, M.; Schwandt, M.L.; Jophlin, L.L.; Feng, W.; Barve, A.J.; Vatsalya, V. Theragnostic efficacy of K18 response in alcohol use disorder with clinically significant fibrosis using gut-liver axis. Int. J. Mol. Sci. 2022, 23, 5852. [Google Scholar] [CrossRef] [PubMed]
- Gala, K.S.; Vatsalya, V. Emerging noninvasive biomarkers, and medical management strategies for alcoholic hepatitis: Present understanding and scope. Cells 2020, 9, 524. [Google Scholar] [CrossRef]
- Elisaf, M.; Merkouropoulos, M.; Tsianos, E.; Siamopoulos, K. Pathogenetic mechanisms of hypomagnesemia in alcoholic patients. J. Trace Elem. Med. Biol. 1995, 9, 210–214. [Google Scholar] [CrossRef]
- Vandemergel, X.; Simon, F. Evolution of metabolic abnormalities in alcoholic patients during withdrawal. J. Addict. 2015, 2015, 541536. [Google Scholar] [CrossRef]
- Young, A.; Cefaratti, C.; Romani, A. Chronic EtOH administration alters liver Mg2+ homeostasis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 284, G57–G67. [Google Scholar] [CrossRef]
- Romani, A.M. Magnesium homeostasis and alcohol consumption. Magnes. Res. 2008, 21, 197–204. [Google Scholar]
- Papierkowski, A.; Pasternak, K. The effect of a single dose of morphine and ethanol on magnesium level in blood serum and tissues in mice. Magnes. Res. 1998, 11, 85–89. [Google Scholar]
- Aagaard, N.; Andersen, H.; Vilstrup, H.; Clausen, T.; Jakobsen, J.; Dørup, I. Muscle strength, Na, K-pumps, magnesium and potassium in patients with alcoholic liver cirrhosis–relation to spironolactone. J. Intern. Med. 2002, 252, 56–63. [Google Scholar] [CrossRef]
- Vatsalya, V.; Gala, K.S.; Mishra, M.; Schwandt, M.L.; Umhau, J.; Cave, M.C.; Parajuli, D.; Ramchandani, V.A.; McClain, C.J. Lower serum magnesium concentrations are associated with specific heavy drinking markers, pro-inflammatory response and early-stage alcohol-associated liver injury. Alcohol Alcohol. 2020, 55, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhu, X.; Fan, L.; Kabagambe, E.K.; Song, Y.; Tao, M.; Zhong, X.; Hou, L.; Shrubsole, M.J.; Liu, J. Magnesium intake and mortality due to liver diseases: Results from the Third National Health and Nutrition Examination Survey Cohort. Sci. Rep. 2017, 7, 17913. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Mueller, S.; Szabo, G. Non-invasive diagnosis and biomarkers in alcohol-related liver disease. J. Hepatol. 2019, 70, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Lippai, D. Converging actions of alcohol on liver and brain immune signaling. Int. Rev. Neurobiol. 2014, 118, 359–380. [Google Scholar]
- Vatsalya, V.; Cave, M.C.; Kong, M.; Gobejishvili, L.; Falkner, K.C.; Craycroft, J.; Mitchell, M.; Szabo, G.; McCullough, A.; Dasarathy, S.; et al. Keratin 18 is a diagnostic and prognostic factor for acute alcoholic hepatitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2046–2054. [Google Scholar] [CrossRef]
- Vatsalya, V.; Bin Liaquat, H.; Ghosh, K.; Prakash Mokshagundam, S.; McClain, C.J. A review on the sex differences in organ and system pathology with alcohol drinking. Curr. Drug Abus. Rev. 2016, 9, 87–92. [Google Scholar] [CrossRef]
- Elin, R.J. Magnesium metabolism in health and disease. Disease-A-Month 1988, 34, 166–218. [Google Scholar] [CrossRef]
- Kirpich, I.A.; McClain, C.J.; Vatsalya, V.; Schwandt, M.; Phillips, M.; Falkner, K.C.; Zhang, L.; Harwell, C.; George, D.T.; Umhau, J.C. Liver injury and endotoxemia in male and female alcohol-dependent individuals admitted to an alcohol treatment program. Alcohol. Clin. Exp. Res. 2017, 41, 747–757. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Bridges, B.W.; Dunn, W.; Olson, J.C.; Weinman, S.A.; Jaeschke, H. Cell death and prognosis of mortality in alcoholic hepatitis patients using plasma keratin-18. Gene Expr. 2017, 17, 301–312. [Google Scholar] [CrossRef]
- Pasqualetti, P.; Casale, R.; Colantonio, D.; Di Lauro, G.; Festuccia, V.; Natali, L.; Natali, G. Serum levels of magnesium in hepatic cirrhosis. Quad. Sclavo Diagn. Clin. Lab. 1987, 23, 12–17. [Google Scholar]
- McClain, C.J.; Marsano, L.; Burk, R.F.; Bacon, B. Trace Metals in Liver Disease; Seminars in liver Disease; Thieme Medical Publishers, Inc.: New York, NY, USA, 1991; Volume 11, pp. 321–339. [Google Scholar]
- Shane, S.; Flink, E. Magnesium deficiency in alcohol addiction and withdrawal. Magnes. Trace Elem. 1991, 10, 263–268. [Google Scholar] [PubMed]
- Agus, Z.S. Mechanisms and causes of hypomagnesemia. Curr. Opin. Nephrol. Hypertens. 2016, 25, 301–307. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Markiewicz-Górka, I.; Zawadzki, M.; Januszewska, L.; Hombek-Urban, K.; Pawlas, K. Influence of selenium and/or magnesium on alleviation alcohol induced oxidative stress in rats, normalization function of liver and changes in serum lipid parameters. Hum. Exp. Toxicol. 2011, 30, 1811–1827. [Google Scholar] [CrossRef]
- El-Tantawy, W.H.; Sabry, D.; Abd Al Haleem, E.N. Comparative study of antifibrotic activity of some magnesium-containing supplements on experimental liver toxicity. Mol. Study. Drug Chem. Toxicol. 2017, 40, 47–56. [Google Scholar] [CrossRef]
- Vatsalya, V.; Gala, K.S.; Mishra, M.; Schwandt, M.L.; Umhau, J.; Cave, M.C.; Parajuli, D.; Ramchandani, V.A.; McClain, C.J. Lowering of Magnesium is associated with specific heavy drinking markers, pro-inflammatory response and early-stage liver injury in heavy drinkers [Abstract]. Gastroenterology 2019, 156, S1230. [Google Scholar]
- Poikolainen, K.; Alho, H. Magnesium treatment in alcoholics: A randomized clinical trial. Subst. Abus. Treat. Prev. Policy 2008, 3, 1. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, J.; Chen, F.; Lin, K.; Zhu, M.; Chen, L.; Zhou, X.; Li, C.; Zhu, H. Protective role of magnesium isoglycyrrhizinate in non-alcoholic fatty liver disease and the associated molecular mechanisms. Int. J. Mol. Med. 2016, 38, 275–282. [Google Scholar] [CrossRef]
- DiCecco, S.R.; Francisco-Ziller, N. Nutrition in alcoholic liver disease. Nutr. Clin. Pract. 2006, 21, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Eshraghian, A.; Nikeghbalian, S.; Geramizadeh, B.; Malek-Hosseini, S.A. Serum magnesium concentration is independently associated with non-alcoholic fatty liver and non-alcoholic steatohepatitis. United Eur. Gastroenterol. J. 2018, 6, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, H.; Mao, Y. Magnesium and liver disease. Ann. Transl. Med. 2019, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Segal, D.L. Diagnostic and statistical manual of mental disorders (DSM-IV-TR). In The Corsini Encyclopedia of Psychology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 1–3. [Google Scholar]
- Klepp, T.; Sloan, M.; Soundararajan, S.; Ramsden, C.; Cinar, R.; Schwandt, M.; Diazgranados, N.; Vatsalya, V.; Ramchandani, V. Elevated stearoyl-coA desaturase 1 activity is associated with alcohol-associated liver disease. Alcohol 2022, 102, 51–57. [Google Scholar] [CrossRef]
- Vatsalya, V.; Gala, K.S.; Hassan, A.Z.; Frimodig, J.; Kong, M.; Sinha, N.; Schwandt, M.L. Characterization of early-stage alcoholic liver disease with hyperhomocysteinemia and gut dysfunction and associated immune response in alcohol use disorder patients. Biomedicines 2020, 9, 7. [Google Scholar] [CrossRef]
- Umhau, J.C.; Momenan, R.; Schwandt, M.L.; Singley, E.; Lifshitz, M.; Doty, L.; Adams, L.J.; Vengeliene, V.; Spanagel, R.; Zhang, Y. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: A randomized controlled experimental medicine study. Arch. Gen. Psychiatry 2010, 67, 1069–1077. [Google Scholar] [CrossRef]
- Sobell, L.C.; Sobell, M.B.; Connors, G.J.; Agrawal, S. Assessing drinking outcomes in alcohol treatment efficacy studies: Selecting a yardstick of success. Alcohol. Clin. Exp. Res. 2003, 27, 1661–1666. [Google Scholar] [CrossRef]
- Skinner, H.A.; Sheu, W.-J. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J. Stud. Alcohol. 1982, 43, 1157–1170. [Google Scholar] [CrossRef]
- Fukushima, K.; Ueno, Y.; Kawagishi, N.; Kondo, Y.; Inoue, J.; Kakazu, E.; Ninomiya, M.; Wakui, Y.; Saito, N.; Satomi, S. The nutritional index ‘CONUT’is useful for predicting long-term prognosis of patients with end-stage liver diseases. Tohoku J. Exp. Med. 2011, 224, 215–219. [Google Scholar] [CrossRef]

| Measures | Group 1 (Normal Initial ALT, Gr.1) | Group 2 (Elevated Initial ALT, Gr.2) | Between Group p-Value | ||||
|---|---|---|---|---|---|---|---|
| Normal Mg (n = 7; 46.67%) | Low Mg (n = 8; 53.33%) | Total (n = 15; 31.25%) | Normal Mg (n = 16; 48.48%) | Low Mg (n = 17; 51.52%) | Total (n = 33; 68.75%) | ||
| Age (years) | 37.64 ± 10.5 | 42.9 ± 12.4 | 40.45 ± 11.4 | 41.4 ± 9.5 | 47.3 ± 9.5 | 44.45 ± 9.8 | NS |
| BMI (kg/m2) | 31.31 ± 8.2 | 25.9 ± 7.4 | 28.45 ± 8.0 | 25.9 ± 4.7 | 26.1 ± 2.9 | 25.99 ± 3.7 | NS |
| Sex (F/M) | 2/5 | 6/2 | 8/7 | 2/14 | 4/13 | 6/27 | NA |
| Baseline Drinking History | |||||||
| TD90 | 1511.2 ± 653.3 | 809.2 ± 598.0 | 1160.21 ± 703.33 | 1111.2 ± 593.0 | 1048.65 ± 440.7 | 1078.98 ± 512.80 | NS |
| HDD90 c | 75.4 ± 14.0 | 54.57 ± 21.9 | 65.00 ± 20.71 | 68.94 ± 22.2 | 76.24 ± 21.9 | 72.69 ± 21.18 | NS |
| AvgDPD90 | 19.36 ± 6.54 | 14.19 ± 7.6 | 16.78 ± 7.33 | 15.46 ± 6.5 | 13.18 ± 4.5 | 14.29 ± 5.60 | NS |
| NDD90 c | 76.86 ± 56.86 | 56.86 ± 23.25 | 66.86 ± 21.48 | 72.00 ± 20.71 | 78.49 ± 15.85 | 75.49 ± 18.40 | NS |
| LTDH c | 12.5 ± 6.7 | 9.63 ± 5.3 | 10.86 ± 5.86 | 17.3 ± 10.1 | 17.8 ± 10.4 | 17.56 ± 10.06 | 0.025 |
| Baseline Liver Injury Markers | |||||||
| ALT (IU/L) c,d | 27.86 ± 7.0 | 25.25 ± 10.5 | 26.47 ± 8.86 | 87.19 ± 48.13 | 109.24 ± 62.8 | 98.55 ± 56.43 | NA |
| AST (IU/L) c,d | 31.14 ± 9.8 | 37.50 ± 26.5 | 34.53 ± 20.09 | 115.56 ± 95.5 | 144.94 ± 105.8 | 130.70 ± 100.45 | <0.01 |
| AST: ALT | 1.16 ± 0.4 | 1.43 ± 0.6 | 1.30 ± 0.51 | 1.23 ± 0.6 | 1.35 ± 0.8 | 1.29 ± 0.70 | NS |
| Baseline Magnesium Levels | |||||||
| Serum Mg mmol/L a,b | 0.9 ± 0.09 | 0.76 ± 0.04 | 0.83 ± 0.10 | 0.93 ± 0.07 | 0.77 ± 0.06 | 0.85 ± 0.10 | NS |
| Baseline Nutritional Status | |||||||
| CONUT | 1.00 ± 1.0 | 1.00 ± 1.7 | 1.00 ± 1.36 | 0.75 ± 0.9 | 1.18 ± 1.1 | 0.97 ± 1.05 | NS |
| Baseline Blood Cell Types | |||||||
| WBC (K/uL) c | 7.03 ± 3.2 | 7.93 ± 3.05 | 7.51 ± 3.03 | 6.42 ± 2.3 | 5.65 ± 2.1 | 6.03 ± 2.19 | 0.061 |
| AMC (K/uL) | 0.62 ± 0.26 | 0.46 ± 0.21 | 0.53 ± 0.24 | 0.58 ± 0.28 | 0.46 ± 0.16 | 0.52 ± 0.23 | NS |
| ANC (K/uL) | 4.29 ± 2.4 | 4.66 ± 2.5 | 4.49 ± 2.37 | 3.64 ± 1.7 | 3.49 ± 1.7 | 3.56 ± 1.68 | NS |
| Baseline Candidate Cytokine Response | |||||||
| IL-1β (pg/mL) a | 0.66 ± 0.50 | 0.57 ± 0.60 | 0.62 ± 0.53 | 0.50 ± 0.29 | 0.45 ± 0.23 | 0.47 ± 0.26 | NS |
| IL-6 (pg/mL) a,c,d | 2.25 ± 1.15 | 3.68 ± 4.41 | 2.97 ± 3.18 | 3.64 ± 2.16 | 3.98 ± 3.94 | 3.82 ± 3.19 | NS |
| TNF-α (pg/mL) | 1.74 ± 0.77 | 1.17 ± 0.52 | 1.45 ± 0.70 | 1.93 ± 0.59 | 2.26 ± 1.15 | 2.11 ± 0.93 | 0.025 |
| IL-8 (pg/mL) a | 13.25 ± 26.19 | 4.63 ± 5.23 | 8.94 ± 18.68 | 4.05 ± 2.09 | 9.00 ± 13.92 | 6.69 ± 10.42 | NS |
| MCP-1 (pg/mL) a | 96.03 ± 29.75 | 110.87 ± 67.01 | 103.45 ± 50.41 | 115.91 ± 56.43 | 115.66 ± 72.96 | 115.78 ± 64.66 | NS |
| Baseline Candidate Gut-dysfunction Markers | |||||||
| LPS (EU/mL) | 0.078 ± 0.06 | 0.080 ± 0.05 | 0.08 0.05 | 0.106 ± 0.06 | 0.110 ± 0.06 | 0.11 ± 0.06 | NS |
| LBP (ng/mL) | 624.51 ± 742.92 | 2009.87 ± 3374.97 | 1317.19 ± 2455.31 | 2497.59 ± 3096.29 | 1759.19 ± 2750.63 | 2092.66 ± 2886.01 | NS |
| +sCD14 (×10 pg/mL) | 8865.95 ± 2238.92 | 8962.71 ± 1509.51 | 8917.56 ± 1813.29 | 9193.12 ± 1997.19 | 9744.99 ± 1614.74 | 9477.42 ± 1803.29 | NS |
| Baseline Liver Cell Death Markers | |||||||
| K18M65 (IU/L) | 138.62 ± 63.85 | 456.42 ± 528.24 | 308.11 ± 410.12 | 856.21 ± 1083.77 | 922.37 ± 827.66 | 890.29 ± 945.63 | 0.027 |
| K18M30 (IU/L) | 514.08 ± 854.73 | 278.89 ± 165.09 | 388.65 ± 584.36 | 361.13 ± 415.01 | 378.20 ± 342.46 | 378.20 ± 342.46 | NS |
| M65:M30 | 0.682 ± 0.59 | 1.333 ± 0.78 | 1.03 ± 0.76 | 2.325 ± 1.50 | 2.214 ± 1.26 | 2.27 ± 1.36 | 0.002 |
| Measures | Group 1 (Normal Initial ALT, Gr.1) | Group 2 (Elevated Initial ALT, Gr.2) | Between Group p-Value | ||||
|---|---|---|---|---|---|---|---|
| Normal Mg | Low Mg | Total | Normal Mg | Low Mg | Total | ||
| Post-Study Liver Injury Markers | |||||||
| ALT (IU/L) | 58.33 ± 34.7 | 50.67 ± 36.7 | 54.50 ± 32.24 | 68.75 ± 18.4 | 56.00 ± 22.3 | 61.67 ± 20.50 | NS |
| AST (IU/L) | 78.0 ± 79.98 | 68.67 ± 58.53 | 73.33 ± 62.89 | 36.00 ± 6.98 | 30.40 ± 7.09 | 32.89 ± 7.22 | 0.073 |
| AST: ALT | 1.16 ± 0.56 | 1.27 ± 0.18 | 1.22 ± 0.38 | 0.54 ± 0.13 | 0.61 ± 0.28 | 0.58 ± 0.22 | 0.001 |
| Post-Study Magnesium Levels | |||||||
| Serum Mg mmol/L | 0.90 ± 0.06 | 0.80 ± 0.13 | 0.85 ± 0.10 | 0.92 ± 0.07 | 0.85 ± 0.05 | 0.88 ± 0.07 | NS |
| Post-Study Candidate Cytokine Response | |||||||
| IL-1β (pg/mL) | 0.64 ± 0.39 | 0.62 ± 0.93 | 0.63 ± 0.68 | 0.38 ± 0.25 | 0.78 ± 0.96 | 0.59 ± 0.74 | NS |
| IL-6 (pg/mL) | 2.44 ± 1.02 | 3.44 ± 3.56 | 2.94 ± 2.57 | 3.30 ± 2.29 | 2.95 ± 0.95 | 3.11 ± 1.69 | NS |
| TNF-α (pg/mL) a | 2.03 ± 0.68 | 1.49 ± 0.64 | 1.76 ± 0.69 | 2.45 ± 0.85 | 2.61 ± 0.78 | 2.53 ± 0.85 | 0.004 |
| IL-8 (pg/mL) | 2.36 ± 0.47 | 4.35 ± 6.02 | 3.36 ± 4.23 | 2.69 ± 1.27 | 3.68 ± 2.57 | 3.21 ± 2.09 | NS |
| MCP-1 (pg/mL) | 120.63 ± 36.19 | 94.98 ± 51.76 | 107.81 ± 44.92 | 120.97 ± 44.50 | 124.60 ± 57.67 | 122.91 ± 51.10 | NS |
| Post-Study Candidate Gut-dysfunction Markers | |||||||
| LPS (EU/mL) | 0.07 ± 0.047 | 0.08 ± 0.057 | 0.07 ± 0.05 | 0.07 ± 0.02 | 0.06 ± 0.03 | 0.06 ± 0.03 | NS |
| LBP (ng/mL) | 2201.25 ± 2823.20 | 1630.45 ± 2287.74 | 1896.82 ± 2473.80 | 2484.89 ± 3406.92 | 1917.26 ± 2334.62 | 2191.92 0 ± 2867.93 | NS |
| +sCD14 (×10 pg/mL) | 6318.33 ± 1845.33 | 7393.96 ± 1103.19 | 6892.00 ± 1541.57 | 6851.46 ± 1855.94 | 7739.69 ± 1778.08 | 7295.57 ± 1843.93 | NS |
| Post-Study Liver Cell Death Markers | |||||||
| K18M65 (IU/L) b | 239.57 ± 106.56 | 373.50 ± 483.61 | 311.00 ± 355.79 | 357.25 ± 129.83 | 726.00 ± 1678.11 | 541.62 ± 1185.69 | NS |
| K18M30 (IU/L) | 508.47 ± 794.91 | 247.45 ± 154.50 | 369.26 ± 548.55 | 239.59 ± 76.14 | 246.68 ± 148.57 | 243.02 ± 114.95 | NS |
| M65:M30 b | 0.98 ± 0.66 | 1.16 ± 0.80 | 1.08 ± 0.72 | 1.56 ± 0.52 | 1.35 ± 0.56 | 1.46 ± 0.54 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winrich, E.J.; Gala, K.S.; Rajhans, A.; Rios-Perez, C.D.; Royer, A.J.; Zamani, Z.; Parthasarathy, R.; Marsano-Obando, L.S.; Barve, A.J.; Schwandt, M.L.; et al. Association of Hypomagnesemia and Liver Injury, Role of Gut-Barrier Dysfunction and Inflammation: Efficacy of Abstinence, and 2-Week Medical Management in Alcohol Use Disorder Patients. Int. J. Mol. Sci. 2022, 23, 11332. https://doi.org/10.3390/ijms231911332
Winrich EJ, Gala KS, Rajhans A, Rios-Perez CD, Royer AJ, Zamani Z, Parthasarathy R, Marsano-Obando LS, Barve AJ, Schwandt ML, et al. Association of Hypomagnesemia and Liver Injury, Role of Gut-Barrier Dysfunction and Inflammation: Efficacy of Abstinence, and 2-Week Medical Management in Alcohol Use Disorder Patients. International Journal of Molecular Sciences. 2022; 23(19):11332. https://doi.org/10.3390/ijms231911332
Chicago/Turabian StyleWinrich, Evan J., Khushboo S. Gala, Abhas Rajhans, Christian D. Rios-Perez, Amor J. Royer, Zarlakhta Zamani, Ranganathan Parthasarathy, Luis S. Marsano-Obando, Ashutosh J. Barve, Melanie L. Schwandt, and et al. 2022. "Association of Hypomagnesemia and Liver Injury, Role of Gut-Barrier Dysfunction and Inflammation: Efficacy of Abstinence, and 2-Week Medical Management in Alcohol Use Disorder Patients" International Journal of Molecular Sciences 23, no. 19: 11332. https://doi.org/10.3390/ijms231911332
APA StyleWinrich, E. J., Gala, K. S., Rajhans, A., Rios-Perez, C. D., Royer, A. J., Zamani, Z., Parthasarathy, R., Marsano-Obando, L. S., Barve, A. J., Schwandt, M. L., & Vatsalya, V. (2022). Association of Hypomagnesemia and Liver Injury, Role of Gut-Barrier Dysfunction and Inflammation: Efficacy of Abstinence, and 2-Week Medical Management in Alcohol Use Disorder Patients. International Journal of Molecular Sciences, 23(19), 11332. https://doi.org/10.3390/ijms231911332






