Dynamic Assessment of Tissue and Plasma EGFR-Activating and T790M Mutations with Droplet Digital PCR Assays for Monitoring Response and Resistance in Non-Small Cell Lung Cancers Treated with EGFR-TKIs
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Detection of Tissue and Plasma EGFR Mutations at Baseline Using the ddPCR Platform
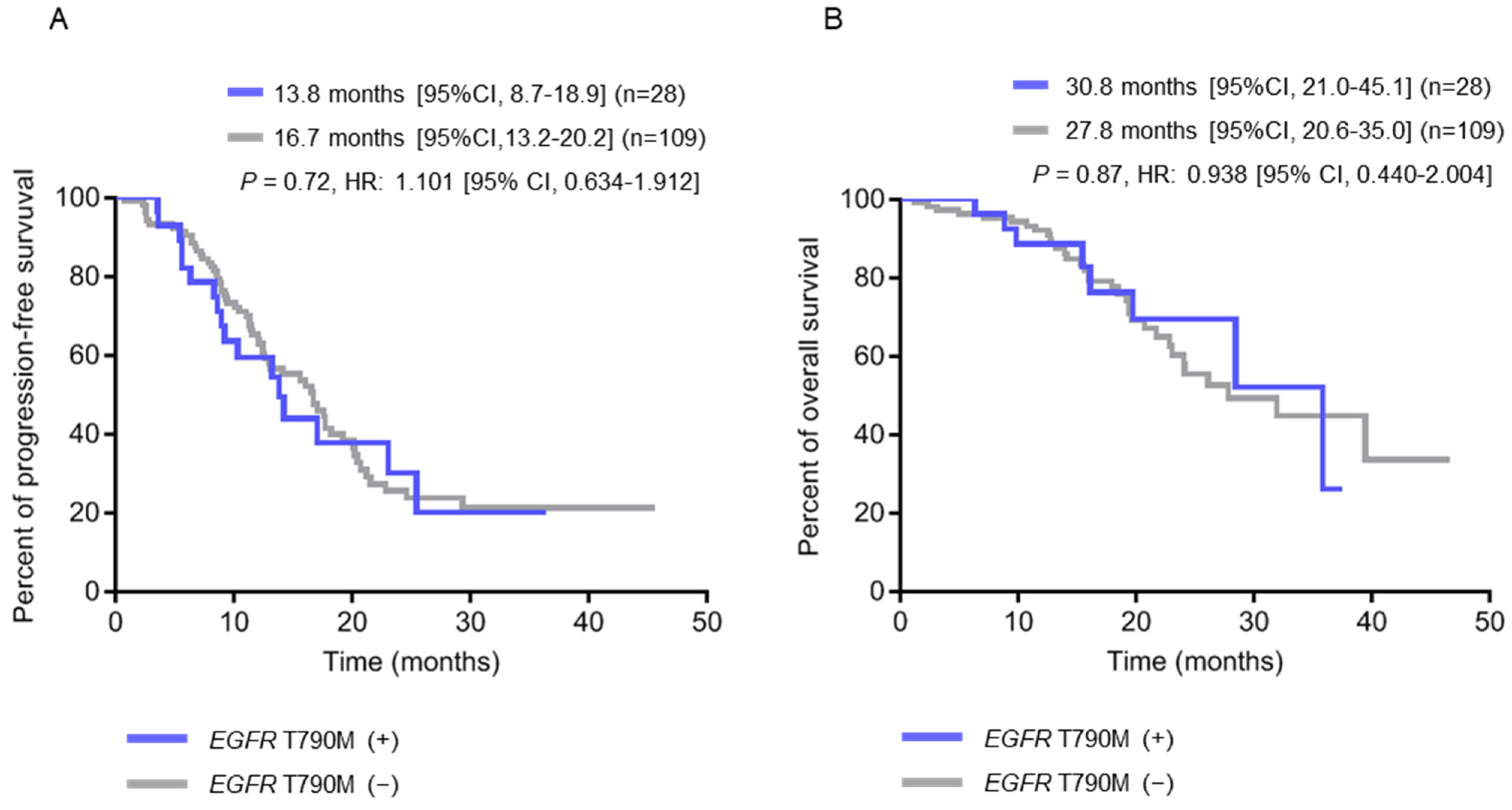
2.3. Clinical Characteristics of Patients with Pre-Treatment Tissue EGFR T790M and Plasma EGFR-Activating Mutations
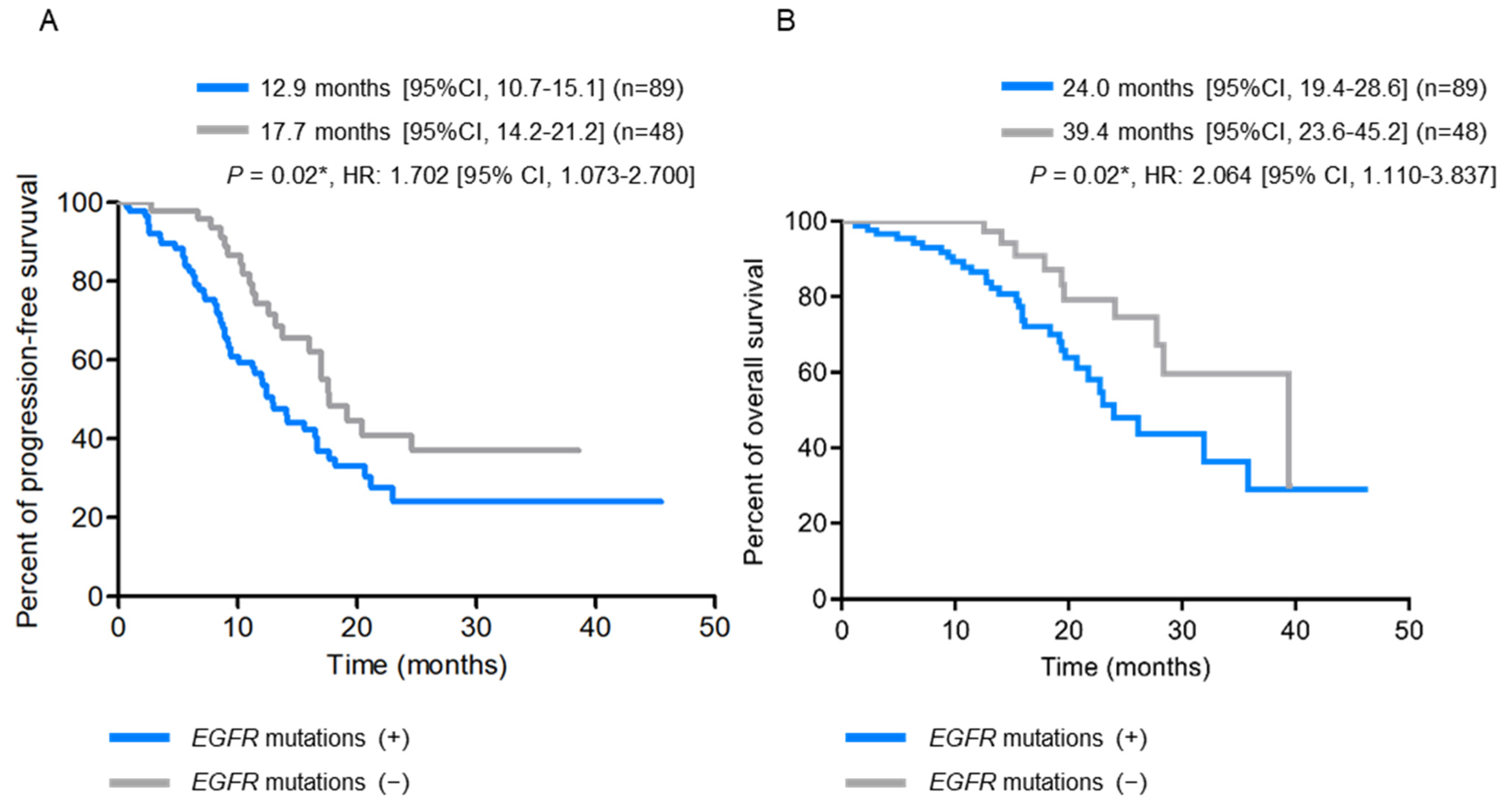
2.4. Detection of Tissue and Plasma EGFR Mutations at Disease Progression Using the ddPCR Platform
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. DNA Isolation from Plasma and Formalin-Fixed Paraffin-Embedded Tissues
4.3. EGFR Mutation Analysis Using Droplet Digital PCR
4.4. ddPCR Data Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V.; Bell, D.W.; Settleman, J.; Haber, D.A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 2007, 7, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Au, J.S.; Thongprasert, S.; Srinivasan, S.; Tsai, C.M.; Khoa, M.T.; Heeroma, K.; Itoh, Y.; Cornelio, G.; Yang, P.C. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Fukuoka, M.; Wu, Y.L.; Thongprasert, S.; Sunpaweravong, P.; Leong, S.S.; Sriuranpong, V.; Chao, T.Y.; Nakagawa, K.; Chu, D.T.; Saijo, N.; et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 2011, 29, 2866–2874. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.M.; Boyer, M.; et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Onozato, R.; Yatabe, Y.; Mitsudomi, T. EGFR T790M mutation: A double role in lung cancer cell survival? J. Thorac. Oncol. 2009, 4, 1–4. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Pirker, R.; Herth, F.J.; Kerr, K.M.; Filipits, M.; Taron, M.; Gandara, D.; Hirsch, F.R.; Grunenwald, D.; Popper, H.; Smit, E.; et al. Consensus for EGFR mutation testing in non-small cell lung cancer: Results from a European workshop. J. Thorac. Oncol. 2010, 5, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Karachaliou, N. Implications of Blood-Based T790M Genotyping and Beyond in Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 3361–3362. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.M.; de Petris, G.; Lopez, J.I. Detection of Intratumor Heterogeneity in Modern Pathology: A Multisite Tumor Sampling Perspective. Front. Med. 2017, 4, 25. [Google Scholar] [CrossRef]
- Esposito, A.; Criscitiello, C.; Locatelli, M.; Milano, M.; Curigliano, G. Liquid biopsies for solid tumors: Understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol. Ther. 2016, 157, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.K.; Pandey, T.; Dey, P. Liquid biopsy: A new avenue in pathology. Cytopathology 2019, 30, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Malapelle, U.; Sirera, R.; Jantus-Lewintre, E.; Reclusa, P.; Calabuig-Farinas, S.; Blasco, A.; Pisapia, P.; Rolfo, C.; Camps, C. Profile of the Roche cobas(R) EGFR mutation test v2 for non-small cell lung cancer. Expert Rev. Mol. Diagn. 2017, 17, 209–215. [Google Scholar] [CrossRef]
- Pekin, D.; Skhiri, Y.; Baret, J.C.; Le Corre, D.; Mazutis, L.; Salem, C.B.; Millot, F.; El Harrak, A.; Hutchison, J.B.; Larson, J.W.; et al. Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab Chip 2011, 11, 2156–2166. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.; Lu, H.; Garlan, F.; Taly, V. Droplet-Based Digital PCR: Application in Cancer Research. Adv. Clin. Chem. 2017, 79, 43–91. [Google Scholar] [PubMed]
- Taylor, S.C.; Laperriere, G.; Germain, H. Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: From variable nonsense to publication quality data. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.L.; Sauzade, M.; Brouzes, E. dPCR: A Technology Review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef]
- Yoon, B.W.; Kim, J.H.; Lee, S.H.; Choi, C.M.; Rho, J.K.; Yoon, S.; Lee, D.H.; Kim, S.W.; Jang, T.W.; Lee, J.C. Comparison of T790M Acquisition Between Patients Treated with Afatinib and Gefitinib as First-Line Therapy: Retrospective Propensity Score Matching Analysis. Transl. Oncol. 2019, 12, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular Testing Guideline for the Selection of Patients with Lung Cancer for Treatment with Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 911–919. [Google Scholar] [PubMed]
- Kim, H.R.; Lee, S.Y.; Hyun, D.S.; Lee, M.K.; Lee, H.K.; Choi, C.M.; Yang, S.H.; Kim, Y.C.; Lee, Y.C.; Kim, S.Y.; et al. Detection of EGFR mutations in circulating free DNA by PNA-mediated PCR clamping. J. Exp. Clin. Cancer Res. 2013, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E.; Okamoto, I.; Sriuranpong, V.; Vansteenkiste, J.; Imamura, F.; Lee, J.S.; Pang, Y.K.; Cobo, M.; Kasahara, K.; Cheng, Y.; et al. Tissue and Plasma EGFR Mutation Analysis in the FLAURA Trial: Osimertinib versus Comparator EGFR Tyrosine Kinase Inhibitor as First-Line Treatment in Patients with EGFR-Mutated Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 6644–6652. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, T.; Saito, H.; Furuya, N.; Watanabe, K.; Sugawara, S.; Iwasawa, S.; Tsunezuka, Y.; Yamaguchi, O.; Okada, P.M.; Yoshimori, K.; et al. Evaluation of plasma EGFR mutation as an early predictor of response of erlotinib plus bevacizumab treatment in the NEJ026 study. EBioMedicine 2020, 57, 102861. [Google Scholar] [CrossRef]
- Wu, Y.L.; Lee, V.; Liam, C.K.; Lu, S.; Park, K.; Srimuninnimit, V.; Wang, J.; Zhou, C.; Appius, A.; Button, P.; et al. Clinical utility of a blood-based EGFR mutation test in patients receiving first-line erlotinib therapy in the ENSURE, FASTACT-2, and ASPIRATION studies. Lung Cancer 2018, 126, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J. Thorac. Oncol. 2018, 13, 323–358. [Google Scholar] [PubMed]
- Fujita, Y.; Suda, K.; Kimura, H.; Matsumoto, K.; Arao, T.; Nagai, T.; Saijo, N.; Yatabe, Y.; Mitsudomi, T.; Nishio, K. Highly sensitive detection of EGFR T790M mutation using colony hybridization predicts favorable prognosis of patients with lung cancer harboring activating EGFR mutation. J. Thorac. Oncol. 2012, 7, 1640–1644. [Google Scholar] [CrossRef]
- Ma, C.; Wei, S.; Song, Y. T790M and acquired resistance of EGFR TKI: A literature review of clinical reports. J. Thorac. Dis. 2011, 3, 10–18. [Google Scholar] [PubMed]
- Su, K.Y.; Chen, H.Y.; Li, K.C.; Kuo, M.L.; Yang, J.C.; Chan, W.K.; Ho, B.C.; Chang, G.C.; Shih, J.Y.; Yu, S.L.; et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 433–440. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, J.; Yin, L.; Jiang, H.; Zhang, W.; Song, Y.; Liu, M. The prognostic role of pretreatment epidermal growth factor receptor T790M mutation in advanced non-small cell lung cancer patients treated with EGFR tyrosine kinase inhibitors. Oncotarget 2017, 8, 50941–50948. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.L.; Heideman, D.A.; Thunnissen, E.; Paul, M.A.; van Wijk, A.W.; Postmus, P.E.; Smit, E.F. Incidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC-patients. Lung Cancer 2014, 85, 19–24. [Google Scholar] [CrossRef]
- Ballard, P.; Yates, J.W.; Yang, Z.; Kim, D.W.; Yang, J.C.; Cantarini, M.; Pickup, K.; Jordan, A.; Hickey, M.; Grist, M.; et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin. Cancer Res. 2016, 22, 5130–5140. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet. Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Furugaki, K.; Moriya, Y.; Iwai, T.; Yorozu, K.; Yanagisawa, M.; Kondoh, K.; Fujimoto-Ohuchi, K.; Mori, K. Erlotinib inhibits osteolytic bone invasion of human non-small-cell lung cancer cell line NCI-H292. Clin. Exp. Metastasis 2011, 28, 649–659. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Taly, V.; Pekin, D.; Benhaim, L.; Kotsopoulos, S.K.; Le Corre, D.; Li, X.; Atochin, I.; Link, D.R.; Griffiths, A.D.; Pallier, K.; et al. Multiplex picodroplet digital PCR to detect KRAS mutations in circulating DNA from the plasma of colorectal cancer patients. Clin. Chem. 2013, 59, 1722–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Cobas EGFR Mutation Test | ddPCR | |||
|---|---|---|---|---|
| No. | (%) | No. | (%) | |
| L858R | 77 | (56.2) | 61 | (44.5) |
| L858R/T790M | 0 | (0.0) | 16 | (11.7) |
| E19D | 60 | (43.8) | 48 | (35.0) |
| E19D/T790M | 0 | (0.0) | 12 | (8.8) |
| Total | 137 | (100.0) | 137 | (100.0) |
| Tumor Tissues | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L858R | L858R/T790M | E19D | E19D/T790M | Total | ||||||
| Plasma | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) |
| L858R | 37 | (27.0) | 10 | (7.3) | 0 | (0.0) | 0 | (0.0) | 47 | (34.3) |
| L858R/T790M | 1 | (0.7) | 1 | (0.7) | 0 | (0.0) | 0 | (0.0) | 2 | (1.5) |
| E19D | 0 | (0.0) | 0 | (0.0) | 32 | (23.4) | 6 | (4.4) | 38 | (27.7) |
| E19D/T790M | 0 | (0.0) | 0 | (0.0) | 1 | (0.7) | 1 | (0.7) | 2 | (1.5) |
| Not detected | 23 | (16.8) | 5 | (3.6) | 15 | (10.9) | 5 | (3.6) | 48 | (35.0) |
| Total | 61 | (44.5) | 16 | (11.7) | 48 | (35.0) | 12 | (8.8) | 137 | (100.0) |
| All | Tissue | Plasma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T790M+ | T790M− | p Value | EGFR m+ | EGFR m− | p Value | |||||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |||
| Number of cases | 137 | (100.0) | 28 | (20.4) | 109 | (79.6) | 89 | (65.0) | 48 | (35.0) | ||
| Stage a | 0.99 | 0.54 | ||||||||||
| IIIB | 2 | (1.5) | 0 | (0.0) | 2 | (1.8) | 2 | (2.2) | 0 | (0.0) | ||
| IV | 135 | (98.5) | 28 | (100.0) | 107 | (98.2) | 87 | (97.8) | 48 | (100.0) | ||
| Sex | 0.82 | 0.68 | ||||||||||
| Male | 61 | (44.5) | 13 | (46.4) | 48 | (44.0) | 40 | (44.9) | 21 | (43.8) | ||
| Female | 76 | (55.5) | 15 | (53.6) | 61 | (56.0) | 49 | (55.1) | 27 | (56.2) | ||
| Age | ||||||||||||
| Mean | 64.3 | 63.0 | 64.7 | 0.49 | 63.7 | 65.5 | 0.37 | |||||
| Range | 37.3–90.7 | 37.3–83.2 | 45.4–90.7 | 37.3–90.7 | 45.4–90.6 | |||||||
| Smoking history | 0.20 | 0.51 | ||||||||||
| Never-smoker | 101 | (73.7) | 18 | (64.3) | 83 | (76.1) | 64 | (71.9) | 37 | (77.1) | ||
| Smoker | 36 | (26.3) | 10 | (35.7) | 26 | (23.9) | 25 | (28.1) | 11 | (22.9) | ||
| First-line therapy | 0.33 | 0.11 | ||||||||||
| Afatinib | 74 | (54.0) | 16 | (57.1) | 58 | (53.2) | 51 | (57.3) | 23 | (47.9) | ||
| Erlotinib | 41 | (29.9) | 10 | (35.7) | 31 | (28.4) | 28 | (31.5) | 13 | (27.1) | ||
| Gefitinib | 22 | (16.1) | 2 | (7.1) | 20 | (18.3) | 10 | (11.2) | 12 | (25.0) | ||
| Primary tumor size, mm | 0.30 | 0.004 b | ||||||||||
| Mean | 40.0 | 43.9 | 39.0 | 42.9 | 34.2 | |||||||
| Range | 12–159 | 14–76 | 12–159 | 12–159 | 16–76 | |||||||
| Tumor category | 0.68 | 0.69 | ||||||||||
| T1 | 25 | (18.2) | 3 | (10.7) | 22 | (20.2) | 16 | (18.0) | 9 | (18.8) | ||
| T2 | 50 | (36.5) | 12 | (42.9) | 38 | (34.9) | 33 | (37.0) | 17 | (35.4) | ||
| T3 | 20 | (14.6) | 4 | (14.3) | 16 | (14.7) | 15 | (16.9) | 5 | (10.4) | ||
| T4 | 42 | (30.7) | 9 | (32.1) | 33 | (30.3) | 25 | (28.1) | 17 | (35.4) | ||
| Nodal status | 0.42 | 0.04 b | ||||||||||
| N0 | 43 | (31.4) | 11 | (39.3) | 32 | (29.4) | 23 | (25.9) | 20 | (41.7) | ||
| N1 | 15 | (10.9) | 3 | (10.7) | 12 | (11.0) | 10 | (11.2) | 5 | (10.4) | ||
| N2 | 43 | (31.4) | 10 | (35.7) | 33 | (30.3) | 26 | (29.2) | 17 | (35.4) | ||
| N3 | 36 | (26.3) | 4 | (14.3) | 32 | (29.4) | 30 | (33.7) | 6 | (12.5) | ||
| Metastasis status a | 0.54 | 0.05 | ||||||||||
| M0 | 3 | (2.3) | 0 | (0.0) | 3 | (2.8) | 2 | (2.2) | 1 | (2.1) | ||
| M1a | 35 | (25.5) | 5 | (17.9) | 30 | (27.5) | 16 | (18.0) | 19 | (39.5) | ||
| M1b | 75 | (54.7) | 18 | (64.3) | 57 | (52.3) | 54 | (60.7) | 21 | (43.8) | ||
| M1c | 24 | (17.5) | 5 | (17.9) | 19 | (17.4) | 17 | (19.1) | 7 | (14.6) | ||
| EGFR mutation | 0.91 | N/A | ||||||||||
| L858R | 77 | (56.2) | 16 | (57.1) | 61 | (56.0) | 49 | (55.1) | 0 | (0.0) | ||
| E19D | 60 | (43.8) | 12 | (42.9) | 48 | (44.0) | 40 | (44.9) | 0 | (0.0) | ||
| Brain-Metastasis | 0.005 b | 0.34 | ||||||||||
| Yes | 56 | (40.9) | 18 | (64.3) | 38 | (34.9) | 39 | (43.8) | 17 | (35.4) | ||
| No | 81 | (59.1) | 10 | (35.7) | 71 | (65.1) | 50 | (56.2) | 31 | (64.6) | ||
| Bone-Metastasis | 0.84 | 0.002 b | ||||||||||
| Yes | 71 | (51.8) | 15 | (53.6) | 56 | (51.4) | 55 | (61.8) | 16 | (33.3) | ||
| No | 66 | (48.2) | 13 | (46.4) | 53 | (48.6) | 34 | (38.2) | 32 | (66.7) | ||
| Pleura-Metastasis | 0.15 | 0.65 | ||||||||||
| Yes | 34 | (24.8) | 4 | (14.3) | 30 | (27.5) | 21 | (23.6) | 13 | (27.1) | ||
| No | 103 | (75.2) | 24 | (85.7) | 79 | (72.5) | 68 | (76.4) | 35 | (72.9) | ||
| Liver-Metastasis a | 0.23 | 0.14 | ||||||||||
| Yes | 13 | (9.5) | 1 | (3.6) | 12 | (11.0) | 11 | (12.4) | 2 | (4.2) | ||
| No | 124 | (90.5) | 27 | (96.4) | 97 | (89.0) | 78 | (87.6) | 46 | (95.8) | ||
| Plasma | Tumor Rebiopsy | |||
|---|---|---|---|---|
| No. | % | No. | % | |
| No. of patients | 79 | 100.0 | 35 | 100.0 |
| L858R | 17 | 21.5 | 8 | 22.9 |
| L858R/T790M | 9 | 11.4 | 6 | 17.1 |
| E19D | 7 | 8.9 | 10 | 28.6 |
| E19D/T790M | 12 | 15.2 | 8 | 22.9 |
| T790M | 0 | 0.0 | 1 | 2.9 |
| No variation detected | 34 | 43.0 | 2 | 5.7 |
| Tumor Rebiopsy | ||||||
|---|---|---|---|---|---|---|
| T790M+ | T790M− | Total | ||||
| No. | (%) | No. | (%) | No. | (%) | |
| Plasma | ||||||
| T790M+ | 8 | (22.9) | 3 | (8.5) | 11 | (31.4) |
| T790M− | 7 | (20.0) | 17 | (48.6) | 24 | (68.6) |
| Total | 15 | (42.9) | 20 | (57.1) | 35 | (100.0) |
| Tissue at Baseline | |||||
|---|---|---|---|---|---|
| T790M+ | T790M− | p Value | |||
| No. | (%) | No. | (%) | ||
| No. of patients | 28 | (100.0) | 109 | (100.0) | |
| Plasma at baseline a | 0.19 | ||||
| T790M+ | 2 | (7.1) | 2 | (1.8) | |
| T790M− | 26 | (92.9) | 107 | (98.2) | |
| Plasma at progression | 0.36 | ||||
| T790M+ | 6 | (21.4) | 15 | (13.8) | |
| T790M− | 11 | (39.3) | 47 | (43.1) | |
| N/A | 11 | (39.3) | 47 | (43.1) | |
| Tumor Rebiopsy at progression a | 0.45 | ||||
| T790M+ | 5 | (17.9) | 10 | (9.2) | |
| T790M− | 4 | (14.3) | 16 | (14.6) | |
| N/A | 19 | (67.8) | 83 | (76.2) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, H.-L.; Wang, F.-Y.; Chiang, C.-L.; Tsai, C.-M.; Chiu, C.-H.; Chou, T.-Y. Dynamic Assessment of Tissue and Plasma EGFR-Activating and T790M Mutations with Droplet Digital PCR Assays for Monitoring Response and Resistance in Non-Small Cell Lung Cancers Treated with EGFR-TKIs. Int. J. Mol. Sci. 2022, 23, 11353. https://doi.org/10.3390/ijms231911353
Ho H-L, Wang F-Y, Chiang C-L, Tsai C-M, Chiu C-H, Chou T-Y. Dynamic Assessment of Tissue and Plasma EGFR-Activating and T790M Mutations with Droplet Digital PCR Assays for Monitoring Response and Resistance in Non-Small Cell Lung Cancers Treated with EGFR-TKIs. International Journal of Molecular Sciences. 2022; 23(19):11353. https://doi.org/10.3390/ijms231911353
Chicago/Turabian StyleHo, Hsiang-Ling, Fang-Yu Wang, Chi-Lu Chiang, Chun-Ming Tsai, Chao-Hua Chiu, and Teh-Ying Chou. 2022. "Dynamic Assessment of Tissue and Plasma EGFR-Activating and T790M Mutations with Droplet Digital PCR Assays for Monitoring Response and Resistance in Non-Small Cell Lung Cancers Treated with EGFR-TKIs" International Journal of Molecular Sciences 23, no. 19: 11353. https://doi.org/10.3390/ijms231911353
APA StyleHo, H.-L., Wang, F.-Y., Chiang, C.-L., Tsai, C.-M., Chiu, C.-H., & Chou, T.-Y. (2022). Dynamic Assessment of Tissue and Plasma EGFR-Activating and T790M Mutations with Droplet Digital PCR Assays for Monitoring Response and Resistance in Non-Small Cell Lung Cancers Treated with EGFR-TKIs. International Journal of Molecular Sciences, 23(19), 11353. https://doi.org/10.3390/ijms231911353






