PD-L1, a Potential Immunomodulator Linking Immunology and Orthodontically Induced Inflammatory Root Resorption (OIIRR): Friend or Foe?
Abstract
:1. Introduction
2. OIIRR
2.1. Pathophysiology of OIIRR
2.2. Resorptive and Immune Cells in OIIRR
2.3. Cementum Repair in OIIRR
3. Immunological Aspects in OIIRR
3.1. Possible Immunological Responses to OIIRR
3.2. The Molecular Immunological Change in OIIRR
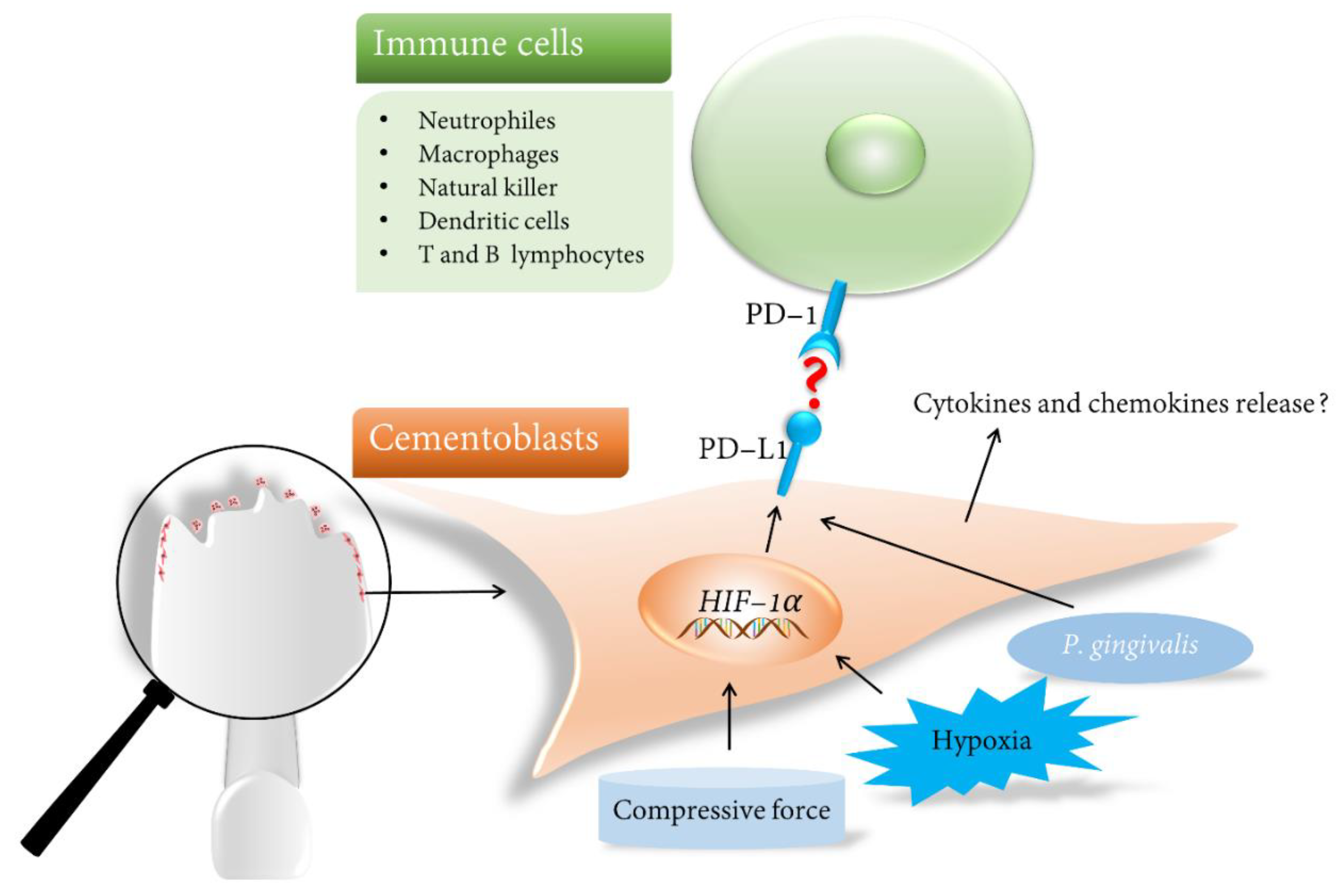
4. PD-L1 and OIIRR
4.1. Immunomodulator PD-L1
4.2. Regulation of PD-L1 in Orthodontic-Induced Microenvironments
4.3. Potential Clinical Perspective of Targeting PD-L1 Treatment for OIIRR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yong, J.; Groeger, S.; Meyle, J.; Ruf, S. MAPK and β-Catenin signaling: Implication and interplay in orthodontic tooth movement. Front. Biosci. (Landmark Ed.) 2022, 27, 54. [Google Scholar] [CrossRef] [PubMed]
- Diercke, K.; Kohl, A.; Lux, C.J.; Erber, R. Compression of human primary cementoblasts leads to apoptosis: A possible cause of dental root resorption? J. Orofac. Orthop. 2014, 75, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; von Bremen, J.; Ruiz-Heiland, G.; Ruf, S. Adiponectin as Well as Compressive Forces Regulate in vitro beta-Catenin Expression on Cementoblasts via Mitogen-Activated Protein Kinase Signaling Activation. Front. Cell Dev. Biol. 2021, 9, 645005. [Google Scholar] [CrossRef] [PubMed]
- Brezniak, N.; Wasserstein, A. Orthodontically induced inflammatory root resorption. Part I: The basic science aspects. Angle Orthod. 2002, 72, 175–179. [Google Scholar]
- Artun, J.; Van ‘t Hullenaar, R.; Doppel, D.; Kuijpers-Jagtman, A.M. Identification of orthodontic patients at risk of severe apical root resorption. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 448–455. [Google Scholar] [CrossRef]
- Silva, L.B.; Guimaraes, C.S.; Santos, R.A. Immunology of root resorption: A literature review. Indian J. Dent. Res. 2008, 19, 340–343. [Google Scholar] [CrossRef]
- Haug, S.R.; Brudvik, P.; Fristad, I.; Heyeraas, K.J. Sympathectomy causes increased root resorption after orthodontic tooth movement in rats: Immunohistochemical study. Cell Tissue Res. 2003, 313, 167–175. [Google Scholar] [CrossRef]
- Alhashimi, N.; Frithiof, L.; Brudvik, P.; Bakhiet, M. CD40-CD40L expression during orthodontic tooth movement in rats. Angle Orthod. 2004, 74, 100–105. [Google Scholar]
- Yan, Y.; Liu, F.; Kou, X.; Liu, D.; Yang, R.; Wang, X.; Song, Y.; He, D.; Gan, Y.; Zhou, Y. T Cells Are Required for Orthodontic Tooth Movement. J. Dent. Res. 2015, 94, 1463–1470. [Google Scholar] [CrossRef]
- Spitz, A.; Christovam, I.O.; Maranon-Vasquez, G.A.; Masterson, D.F.; Adesse, D.; Maia, L.C.; Bolognese, A.M. Global gene expression profile of periodontal ligament cells submitted to mechanical loading: A systematic review. Arch. Oral. Biol. 2020, 118, 104884. [Google Scholar] [CrossRef]
- Iglesias-Linares, A.; Hartsfield, J.K., Jr. Cellular and Molecular Pathways Leading to External Root Resorption. J. Dent. Res. 2017, 96, 145–152. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of human cementum: Its structure, function, and development. Jpn. Dent. Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Yong, J.; von Bremen, J.; Ruiz-Heiland, G.; Ruf, S. Adiponectin Interacts In-Vitro with Cementoblasts Influencing Cell Migration, Proliferation and Cementogenesis Partly Through the MAPK Signaling Pathway. Front. Pharmacol. 2020, 11, 585346. [Google Scholar] [CrossRef]
- Klein, Y.; Fleissig, O.; Polak, D.; Barenholz, Y.; Mandelboim, O.; Chaushu, S. Immunorthodontics: In vivo gene expression of orthodontic tooth movement. Sci. Rep. 2020, 10, 8172. [Google Scholar] [CrossRef]
- Zeichner-David, M.; Oishi, K.; Su, Z.; Zakartchenko, V.; Chen, L.S.; Arzate, H.; Bringas, P., Jr. Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev. Dyn. 2003, 228, 651–663. [Google Scholar] [CrossRef]
- Hidalgo, M.M.; Itano, E.N.; Consolaro, A. Humoral immune response of patients with dental trauma and consequent replacement resorption. Dent. Traumatol. 2005, 21, 218–221. [Google Scholar] [CrossRef]
- Horita, H.; Law, A.; Hong, S.; Middleton, K. Identifying Regulatory Posttranslational Modifications of PD-L1: A Focus on Monoubiquitinaton. Neoplasia 2017, 19, 346–353. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Zheng, Y.; Fang, Y.C.; Li, J. PD-L1 expression levels on tumor cells affect their immunosuppressive activity. Oncol. Lett. 2019, 18, 5399–5407. [Google Scholar] [CrossRef]
- Groeger, S.; Howaldt, H.P.; Raifer, H.; Gattenloehner, S.; Chakraborty, T.; Meyle, J. Oral Squamous Carcinoma Cells Express B7-H1 and B7-DC Receptors in Vivo. Pathol. Oncol. Res. 2017, 23, 99–110. [Google Scholar] [CrossRef]
- Adel-Khattab, D.; Groeger, S.; Domann, E.; Chakraborty, T.; Lochnit, G.; Meyle, J. Porphyromonas gingivalis induced up-regulation of PD-L1 in colon carcinoma cells. Mol. Oral. Microbiol. 2021, 36, 172–181. [Google Scholar] [CrossRef]
- Groeger, S.; Wu, F.; Wagenlehner, F.; Dansranjav, T.; Ruf, S.; Denter, F.; Meyle, J. PD-L1 Up-Regulation in Prostate Cancer Cells by Porphyromonas gingivalis. Front. Cell Infect. Microbiol. 2022, 12, 935806. [Google Scholar] [CrossRef]
- Juneja, V.R.; McGuire, K.A.; Manguso, R.T.; LaFleur, M.W.; Collins, N.; Haining, W.N.; Freeman, G.J.; Sharpe, A.H. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 2017, 214, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The implication of the PD-1/PD-L1 checkpoint in chronic periodontitis suggests novel therapeutic opportunities with natural products. Jpn. Dent. Sci. Rev. 2020, 56, 90–96. [Google Scholar] [CrossRef]
- Motokawa, M.; Sasamoto, T.; Kaku, M.; Kawata, T.; Matsuda, Y.; Terao, A.; Tanne, K. Association between root resorption incident to orthodontic treatment and treatment factors. Eur. J. Orthod. 2012, 34, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Fukasawa, S. Is Inflammation a Friend or Foe for Orthodontic Treatment?: Inflammation in Orthodontically Induced Inflammatory Root Resorption and Accelerating Tooth Movement. Int. J. Mol. Sci. 2021, 22, 2388. [Google Scholar] [CrossRef]
- Al-Ghurabi, B.; Al-Hindawi, S.; Mohammed, I. Physiological Role of Immune System Elements in Orthodontic Treatment. Med.-Leg. Update 2020, 20, 6767–6772. [Google Scholar]
- Hammarstrom, L.; Lindskog, S. General morphological aspects of resorption of teeth and alveolar bone. Int. Endod. J. 1985, 18, 93–108. [Google Scholar] [CrossRef]
- Krishnan, V. Root Resorption with Orthodontic Mechanics: Pertinent Areas Revisited. Aust. Dent. J. 2017, 62 (Suppl. S1), 71–77. [Google Scholar] [CrossRef] [PubMed]
- Blaushild, N.; Michaeli, Y.; Steigman, S. Histomorphometric study of the periodontal vasculature of the rat incisor. J. Dent. Res. 1992, 71, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- Mavridou, A.M.; Pyka, G.; Kerckhofs, G.; Wevers, M.; Bergmans, L.; Gunst, V.; Huybrechts, B.; Schepers, E.; Hauben, E.; Lambrechts, P. A novel multimodular methodology to investigate external cervical tooth resorption. Int. Endod. J. 2016, 49, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Gratz, E.M.; Widbiller, M.; Buchalla, W.; Knuttel, H. Pathophysiological mechanisms of root resorption after dental trauma: A systematic scoping review. BMC Oral. Health 2021, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Brudvik, P.; Rygh, P. The initial phase of orthodontic root resorption incident to local compression of the periodontal ligament. Eur. J. Orthod. 1993, 15, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Goz, G.R.; Rahn, B.A.; Schulte-Monting, J. The effects of horizontal tooth loading on the circulation and width of the periodontal ligament—An experimental study on beagle dogs. Eur. J. Orthod. 1992, 14, 21–25. [Google Scholar] [CrossRef]
- Hellsing, E.; Hammarstrom, L. The hyaline zone and associated root surface changes in experimental orthodontics in rats: A light and scanning electron microscope study. Eur. J. Orthod. 1996, 18, 11–18. [Google Scholar] [CrossRef]
- Georgess, D.; Machuca-Gayet, I.; Blangy, A.; Jurdic, P. Podosome organization drives osteoclast-mediated bone resorption. Cell Adhes. Migr. 2014, 8, 191–204. [Google Scholar] [CrossRef]
- Winter, B.U.; Stenvik, A.; Vandevska-Radunovic, V. Dynamics of orthodontic root resorption and repair in human premolars: A light microscopy study. Eur. J. Orthod. 2009, 31, 346–351. [Google Scholar] [CrossRef]
- Brezniak, N.; Wasserstein, A. Orthodontically induced inflammatory root resorption. Part II: The clinical aspects. Ang. Orthod. 2002, 72, 180–184. [Google Scholar]
- Wu, A.T.; Turk, T.; Colak, C.; Elekdag-Turk, S.; Jones, A.S.; Petocz, P.; Darendeliler, M.A. Physical properties of root cementum: Part 18. The extent of root resorption after the application of light and heavy controlled rotational orthodontic forces for 4 weeks: A microcomputed tomography study. Am. J. Orthod. Dentofac. Orthop. 2011, 139, e495–e503. [Google Scholar] [CrossRef]
- Wang, Z.; McCauley, L.K. Osteoclasts and odontoclasts: Signaling pathways to development and disease. Oral. Dis. 2011, 17, 129–142. [Google Scholar] [CrossRef]
- Limeback, H. Molecular mechanisms in dental hard tissue mineralization. Curr. Opin. Dent. 1991, 1, 826–835. [Google Scholar]
- Kamat, M.; Puranik, R.; Vanaki, S.; Kamat, S. An insight into the regulatory mechanisms of cells involved in resorption of dental hard tissues. J. Oral. Maxillofac. Pathol. 2013, 17, 228–233. [Google Scholar] [CrossRef]
- Kumar, G. Orban’s Oral Histology & Embryology-E-BOOK; Elsevier Health Sciences: Philadelphia, PA, USA, 2015. [Google Scholar]
- Chaushu, S.; Klein, Y.; Mandelboim, O.; Barenholz, Y.; Fleissig, O. Immune Changes Induced by Orthodontic Forces: A Critical Review. J. Dent. Res. 2022, 101, 11–20. [Google Scholar] [CrossRef]
- Ne, R.F.; Witherspoon, D.E.; Gutmann, J.L. Tooth resorption. Quintessence Int. 1999, 30, 9–25. [Google Scholar]
- Nanci, A. Physiologic tooth movement: Eruption and shedding. In Ten Cate’s Oral Histology—Development, Structure, and Function; Elsevier: Philadelphia, PA, USA, 2007; pp. 268–289. [Google Scholar]
- Hidalgo, M. Study About the Immunogenic Potential of Dentin: A Contribution to the Etiopathogeny of Root Resorption. Ph.D. Thesis, Faculdade de Odontologia de Bauru, Universidade de São Paulo, Bauru, Brazil, 2001. [Google Scholar]
- Sismanidou, C.; Hilliges, M.; Lindskog, S. Healing of the root surface-associated periodontium: An immunohistochemical study of orthodontic root resorption in man. Eur. J. Orthod. 1996, 18, 435–444. [Google Scholar] [CrossRef]
- Brudvik, P.; Rygh, P. Transition and determinants of orthodontic root resorption-repair sequence. Eur. J. Orthod. 1995, 17, 177–188. [Google Scholar] [CrossRef]
- Brudvik, P.; Rygh, P. The repair of orthodontic root resorption: An ultrastructural study. Eur. J. Orthod. 1995, 17, 189–198. [Google Scholar] [CrossRef]
- Jager, A.; Kunert, D.; Friesen, T.; Zhang, D.; Lossdorfer, S.; Gotz, W. Cellular and extracellular factors in early root resorption repair in the rat. Eur. J. Orthod. 2008, 30, 336–345. [Google Scholar] [CrossRef]
- Weltman, B.; Vig, K.W.; Fields, H.W.; Shanker, S.; Kaizar, E.E. Root resorption associated with orthodontic tooth movement: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 462–476, discussion 12A. [Google Scholar] [CrossRef]
- de Freitas, M.R.; Beltrao, R.T.; Janson, G.; Henriques, J.F.; Chiqueto, K. Evaluation of root resorption after open bite treatment with and without extractions. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 143.e15–143.e22. [Google Scholar] [CrossRef]
- Wald, S.; Leibowitz, A.; Aizenbud, Y.; Saba, Y.; Zubeidat, K.; Barel, O.; Koren, N.; Heyman, O.; Wilharm, A.; Sandrock, I.; et al. γδT Cells Are Essential for Orthodontic Tooth Movement. J. Dent. Res. 2021, 100, 731–738. [Google Scholar] [CrossRef]
- Novak, M.L.; Koh, T.J. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 2013, 183, 1352–1363. [Google Scholar] [CrossRef]
- He, D.; Kou, X.; Yang, R.; Liu, D.; Wang, X.; Luo, Q.; Song, Y.; Liu, F.; Yan, Y.; Gan, Y.; et al. M1-like Macrophage Polarization Promotes Orthodontic Tooth Movement. J. Dent. Res. 2015, 94, 1286–1294. [Google Scholar] [CrossRef]
- He, D.; Kou, X.; Luo, Q.; Yang, R.; Liu, D.; Wang, X.; Song, Y.; Cao, H.; Zeng, M.; Gan, Y.; et al. Enhanced M1/M2 macrophage ratio promotes orthodontic root resorption. J. Dent. Res. 2015, 94, 129–139. [Google Scholar] [CrossRef]
- Vandevska-Radunovic, V.; Kvinnsland, I.H.; Kvinnsland, S.; Jonsson, R. Immunocompetent cells in rat periodontal ligament and their recruitment incident to experimental orthodontic tooth movement. Eur. J. Oral. Sci. 1997, 105, 36–44. [Google Scholar] [CrossRef]
- Schroder, A.; Kappler, P.; Nazet, U.; Jantsch, J.; Proff, P.; Cieplik, F.; Deschner, J.; Kirschneck, C. Effects of Compressive and Tensile Strain on Macrophages during Simulated Orthodontic Tooth Movement. Mediat. Inflamm. 2020, 2020, 2814015. [Google Scholar] [CrossRef]
- Hunter, M.M.; Wang, A.; Parhar, K.S.; Johnston, M.J.; Van Rooijen, N.; Beck, P.L.; McKay, D.M. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology 2010, 138, 1395–1405. [Google Scholar] [CrossRef]
- Yamasaki, K.; Shibasaki, Y.; Fukuhara, T. Behavior of mast cells in periodontal ligament associated with experimental tooth movement in rats. J. Dent. Res. 1982, 61, 1447–1450. [Google Scholar] [CrossRef]
- Li, J.; Yu, T.T.; Yan, H.C.; Qiao, Y.Q.; Wang, L.C.; Zhang, T.; Li, Q.; Zhou, Y.H.; Liu, D.W. T cells participate in bone remodeling during the rapid palatal expansion. FASEB J. 2020, 34, 15327–15337. [Google Scholar] [CrossRef]
- Kook, S.H.; Jang, Y.S.; Lee, J.C. Human periodontal ligament fibroblasts stimulate osteoclastogenesis in response to compression force through TNF-alpha-mediated activation of CD4+ T cells. J. Cell. Biochem. 2011, 112, 2891–2901. [Google Scholar] [CrossRef]
- Lacy, P. Editorial: Secretion of cytokines and chemokines by innate immune cells. Front. Immunol. 2015, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.C.; Everts, V.; Pavasant, P.; Ampornaramveth, R.S. Interleukin-1beta induces human cementoblasts to support osteoclastogenesis. Int. J. Oral. Sci. 2017, 9, e5. [Google Scholar] [CrossRef] [PubMed]
- Andrade, I., Jr.; Taddei, S.R.A.; Souza, P.E.A. Inflammation and Tooth Movement: The Role of Cytokines, Chemokines, and Growth Factors. Semin. Orthod. 2012, 18, 257–269. [Google Scholar] [CrossRef]
- Taddei, S.R.; Andrade, I., Jr.; Queiroz-Junior, C.M.; Garlet, T.P.; Garlet, G.P.; Cunha Fde, Q.; Teixeira, M.M.; da Silva, T.A. Role of CCR2 in orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 153–160. [Google Scholar] [CrossRef]
- Alansari, S.; Sangsuwon, C.; Vongthongleur, T.; Kwal, R.; Teo, M.C.; Lee, Y.B.; Nervina, J.; Teixeira, C.; Alikhani, M. Biological principles behind accelerated tooth movement. Semin. Orthod. 2015, 21, 151–161. [Google Scholar] [CrossRef]
- Yong, J.; Groeger, S.; von Bremen, J.; Ruf, S. Ciliary Neurotrophic Factor (CNTF) and Its Receptors Signal Regulate Cementoblasts Apoptosis through a Mechanism of ERK1/2 and Caspases Signaling. Int. J. Mol. Sci. 2022, 23, 8335. [Google Scholar] [CrossRef]
- Keir, M.E.; Francisco, L.M.; Sharpe, A.H. PD-1 and its ligands in T-cell immunity. Curr. Opin. Immunol. 2007, 19, 309–314. [Google Scholar] [CrossRef]
- Groeger, S.; Domann, E.; Gonzales, J.R.; Chakraborty, T.; Meyle, J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology 2011, 216, 1302–1310. [Google Scholar] [CrossRef]
- Groeger, S.; Jarzina, F.; Mamat, U.; Meyle, J. Induction of B7-H1 receptor by bacterial cells fractions of Porphyromonas gingivalis on human oral epithelial cells: B7-H1 induction by Porphyromonas gingivalis fractions. Immunobiology 2017, 222, 137–147. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, C.; Ohno, T.; Azuma, M. Unique B7-H1 expression on masticatory mucosae in the oral cavity and trans-coinhibition by B7-H1-expressing keratinocytes regulating CD4(+) T cell-mediated mucosal tissue inflammation. Mucosal Immunol. 2017, 10, 650–660. [Google Scholar] [CrossRef]
- Shen, J.K.; Cote, G.M.; Choy, E.; Yang, P.; Harmon, D.; Schwab, J.; Nielsen, G.P.; Chebib, I.; Ferrone, S.; Wang, X.; et al. Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol. Res. 2014, 2, 690–698. [Google Scholar] [CrossRef]
- Wang, K.; Gu, Y.; Liao, Y.; Bang, S.; Donnelly, C.R.; Chen, O.; Tao, X.; Mirando, A.J.; Hilton, M.J.; Ji, R.R. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J. Clin. Investig. 2020, 130, 3603–3620. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.M.; Zhang, P.; Wang, X.; Chen, J.; Yang, J.; Lu, W.; Zhou, W.; Yuan, W.; Feng, Y. Expression of programmed death 1 ligand 1 on periodontal tissue cells as a possible protective feedback mechanism against periodontal tissue destruction. Mol. Med. Rep. 2016, 13, 2423–2430. [Google Scholar] [CrossRef]
- Zhou, K.; Sun, M.; Xia, Y.; Xie, Y.; Shu, R. LPS Stimulates Gingival Fibroblasts to Express PD-L1 via the p38 Pathway under Periodontal Inflammatory Conditions. Arch. Oral. Biol. 2021, 129, 105161. [Google Scholar] [CrossRef]
- Ma, W.; Gilligan, B.M.; Yuan, J.; Li, T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J. Hematol. Oncol. 2016, 9, 47. [Google Scholar] [CrossRef]
- Meyle, J.; Domann, E.; Chakrabborty, T.; Gröger, S. B7-H1 Receptor Induced T-Helper Cell Differentiation in vitro. Blood 2008, 111, 3635–3643. [Google Scholar]
- Zhu, D.; Liu, F.; Dai, F.; Luo, X.; Hong, B. Expression of programmed death-1 and programmed death ligand-1 in the peripheral T-lymphocytes from patients with chronic periodontitis. Zhonghua Kou Qiang Yi Xue Za Zhi Zhonghua Kouqiang Yixue Zazhi Chin. J. Stomatol. 2014, 49, 216–219. [Google Scholar]
- Delgado, R.J.R.; Pinheiro, C.R.; Gasparoto, T.H.; Sipert, C.R.; De Moraes, I.G.; Garcia, R.B.; Bramante, C.M.; Bernardineli, N.; Nishiyama, C.K.; Da Silva, J.S. Programmed death 1 (PD-1) and PD-1 ligand (PD-L1) expression in chronic apical periodontitis. Eur. Endod. J. 2019, 4, 3. [Google Scholar] [CrossRef]
- la Rosa-Ruiz, D.; del Pilar, M.; Álvarez-Pérez, M.A.; Cortés-Morales, V.A.; Monroy-García, A.; Mayani, H.; Fragoso-González, G.; Caballero-Chacón, S.; Diaz, D.; Candanedo-González, F. Mesenchymal stem/stromal cells derived from dental tissues: A comparative in vitro evaluation of their immunoregulatory properties against T cells. Cells 2019, 8, 1491. [Google Scholar] [CrossRef]
- Ritprajak, P.; Azuma, M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral. Oncol. 2015, 51, 221–228. [Google Scholar] [CrossRef]
- Yong, J.; Gröger, S.; Meyle, J.; Ruf, S. Immunorthodontics: Role of HIF-1α in the Regulation of (Peptidoglycan-Induced) PD-L1 Expression in Cementoblasts under Compressive Force. Int. J. Mol. Sci. 2022, 23, 6977. [Google Scholar] [CrossRef]
- Yong, J.; Gröger, S.; von Bremen, J.; Meyle, J.; Ruf, S. Immunorthodontics: PD-L1, a Novel Immunomodulator in Cementoblasts, Is Regulated by HIF-1α under Hypoxia. Cells 2022, 11, 2350. [Google Scholar] [CrossRef]
- Ruf, M.; Moch, H.; Schraml, P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int. J. Cancer 2016, 139, 396–403. [Google Scholar] [CrossRef]
- Shelby, A.; Pendleton, C.; Thayer, E.; Johnson, G.K.; Xie, X.J.; Brogden, K.A. PD-L1 correlates with chemokines and cytokines in gingival crevicular fluid from healthy and diseased sites in subjects with periodontitis. BMC Res. Notes 2020, 13, 532. [Google Scholar] [CrossRef]
- Wongtim, K.; Ikeda, E.; Ohno, T.; Nagai, S.; Okuhara, S.; Kure, K.; Azuma, M. Overexpression of PD-L1 in gingival basal keratinocytes reduces periodontal inflammation in a ligature-induced periodontitis model. J. Periodontol. 2021, 93, 146–155. [Google Scholar] [CrossRef]
- Lu, D.; Ni, Z.; Liu, X.; Feng, S.; Dong, X.; Shi, X.; Zhai, J.; Mai, S.; Jiang, J.; Wang, Z.; et al. Beyond T Cells: Understanding the Role of PD-1/PD-L1 in Tumor-Associated Macrophages. J. Immunol. Res. 2019, 2019, 1919082. [Google Scholar] [CrossRef]
- Seliger, B. Basis of PD1/PD-L1 Therapies. J. Clin. Med. 2019, 8, 2168. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr. Opin. Immunol. 2012, 24, 207–212. [Google Scholar] [CrossRef]
- Bajwa, R.; Cheema, A.; Khan, T.; Amirpour, A.; Paul, A.; Chaughtai, S.; Patel, S.; Patel, T.; Bramson, J.; Gupta, V.; et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J. Clin. Med. Res. 2019, 11, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Akella, P.; Loganathan, S.; Jindal, V.; Akhtar, J.; Lal, A. Anti PD-1 immunotherapy related interstitial lung disease presenting as respiratory failure—A review with case series. Respir. Med. Case Rep. 2019, 26, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Celli, R.; Kluger, H.M.; Zhang, X. Anti-PD-1 Therapy-Associated Perforating Colitis. Case Rep. Gastrointest. Med. 2018, 2018, 3406437. [Google Scholar] [CrossRef]
- Frigeri, M.; Meyer, P.; Banfi, C.; Giraud, R.; Hachulla, A.L.; Spoerl, D.; Friedlaender, A.; Pugliesi-Rinaldi, A.; Dietrich, P.Y. Immune Checkpoint Inhibitor-Associated Myocarditis: A New Challenge for Cardiologists. Can. J. Cardiol. 2018, 34, 92.e1–92.e3. [Google Scholar] [CrossRef]
- de Filette, J.M.K.; Pen, J.J.; Decoster, L.; Vissers, T.; Bravenboer, B.; Van der Auwera, B.J.; Gorus, F.K.; Roep, B.O.; Aspeslagh, S.; Neyns, B.; et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: A case report and systematic review. Eur. J. Endocrinol. 2019, 181, 363–374. [Google Scholar] [CrossRef]
- Sibaud, V.; Eid, C.; Belum, V.R.; Combemale, P.; Barres, B.; Lamant, L.; Mourey, L.; Gomez-Roca, C.; Estilo, C.L.; Motzer, R.; et al. Oral lichenoid reactions associated with anti-PD-1/PD-L1 therapies: Clinicopathological findings. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e464–e469. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, J.; Gröger, S.; von Bremen, J.; Meyle, J.; Ruf, S. PD-L1, a Potential Immunomodulator Linking Immunology and Orthodontically Induced Inflammatory Root Resorption (OIIRR): Friend or Foe? Int. J. Mol. Sci. 2022, 23, 11405. https://doi.org/10.3390/ijms231911405
Yong J, Gröger S, von Bremen J, Meyle J, Ruf S. PD-L1, a Potential Immunomodulator Linking Immunology and Orthodontically Induced Inflammatory Root Resorption (OIIRR): Friend or Foe? International Journal of Molecular Sciences. 2022; 23(19):11405. https://doi.org/10.3390/ijms231911405
Chicago/Turabian StyleYong, Jiawen, Sabine Gröger, Julia von Bremen, Joerg Meyle, and Sabine Ruf. 2022. "PD-L1, a Potential Immunomodulator Linking Immunology and Orthodontically Induced Inflammatory Root Resorption (OIIRR): Friend or Foe?" International Journal of Molecular Sciences 23, no. 19: 11405. https://doi.org/10.3390/ijms231911405
APA StyleYong, J., Gröger, S., von Bremen, J., Meyle, J., & Ruf, S. (2022). PD-L1, a Potential Immunomodulator Linking Immunology and Orthodontically Induced Inflammatory Root Resorption (OIIRR): Friend or Foe? International Journal of Molecular Sciences, 23(19), 11405. https://doi.org/10.3390/ijms231911405




