Selaginella tamariscina Inhibits Glutamate-Induced Autophagic Cell Death by Activating the PI3K/AKT/mTOR Signaling Pathways
Abstract
1. Introduction
2. Results
2.1. STE Attenuates Glutamate-Induced Cytotoxicity in HT22 Cells
2.2. STE Reduced Intracellular ROS Produced by Glutamate
2.3. STE Inhibited Glutamate-Induced Neuronal Cell Death
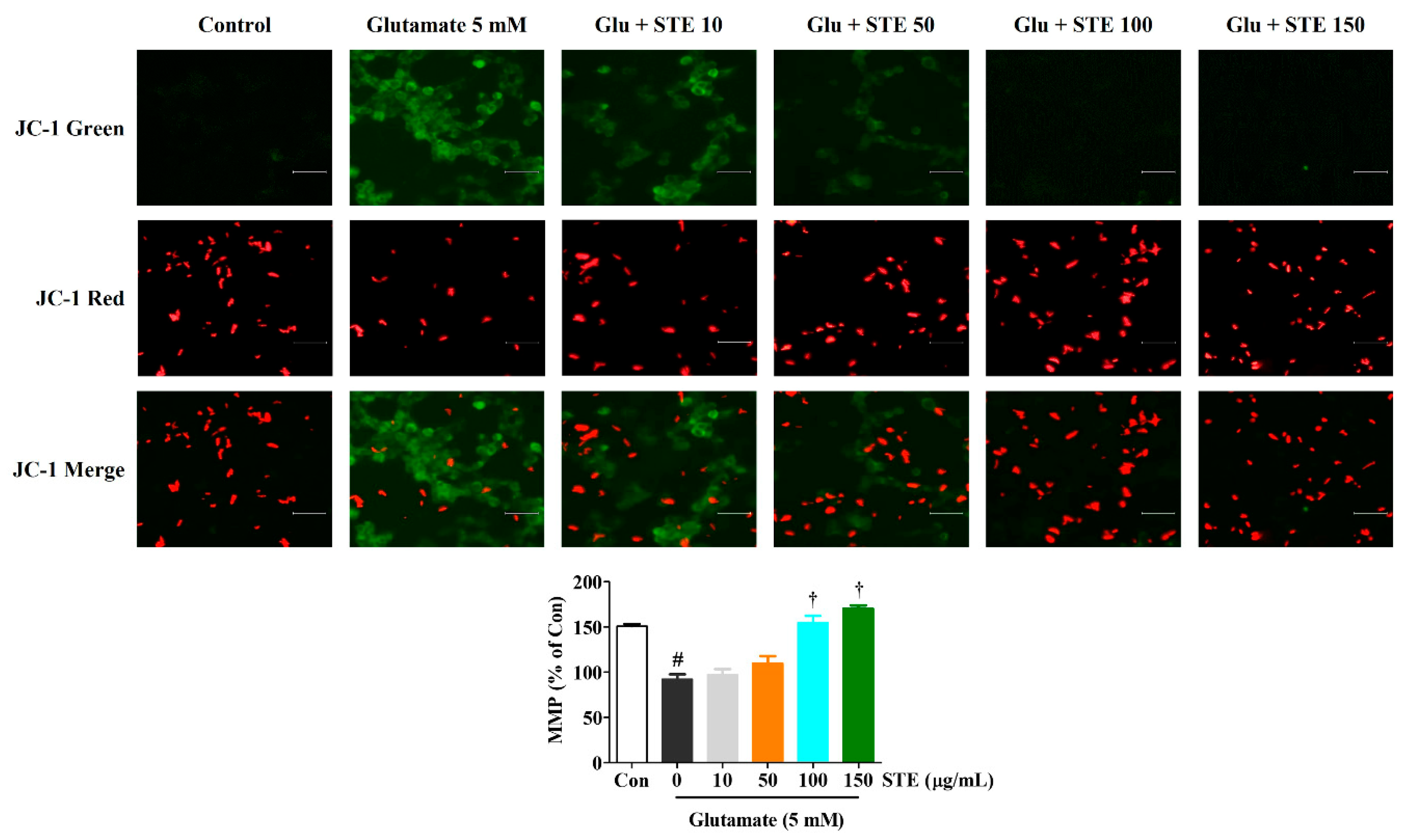
2.4. STE Prevents Glutamate-Induced Loss of MMP
2.5. STE Inhibited Glutamate-Induced Autophagy and Downregulated the Related Molecular Mechanism in HT22 Cells
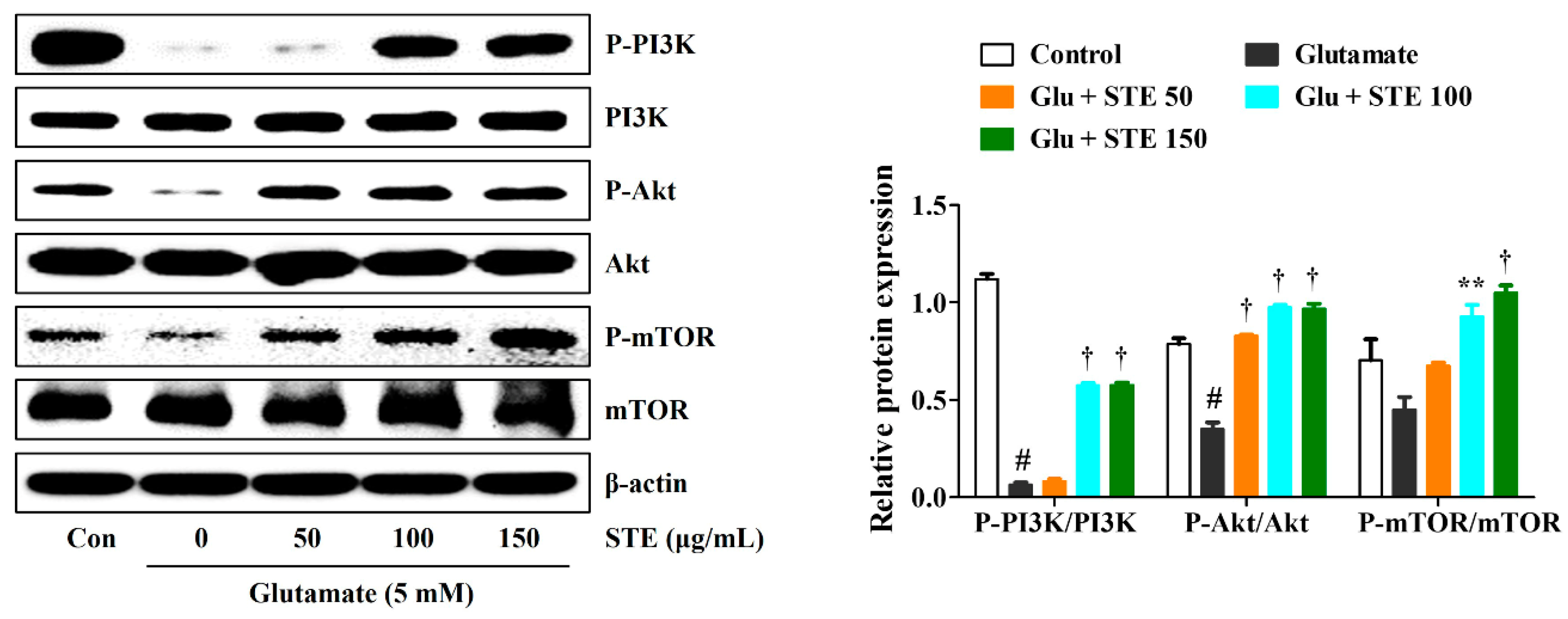
2.6. STE Prevented Glutamate-Induced Autophagy via the Activation of the PI3K/Akt/mTOR Signaling Pathway
2.7. LY294002 Inhibits the Neuroprotective Effects of STE
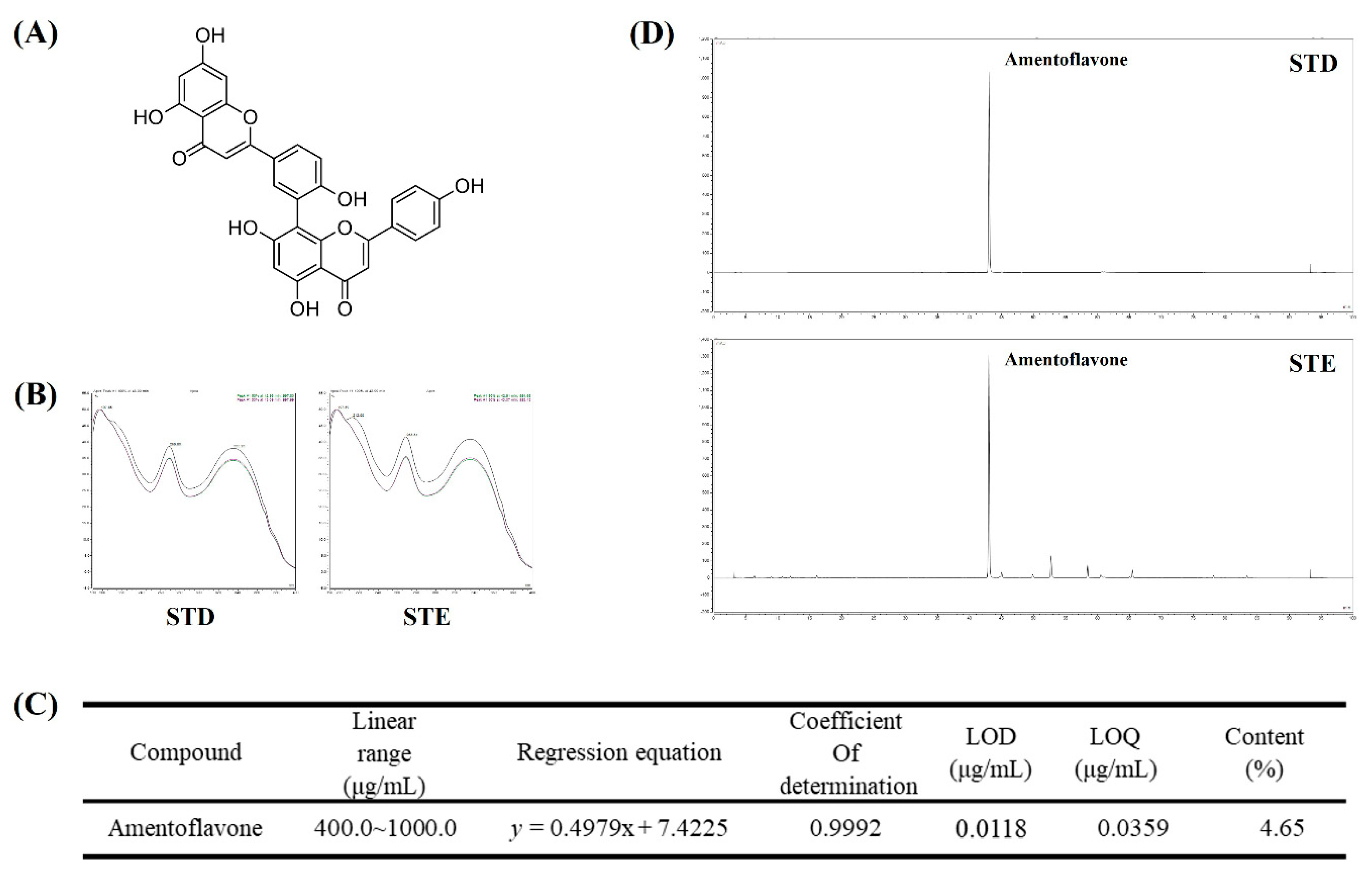
2.8. Identification and Quantitative Analysis of the Chemical Constituents of STE
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. STE Preparation
4.3. Cell Culture
4.4. Measurement of Cell Viability
4.5. Cytotoxicity Assay
4.6. Measurement of Intracellular ROS Levels
4.7. Measurement of the MMP
4.8. Cell Death Assessment by Flow Cytometry
4.9. Cyto-ID Autophagy Detection Assay
4.10. Nuclear and Cytosolic Protein Extraction
4.11. Western Blot Analysis
4.12. Preparation of STE and Standard Solution
4.13. HPLC Conditions
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollari, E.; Goldsteins, G.; Bart, G.; Koistinaho, J.; Giniatullin, R. The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2014, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.F.; Sun, Q.; Chen, S.D. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef]
- Amaro, S.; Llull, L.; Renú, A.; Laredo, C.; Perez, B.; Vila, E.; Torres, F.; Planas, A.M.; Chamorro, Á. Uric acid improves glucose-driven oxidative stress in human ischemic stroke. Ann. Neurol. 2015, 77, 775–783. [Google Scholar] [CrossRef]
- Kochergin, I.A.; Zakharova, M.N. The role of autophagy in neurodegenerative diseases. Neurochem. J. 2016, 10, 7–18. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, D.W.; Jung, B.H.; Lee, J.H.; Lee, H.; Hwang, G.S.; Kang, K.S.; Lee, J.W. Ginsenoside Rb2 suppresses the glutamate-mediated oxidative stress and neuronal cell death in HT22 cells. J. Ginseng Res. 2019, 43, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Song, J.H.; Kim, S.N.; Lee, J.H.; Lee, H.J.; Kang, K.S.; Lim, H.H. Neuroprotective effects of tetrahydrocurcumin against glutamate-induced oxidative stress in hippocampal HT22 cells. Molecules 2019, 25, 144. [Google Scholar] [CrossRef]
- Naranmandura, H.; Xu, S.; Sawata, T.; Hao, W.H.; Liu, H.; Bu, N.; Ogra, Y.; Lou, Y.J.; Suzuki, N. Mitochondria are the main target organelle for trivalent monomethylarsonous acid (MMAIII)-induced cytotoxicity. Chem. Res. Toxicol. 2011, 24, 1094–1103. [Google Scholar] [CrossRef]
- Baillet, A.; Chanteperdrix, V.; Trocme, C.; Casez, P.; Garrel, C.; Besson, G. The Role of Oxidative Stress in Amyotrophic Lateral Sclerosis and Parkinson’s Disease. Neurochem. Res. 2010, 35, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.A. The role of peroxidation of mitochondrial membrane phospholipids in pancreatic β-cell failure. Curr. Diabetes Rev. 2012, 8, 69–75. [Google Scholar] [CrossRef]
- Wang, X.; Iyaswamy, A.; Xu, D.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Yang, Y.; Li, Y.; Chen, C.; Li, M.; Li, H.W.; et al. Real-Time Detection and Visualization of Amyloid-β Aggregates Induced by Hydrogen Peroxide in Cell and Mouse Models of Alzheimer’s Disease. ACS Appl. Mater. Interfaces 2022, in press. [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in Mammalian Autophagy Research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Cuervo, A.M. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 2010, 13, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Li, X.H.; Zeng, X.C.; Li, J.; Zhou, J.; Xiao, B.; Hu, K. Curcumin protects neuronal cells against status-epilepticus-induced hippocampal damage through induction of autophagy and inhibition of necroptosis. Can. J. Physiol. Pharmacol. 2017, 95, 501–509. [Google Scholar] [CrossRef]
- Hu, X.; Song, Q.; Li, X.; Li, D.; Zhang, Q.; Meng, W.; Zhao, Q. Neuroprotective effects of Kukoamine A on neurotoxin-induced Parkinson’s model through apoptosis inhibition and autophagy enhancement. Neuropharmacology 2017, 117, 352–363. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, X.; Liang, Y.; Liu, Q.; Chen, S.; Guo, Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017, 91, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Iyaswamy, A.; Wang, X.; Krishnamoorthi, S.; Kaliamoorthy, V.; Sreenivasmurthy, S.G.; Kumar Durairajan, S.S.; Song, J.X.; Tong, B.C.; Zhu, Z.; Su, C.F.; et al. Theranostic F-SLOH mitigates Alzheimer’s disease pathology involving TFEB and ameliorates cognitive functions in Alzheimer’s disease models. Redox Biol. 2022, 51, 102280. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, L.F.; Su, C.F.; Liu, J.; Tong, B.C.; Iyaswamy, A.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Guan, X.J.; Kan, Y.X.; et al. Corynoxine B derivative CB6 prevents Parkinsonian toxicity in mice by inducing PIK3C3 complex-dependent autophagy. Acta Pharm. Sin. 2022, 43, 2511–2526. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Su, C.; Iyaswamy, A.; Krishnamoorthi, S.K.; Zhu, Z.; Yang, S.; Tong, B.C.; Liu, J.; Sreenivasmurthy, S.G.; Guan, X.; et al. Celastrol enhances transcription factor EB (TFEB)-mediated autophagy and mitigates Tau pathology: Implications for Alzheimer’s disease therapy. Acta Pharm. Sin. B 2022, 12, 1707–1722. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef]
- do Nascimento-Neto, L.G.; Cabral, M.G.; Carneiro, R.F.; Silva, Z.; Arruda, F.V.S.; Nagano, C.S.; Fernandes, A.R.; Sampaio, A.H.; Teixeira, E.H.; Videira, P.A. Halilectin-3, a Lectin from the Marine Sponge Haliclona caerulea, Induces Apoptosis and Autophagy in Human Breast Cancer MCF7 Cells Through Caspase-9 Pathway and LC3-II Protein Expression. Anticancer Agents Med. Chem. 2018, 18, 521–528. [Google Scholar] [CrossRef]
- Wei, H.P.; Li, Y.; Han, S.; Liu, S.Q.; Zhang, N.; Zhao, L.; Li, S.J.; Li, J.F. cPKC gamma-Modulated Autophagy in Neurons Alleviates Ischemic Injury in Brain of Mice with Ischemic Stroke Through Akt-mTOR Pathway. Transl. Stroke Res. 2016, 7, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Y.; Zhou, H.H.; Li, X.; Liu, Z.Q. Huperzine A Alleviates Oxidative Glutamate Toxicity in Hippocampal HT22 Cells via Activating BDNF/TrkB-Dependent PI3K/Akt/mTOR Signaling Pathway. Cell Mol. Neurobiol. 2016, 36, 915–925. [Google Scholar] [CrossRef]
- Harris, H.; Rubinsztein, D.C. Control of autophagy as a therapy for neurodegenerative disease. Nat. Rev. Neurol. 2012, 8, 108–117. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, E.H.; Lee, C.G.; Rhee, K.J.; Jung, W.S.; Choi, Y.; Pan, C.H.; Kang, K. AKR1B10-inhibitory Selaginella tamariscina extract and amentoflavone decrease the growth of A549 human lung cancer cells in vitro and in vivo. J. Ethnopharmacol. 2017, 202, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.K.; Wang, W.W.; Zhang, L.; Su, C.F.; Wu, Y.Y.; Ke, Y.Y.; Hou, Q.W.; Liu, Z.Y.; Gao, A.S.; Feng, W.S. Antihyperlipidaemic and antioxidant effect of the total flavonoids in Selaginella tamariscina (Beauv.) Spring in diabetic mice. J. Pharm. Pharmacol. 2013, 65, 757–766. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.L.; Gao, D.; Cai, L.; Yang, Y.Y.; Hu, Y.J.; Yang, F.Q.; Chen, H.; Xia, Z.N. Comparing coagulation activity of Selaginella tamariscina before and after stir-frying process and determining the possible active constituents based on compositional variation. Pharm. Biol. 2018, 56, 67–75. [Google Scholar] [CrossRef]
- Hsin, C.H.; Wu, B.C.; Chuang, C.Y.; Yang, S.F.; Hsieh, Y.H.; Ho, H.Y.; Lin, H.P.; Chen, M.K.; Lin, C.W. Selaginella tamariscina extract suppresses TPA-induced invasion and metastasis through inhibition of MMP-9 in human nasopharyngeal carcinoma HONE-1 cells. BMC Complement. Altern. Med. 2013, 13, 234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jung, H.J.; Sung, W.S.; Yeo, S.H.; Kim, H.S.; Lee, I.S.; Woo, E.R.; Lee, D.G. Antifungal effect of amentoflavone derived from Selaginella tamariscina. Arch. Pharm. Res. 2006, 29, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Zhao, B.T.; Ali, M.Y.; Choi, J.S.; Rhyu, D.Y.; Min, B.S.; Woo, M.H. Insulin-Mimetic Selaginellins from Selaginella tamariscina with Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitory Activity. J. Nat. Prod. 2015, 78, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Jin, M.F.; Zhao, D.J.; Ni, H. Reduction of Mitophagy-Related Oxidative Stress and Preservation of Mitochondria Function Using Melatonin Therapy in an HT22 Hippocampal Neuronal Cell Model of Glutamate-Induced Excitotoxicity. Front. Endocrinol. 2019, 10, 550. [Google Scholar] [CrossRef]
- Yang, C.B.; Liu, J.; Tong, B.C.; Wang, Z.Y.; Zhu, Z.; Su, C.F.; Sreenivasmurthy, S.G.; Wu, J.X.; Iyaswamy, A.; Krishnamoorthi, S.; et al. TFEB, a master regulator of autophagy and biogenesis, unexpectedly promotes apoptosis in response to the cyclopentenone prostaglandin 15d-PGJ2. Acta Pharmacol. Sin. 2022, 43, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.H.; Zhang, Y.S.; Li, J.J.; Zhang, J.; Li, Y.L.; Dang, C.; Li, C.; Fan, Y.H.; Yu, J.; Pei, Z.; et al. Beclin 1 knockdown inhibits autophagic activation and prevents secondary neurodegenerative damage in the ipsilateral thalamus following focal cerebral infarction. Autophagy 2012, 8, 63–76. [Google Scholar] [CrossRef]
- Zhang, Z.; Singh, R.; Aschner, M. Methods for the detection of autophagy in mammalian cells. Curr. Protoc. Toxicol. 2016, 69, 20.12.1–20.12.26. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Na, L.X.; Li, Y.F.; Chen, L.N. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020, 10, 54. [Google Scholar] [CrossRef]
- Cao, B.; Zeng, M.; Zhang, Q.; Zhang, B.; Cao, Y.; Wu, Y.; Feng, W.; Zheng, X. Amentoflavone Ameliorates Memory Deficits and Abnormal Autophagy in Aβ25–35-Induced Mice by mTOR Signaling. Neurochem. Res. 2021, 46, 921–934. [Google Scholar] [CrossRef]
- Rong, S.; Wan, D.; Fan, Y.; Liu, S.; Sun, K.; Huo, J.; Zhang, P.; Li, X.; Xie, X.; Wang, F.; et al. Amentoflavone Affects Epileptogenesis and Exerts Neuroprotective Effects by Inhibiting NLRP3 Inflammasome. Front. Pharmacol. 2019, 10, 856. [Google Scholar] [CrossRef]
- Shin, D.H.; Bae, Y.C.; Kim-Han, J.S.; Lee, J.H.; Choi, I.Y.; Son, K.H.; Kang, S.S.; Kim, W.K.; Han, B.H. Polyphenol amentoflavone affords neuroprotection against neonatal hypoxic-ischemic brain damage via multiple mechanisms. J. Neurochem. 2006, 96, 561–572. [Google Scholar] [CrossRef]





| Antibody | Corporation | Product No. | RRID | Dilution Rate |
|---|---|---|---|---|
| BAX | Cell Signaling | #2772 | AB_10695870 | 1:1000 |
| Bcl-2 | Cell Signaling | #3498 | AB_1903907 | 1:1000 |
| PARP | Cell Signaling | #9532 | AB_659884 | 1:1000 |
| β-actin | Cell Signaling | #4970 | AB_2223172 | 1:1000 |
| LC3 | Cell Signaling | #4108 | AB_2137703 | 1:1000 |
| beclin-1 | Cell Signaling | #3738 | AB_490837 | 1:1000 |
| p62 | Cell Signaling | #39749 | AB_2799160 | 1:1000 |
| P-PI3K | Cell Signaling | #17366 | AB_2895293 | 1:1000 |
| PI3K | Cell Signaling | #4257 | AB_659889 | 1:1000 |
| P-Akt | Cell Signaling | #4060 | AB_2315049 | 1:1000 |
| Akt | Cell Signaling | #4691 | AB_915783 | 1:1000 |
| P-mTOR | Cell Signaling | #5536 | AB_10691552 | 1:1000 |
| mTOR | Cell Signaling | #2983 | AB_2105622 | 1:1000 |
| 2nd anti-mouse | Cell Signaling | #7076 | AB_330924 | 1:5000 |
| 2nd anti-rabbit | Cell Signaling | #7074 | AB_2099233 | 1:5000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.H.; Kim, T.I.; Oh, Y.-C.; Ma, J.Y. Selaginella tamariscina Inhibits Glutamate-Induced Autophagic Cell Death by Activating the PI3K/AKT/mTOR Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 11445. https://doi.org/10.3390/ijms231911445
Jeong YH, Kim TI, Oh Y-C, Ma JY. Selaginella tamariscina Inhibits Glutamate-Induced Autophagic Cell Death by Activating the PI3K/AKT/mTOR Signaling Pathways. International Journal of Molecular Sciences. 2022; 23(19):11445. https://doi.org/10.3390/ijms231911445
Chicago/Turabian StyleJeong, Yun Hee, Tae In Kim, You-Chang Oh, and Jin Yeul Ma. 2022. "Selaginella tamariscina Inhibits Glutamate-Induced Autophagic Cell Death by Activating the PI3K/AKT/mTOR Signaling Pathways" International Journal of Molecular Sciences 23, no. 19: 11445. https://doi.org/10.3390/ijms231911445
APA StyleJeong, Y. H., Kim, T. I., Oh, Y.-C., & Ma, J. Y. (2022). Selaginella tamariscina Inhibits Glutamate-Induced Autophagic Cell Death by Activating the PI3K/AKT/mTOR Signaling Pathways. International Journal of Molecular Sciences, 23(19), 11445. https://doi.org/10.3390/ijms231911445





