Early Detection of Cervical Cancer by Fluorescence Lifetime Imaging Microscopy Combined with Unsupervised Machine Learning
Abstract
:1. Introduction
2. Results and Discussion
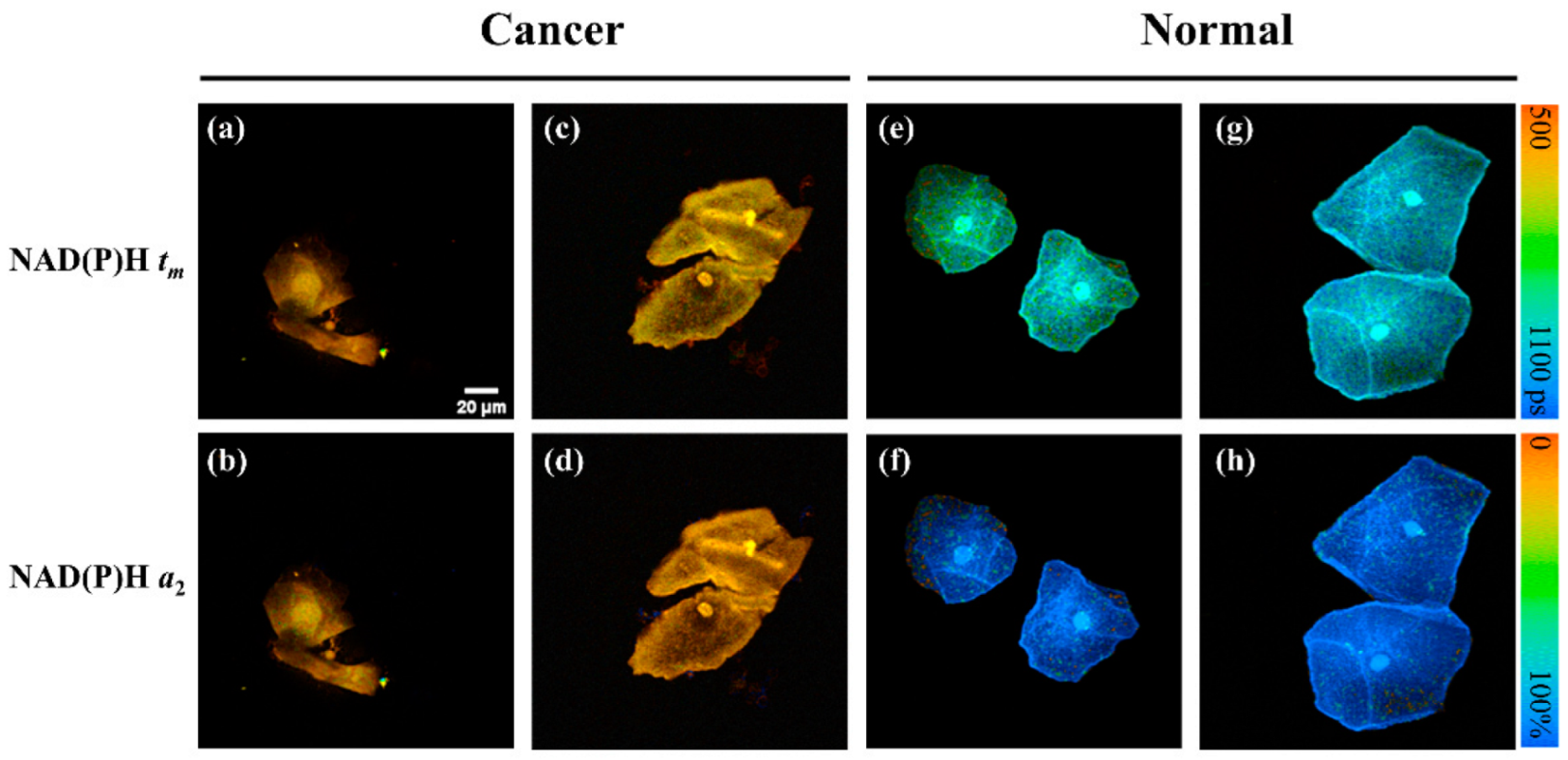
2.1. NAD(P)H FLIM Images of Exfoliated Cervical Cells
2.2. Statistical Analysis of FLIM Images and Dataset Selection
2.3. Result of Feature Extraction and PCA
2.4. Results of Clustering and the FLIM-ML Model
2.5. Results of FLIM-ML and Its Comparison with LBC
3. Materials and Methods
3.1. Participants and Exfoliated Cervical Cell Samples
3.2. Fluorescence Lifetime Imaging and Analysis
3.3. FLIM Images Preprocessing
3.4. Unsupervised Machine Learning Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, G.; Nyaga, V.N.; Santesso, N.; Bryant, A.; Martin-Hirsch, P.P.; Mustafa, R.A.; Schünemann, H.; Paraskevaidis, E.; Arbyn, M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst. Rev. 2017, 8, CD008587. [Google Scholar] [CrossRef]
- Saslow, D.; Solomon, D.; Lawson, H.W.; Killackey, M.; Kulasingam, S.L.; Cain, J.; Garcia, F.A.; Moriarty, A.T.; Waxman, A.G.; Wilbur, D.C. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am. J. Clin. Pathol. 2012, 137, 516–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, Q.; Zhang, M.; Wu, Z.; Xue, P. Fluorescence lifetime imaging microscopy and its applications in skin cancer diagnosis. J. Innov. Opt. Health Sci. 2019, 12, 1930004. [Google Scholar] [CrossRef]
- Luo, T.; Lu, Y.; Liu, S.; Lin, D.; Qu, J. Phasor–FLIM as a Screening Tool for the Differential Diagnosis of Actinic Keratosis, Bowen’s Disease, and Basal Cell Carcinoma. Anal. Chem. 2017, 89, 8104–8111. [Google Scholar] [CrossRef]
- Qian, T.; Heaster, T.M.; Houghtaling, A.R.; Sun, K.; Samimi, K.; Skala, M.C. Label-free imaging for quality control of cardiomyocyte differentiation. Nat. Commun. 2021, 12, 4580. [Google Scholar] [CrossRef]
- Alfonso-García, A.; Smith, T.D.; Datta, R.; Luu, T.U.; Gratton, E.; Potma, E.O.; Liu, W.F. Label-free identification of macrophage phenotype by fluorescence lifetime imaging microscopy. J. Biomed. Opt. 2016, 21, 046005. [Google Scholar] [CrossRef]
- Kolenc, O.I.; Quinn, K.P. Evaluating Cell Metabolism Through Autofluorescence Imaging of NAD(P)H and FAD. Antioxid. Redox Signal. 2017, 30, 875–889. [Google Scholar] [CrossRef]
- Wang, Y.; Song, C.; Wang, M.; Xie, Y.; Mi, L.; Wang, G. Rapid, label-free, and highly sensitive detection of cervical cancer with fluorescence lifetime imaging microscopy. IEEE J. Sel. Top. Quantum Electron. 2015, 22, 228–234. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Z.; Wang, X.; Xie, Y.; Fei, Y.; Ma, J.; Wang, J.; Chen, L.; Mi, L.; Wang, Y. Detecting benign uterine tumors by autofluorescence lifetime imaging microscopy through adjacent healthy cervical tissues. J. Innov. Opt. Health Sci. 2019, 12, 1940006. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, Z.; Huang, M.; Fei, Y.; Ma, J.; Mi, L. Discriminating different grades of cervical intraepithelial neoplasia based on label-free phasor fluorescence lifetime imaging microscopy. Biomed. Opt. Express 2020, 11, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, B.; Park, B.; Won, Y.; Kim, S.Y.; Lee, S. Real-time cancer diagnosis of breast cancer using fluorescence lifetime endoscopy based on the pH. Sci. Rep. 2021, 11, 16864. [Google Scholar] [CrossRef] [PubMed]
- Mannam, V.; Zhang, Y.; Yuan, X.; Ravasio, C.; Howard, S.S. Machine learning for faster and smarter fluorescence lifetime imaging microscopy. Phys. Photonics 2020, 2, 042005. [Google Scholar] [CrossRef]
- Wu, G.; Nowotny, T.; Zhang, Y.; Yu, H.; Li, D. Artificial neural network approaches for fluorescence lifetime imaging techniques. Opt. Lett. 2016, 41, 2561–2564. [Google Scholar] [CrossRef]
- Jason, T.S.; Yao, R.; Sinsuebphon, N.; Rudkouskaya, A.; Un, N.; Mazurkiewicz, J.; Barroso, M.; Yan, P.; Intes, X. Fast fit-free analysis of fluorescence lifetime imaging via deep learning. Proc. Natl. Acad. Sci. USA 2019, 116, 24019–24030. [Google Scholar]
- Zhang, Y.; Hato, T.; Dagher, P.C.; Nichols, E.L.; Smith, C.J.; Dunn, K.W.; Howard, S.S. Automatic segmentation of intravital fluorescence microscopy images by K-means clustering of FLIM phasors. Opt. Lett. 2019, 44, 3928–3931. [Google Scholar] [CrossRef]
- Ma, N.; Mochel, N.R.D.; Pham, P.D.; Yoo, T.Y.; Cho, K.W.Y.; Digman, M.A. Label-free assessment of pre-implantation embryo quality by the Fluorescence Lifetime Imaging Microscopy (FLIM)-phasor approach. Sci. Rep. 2019, 9, 132036. [Google Scholar] [CrossRef]
- Sagar, M.A.K.; Cheng, K.P.; Ouellette, J.N.; Williams, J.C.; Watters, J.J.; Eliceiri, K.W. Machine Learning Methods for Fluorescence Lifetime Imaging (FLIM) Based Label-Free Detection of Microglia. Front. Neurosci. 2020, 14, 931. [Google Scholar] [CrossRef]
- Gu, J.; Fu, C.Y.; Ng, B.K.; Liu, L.B.; Lim, B.K.; Lee, C.G.L. Enhancement of early cervical cancer diagnosis with epithelial layer analysis of fluorescence lifetime images. PLoS ONE 2015, 10, e0125706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhruvajyoti, R.; Anthony, L.; Michail, I.; Stefanie, S.J. Cell-free circulating tumor DNA profiling in cancer management. Trends Mol. Med. 2021, 14, 00182–00189. [Google Scholar]
- Echle, A.; Rindtorff, N.T.; Brinker, T.J.; Luedde, T.; Pearson, A.T.; Kather, J.N. Deep learning in cancer pathology: A new generation of clinical biomarkers. Br. J. Cancer 2021, 124, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Tiirikainen, M. Diagnostic Power of DNA Methylation Classifiers for Early Detection of Cancer. Trends Cancer 2020, 6, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, Y.; Lu, F. Differentiation of different antifungals with various mechanisms using dynamic surface-enhanced Raman spectroscopy combined with machine learning. J. Innov. Opt. Health Sci. 2021, 14, 2141002. [Google Scholar] [CrossRef]
- Cohn, R.; Holm, E. Unsupervised Machine Learning Via Transfer Learning and k-Means Clustering to Classify Materials Image Data. Integr. Mater. Manuf. Innov. 2021, 10, 231–244. [Google Scholar] [CrossRef]
- Dong, N.; Zhai, M.-d.; Zhao, L.; Wu, C.H. Cervical cell classification based on the CART feature selection algorithm. J. Ambient. Intell. Humaniz. Comput. 2021, 12, 1837–1849. [Google Scholar] [CrossRef]
- Sabeena, K.; Gopakumar, C. A hybrid model for efficient cervical cell classification. Biomed. Signal Process. Control. 2022, 72, 103288. [Google Scholar]
- Damayanti, N.P.; Craig, A.P.; Irudayaraj, J. A hybrid FLIM-elastic net platform for label free profiling of breast cancer. Analyst 2013, 138, 7127–7134. [Google Scholar] [CrossRef]
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The Warburg Effect 97 Years after Its Discovery. Cancers 2020, 12, 2819. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We need to talk about the Warburg effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Nichols, E.; Wang, Q.; Zhang, S.; Smith, C.; Howard, S. A Poisson-Gaussian denoising dataset with real fluorescence microscopy images. In Proceedings of the 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Long Beach, CA, USA, 16–19 June 2019; pp. 11702–11710. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Maaten, L.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Chen, B.; Lu, Y.; Pan, W.; Xiong, J.; Yang, Z.; Yan, W.; Liu, L.; Qu, J. Support Vector Machine Classification of Nonmelanoma Skin Lesions Based on Fluorescence Lifetime Imaging Microscopy. Anal. Chem. 2019, 91, 10640–10647. [Google Scholar] [CrossRef] [PubMed]
- Balaji, K. Machine learning algorithm for feature space clustering of mixed data with missing information based on molecule similarity. J. Biomed. Inform. 2022, 125, 103954. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.; Sutin, J.; Wu, W.; Fu, B.; Uhlirova, H.; Devor, A.; Boas, D.A.; Sakadzic, S. Fluorescence lifetime microscopy of NADH distinguishes alterations in cerebral metabolism in vivo. Biomed. Opt. Express 2017, 8, 2368–2385. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.; Salma, N.; Osseiran, S.; Birngruber, M.C.R.; Evans, C.L.; Manstein, D. Enhanced quantification of metabolic activity for individual adipocytes by label-free FLIM. Sci. Rep. 2018, 8, 8757. [Google Scholar] [CrossRef]
- Mehmood, S.; Ghazal, T.M.; Khan, M.A.; Zubair, M.; Naseem, M.T.; Faiz, T.; Ahmad, M. Malignancy Detection in Lung and Colon Histopathology Images Using Transfer Learning with Class Selective Image Processing. IEEE Access 2022, 10, 25657–25668. [Google Scholar] [CrossRef]
- Chen, J.; Wan, Z.; Zhang, J.; Li, W.; Chen, Y.; Li, Y.; Duan, Y. Medical image segmentation and reconstruction of prostate tumor based on 3D AlexNet. Comput. Meth. Prog. Bio. 2021, 200, 105878. [Google Scholar] [CrossRef]
- Seo, H.; Khuzani, M.B.; Vasudevan, V.; Huang, C.; Ren, H.; Xiao, R.; Jia, X.; Xing, L. Machine learning techniques for biomedical image segmentation: An overview of technical aspects and introduction to state-of-art applications. Med. Phys. 2020, 47, e148–e167. [Google Scholar] [CrossRef] [PubMed]
- Arthur, D.; Vassilvitskii, S. K-Means++: The Advantages of Careful Seeding. In Proceedings of the Eighteenth Annual ACM-SIAM Symposium on Discrete Algorithms, New Orleans, LA, USA, 7–9 January 2007. [Google Scholar]






| Clinical Diagnosis | Training Dataset | Validation Dataset |
|---|---|---|
| Cervical cancer | 5 | 6 |
| CINII/III | 4 | 3 |
| Benign | 0 | 18 |
| Normal | 14 | 9 |
| Follow-up | 0 | 12 |
| Total number | 23 | 48 |
| Input Images | Group | Cluster 1 | Cluster 2 |
|---|---|---|---|
| tm images | CC/CINII-III | 114/151 (75.5%) | 37/151 (24.5%) |
| Normal | 5/217 (2.3%) | 212/217 (97.7%) | |
| a2 images | CC/CINII-III | 114/151 (75.5%) | 37/151 (24.5%) |
| Normal | 0/217 (0%) | 217/217 (100%) | |
| tm & a2 images | CC/CINII-III | 114/151 (75.5%) | 37/151 (24.5%) |
| Normal | 0/217 (0%) | 217/217 (100%) |
| Patient No. | Percentage of Abnormal Images | FLIM-ML | LBC Test | ||
|---|---|---|---|---|---|
| tm Images | a2 Images | tm & a2 Images | |||
| CC-2 (stage IB3) | 100.0 | 100.0 | 100.0 | + | + |
| CC-4 (stage IB2) | 100.0 | 100.0 | 95.6 | + | + |
| CC-6 (stage IIB) | 100.0 | 100.0 | 100.0 | + | + |
| CC-8 (stage IA1) | 2.5 | 0.0 | 0.0 | −(FN) | + |
| CC-10 (stage IIA1) | 100.0 | 100.0 | 100.0 | + | + |
| CC-11 (stage IIB) | 73.7 | 100.0 | 89.5 | + | + |
| CINII-2 | 83.3 | 83.3 | 58.3 | + | −(FN) |
| CINII-4 | 50.0 | 80.0 | 50.0 | + | + |
| CINII-6 | 78.3 | 91.3 | 78.3 | + | + |
| Benign-1 | 0.0 | 0.0 | 0.0 | − | − |
| Benign-2 | 0.0 | 0.0 | 0.0 | − | − |
| Benign-3 | 8.3 | 8.3 | 8.3 | − | − |
| Benign-4 | 4.5 | 0.0 | 0.0 | − | − |
| Benign-5 | 0.0 | 0.0 | 0.0 | − | − |
| Benign-6 | 0.0 | 0.0 | 0.0 | − | − |
| Benign-7 | 45.5 | 0.0 | 0.0 | − | − |
| Benign-8 | 73.3 | 40.0 | 40.0 | − | − |
| Benign-9 | 0.0 | 0.0 | 0.0 | − | − |
| Benign-10 | 0.0 | 0.0 | 0.0 | − | − |
| Benign-11 | 36.4 | 9.1 | 9.1 | − | − |
| Benign-12 | 0.0 | 0.0 | 0.0 | − | − |
| Benign-13 | 0.0 | 11.1 | 0.0 | − | − |
| Benign-14 | 20.0 | 20.0 | 20.0 | − | − |
| Benign-15 | 54.5 | 9.1 | 0.0 | − | − |
| Benign-16 | 0.0 | 0.0 | 0.0 | − | − |
| Benign-17 | 36.4 | 45.5 | 36.4 | − | − |
| Benign-18 | 58.3 | 0.0 | 0.0 | − | +(FP) |
| Normal-15 | 0.0 | 0.0 | 0.0 | − | − |
| Normal-16 | 0.0 | 0.0 | 0.0 | − | − |
| Normal-17 | 0.0 | 0.0 | 0.0 | − | − |
| Normal-18 | 0.0 | 0.0 | 0.0 | − | − |
| Normal-19 | 50.0 | 0.0 | 15.4 | − | +(FP) |
| Normal-20 | 0.0 | 23.1 | 0.0 | − | +(FP) |
| Normal-21 | 36.4 | 0.0 | 0.0 | − | +(FP) |
| Normal-22 | 0.0 | 0.0 | 0.0 | − | +(FP) |
| Normal-23 | 0.0 | 0.0 | 0.0 | − | − |
| Follow-up-1 | 10.0 | 0.0 | 0.0 | − | − |
| Follow-up-2 | 0.0 | 0.0 | 0.0 | − | − |
| Follow-up-3 | 10.0 | 0.0 | 0.0 | − | − |
| Follow-up-4 | 13.3 | 13.3 | 13.3 | − | − |
| Follow-up-5 (VINII-III) | 85.0 | 100.0 | 70.0 | + | + |
| Follow-up-6 | 0.0 | 37.5 | 0.0 | − | − |
| Follow-up-7 (VAINIII) | 100.0 | 88.0 | 76.0 | + | −(FN) |
| Follow-up-8 | 5.0 | 55.0 | 10.0 | − | − |
| Follow-up-9 | 0.0 | 45.0 | 10.0 | − | − |
| Follow-up-10 | 5.0 | 0.0 | 0.0 | − | − |
| Follow-up-11 | 15.0 | 40.0 | 15.0 | − | − |
| Follow-up-12 | 5.0 | 5.0 | 0.0 | − | − |
| Method | Sensitivity (%) | Specificity (%) |
|---|---|---|
| LBC | 81.8 | 86.5 |
| FLIM-ML | 90.9 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, M.; Zhong, J.; Xue, R.; Su, W.; Kong, Y.; Fei, Y.; Ma, J.; Wang, Y.; Mi, L. Early Detection of Cervical Cancer by Fluorescence Lifetime Imaging Microscopy Combined with Unsupervised Machine Learning. Int. J. Mol. Sci. 2022, 23, 11476. https://doi.org/10.3390/ijms231911476
Ji M, Zhong J, Xue R, Su W, Kong Y, Fei Y, Ma J, Wang Y, Mi L. Early Detection of Cervical Cancer by Fluorescence Lifetime Imaging Microscopy Combined with Unsupervised Machine Learning. International Journal of Molecular Sciences. 2022; 23(19):11476. https://doi.org/10.3390/ijms231911476
Chicago/Turabian StyleJi, Mingmei, Jiahui Zhong, Runzhe Xue, Wenhua Su, Yawei Kong, Yiyan Fei, Jiong Ma, Yulan Wang, and Lan Mi. 2022. "Early Detection of Cervical Cancer by Fluorescence Lifetime Imaging Microscopy Combined with Unsupervised Machine Learning" International Journal of Molecular Sciences 23, no. 19: 11476. https://doi.org/10.3390/ijms231911476
APA StyleJi, M., Zhong, J., Xue, R., Su, W., Kong, Y., Fei, Y., Ma, J., Wang, Y., & Mi, L. (2022). Early Detection of Cervical Cancer by Fluorescence Lifetime Imaging Microscopy Combined with Unsupervised Machine Learning. International Journal of Molecular Sciences, 23(19), 11476. https://doi.org/10.3390/ijms231911476






