Jellyfish Polysaccharides for Wound Healing Applications
Abstract
:1. Introduction
2. Results
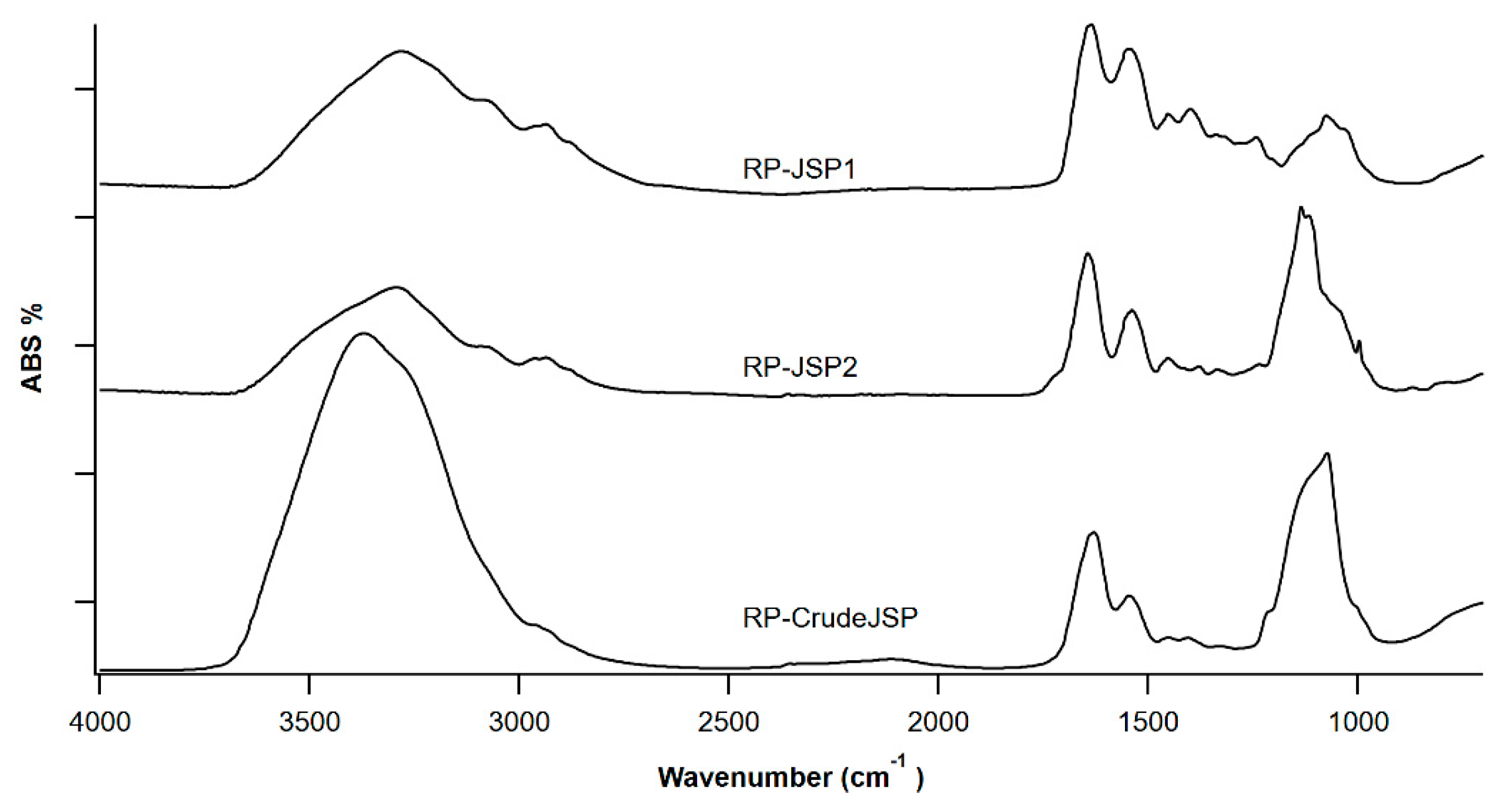
2.1. Characteristics of the Extracted Polysaccharides
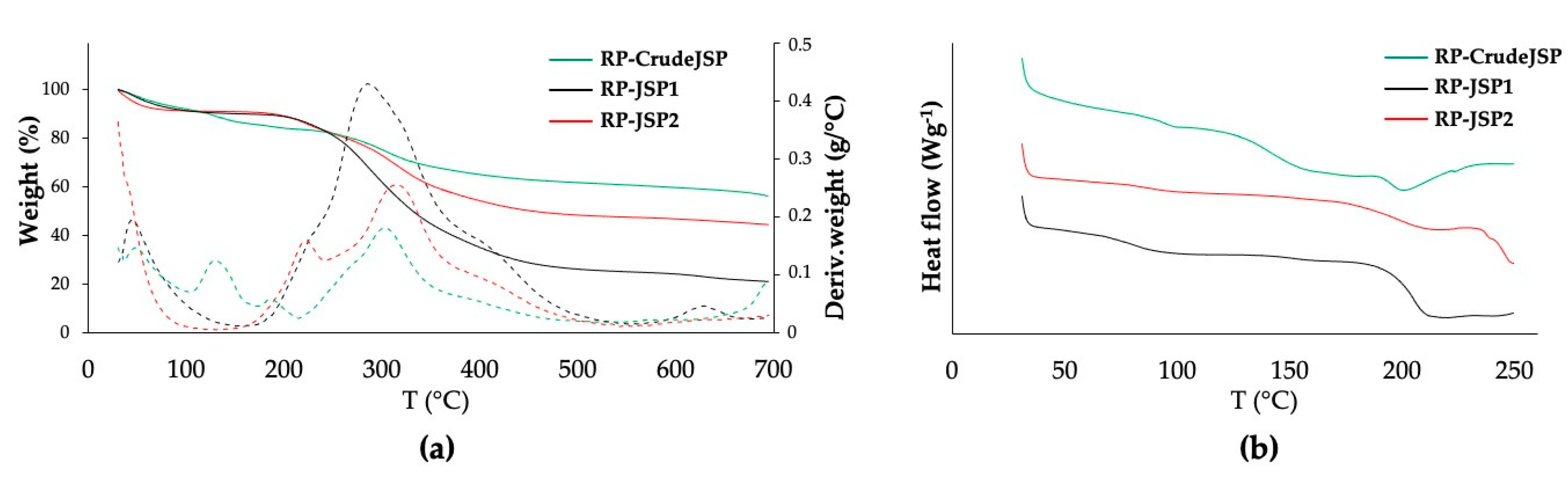
2.2. Thermal Characterization
2.3. Biological Evaluation of Extracted Polysaccharides for Wound Healing Application
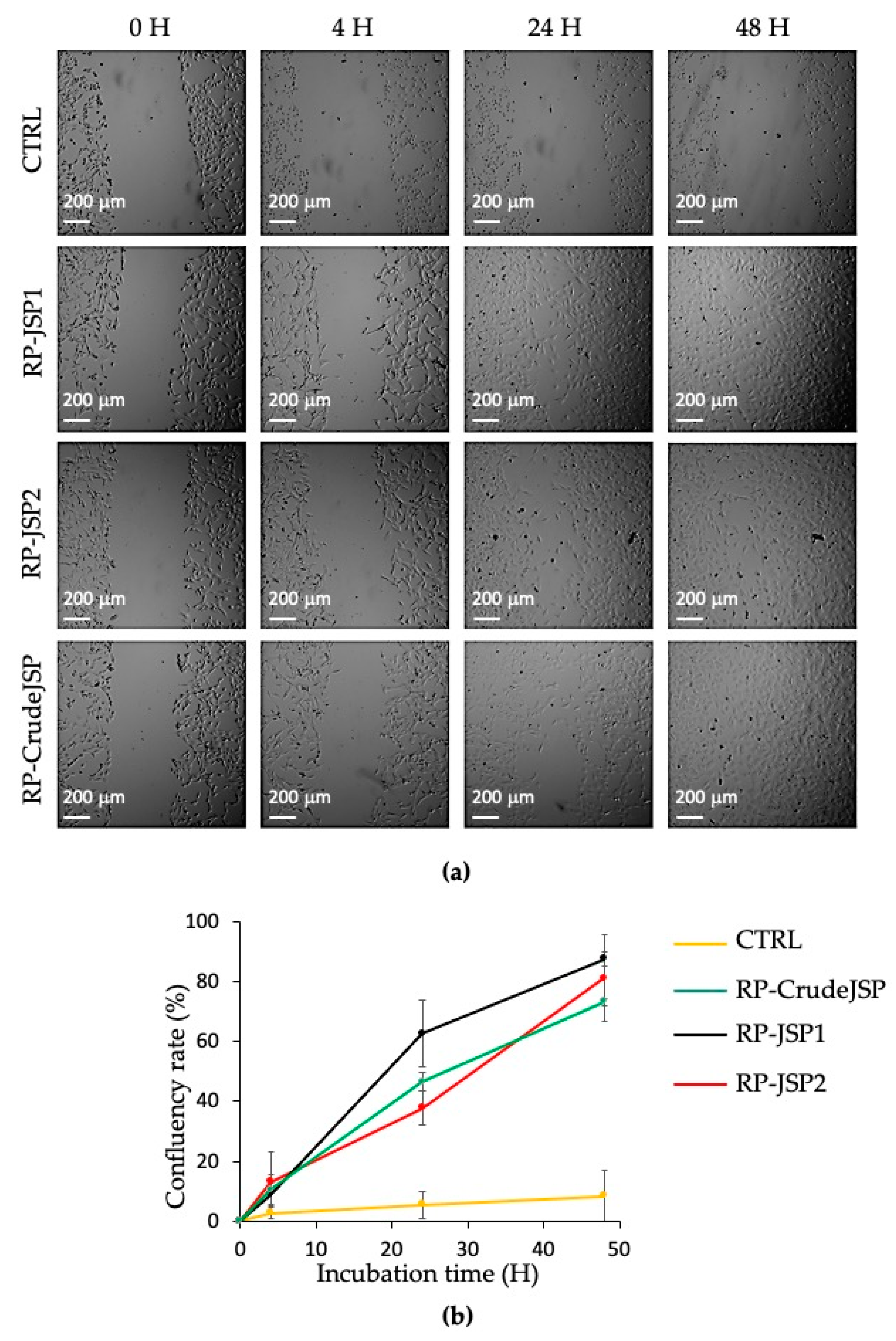
2.3.1. Scratch Test and Protective Effect from Oxidative Stress on Fibroblast BALB/3T3 Cells
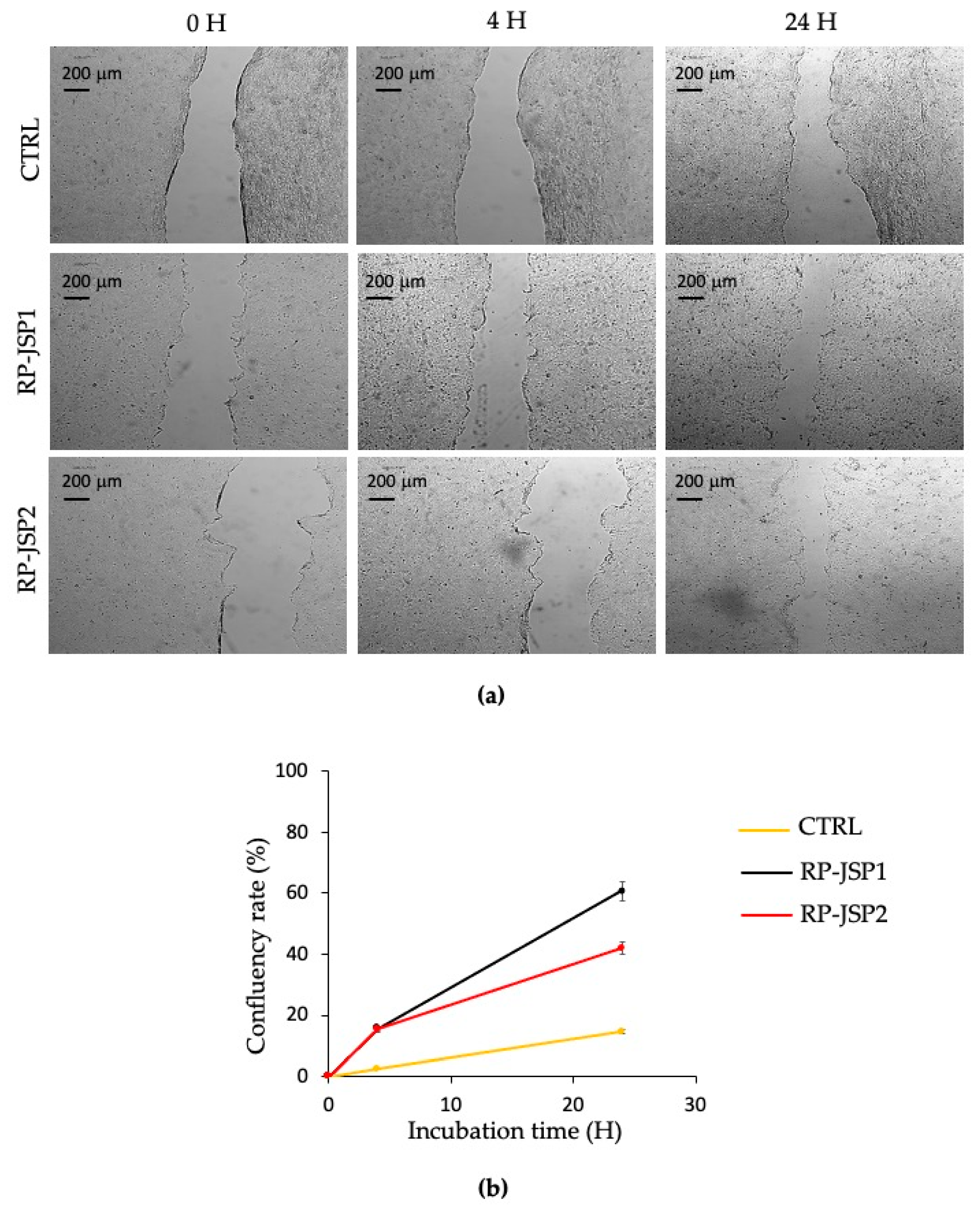
2.3.2. Keratinocytes Scratch Wound Healing Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction and Purification of Polysaccharides
4.3. Instruments
4.4. Molecular Weight Determination
4.5. Determination of Protein Content
4.6. Determination of Sulphate Groups’ Content
4.7. Monosaccharide Characterization
4.7.1. Polysaccharide Hydrolysis and Derivatization
4.7.2. UHPLC-HR-ESI-Orbitrap/MS Analysis
4.8. Thermal Analysis
4.9. Biological Investigation
4.9.1. Cell Culture Condition
4.9.2. Cell Viability
4.9.3. Scratch Test on BALB/3T3 Cells and HaCat Cells
4.9.4. Protection against Oxidative Damage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BeMiller, J.N. Polysaccharides; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Chen, L.; Ge, M.D.; Zhu, Y.J.; Song, Y.; Cheung, P.C.K.; Zhang, B.B.; Liu, L.M. Structure, bioactivity and applications of natural hyperbranched polysaccharides. Carbohydr. Polym. 2019, 223, 115076. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Cao, Q.; Ye, L.; Wang, J.; Guo, M. The Function of Natural Polysaccharides in the Treatment of Ulcerative Colitis. Front. Pharmacol. 2022, 13, 927855. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Yang, W.; Huang, G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2002, 19, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Chandika, P.; Ko, S.C.; Jung, W.K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int. J. Biol. Macromol. 2015, 77, 24–35. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. Fucoidan-loaded hydrogels facilitates wound healing using photodynamic therapy by in vitro and in vivo evaluation. Carbohydr. Polym. 2020, 247, 116624. [Google Scholar] [CrossRef]
- Massironi, A.; Franco, A.R.; Babo, P.S.; Puppi, D.; Chiellini, F.; Reis, R.L.; Gomes, M.E. Development and Characterization of Highly Stable Silver NanoParticles as Novel Potential Antimicrobial Agents for Wound Healing Hydrogels. Int. Mol. Sci. 2022, 23, 2161. [Google Scholar] [CrossRef]
- Chiellini, F.; Puppi, D.; Piras, A.M.; Morelli, A.; Bartoli, C.; Migone, C. Modelling of pancreatic ductal adenocarcinoma in vitro with three-dimensional microstructured hydrogels. RSC Adv. 2016, 6, 54226–54235. [Google Scholar] [CrossRef]
- Fabiano, A.; Migone, C.; Cerri, L.; Piras, A.M.; Mezzetta, A.; Maisetta, G.; Esin, S.; Batoni, G.; Di Stefano, R.; Zambito, Y. Combination of Two Kinds of Medicated Microparticles Based on Hyaluronic Acid or Chitosan for a Wound Healing Spray Patch. Pharmaceutics 2021, 13, 2195. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X.; Dong, H. Current advances in the anti-inflammatory effects and mechanisms of natural polysaccharides. Crit. Rev. Food Sci. Nutr. 2022, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.M.; Fernandez, N.; Matias, A.A.; Bronze, M.D.R. Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Capillo, G.; Albergamo, A.; Li Volsi, R.; Bartolomeo, G.; Bua, G.; Ferracane, A.; Savoca, S.; Gervasi, T.; Rando, R.; et al. A Multi-screening Evaluation of the Nutritional and Nutraceutical Potential of the Mediterranean Jellyfish Pelagia noctiluca. Mar. Drugs 2019, 17, 172. [Google Scholar] [CrossRef]
- Zhang, H.L.; Cui, S.H.; Zha, X.Q.; Bansal, V.; Xue, L.; Li, X.L.; Hao, R.; Pan, L.H.; Luo, J.P. Jellyfish skin polysaccharides: Extraction and inhibitory activity on macrophage-derived foam cell formation. Carbohydr. Polym. 2014, 106, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.M.; Wang, J.F.; Zha, X.Q.; Pan, L.H.; Zhang, H.L.; Luo, J.P. Structural characterization and immunomodulatory activity of a new polysaccharide from jellyfish. Carbohydr. Polym. 2017, 159, 188–194. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, J.; Zhang, L.; Qin, N.; Zhu, B.; Xia, X. Jellyfish skin polysaccharides enhance intestinal barrier function and modulate the gut microbiota in mice with DSS-induced colitis. Food Funct. 2021, 12, 10121–10135. [Google Scholar] [CrossRef] [PubMed]
- Marambio, M.; Canepa, A.; Lòpez, L.; Gauci, A.; Gueroun, S.; Zampardi, S.; Boero, F.; Yahia, O.; Yahia, M.; Fuentes, V.; et al. Unfolding Jellyfish Bloom Dynamics along the Mediterranean Basin by Transnational Citizen Science Initiatives. Diversity 2021, 13, 274. [Google Scholar] [CrossRef]
- Gravili, C. Jelly surge in the Mediterranean Sea: Threat or opportunity? Mediterr. Mar. Sci. 2020, 21, 11–21. [Google Scholar] [CrossRef]
- Stabili, L.; Rizzo, L.; Basso, L.; Marzano, M.; Fosso, B.; Pesole, G.; Piraino, S. The Microbial Community Associated with Rhizostoma pulmo: Ecological Significance and Potential Consequences for Marine Organisms and Human Health. Mar. Drugs 2020, 18, 437. [Google Scholar] [CrossRef]
- Edwards, M.; Atkinson, A.; Bresnan, E.; Helaouet, P.; McQuatters-Gollop, A.; Ostle, C.; Pitois, S.; Widdicombe, C. Plankton, jellyfish and climate in the North-East Atlantic. MCCIP Sci. Rev. 2020, 322–353. [Google Scholar] [CrossRef]
- Clinton, M.; Ferrier, D.E.K.; Martin, S.A.M.; Brierley, A.S. Impacts of jellyfish on marine cage aquaculture: An overview of existing knowledge and the challenges to finfish health. ICES J. Mar. Sci. 2021, 78, 1557–1573. [Google Scholar] [CrossRef]
- Toida, T.; Toyoda, H.; Imanari, T. High-Resolution Proton Nuclear Magnetic Resonance Studies on Chondroitin Sulfates. Anal. Sci. 1993, 9, 53–58. [Google Scholar] [CrossRef]
- Sundaresan, G.; Abraham, R.J.; Appa Rao, V.; Narendra Babu, R.; Govind, V.; Meti, M.F. Established method of chondroitin sulphate extraction from buffalo (Bubalus bubalis) cartilages and its identification by FTIR. J. Food Sci. Technol. 2018, 55, 3439–3445. [Google Scholar] [CrossRef]
- Pomin, V.H. NMR Chemical Shifts in Structural Biology of Glycosaminoglycans. Anal. Chem. 2014, 86, 65–94. [Google Scholar] [CrossRef]
- Fajardo, A.R.; Lopes, L.C.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Polyelectrolyte complexes based on pectin–NH2 and chondroitin sulfate. Carbohydr. Polym. 2012, 87, 1950–1955. [Google Scholar] [CrossRef]
- ISO-10993-1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process. International Organization for Standardisation: Geneva, Switzerland, 2018.
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–411. [Google Scholar] [PubMed]
- Morgner, B.; Husmark, J.; Arvidsson, A.; Wiegand, C. Effect of a DACC-coated dressing on keratinocytes and fibroblasts in wound healing using an in vitro scratch model. J. Mater. Sci. Mater. Med. 2022, 33, 22. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e1–e16. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidationsubstrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean Jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef] [Green Version]
- Amreen Nisa, S.; Vinu, D.; Krupakar, P.; Govindaraju, K.; Sharma, D.; Vivek, R. Jellyfish venom proteins and their pharmacological potentials: A review. Int. J. Biol. Macromol. 2021, 176, 424–436. [Google Scholar] [CrossRef]
- Long, X.; Yan, Q.; Cai, L.; Li, G.; Luo, X. Box-Behnken design-based optimization for deproteinization of crude polysaccharides in Lycium barbarum berry residue using the Sevag method. Heliyon 2020, 6, e03888. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. Protein–glycosaminoglycan interaction networks: Focus on heparan sulfate. Perspect. Sci. 2017, 11, 62–69. [Google Scholar] [CrossRef]
- Konovalova, I.; Novikov, V.; Kuchina, Y.; Dolgopiatova, N. Technology and Properties of Chondroitin Sulfate from Marine Hydrobionts. KnE Life Sci. 2020, 5, 305–314. [Google Scholar] [CrossRef]
- Oliveira, A.P.V.; de Abreu Feitosa, V.; de Oliveira, J.M.; Coelho, A.L.; Lídia de Araújo, P.V.; da Silva, F.D.A.R.; Sobrinho, F.D.A.A.F.; Duarte, E.B.; de Souza, B.W. Characteristics of Chondroitin Sulfate Extracted of Tilapia (Oreochromis niloticus) Processing. Procedia Eng. 2017, 200, 193–199. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, G.; Zheng, Y.; Qu, M.; Jin, Q.; Tong, C.; Li, W.J.P. Effect of drying procedures on the physicochemical properties and antioxidant activities of polysaccharides from Crassostrea gigas. PLoS ONE 2017, 12, 0188536. [Google Scholar] [CrossRef]
- Benešová, K.; Pekař, M.; Lapčík, L.; Kučerík, J.J.J. calorimetry. Stability evaluation of n-alkyl hyaluronic acid derivates by DSC and TG measurement. J. Therm. Anal. Calorim. 2006, 83, 341–348. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.-H.; Levchenko, A.J.B.J. Topotaxis: A new mechanism of directed cell migration in topographic ECM gradients. Biophys. J. 2018, 114, 1257–1263. [Google Scholar] [CrossRef]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.; Costa, L.S.; Rocha, H.A.O. The Protective Role of Sulfated Polysaccharides from Green Seaweed Udotea flabellum in Cells Exposed to Oxidative Damage. Mar. Drugs 2018, 16, 135. [Google Scholar] [CrossRef]
- De Domenico, S.; De Rinaldis, G.; Paulmery, M.; Piraino, S.; Leone, A.J.M.d. Barrel jellyfish (Rhizostoma pulmo) as source of antioxidant peptides. Mar. Drugs 2019, 17, 134. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xie, Y.; Yang, W.; Yang, Z.; Jiang, S.; Zhang, C.; Zhang, G. Alfalfa polysaccharide prevents H2O2-induced oxidative damage in MEFs by activating MAPK/Nrf2 signaling pathways and suppressing NF-kappaB signaling pathways. Sci. Rep. 2019, 9, 1782. [Google Scholar] [CrossRef]
- Parsons, B.J. Oxidation of glycosaminoglycans by free radicals and reactive oxidative species: A review of investigative methods. Free Radic. Res. 2015, 49, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Bektas, N.; Senel, B.; Yenilmez, E.; Ozatik, O.; Arslan, R. Evaluation of wound healing effect of chitosan-based gel formulation containing vitexin. Saudi Pharm. J. 2020, 28, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Mir-Palomo, S.; Caddeo, C.; Nacher, A.; Díez-Sales, O.; Peris, J.E.; Pedraz, J.L.; Fadda, A.M.; Manconi, M.J.I. Sorbitol-penetration enhancer containing vesicles loaded with baicalin for the protection and regeneration of skin injured by oxidative stress and UV radiation. Int. J. Pharm. 2019, 555, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Veeraperumal, S.; Qiu, H.M.; Zeng, S.S.; Yao, W.Z.; Wang, B.P.; Liu, Y.; Cheong, K.L. Polysaccharides from Gracilaria lemaneiformis promote the HaCaT keratinocytes wound healing by polarised and directional cell migration. Carbohydr. Polym. 2020, 241, 116310. [Google Scholar] [CrossRef] [PubMed]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Thippraphan, P.; Yodkeeree, S.; Limtrakul, P. Skin Wound-Healing Potential of Polysaccharides from Medicinal Mushroom Auricularia auricula-judae (Bull.). J. Fungi 2021, 7, 247. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, W.; Yu, Q.; Qu, W.; Wang, Y.; Li, R. Functional biomaterials for treatment of chronic wound. Front. Bioeng. Biotechnol. 2020, 8, 516. [Google Scholar] [CrossRef]
- Staub, A.M. Removal of Proteins from Polysaccharides. J. Chem. Educ. 1965, 5, 5–7. [Google Scholar]
- Tumolo, T.; Angnes, L.; Baptista, M.S. Determination of the refractive index increment (dn/dc) of molecule and macromolecule solutions by surface plasmon resonance. Anal. Biochem. 2004, 333, 273–279. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Jiang, W.; Lu, J.; Yu, Y.; Wu, B. Analysis of the monosaccharide composition of water-soluble polysaccharides from Sargassum fusiforme by high performance liquid chromatography/electrospray ionisation mass spectrometry. Food Chem. 2014, 145, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, M.; Bianchi, A.; Sanmartin, C.; Taglieri, I.; Venturi, F.; Testai, L.; Flori, L.; Calderone, V.; De Leo, M.; Braca, A.; et al. By-Products from Winemaking and Olive Mill Value Chains for the Enrichment of Refined Olive Oil: Technological Challenges and Nutraceutical Features. Foods 2020, 9, 1390. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]


| Samples | Molecular Weight (kDa) | Protein % wt | Sulphate Groups % wt |
|---|---|---|---|
| RP-CrudeJSP | N.D. * | 18.42 ± 0.71 | 24.20 ± 0.11 |
| RP-JSP1 | 121 ± 6.33 | 25.13 ± 0.78 | 3.99 ± 0.22 |
| RP-JSP2 | 590 ± 13.5 | 17.22 ± 0.42 | 25.92 ± 0.02 |
| Peak a | Compound | tR (min) | HR- [M − H]−(m/z) | HR-MS/MS Product Ions (m/z) c | Molecular Formula | Error (ppm) | Fraction |
|---|---|---|---|---|---|---|---|
| 1 | Glucosamine-PMP b | 5.4 | 508.2199 | 214.10, 173.07, 157.04 | C26H30N5O6 | −0.590 | RP-JSP1, RP-JSP2, RP-CrudeJSP |
| 2 | Hexose1-PMP | 7.4 | 509.2041 | 215.08, 173.07, 92.05 | C26H30N4O7 | −0.196 | RP-CrudeJSP, |
| 3 | Galactosamine-PMP b | 7.9 | 508.2199 | 214.10, 173.07, 157.04 | C26H30N5O6 | −0.590 | RP-JSP1, RP-JSP2, RP-CrudeJSP, |
| 4 | Hexose2-PMP | 8.4 | 509.2041 | 215.08, 173.07, 92.05 | C26H30N4O7 | −0.196 | RP-JSP1, RP-JSP2, RP-CrudeJSP, |
| 5 | Pentose1-PMP | 8.8 | 479.1938 | 215.08, 173.07, 92.05 | C25H28N4O6 | +0.417 | RP-CrudeJSP |
| 6 | Pentose2-PMP | 9.3 | 479.1938 | 215.08, 173.07, 92.05 | C25H28N4O6 | +0.417 | RP-JSP2 |
| 7 | Deoxyhexose-PMP | 9.6 | 493.2094 | 215.08, 173.07, 92.05 | C26H29N4O6 | +0.203 | RP-JSP1 |
| 8 | Pentose3-PMP | 9.7 | 479.1938 | 215.08, 173.07, 92.05 | C25H28N4O6 | +0.417 | RP-JSP1, RP-JSP2, RP-CrudeJSP |
| 9 | Glucose-PMP b | 12.2 | 509.2040 | 215.08, 173.07, 92.05 | C26H30N4O7 | −0.393 | RP-JSP1, RP-JSP2, RP-CrudeJSP |
| 10 | Galactose-PMP b | 12.5 | 509.2040 | 215.08, 173.07, 92.05 | C26H30N4O7 | −0.393 | RP-JSP1, RP-JSP2, RP-CrudeJSP |
| 11 | Pentose4-PMP | 12.6 | 479.1938 | 215.08, 173.07, 92.05 | C25H28N4O6 | +0.417 | RP-JSP1, RP-JSP2, RP-CrudeJSP |
| Sample | Tmax (°C) | Residue (%) | Tg (°C) |
|---|---|---|---|
| RP-JSP1 | 285.1 | 20.9 | 81.9 |
| RP-JSP2 | 313.5 | 44.3 | 88.8 |
| RP-CrudeJSP | 302.6 | 56.0 | 94.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migone, C.; Scacciati, N.; Grassiri, B.; De Leo, M.; Braca, A.; Puppi, D.; Zambito, Y.; Piras, A.M. Jellyfish Polysaccharides for Wound Healing Applications. Int. J. Mol. Sci. 2022, 23, 11491. https://doi.org/10.3390/ijms231911491
Migone C, Scacciati N, Grassiri B, De Leo M, Braca A, Puppi D, Zambito Y, Piras AM. Jellyfish Polysaccharides for Wound Healing Applications. International Journal of Molecular Sciences. 2022; 23(19):11491. https://doi.org/10.3390/ijms231911491
Chicago/Turabian StyleMigone, Chiara, Noemi Scacciati, Brunella Grassiri, Marinella De Leo, Alessandra Braca, Dario Puppi, Ylenia Zambito, and Anna Maria Piras. 2022. "Jellyfish Polysaccharides for Wound Healing Applications" International Journal of Molecular Sciences 23, no. 19: 11491. https://doi.org/10.3390/ijms231911491
APA StyleMigone, C., Scacciati, N., Grassiri, B., De Leo, M., Braca, A., Puppi, D., Zambito, Y., & Piras, A. M. (2022). Jellyfish Polysaccharides for Wound Healing Applications. International Journal of Molecular Sciences, 23(19), 11491. https://doi.org/10.3390/ijms231911491












