Nitric Oxide Acts as an Inhibitor of Postharvest Senescence in Horticultural Products
Abstract
:1. Introduction
2. NO Production in Plants
3. NO Delays Postharvest Senescence
3.1. Exogenous NO Delays Postharvest Senescence
3.2. Endogenous NO Production during Postharvest Senescence Process
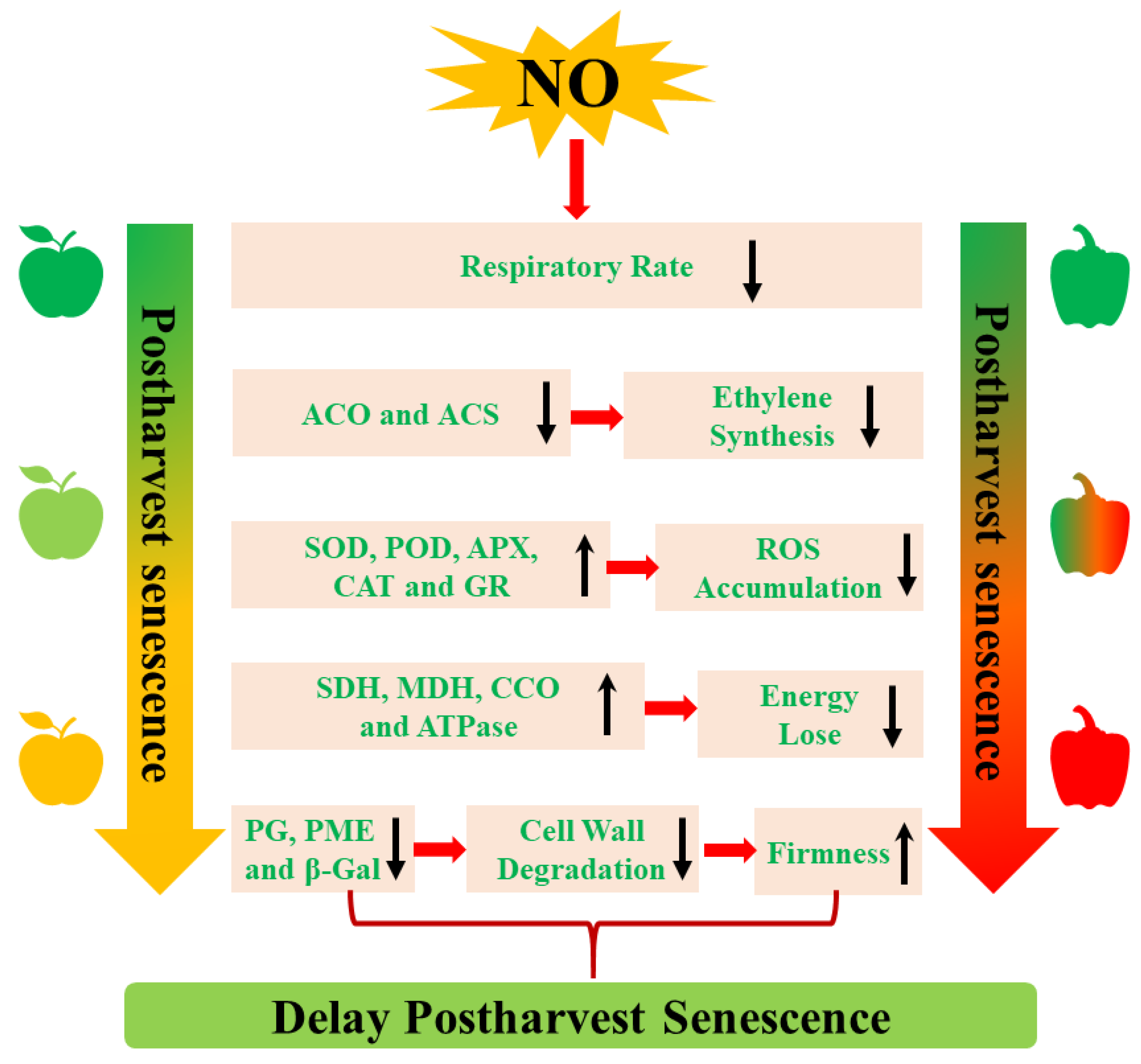
4. The Mechanism of NO-Regulated Postharvest Senescence
4.1. The Inhibition of Ethylene (ETH) Biosynthesis
4.2. The Decrease of Respiratory Metabolism
4.3. The Activation of Cell Wall Metabolism
4.4. The Regulation of ROS Metabolism
4.5. The Promotion of Energy Metabolism
4.6. The Induction of SAGs
5. Crosstalk between NO and Plant Growth Regulators during Postharvest Senescence
5.1. Crosstalk between NO and ETH
5.2. Crosstalk between NO and ABA
5.3. Crosstalk between NO and MT
5.4. Crosstalk between NO and H2
5.5. Crosstalk between NO and H2S
5.6. Crosstalk between NO and H2O2
5.7. Crosstalk between NO and Ca2+
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, S.; Kumari, A.; Shree, M.; Kumar, V.; Singh, P.; Bharadwaj, C.; Loake, G.J.; Parida, S.K.; Masakapalli, S.K.; Gupta, K.J. Nitric oxide accelerates germination via the regulation of respiration in chickpea. J. Exp. Bot. 2019, 70, 4539–4555. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, W.; Wang, M.; Niu, L.; Xu, Q.; Jin, X. Nitric oxide is required for hydrogen gas-induced adventitious root formation in cucumber. J. Plant Physiol. 2016, 195, 50–58. [Google Scholar] [CrossRef] [PubMed]
- González-Gordo, S.; Bautista, R.; Claros, M.G.; Cañas, A.; Palma, J.M.; Corpas, F.J. Nitric oxide-dependent regulation of sweet pepper fruit ripening. J. Exp. Bot. 2019, 70, 4557–4570. [Google Scholar] [CrossRef]
- Kong, W.; Huang, C.; Chen, Q.; Zou, Y.; Zhang, J. Nitric oxide alleviates heat stress-induced oxidative damage in Pleurotus eryngii var. tuoliensis. Fungal Genet. Biol. 2012, 49, 15–20. [Google Scholar] [CrossRef]
- Kaya, C.; Polat, T.; Ashraf, M.; Kaushik, P.; Alyemeni, M.N.; Ahmad, P. Endogenous nitric oxide and its potential sources regulate glutathione-induced cadmium stress tolerance in maize plants. Plant Physiol. Biochem. 2021, 167, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd Allah, E.F.; Gucel, S.; Tran, L.P. Nitric oxide mitigates salt stress by regulating levels of ssmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef]
- Wang, C.; Wei, L.; Zhang, J.; Hu, D.; Gao, R.; Liu, Y.; Feng, L.; Gong, W.; Liao, W. Nitric oxide enhances salt tolerance in tomato seedlings by regulating endogenous S-nitrosylation levels. J. Plant Growth Regul. 2022, 1–19. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Zeng, K. Exogenous nitric oxide-induced postharvest disease resistance in citrus fruit to colletotrichum gloeosporioides. J. Sci. Food Agr. 2016, 96, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y. Nitric oxide fumigation for postharvest pest control on lettuce. Pest Manag. Sci. 2019, 75, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Gapper, N.E.; McQuinn, R.P.; Giovannoni, J.J. Molecular and genetic regulation of fruit ripening. Plant Mol. Biol. 2013, 82, 575–591. [Google Scholar] [CrossRef]
- Barsan, C.; Zouine, M.; Maza, E.; Bian, W.; Egea, I.; Rossignol, M.; Bouyssie, D.; Pichereaux, C.; Purgatto, E.; Bouzayen, M.; et al. Proteomic analysis of chloroplast-to-chromoplast transition in tomato reveals metabolic shifts coupled with disrupted thylakoid biogenesis machinery and elevated energy-production components. Plant Physiol. 2012, 160, 708–725. [Google Scholar] [CrossRef] [PubMed]
- Seraglio, S.; Schulz, M.; Nehring, P.; Della, B.F.; Valese, A.C.; Daguer, H.; Gonzaga, L.V.; Fett, R.; Costa, A. Nutritional and bioactive potential of Myrtaceae fruits during ripening. Food Chem. 2018, 239, 649–656. [Google Scholar] [CrossRef]
- Zhu, X.; Song, Z.; Li, Q.; Li, J.; Chen, W.; Li, X. Physiological and transcriptomic analysis reveals the roles of 1-MCP in the ripening and fruit aroma quality of banana fruit (Fenjiao). Food Res. Int. 2020, 130, 108968. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S. Recent advancements in the mechanism of nitric oxide signaling associated with hydrogen sulfide and melatonin crosstalk during ethylene-induced fruit ripening in plants. Nitric Oxide 2019, 82, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fang, H.; Huo, J.; Huang, D.; Wang, B.; Liao, W. Involvement of calcium and calmodulin in nitric oxide-regulated senescence of cut lily flowers. Front. Plant Sci. 2018, 9, 1284. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, M.; Yu, J. Role of nitric oxide in delaying senescence of cut rose flowers and its interaction with ethylene. Sci. Hortic. 2013, 155, 30–38. [Google Scholar] [CrossRef]
- Qian, C.; Ji, Z.; Lin, C.; Zhang, M.; Zhang, J.; Kan, J.; Liu, J.; Jin, C.; Xiao, L.; Qi, X. Nitric oxide extends the postharvest life of water bamboo shoots partly by maintaining mitochondrial structure and energy metabolism. Int. J. Mol. Sci. 2022, 23, 1607. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, M.; Liu, Z.; Yuan, H.; Lv, T.; Li, H.; Xu, Y.; Si, Y.; Wang, A. NUCLEOCYTOPLASMIC shuttling of ETHYLENE RESPONSE FACTOR 5 mediated by nitric oxide suppresses ethylene biosynthesis in apple fruit. New Phytol. 2022, 234, 1714–1734. [Google Scholar] [CrossRef]
- Rabiei, V.; Kakavand, F.; Nahandi, F.Z.; Razavi, F.; Aghdam, M.S. Nitric oxide and γ-aminobutyric acid treatments delay senescence of cornelian cherry fruits during postharvest cold storage by enhancing antioxidant system activity. Sci. Hortic. 2019, 243, 268–273. [Google Scholar] [CrossRef]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the origins of nitric oxide. Trends Plant Sci. 2011, 16, 160–168. [Google Scholar] [CrossRef]
- Tian, Q.Y.; Sun, D.H.; Zhao, M.G.; Zhang, W.H. Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytol. 2007, 174, 322–331. [Google Scholar] [CrossRef]
- Harrison, R. Structure and function of xanthine oxidoreductase: Where are we now? Free Radic. Biol. Med. 2002, 33, 774–797. [Google Scholar] [CrossRef]
- Cooney, R.V.; Harwood, P.J.; Custer, L.J.; Franke, A.A. Light-mediated conversion of nitrogen dioxide to nitric oxide by carotenoids. Environ. Health Perspect. 1994, 102, 460–462. [Google Scholar] [CrossRef]
- Wang, X.; Hargrove, M.S. Nitric oxide in plants: The roles of ascorbate and hemoglobin. PLoS ONE 2013, 8, e82611. [Google Scholar] [CrossRef]
- Wojtaszek, P. Nitric oxide in plants. To NO or not to NO. Phytochemistry 2000, 54, 1–4. [Google Scholar] [CrossRef]
- Gu, C.; Xu, H.; Zhou, Y.; Yao, J.; Xie, Z.; Chen, Y.; Zhang, S. Multiomics analyses unveil the involvement of microRNAs in pear fruit senescence under high- or low-temperature conditions. Hortic. Res. 2020, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Vicente, A.R.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biol. Technol. 2006, 39, 204–210. [Google Scholar] [CrossRef]
- Büchert, A.M.; Gómez Lobato, M.E.; Villarreal, N.M.; Civello, P.M.; Martínez, G.A. Effect of visible light treatments on postharvest senescence of broccoli (Brassica oleracea L.). J. Sci. Food Agr. 2011, 91, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Imran, H.; Zhang, Y.; Du, G.; Wang, G.; Zhang, J. Effect of salicylic acid (SA) on delaying fruit senescence of Huang Kum pear. Front. Agric. China 2007, 1, 456–459. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Zuo, J.; Gao, L.; Lv, J.; Wang, Q. Methyl jasmonate delays postharvest ripening and senescence in the non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 2016, 120, 76–83. [Google Scholar] [CrossRef]
- Zaharah, S.S.; Singh, Z. Mode of action of nitric oxide in inhibiting ethylene biosynthesis and fruit softening during ripening and cool storage of ‘Kensington Pride’ mango. Postharvest Biol. Technol. 2011, 62, 258–266. [Google Scholar] [CrossRef]
- Han, S.; Cai, H.; An, X.; Huan, C.; Wu, X.; Jiang, L.; Yu, M.; Ma, R.; Yu, Z. Effect of nitric oxide on sugar metabolism in peach fruit (cv. Xiahui 6) during cold storage. Postharvest Biol. Technol. 2018, 142, 72–80. [Google Scholar] [CrossRef]
- Lai, T.; Wang, Y.; Li, B.; Qin, G.; Tian, S. Defense responses of tomato fruit to exogenous nitric oxide during postharvest storage. Postharvest Biol. Technol. 2011, 62, 127–132. [Google Scholar] [CrossRef]
- Shi, K.; Liu, Z.; Wang, J.; Zhu, S.; Huang, D. Nitric oxide modulates sugar metabolism and maintains the quality of red raspberry during storage. Sci. Hortic. 2019, 256, 108611. [Google Scholar] [CrossRef]
- Gergoff Grozeff, G.E.; Alegre, M.L.; Senn, M.E.; Chaves, A.R.; Simontacchi, M.; Bartoli, C.G. Combination of nitric oxide and 1-MCP on postharvest life of the blueberry (Vaccinium spp.) fruit. Postharvest Biol. Technol. 2017, 133, 72–80. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Zhang, H.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Melatonin inhibits ethylene synthesis via nitric oxide regulation to delay postharvest senescence in pears. J. Agric. Food Chem. 2019, 67, 2279–2288. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, J. Effect of nitric oxide on ethylene production in strawberry fruit during storage. Food Chem. 2007, 100, 1517–1522. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, L.; Xu, G. The physiological responses of carnation cut flowers to exogenous nitric oxide. Sci. Hortic. 2011, 127, 424–430. [Google Scholar] [CrossRef]
- Ul Haq, A.; Lateef Lone, M.; Farooq, S.; Parveen, S.; Altaf, F.; Tahir, I.; Ingo Hefft, D.; Ahmad, A.; Ahmad, P. Nitric oxide effectively orchestrates postharvest flower senescence: A case study of Consolida ajacis. Funct. Plant Biol. 2021. [Google Scholar] [CrossRef]
- Lone, M.L.; Haq, A.U.; Farooq, S.; Altaf, F.; Tahir, I. Nitric oxide effectively curtails neck bending and mitigates senescence in isolated flowers of Calendula officinalis L. Physiol. Mol. Biol. Plants 2021, 27, 835–845. [Google Scholar] [CrossRef]
- Iakimova, E.T.; Woltering, E.J. Nitric oxide prevents wound-induced browning and delays senescence through inhibition of hydrogen peroxide accumulation in fresh-cut lettuce. Innov. Food Sci. Emerg. 2015, 30, 157–169. [Google Scholar] [CrossRef]
- Ruan, J.; Li, M.; Jin, H.; Sun, L.; Zhu, Y.; Xu, M.; Dong, J. UV-B irradiation alleviates the deterioration of cold-stored mangoes by enhancing endogenous nitric oxide levels. Food Chem. 2015, 169, 417–423. [Google Scholar] [CrossRef]
- Tian, W.; Huang, D.; Geng, B.; Zhang, Q.; Feng, J.; Zhu, S. Regulation of the biosynthesis of endogenous nitric oxide and abscisic acid in stored peaches by exogenous nitric oxide and abscisic acid. J. Sci. Food Agric. 2020, 100, 2136–2144. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Wang, S.; Liu, Y.; Zou, J.; Ding, W.; Du, H.; Shen, W. Magnesium hydride acts as a convenient hydrogen supply to prolong the vase life of cut roses by modulating nitric oxide synthesis. Postharvest Biol. Technol. 2021, 177, 111526. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Z.; Swinny, E.E. Postharvest nitric oxide fumigation delays fruit ripening and alleviates chilling injury during cold storage of Japanese plums (Prunus salicina Lindell). Postharvest Biol. Technol. 2009, 53, 101–108. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Hou, Y.; Wang, X.; Li, X. Postharvest nitric oxide treatment delays the senescence of winter jujube (Zizyphus jujuba Mill cv. Dongzao) fruit during cold storage by regulating reactive oxygen species metabolism. Sci. Hortic. 2020, 261, 109009. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Kakavand, F.; Rabiei, V.; Zaare-Nahandi, F.; Razavi, F. γ-Aminobutyric acid and nitric oxide treatments preserve sensory and nutritional quality of cornelian cherry fruits during postharvest cold storage by delaying softening and enhancing phenols accumulation. Sci. Hortic. 2019, 246, 812–817. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Hou, Y.; Wang, X.; Li, X. Effects of nitric oxide fumigation treatment on retarding cell wall degradation and delaying softening of winter jujube (Ziziphus jujuba Mill cv. Dongzao) fruit during storage. Postharvest Biol. Technol. 2019, 156, 110954. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Chen, Y.; Wei, J.; Wu, B. Nitric oxide treatment maintains postharvest quality of table grapes by mitigation of oxidative damage. Postharvest Biol. Technol. 2019, 152, 9–18. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Y.; Qu, H.; Duan, X.; Luo, Y.; Jiang, W. Energy aspects in ripening and senescence of harvested horticultural crops. Stewart Postharvest Rev. 2007, 3, 1–5. [Google Scholar]
- Huo, J.; Huang, D.; Zhang, J.; Fang, H.; Wang, B.; Wang, C.; Ma, Z.; Liao, W. Comparative proteomic analysis during the involvement of nitric oxide in hydrogen gas-improved postharvest freshness in cut lilies. Int. J. Mol. Sci. 2018, 19, 3955. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Gong, D.; Xu, H.; Wang, S.; Jia, Z.; Chen, J.; Zhang, L. Effects of salicylic acid and nitric oxide pretreatment on the expression of genes involved in the ethylene signalling pathway and the quality of postharvest mango fruit. N. Z. J. Crop Hortic. Sci. 2014, 3, 205–216. [Google Scholar] [CrossRef]
- Yang, R.; Lin, X.; Dou, Y.; Zhang, W.; Du, H.; Wan, C.; Chen, J.; Zhang, L.; Zhu, L. Transcriptome profiling of postharvest kiwifruit in response to exogenous nitric oxide. Sci. Hortic. 2021, 277, 109788. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, F.; Wu, G.; Gao, W. Impact of postharvest nitric oxide treatment on lignin biosynthesis-related genes in wax apple (Syzygium samarangense) fruit. J. Agric. Food Chem. 2016, 64, 8483–8490. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, J.; Wang, X.; Yu, M.; Ma, R.; Yu, Z. Synergy of nitric oxide and 1-methylcyclopropene treatment in prolong ripening and senescence of peach fruit. Foods 2021, 10, 2956. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Arora, A.; Singh, V.P.; Sairam, R.; Bhattacharya, R.C. Effect of sodium nitroprusside on differential activity of antioxidants and expression of SAGs in relation to vase life of gladiolus cut flowers. Sci. Hortic. 2016, 210, 158–165. [Google Scholar] [CrossRef]
- Mortazavi, S.N.; Talebi, S.; Naderi, R.; Sharafi, Y. Regulation of ethylene biosynthesis by nitric oxide and thidiazuron during postharvest of rose. J. Med. Plants Res. 2011, 5, 5177–5183. [Google Scholar]
- Steelheart, C.; Alegre, M.L.; Vera Bahima, J.; Senn, M.E.; Simontacchi, M.; Bartoli, C.G.; Gergoff Grozeff, G.E. Nitric oxide improves the effect of 1-methylcyclopropene extending the tomato (Lycopersicum esculentum L.) fruit postharvest life. Sci. Hortic. 2019, 255, 193–201. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, C.; Huo, J.; Hu, W.; Liao, W. The involvement of NO in ABA-delayed the senescence of cut roses by maintaining water content and antioxidant enzymes activity. Sci. Hortic. 2019, 247, 35–41. [Google Scholar] [CrossRef]
- Miret, J.A.; Munné-Bosch, S.; Dijkwel, P.P. ABA signalling manipulation suppresses senescence of a leafy vegetable stored at room temperature. Plant Biotechnol. J. 2018, 16, 530–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Cao, S.; Xie, K.; Chi, Z.; Wang, J.; Wang, H.; Wei, Y.; Shao, X.; Zhang, C.; Xu, F.; et al. Melatonin delays yellowing of broccoli during storage by regulating chlorophyll catabolism and maintaining chloroplast ultrastructure. Postharvest Biol. Technol. 2021, 172, 111378. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Lezoul, N.E.H.; Serrano, M.; Ruiz-Aracil, M.C.; Belkadi, M.; Castillo, S.; Valero, D.; Guillén, F. Melatonin as a new postharvest treatment for increasing cut carnation (Dianthus caryophyllus L.) vase life. Postharvest Biol. Technol. 2022, 184, 111759. [Google Scholar] [CrossRef]
- Huang, P.; Li, C.; Liu, H.; Zhao, Z.; Liao, W. hydrogen gas improves seed germination in cucumber by regulating sugar and starch metabolisms. Horticulturae 2021, 7, 456. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, W. A positive role for hydrogen gas in adventitious root development. Plant. Signal. Behav. 2016, 11, e1187359. [Google Scholar] [CrossRef]
- Cao, Z.; Duan, X.; Yao, P.; Cui, W.; Cheng, D.; Zhang, J.; Jin, Q.; Chen, J.; Dai, T.; Shen, W. Hydrogen gas is involved in auxin-induced lateral root formation by modulating nitric oxide synthesis. Int. J. Mol. Sci. 2017, 18, 2084. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, M.; Sun, X. Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS ONE 2013, 8, e71038. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, K.; Cui, W.; Li, L.; Shen, W. Hydrogen-modulated stomatal sensitivity to abscisic acid and drought tolerance via the regulation of apoplastic pH in Medicago sativa. J. Plant Growth Regul. 2016, 35, 565–573. [Google Scholar] [CrossRef]
- Yan, M.; Yao, Y.; Mou, K.; Dan, Y.; Li, W.; Wang, C.; Liao, W. The involvement of abscisic acid in hydrogen gas-enhanced drought resistance in tomato seedlings. Sci. Hortic. 2022, 292, 110631. [Google Scholar] [CrossRef]
- Ren, P.; Jin, X.; Liao, W.; Wang, M.; Niu, L.; Li, X.; Xu, X.; Zhu, Y. Effect of hydrogen-rich water on vase life and quality in cut lily and rose flowers. Hortic. Environ. Biotechnol. 2017, 58, 576–584. [Google Scholar] [CrossRef]
- Fang, H.; Wang, C.; Wang, S.; Liao, W. Hydrogen gas increases the vase life of cut rose ‘Movie star’ by regulating bacterial community in the stem ends. Postharvest Biol. Technol. 2021, 181, 111685. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, S.; Li, P.; Shen, W. Hydrogen gas prolongs the shelf life of kiwifruit by decreasing ethylene biosynthesis. Postharvest Biol. Technol. 2018, 135, 123–130. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Chen, X.; Duan, X.; Jiang, Y. The role of hydrogen water in delaying ripening of banana fruit during postharvest storage. Food Chem. 2022, 373, 131590. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Kuang, Y.; Feng, L.; Liu, Y.; Wang, S.; Du, H.; Shen, W. Molecular hydrogen maintains the storage quality of chinese chive through improving antioxidant capacity. Plants 2021, 10, 1095. [Google Scholar] [CrossRef]
- Hu, H.; Li, P.; Shen, W. Preharvest application of hydrogen-rich water not only affects daylily bud yield but also contributes to the alleviation of bud browning. Sci. Hortic. 2021, 287, 110267. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, W.; Niu, L.; Wang, M.; Ma, Z. Nitric oxide is involved in hydrogen gas-induced cell cycle activation during adventitious root formation in cucumber. BMC Plant Biol. 2016, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mao, Y.; Zhang, W.; Lai, D.; Wang, Q.; Shen, W. Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol. 2014, 165, 759–773. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, S.; Zhang, Z.; Hu, L.; Jiang, C.; Wei, Z.; Liu, J.; Wang, H.; Jiang, S. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol. Technol. 2011, 60, 251–257. [Google Scholar] [CrossRef]
- Hu, L.; Hu, S.; Wu, J.; Li, Y.; Zheng, J.; Wei, Z.; Liu, J.; Wang, H.; Liu, Y.; Zhang, H. Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J. Agric. Food Chem. 2012, 60, 8684–8693. [Google Scholar] [CrossRef]
- Yao, G.; Li, C.; Sun, K.; Tang, J.; Huang, Z.; Yang, F.; Huang, G.; Hu, L.; Jin, P.; Hu, K.; et al. Hydrogen sulfide maintained the good appearance and nutrition in post-harvest tomato fruits by antagonizing the effect of ethylene. Front. Plant Sci. 2020, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, J.; Zhu, L.; Li, C.; Qingguo, W. Cooperative effects of hydrogen sulfide and nitric oxide on delaying softening and decay of strawberry. J. Agric. Biol. Eng. 2014, 7, 114–122. [Google Scholar]
- Zhu, L.; Du, H.; Wang, W.; Zhang, W.; Shen, Y.; Wan, C.; Chen, J. Synergistic effect of nitric oxide with hydrogen sulfide on inhibition of ripening and softening of peach fruits during storage. Sci. Hortic. 2019, 256, 108591. [Google Scholar] [CrossRef]
- Geng, B.; Huang, D.; Zhu, S. Regulation of hydrogen sulfide metabolism by nitric oxide inhibitors and the quality of peaches during cold storage. Antioxidants 2019, 8, 401. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Cañas, A.; López-Jaramillo, J.; Palma, J.M.; Corpas, F.J. Endogenous hydrogen sulfide (H2S) is up-regulated during sweet pepper (Capsicum annuum L.) fruit ripening. In vitro analysis shows that NADP-dependent isocitrate dehydrogenase (ICDH) activity is inhibited by H2S and NO. Nitric. Oxide 2018, 81, 36–45. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, M.; Huang, G.; Yu, J. Hydrogen peroxide in the vase solution increases vase life and keeping quality of cut Oriental×Trumpet hybrid lily ‘Manissa’. Sci. Hortic. 2012, 139, 32–38. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Wang, S.; Zou, J.; Ding, W.; Shen, W. Magnesium hydride-mediated sustainable hydrogen supply prolongs the vase life of cut carnation flowers via hydrogen sulfide. Front. Plant Sci. 2020, 11, 595376. [Google Scholar] [CrossRef]
- Kou, L.; Yang, T.; Luo, Y.; Liu, X.; Huang, L.; Codling, E. Pre-harvest calcium application increases biomass and delays senescence of broccoli microgreens. Postharvest Biol. Technol. 2014, 87, 70–78. [Google Scholar] [CrossRef]



| Species | Treatment | NO-Mediated Effect | References |
|---|---|---|---|
| Pear | 100 μM L−1 SNP | Decreased the transcript levels of cell wall- and ethylene synthetase-related genes; reduced respiration rate and ethylene production | [37] |
| Apple | 100 μM L−1 GSNO | Activated nucleocytoplasmic MdERF5 and suppressed ethylene biosynthesis | [18] |
| Strawberry | 5 μM L−1 SNP | Inhibited ethylene production, respiration rate, and activity of ACC synthase; reduced the content of ACC | [38] |
| Peach | 10 μL L−1 NO | Maintained higher sucrose content but decreased glucose and fructose to lower levels during late storage | [33] |
| Carnation | 0.1 mM L−1 SNP | Maintained water metabolism and antioxidative enzyme activity and mass-eliminated ROS as well as cell membrane stability | [39] |
| Rose | 200 μM L−1 SNP | Decreased ethylene output by inhibiting ACO activity in cut rose flowers | [16] |
| Lily | 100 μM L−1 SNAP | Increased Ca2+/CaM contents, enhanced Ca2+-ATPase activity, and up-regulated gene expression of CaM, CBL1, and CBL3 | [15] |
| Consolida ajacis L. | 40 μM L−1 SNP | Alleviated deteriorative postharvest changes by modulating physiological and biochemical mechanisms underlying senescence | [40] |
| Calendula officinalis L. | 100 μM L−1 SNP | Improved flower longevity by delaying neck bending, inhibited bacterial growth, and increased activities of antioxidant enzymes | [41] |
| Tomato | 1 mM L−1 SNP | Retarded pericarp reddening of tomato fruit, suppressed ethylene production, and influenced quality parameters during storage | [34] |
| Water bamboo shoots | 30 μL L−1 NO | Delayed softness and weight loss and enhanced ATP levels by activating the expression and activity of SDH, MDH, and CCO | [17] |
| Lettuce | 100 and 200 ppm NO | Inhibited the accumulation of H2O2, delayed senescence, and prolonged shelf life | [42] |
| Horticultural Products | Species | SAGs | References |
|---|---|---|---|
| Fruits | Pear | PcPG, PcCel, PcACO1, PcACO2, PcACS1, PcNOS, PcNR1, and PcNR2 | [37] |
| Apple | MdACS1, MdACO1, MdERF5, and MdPP2C57 | [18] | |
| Mango | MiACO, MiACS, MiETR1, MiERS1, MiEIN2, and MiERF | [54] | |
| Table grape | VvSOD, VvCAT, VvPOD2, and VvGR | [51] | |
| Kiwifruit | PG, PL, β-Gal, PE, ACO, ERS1, ETR2, ERF016, ERF7, ERF010, ERF062, ERF110, ERF037, ERF008, ERF113, ERF12, ERF095, CNGC1, CPK1, CIPK2, CML31, CML48, and ZIFL1 | [55] | |
| Wax apple | PAL, POD, GLU, C3H, CA, F5H, 4CL, CCoAOMT, and C4H | [56] | |
| Peach | PpaSOD, PpaCAT, PpaPOD, PpaPOD-1, PpaAPX, and PpaPAL | [57] | |
| Cut flowers | Gladiolus | GgCyP1 and GgDAD1 | [58] |
| Lily | CaM, CBL1, CBL3, and LlatpA | [15,53] | |
| Vegetables | Tomato | LeACS2, LeACS4, LeACO1, LePME, LePG, LePhy1, and LeGAPDH | [34] |
| Water bamboo shoots | ZlH+-ATPase, ZlNa+-K+-ATPase, ZlCa2+-ATPase, ZlMDH, ZlSDH, and ZlCCO | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Du, M.; Jiang, X.; Huang, M.; Zhao, J. Nitric Oxide Acts as an Inhibitor of Postharvest Senescence in Horticultural Products. Int. J. Mol. Sci. 2022, 23, 11512. https://doi.org/10.3390/ijms231911512
Zhu Y, Du M, Jiang X, Huang M, Zhao J. Nitric Oxide Acts as an Inhibitor of Postharvest Senescence in Horticultural Products. International Journal of Molecular Sciences. 2022; 23(19):11512. https://doi.org/10.3390/ijms231911512
Chicago/Turabian StyleZhu, Yongchao, Mei Du, Xianping Jiang, Miao Huang, and Jin Zhao. 2022. "Nitric Oxide Acts as an Inhibitor of Postharvest Senescence in Horticultural Products" International Journal of Molecular Sciences 23, no. 19: 11512. https://doi.org/10.3390/ijms231911512
APA StyleZhu, Y., Du, M., Jiang, X., Huang, M., & Zhao, J. (2022). Nitric Oxide Acts as an Inhibitor of Postharvest Senescence in Horticultural Products. International Journal of Molecular Sciences, 23(19), 11512. https://doi.org/10.3390/ijms231911512








