Nitrate–Nitrite–Nitric Oxide Pathway: A Mechanism of Hypoxia and Anoxia Tolerance in Plants
Abstract
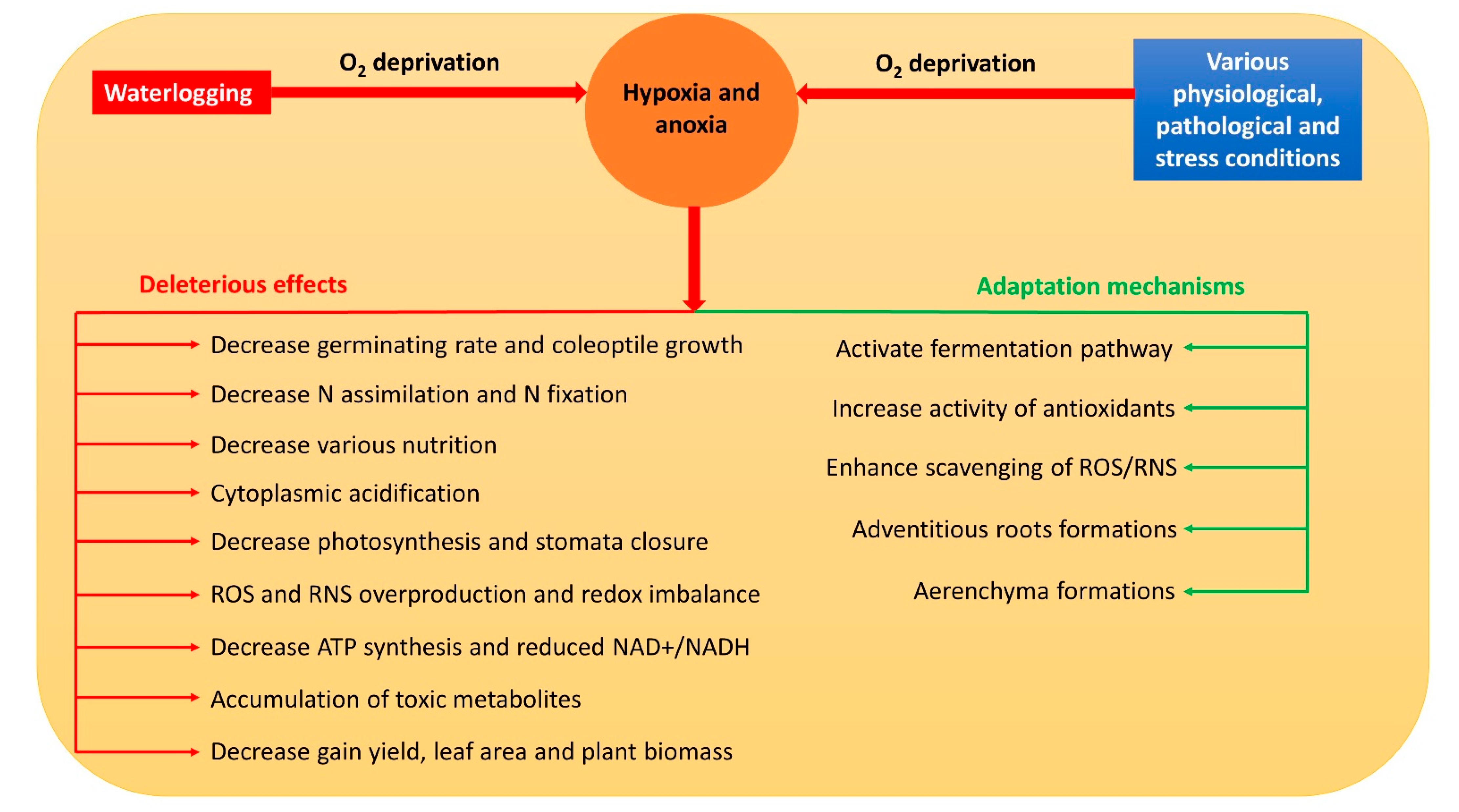
:1. Introduction
2. Pathways of NO Formation during Hypoxia and Anoxia
3. Role of Nitrate and Nitrate Reductase (NR) during Hypoxia and Anoxia Tolerance
4. Role of Nitrite during Hypoxia and Anoxia Tolerance
5. Role of Nitric Oxide during Hypoxia and Anoxia Tolerance
6. Adverse Effects of Nitric Oxide and Role of Nitric Oxide Scavenging on Hypoxia and Anoxia Tolerance
6.1. Nitric Oxide Reduction to Nitrous Oxide
6.2. Phytoglobin-NO Cycle
7. Nitric-Oxide-Mediated Post-Translational Modifications and Their Roles during Hypoxia and Anoxia
7.1. Protein Tyrosine Nitration (PTN)
7.2. S-Nitrosylation
7.3. Metal Nitrosylation
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Greenway, H.; Armstrong, W. Energy-crises in well-aerated and anoxic tissue: Does tolerance require the same specific proteins and energy-efficient transport? Funct. Plant Biol. 2018, 45, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Valeri, M.C.; Novi, G.; Weits, D.A.; Mensuali, A.; Perata, P.; Loreti, E. Botrytis cinerea induces local hypoxia in Arabidopsis leaves. New Phytol. 2021, 229, 173–185. [Google Scholar] [CrossRef]
- Serraj, R.; Roy, G.; Drevon, J.J. Salt stress induces a decrease in the oxygen uptake of soybean nodules and in their permeability to oxygen diffusion. Physiol. Plant. 1994, 91, 161–168. [Google Scholar] [CrossRef]
- Aridhi, F.; Sghaier, H.; Gaitanaros, A.; Khadri, A.; Aschi-Smiti, S.; Brouquisse, R. Nitric oxide production is involved in maintaining energy state in Alfalfa (Medicago sativa L.) nodulated roots under both salinity and flooding. Planta 2020, 252, 22. [Google Scholar] [CrossRef] [PubMed]
- Weits, D.A.; van Dongen, J.T.; Licausi, F. Molecular oxygen as a signaling component in plant development. New Phytol. 2021, 229, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Limami, A.M.; Diab, H.; Lothier, J. Nitrogen metabolism in plants under low oxygen stress. Planta 2014, 239, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.; Witty, J.F.; Mytton, L.R.; Minchin, F.R. The effect of waterlogging on nitrogen fixation and nodule morphology in soil-grown white clover (Trifolium repens L.). J. Exp. Bot. 1995, 46, 285–290. [Google Scholar] [CrossRef]
- Steffens, D.; Hutsch, B.W.; Eschholz, T.; Losak, T.; Schubert, S. Waterlogging may inhibit plant growth primarily by nutrient deficiency rather than nutrient toxicity. Plant Soil Environ. 2005, 51, 545. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Freschi, L.; Sodek, L. Nitrogen metabolism and translocation in soybean plants subjected to root oxygen deficiency. Plant Physiol. Biochem. 2013, 66, 141–149. [Google Scholar] [CrossRef]
- Posso, D.A.; Borella, J.; Reissig, G.N.; Bacarin, M.A. Root flooding-induced changes in the dynamic dissipation of the photosynthetic energy of common bean plants. Acta Physiol. Plant. 2018, 40, 212. [Google Scholar] [CrossRef]
- Shi, K.; Ding, X.-T.; Dong, D.-K.; Zhou, Y.-H.; Yu, J.Q. Putrescine enhancement of tolerance to root-zone hypoxia in Cucumis sativus: A role for increased nitrate reduction. Funct. Plant Biol. 2008, 35, 337–345. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Greenway, H.; Gibbs, J. Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Funct. Plant Biol. 2003, 30, 999–1036. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Yasuda, Y.; Sasaki, R. Soluble sugar availability of aerobically germinated barley, oat and rice coleoptiles in anoxia. J. Plant Physiol. 2010, 167, 1571–1576. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Hill, R.D. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: An alternative to classic fermentation pathways. J. Exp. Bot. 2004, 55, 2473–2482. [Google Scholar] [CrossRef]
- Evans, D.E. Aerenchyma formation. New Phytol. 2004, 161, 35–49. [Google Scholar] [CrossRef]
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tanaka, A.; Mori, H.; Takamure, I.; Kato, K.; Nakazono, M. Ethylene-dependent aerenchyma formation in adventitious roots is regulated differently in rice and maize. Plant Cell Environ. 2016, 39, 2145–2157. [Google Scholar] [CrossRef]
- Chen, T.; Yuan, F.; Song, J.; Wang, B. Nitric oxide participates in waterlogging tolerance through enhanced adventitious root formation in the euhalophyte Suaeda salsa. Funct. Plant Biol. 2016, 43, 244–253. [Google Scholar] [CrossRef]
- Posso, D.A.; Borella, J.; Reissig, G.N.; do Amarante, L.; Bacarin, M.A. Nitrate-mediated maintenance of photosynthetic process by modulating hypoxic metabolism of common bean plants. Acta Physiol. Plant. 2020, 42, 117. [Google Scholar] [CrossRef]
- Groß, F.; Durner, J.; Gaupels, F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 2013, 4, 419. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Mur, L.A.; Wany, A.; Kumari, A.; Fernie, A.R.; Ratcliffe, R.G. The role of nitrite and nitric oxide under low oxygen conditions in plants. New Phytol. 2020, 225, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Timilsina, A.; Zhang, C.; Pandey, B.; Bizimana, F.; Dong, W.; Hu, C. Potential pathway of nitrous oxide formation in plants. Front. Plant Sci. 2020, 11, 1177. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytol. 2013, 199, 633–635. [Google Scholar] [CrossRef]
- Poderoso, J.J.; Helfenberger, K.; Poderoso, C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide 2019, 88, 61–72. [Google Scholar] [CrossRef]
- Wany, A.; Kumari, A.; Gupta, K.J. Nitric oxide is essential for the development of aerenchyma in wheat roots under hypoxic stress. Plant Cell Environ. 2017, 40, 3002–3017. [Google Scholar] [CrossRef]
- Lindermayr, C.; Saalbach, G.; Bahnweg, G.; Durner, J. Differential inhibition of Arabidopsis methionine adenosyltransferases by protein S-nitrosylation. J. Biol. Chem. 2006, 281, 4285–4291. [Google Scholar] [CrossRef]
- Bai, T.; Li, C.; Ma, F.; Shu, H.; Han, M. Exogenous salicylic acid alleviates growth inhibition and oxidative stress induced by hypoxia stress in Malus robusta Rehd. J. Plant Growth Regul. 2009, 28, 358–366. [Google Scholar] [CrossRef]
- Pande, A.; Mun, B.G.; Rahim, W.; Khan, M.; Lee, D.S.; Lee, G.M.; Al Azzawi, T.N.I.; Hussain, A.; Kim, C.K.; Yun, B.W. Phytohormonal Regulation through Protein S-Nitrosylation under Stress. Front. Plant Sci. 2022, 13, 865542. [Google Scholar] [CrossRef]
- Astier, J.; Kulik, A.; Koen, E.; Besson-Bard, A.; Bourque, S.; Jeandroz, S.; Lamotte, O.; Wendehenne, D. Protein S-nitrosylation: What’s going on in plants? Free. Radic. Biol. Med. 2012, 53, 1101–1110. [Google Scholar] [CrossRef]
- Gupta, K.J.; Kaladhar, V.C.; Fitzpatrick, T.B.; Fernie, A.R.; Møller, I.M.; Loake, G.J. Nitric oxide regulation of plant metabolism. Mol. Plant 2022, 15, 228–242. [Google Scholar] [CrossRef]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the origins of nitric oxide. Trends Plant Sci. 2011, 16, 160–168. [Google Scholar] [CrossRef]
- Kumari, A.; Pathak, P.K.; Bulle, M.; Igamberdiev, A.U.; Gupta, K.J. Alternative oxidase is an important player in the regulation of nitric oxide levels under normoxic and hypoxic conditions in plants. J. Exp. Bot. 2019, 70, 4345–4354. [Google Scholar] [CrossRef]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, Á.; Ocaña-Calahorro, F.; Mariscal, V.; Carreras, A.; Barroso, J.B.; Galván, A.; Fernández, E. A dual system formed by the ARC and NR molybdoenzymes mediates nitrite-dependent NO production in Chlamydomonas. Plant Cell Environ. 2016, 39, 2097–2107. [Google Scholar] [CrossRef]
- Valderrama, R.; Corpas, F.J.; Carreras, A.; Fernández-Ocaña, A.; Chaki, M.; Luque, F.; Barroso, J.B. Nitrosative stress in plants. Febs Lett. 2007, 581, 453–461. [Google Scholar] [CrossRef]
- Reda, M.; Golicka, A.; Kabała, K.; Janicka, M. Involvement of NR and PM-NR in NO biosynthesis in cucumber plants subjected to salt stress. Plant Sci. 2018, 267, 55–64. [Google Scholar] [CrossRef]
- Huang, X.; Stettmaier, K.; Michel, C.; Hutzler, P.; Mueller, M.J.; Durner, J. Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta 2004, 218, 938–946. [Google Scholar] [CrossRef]
- Zhao, M.G.; Chen, L.; Zhang, L.L.; Zhang, W.H. Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 2009, 151, 755–767. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Saviani, E.E.; Oliveira, J.F.; Salgado, I. Nitrate reductase-dependent nitric oxide synthesis in the defense response of Arabidopsis thaliana against Pseudomonas syringae. Trop. Plant Pathol. 2010, 35, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Dong, J.-F.; Jin, H.-H.; Sun, L.-N.; Xu, M.-J. Ultraviolet-B-induced flavonoid accumulation in Betula pendula leaves is dependent upon nitrate reductase-mediated nitric oxide signaling. Tree Physiol. 2011, 31, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Monreal, J.A.; Arias-Baldrich, C.; Tossi, V.; Feria, A.B.; Rubio-Casal, A.; García-Mata, C.; García-Mauriño, S. Nitric oxide regulation of leaf phosphoenolpyruvate carboxylase-kinase activity: Implication in sorghum responses to salinity. Planta 2013, 238, 859–869. [Google Scholar] [CrossRef]
- Pissolato, M.D.; Silveira, N.M.; Prataviera, P.J.C.; Machado, E.C.; Seabra, A.B.; Pelegrino, M.T.; Sodek, L.; Ribeiro, R.V. Enhanced nitric oxide synthesis through nitrate supply improves drought tolerance of sugarcane plants. Front. Plant Sci. 2020, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Jasid, S.; Simontacchi, M.; Bartoli, C.G.; Puntarulo, S. Chloroplasts as a nitric oxide cellular source. Effect of reactive nitrogen species on chloroplastic lipids and proteins. Plant Physiol. 2006, 142, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.N.; Sullivan, P.G.; Hall, E.D. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J. Neurosci. Res. 2007, 85, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, F.; Crawford, N.M.; Wang, Y. Molecular regulation of nitrate responses in plants. Int. J. Mol. Sci. 2018, 19, 2039. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Naz, M.; Fan, X.; Xuan, W.; Miller, A.J.; Xu, G. Plant nitrate transporters: From gene function to application. J. Exp. Bot. 2017, 68, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Brandão, A.D.; Sodek, L. Nitrate uptake and metabolism by roots of soybean plants under oxygen deficiency. Braz. J. Plant Physiol. 2009, 21, 13–23. [Google Scholar]
- Oliveira, H.C.; Sodek, L. Effect of oxygen deficiency on nitrogen assimilation and amino acid metabolism of soybean root segments. Amino Acids 2013, 44, 743–755. [Google Scholar]
- De Carvalho, P.A.; de Oliveira, L.E.M.; Sodek, L.; de Carvalho, J.N. Nitrogen metabolism in the roots of rubber tree (Hevea brasiliensis) plants supplied with nitrate or ammonium as nitrogen source during hypoxia. Aust. J. Crop Sci. 2015, 9, 1278. [Google Scholar]
- Liu, B.; Rennenberg, H.; Kreuzwieser, J. Hypoxia affects nitrogen uptake and distribution in young poplar (Populus canescens) trees. PLoS ONE 2015, 10, e0136579. [Google Scholar] [CrossRef]
- Botrel, A.; Magné, C.; Kaiser, W.M. Nitrate reduction, nitrite reduction and ammonium assimilation in barley roots in response to anoxia. Plant Physiol. Biochem. 1996, 34, 645–652. [Google Scholar]
- Benamar, A.; Rolletschek, H.; Borisjuk, L.; Avelange-Macherel, M.H.; Curien, G.; Mostefai, H.A.; Andriantsitohaina, R.; Macherel, D. Nitrite-nitric oxide control of mitochondrial respiration at the frontier of anoxia. Biochim. Biophys. Acta (BBA)-Bioenerg. 2008, 1777, 1268–1275. [Google Scholar] [CrossRef]
- Borisjuk, L.; Rolletschek, H. The oxygen status of the developing seed. New Phytol. 2009, 182, 17–30. [Google Scholar] [CrossRef]
- Pandey, S.; Kumari, A.; Shree, M.; Kumar, V.; Singh, P.; Bharadwaj, C.; Loake, G.J.; Parida, S.K.; Masakapalli, S.K.; Gupta, K.J.; et al. Nitric oxide accelerates germination via the regulation of respiration in chickpea. J. Exp. Bot. 2019, 70, 4539–4555. [Google Scholar]
- Borisjuk, L.; Macherel, D.; Benamar, A.; Wobus, U.; Rolletschek, H. Low oxygen sensing and balancing in plant seeds: A role for nitric oxide. New Phytol. 2007, 176, 813–823. [Google Scholar]
- Hendricks, S.B.; Taylorson, R.B. Promotion of seed germination by nitrates and cyanides. Nature 1972, 237, 169–170. [Google Scholar] [CrossRef]
- Batak, I.; Devic, M.; Gibal, Z.; Grubišic, D.; Poff, K.L.; Konjevic, R. The effects of potassium nitrate and NO-donors on phytochrome A-and phytochrome B-specific induced germination of Arabidopsis thaliana seeds. Seed Sci. Res. 2002, 12, 253. [Google Scholar]
- Lara, T.S.; Lira, J.M.S.; Rodrigues, A.C.; Rakocevi, M.; Alvarenga, A.A. Potassium nitrate priming affects the activity of nitrate reductase and antioxidant enzymes in tomato germination. J. Agric. Sci. 2014, 6, 72. [Google Scholar]
- Vidal, E.A.; Alvarez, J.M.; Araus, V.; Riveras, E.; Brooks, M.D.; Krouk, G.; Ruffel, S.; Lejay, L.; Crawford, N.M.; Coruzzi, G.M.; et al. Nitrate in 2020: Thirty years from transport to signaling networks. Plant Cell 2020, 32, 2094–2119. [Google Scholar]
- Deng, Z.; Song, S. Sodium nitroprusside, ferricyanide, nitrite and nitrate decrease the thermo-dormancy of lettuce seed germination in a nitric oxide-dependent manner in light. S. Afr. J. Bot. 2012, 78, 139–146. [Google Scholar] [CrossRef]
- Dong, T.; Tong, J.; Xiao, L.; Cheng, H.; Song, S. Nitrate, abscisic acid and gibberellin interactions on the thermoinhibition of lettuce seed germination. Plant Growth Regul. 2012, 66, 191–202. [Google Scholar] [CrossRef]
- Nawaz, F.; Naeem, M.; Akram, A.; Ashraf, M.Y.; Ahmad, K.S.; Zulfiqar, B.; Sardar, H.; Shabbir, R.N.; Majeed, S.; Shehzad, M.A.; et al. Seed priming with KNO3-mediates biochemical processes to inhibit lead toxicity in maize (Zea mays L.). J. Sci. Food Agric. 2017, 97, 4780–4789. [Google Scholar] [CrossRef] [PubMed]
- Ikeya, S.; Aoyanagi, T.; Ishizuka, I.; Takeuchi, A.; Kozaki, A. Nitrate promotes germination under inhibition by NaCl or high concentration of glucose. Plants 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dwivedi, P.; Kumar, P.; Singh, B.N.; Pandey, D.K.; Kumar, V.; Bose, B. Mitigation of heat stress responses in crops using nitrate primed seeds. S. Afr. J. Bot. 2021, 140, 25–36. [Google Scholar] [CrossRef]
- Kennedy, R.A.; Rumpho, M.E.; Vanderzee, D. Germination of Echinochloa crus-galli (barnyard grass) seeds under anaerobic conditions: Respiration and response to metabolic inhibitors. Plant Physiol. 1983, 72, 787–794. [Google Scholar]
- Arc, E.; Galland, M.; Godin, B.; Cueff, G.; Rajjou, L. Nitric oxide implication in the control of seed dormancy and germination. Front. Plant Sci. 2013, 4, 346. [Google Scholar] [CrossRef]
- Kemp, K.; Small, J.C. Nitrate and nitrate reductase in Erythrina caffra seeds: Enhancement of induction by anoxia and possible role in germination. Planta 1993, 189, 298–300. [Google Scholar]
- Polyakova, L.I.; Vartapetian, B.B. Exogenous nitrate as a terminal acceptor of electrons in rice (Oryza sativa) coleoptiles and wheat (Triticum aestivum) roots under strict anoxia. Russ. J. Plant Physiol. 2003, 50, 808–812. [Google Scholar] [CrossRef]
- Chen, J.; Strous, M. Denitrification and aerobic respiration, hybrid electron transport chains and co-evolution. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1827, 136–144. [Google Scholar] [CrossRef]
- Board, J.E. Waterlogging effects on plant nutrient concentrations in soybean. J. Plant Nutr. 2008, 31, 828–838. [Google Scholar] [CrossRef]
- Ashraf, M.; Rehman, H. Mineral nutrient status of corn in relation to nitrate and long-term waterlogging. J. Plant Nutr. 1999, 22, 1253–1268. [Google Scholar] [CrossRef]
- Fan, T.W.; Higashi, R.M.; Lane, A.N. An in vivo 1H and 31P NMR investigation of the effect of nitrate on hypoxic metabolism in maize roots. Arch. Biochem. Biophys. 1988, 266, 592–606. [Google Scholar] [CrossRef]
- Libourel, I.G.L.; Van Bodegom, P.M.; Fricker, M.D.; Ratcliffe, R.G. Nitrite reduces cytoplasmic acidosis under anoxia. Plant Physiol. 2006, 142, 1710–1717. [Google Scholar] [CrossRef]
- Da-Silva, C.J.; do Amarante, L. Short-term nitrate supply decreases fermentation and oxidative stress caused by waterlogging in soybean plants. Environ. Exp. Bot. 2020, 176, 104078. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Da-Silva, C.J.; Shimoia, E.P.; Posso, D.A.; Cardoso, A.A.; Batz, T.A.; Oliveira, A.C.B.; do Amarante, L. Nitrate nutrition increases foliar levels of nitric oxide and waterlogging tolerance in soybean. Acta Physiol. Plant. 2021, 43, 116. [Google Scholar] [CrossRef]
- Borella, J.; Becker, R.; Lima, M.C.; Oliveira, D.D.S.C.D.; Braga, E.J.B.; Oliveira, A.C.B.D.; Amarante, L.D. Nitrogen source influences the antioxidative system of soybean plants under hypoxia and re-oxygenation. Sci. Agric. 2019, 76, 51–62. [Google Scholar] [CrossRef]
- Naz, S.; Shen, Q.; Lwalaba, J.L.W.; Zhang, G. Genotypic Difference in the Responses to Nitrogen Fertilizer Form in Tibetan Wild and Cultivated Barley. Plants 2021, 10, 595. [Google Scholar] [CrossRef]
- Nakamura, M.; Noguchi, K. Tolerant mechanisms to O2 deficiency under submergence conditions in plants. J. Plant Res. 2020, 133, 343–371. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Goyal, K.; Arora, K.; Kaur, G. Genotypic variations in nitrate respiration along with potassium nitrate treatment-accountable for water logging tolerance in maize. Biologia 2021, 76, 1651–1660. [Google Scholar] [CrossRef]
- Bacanamwo, M.; Purcell, L.C. Soybean dry matter and N accumulation responses to flooding stress, N sources and hypoxia. J. Exp. Bot. 1999, 50, 689–696. [Google Scholar] [CrossRef]
- Berger, A.; Boscari, A.; Horta Araújo, N.; Maucourt, M.; Hanchi, M.; Bernillon, S.; Rolin, D.; Puppo, A.; Brouquisse, R. Plant nitrate reductases regulate nitric oxide production and nitrogen-fixing metabolism during the Medicago truncatula—Sinorhizobium meliloti symbiosis. Front. Plant Sci. 2020, 11, 1313. [Google Scholar] [CrossRef]
- Berger, A.; Guinand, S.; Boscari, A.; Puppo, A.; Brouquisse, R. Medicago truncatula Phytoglobin 1.1 controls symbiotic nodulation and nitrogen fixation via the regulation of nitric oxide concentration. New Phytol. 2020, 227, 84–98. [Google Scholar] [CrossRef]
- Berger, A.; Boscari, A.; Puppo, A.; Brouquisse, R. Nitrate reductases and hemoglobins control nitrogen-fixing symbiosis by regulating nitric oxide accumulation. J. Exp. Bot. 2021, 72, 873–884. [Google Scholar] [CrossRef]
- Youssef, M.S.; Mira, M.M.; Renault, S.; Hill, R.D.; Stasolla, C. Phytoglobin expression influences soil flooding response of corn plants. Ann. Bot. 2016, 118, 919–931. [Google Scholar] [CrossRef]
- Wany, A.; Gupta, A.K.; Kumari, A.; Mishra, S.; Singh, N.; Pandey, S.; Vanvari, R.; Igamberdiev, A.U.; Fernie, A.R.; Gupta, K.J. Nitrate nutrition influences multiple factors in order to increase energy efficiency under hypoxia in Arabidopsis. Ann. Bot. 2018, 123, 691–705. [Google Scholar] [CrossRef]
- Cochrane, D.W.; Shah, J.K.; Hebelstrup, K.H.; Igamberdiev, A.U. Expression of phytoglobin affects nitric oxide metabolism and energy state of barley plants exposed to anoxia. Plant Sci. 2017, 265, 124–130. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Ratcliffe, R.G.; Gupta, K.J. Plant mitochondria: Source and target for nitric oxide. Mitochondrion 2014, 19, 329–333. [Google Scholar] [CrossRef]
- Hartman, S.; Sasidharan, R.; Voesenek, L.A. The role of ethylene in metabolic acclimations to low oxygen. New Phytol. 2021, 229, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Hartman, S.; Liu, Z.; Van Veen, H.; Vicente, J.; Reinen, E.; Martopawiro, S.; Voesenek, L.A. Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat. Commun. 2019, 10, 4020. [Google Scholar] [CrossRef]
- Moore, M.; Gossmann, N.; Dietz, K.J. Redox regulation of cytosolic translation in plants. Trends Plant Sci. 2016, 21, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Stoimenova, M.; Libourel, I.G.L.; Ratcliffe, R.G.; Kaiser, W.M. The role of nitrate reduction in the anoxic metabolism of roots II. Anoxic metabolism of tobacco roots with or without nitrate reductase activity. Plant Soil 2003, 253, 155–167. [Google Scholar] [CrossRef]
- Horchani, F.; Aschi-Smiti, S.; Brouquisse, R. Involvement of nitrate reduction in the tolerance of tomato (Solanum lycopersicum L.) plants to prolonged root hypoxia. Acta Physiol. Plant. 2010, 32, 1113–1123. [Google Scholar] [CrossRef]
- Shingaki-Wells, R.; Millar, A.H.; Whelan, J.; Narsai, R. What happens to plant mitochondria under low oxygen? An omics review of the responses to low oxygen and reoxygenation. Plant Cell Environ. 2014, 37, 2260–2277. [Google Scholar] [CrossRef]
- Vartapetian, B.B.; Polyakova, L.I. Protective effect of exogenous nitrate on the mitochondrial ultrastructure of Oryza sativa coleoptiles under strict anoxia. Protoplasma 1999, 206, 163–167. [Google Scholar]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef]
- Allègre, A.; Silvestre, J.; Morard, P.; Kallerhoff, J.; Pinelli, E. Nitrate reductase regulation in tomato roots by exogenous nitrate: A possible role in tolerance to long-term root anoxia. J. Exp. Bot. 2004, 55, 2625–2634. [Google Scholar] [CrossRef]
- Horchani, F.; Prévot, M.; Boscari, A.; Evangelisti, E.; Meilhoc, E.; Bruand, C.; Raymond, P.; Boncompagni, E.; Aschi-Smiti, S.; Puppo, A.; et al. Both plant and bacterial nitrate reductases contribute to nitric oxide production in Medicago truncatula nitrogen-fixing nodules. Plant Physiol. 2011, 155, 1023–1036. [Google Scholar]
- Xu, L.; Pan, R.; Zhang, W. Membrane lipids are involved in plant response to oxygen deprivation. Plant Signal. Behav. 2020, 15, 1771938. [Google Scholar] [CrossRef]
- Oberson, J.; Pavelic, D.; Braendle, R.; Rawyler, A. Nitrate increases membrane stability of potato cells under anoxia. J. Plant Physiol. 1999, 155, 792–794. [Google Scholar] [CrossRef]
- Guo, S.R.; Nada, K.; Tachibana, S. A role for nitrate reductase in the high tolerance of cucumber seedlings to root-zone hypoxia. J. Jpn. Soc. Hortic. Sci. 1998, 67, 613–618. [Google Scholar] [CrossRef]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef]
- Brown, G.C.; Borutaite, V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic. Biol. Med. 2002, 33, 1440–1450. [Google Scholar] [CrossRef]
- Costa-Broseta, Á.; Castillo, M.; León, J. Post-translational modifications of nitrate reductases autoregulates nitric oxide biosynthesis in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 549. [Google Scholar] [CrossRef]
- Zheng, P.; Bai, X.; Long, J.; Li, K.; Xu, H. Nitric oxide enhances the nitrate stress tolerance of spinach by scavenging ROS and RNS. Sci. Hortic. 2016, 213, 24–33. [Google Scholar] [CrossRef]
- Gupta, K.J.; Igamberdiev, A.U. The anoxic plant mitochondrion as a nitrite: NO reductase. Mitochondrion 2011, 11, 537–543. [Google Scholar] [CrossRef]
- Bethke, P.C.; Libourel, I.G.; Reinöhl, V.; Jones, R.L. Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 2006, 223, 805–812. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Salgado, I.; Sodek, L. Involvement of nitrite in the nitrate-mediated modulation of fermentative metabolism and nitric oxide production of soybean roots during hypoxia. Planta 2013, 237, 255–264. [Google Scholar] [CrossRef]
- Gupta, K.J.; Lee, C.P.; Ratcliffe, R.G. Nitrite protects mitochondrial structure and function under hypoxia. Plant Cell Physiol. 2017, 58, 175–183. [Google Scholar] [CrossRef]
- Srinivasan, S.; Avadhani, N.G. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic. Biol. Med. 2012, 53, 1252–1263. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Fago, A.; Jensen, F.B. Hypoxia tolerance, nitric oxide, and nitrite: Lessons from extreme animals. Physiology 2015, 30, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Ali, S.N.; Arif, H.; Khan, A.A.; Mahmood, R. Acute oral dose of sodium nitrite induces redox imbalance, DNA damage, metabolic and histological changes in rat intestine. PLoS ONE 2017, 12, e0175196. [Google Scholar] [CrossRef]
- Guo, X.-R.; Chang, B.-W.; Zu, Y.-G.; Tang, Z.-H. The impacts of increased nitrate supply on Catharanthus roseus growth and alkaloid accumulations under ultraviolet-B stress. J. Plant Interact. 2014, 9, 640–646. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Sun, Y.; Gu, Z.; Wang, R.; Saydin, A.; Shen, Q.; Guo, S. Nitrate increased cucumber tolerance to Fusarium wilt by regulating fungal toxin production and distribution. Toxins 2017, 9, 100. [Google Scholar] [CrossRef]
- Stoimenova, M.; Igamberdiev, A.U.; Gupta, K.J.; Hill, R.D. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta 2007, 226, 465–474. [Google Scholar] [CrossRef]
- Floryszak-Wieczorek, J.; Arasimowicz-Jelonek, M.; Izbiańska, K. The combined nitrate reductase and nitrite-dependent route of NO synthesis in potato immunity to Phytophthora infestans. Plant Physiol. Biochem. 2016, 108, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Q.; Li, Y.; Li, J.; Chen, J.; Liu, Z.; Eissa, M.A. Mechanisms of Nitric Oxide in the Regulation of Chilling Stress Tolerance in Camellia sinensis. Horticulturae 2021, 7, 410. [Google Scholar] [CrossRef]
- Guo, X.; Huang, D.; Wen, S.; Bai, Y.; Zhu, S.; Feng, J. Nitric Oxide Regulates Mitochondrial Fatty Acids and Promotes CBF Expression of Peach Fruit during Cold Storage. Phyton 2022, 91, 409. [Google Scholar] [CrossRef]
- Lai, T.; Wang, Y.; Li, B.; Qin, G.; Tian, S. Defense responses of tomato fruit to exogenous nitric oxide during postharvest storage. Postharvest Biol. Technol. 2011, 62, 127–132. [Google Scholar] [CrossRef]
- Wang, H.-H.; Huang, J.-J.; Bi, Y.-R. Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Sci. 2010, 179, 281–288. [Google Scholar] [CrossRef]
- Wang, H.; Hou, J.; Li, Y.; Zhang, Y.; Huang, J.; Liang, W. Nitric oxide-mediated cytosolic glucose-6-phosphate dehydrogenase is involved in aluminum toxicity of soybean under high aluminum concentration. Plant Soil 2017, 416, 39–52. [Google Scholar] [CrossRef]
- Hu, Y.; You, J.; Liang, X. Nitrate reductase-mediated nitric oxide production is involved in copper tolerance in shoots of hulless barley. Plant Cell Rep. 2015, 34, 367–379. [Google Scholar] [CrossRef]
- Khan, M.N.; Alamri, S.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedi, B.; Ali, H.M.; Siddiqui, M.H. Effect of nitric oxide on seed germination and seedling development of tomato under chromium toxicity. J. Plant Growth Regul. 2020, 40, 2358–2370. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, Y.; Lou, W.; Su, J.; Wei, S.; Yang, X.; Shen, W. Nitrate reductase-dependent nitric oxide is crucial for multi-walled carbon nanotube-induced plant tolerance against salinity. Nanoscale 2019, 11, 10511–10523. [Google Scholar] [CrossRef]
- Hayat, S.; Yadav, S.; Wani, A.S.; Irfan, M.; Alyemini, M.N.; Ahmad, A. Impact of sodium nitroprusside on nitrate reductase, proline content, and antioxidant system in tomato under salinity stress. Hortic. Environ. Biotechnol. 2012, 53, 362–367. [Google Scholar] [CrossRef]
- Hao, G.; Du, X.; Zhao, F.; Shi, R.; Wang, J. Role of nitric oxide in UV-B-induced activation of PAL and stimulation of flavonoid biosynthesis in Ginkgo biloba callus. Plant Cell Tissue Organ Cult. 2009, 97, 175–185. [Google Scholar] [CrossRef]
- Silveira, N.M.; Frungillo, L.; Marcos, F.C.; Pelegrino, M.T.; Miranda, M.T.; Seabra, A.B.; Ribeiro, R.V. Exogenous nitric oxide improves sugarcane growth and photosynthesis under water deficit. Planta 2016, 244, 181–190. [Google Scholar] [CrossRef]
- Manoli, A.; Begheldo, M.; Genre, A.; Lanfranco, L.; Trevisan, S.; Quaggiotti, S. NO homeostasis is a key regulator of early nitrate perception and root elongation in maize. J. Exp. Bot. 2014, 65, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, R.; Zhao, M.; Wang, F.; Zhang, N.; Si, H. NO and ABA interaction regulates tuber dormancy and sprouting in potato. Front. Plant Sci. 2020, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Feng, F.; Liu, J.; Zhao, Q. Nitric oxide affects rice root growth by regulating auxin transport under nitrate supply. Front. Plant Sci. 2018, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, J.; Song, W.; Tao, J.; Huang, S.; Chen, S.; Hou, M.; Xu, G.; Zhang, Y. Nitric oxide generated by nitrate reductase increases nitrogen uptake capacity by inducing lateral root formation and inorganic nitrogen uptake under partial nitrate nutrition in rice. J. Exp. Bot. 2015, 66, 2449–2459. [Google Scholar] [CrossRef]
- Jaiswal, A.; Srivastava, J.P. Effect of nitric oxide on some morphological and physiological parameters in maize exposed to waterlogging stress. Afr. J. Agric. Res. 2015, 10, 3462–3471. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Liu, G.; Xu, S.; Dai, J.; Li, W.; Li, Z.; Zhang, D.; Li, C.; Dong, H. Nitric oxide increases the biomass and lint yield of field-grown cotton under temporary waterlogging through physiological and molecular regulation. Field Crops Res. 2021, 261, 107989. [Google Scholar] [CrossRef]
- Safavi-Rizi, V.; Herde, M.; Stöhr, C. Identification of nitric oxide (NO)-responsive genes under hypoxia in tomato (Solanum lycopersicum L.) root. Sci. Rep. 2020, 10, 16509. [Google Scholar] [CrossRef]
- Kopyra, M.; Gwóźdź, E. Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol. Biochem. 2003, 41, 1011–1017. [Google Scholar] [CrossRef]
- Bethke, P.C.; Libourel, I.G.; Jones, R.L. Nitric oxide reduces seed dormancy in Arabidopsis. J. Exp. Bot. 2006, 57, 517–526. [Google Scholar] [CrossRef]
- Gniazdowska, A.; Babańczyk, T.; Krasuska, U. Nitric oxide as germination controlling factor in seeds of various plant species. Phyton 2012, 52, 219–226. [Google Scholar]
- Hayat, S.; Yadav, S.; Alyemeni, M.N.; Ahmad, A. Effect of sodium nitroprusside on the germination and antioxidant activities of tomato (Lycopersicon esculentum Mill). Bulg. J. Agric. Sci. 2014, 20, 140–144. [Google Scholar]
- Ismail, A.M.; Ella, E.S.; Vergara, G.V.; Mackill, D.J. Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa). Ann. Bot. 2009, 103, 197–209. [Google Scholar] [CrossRef]
- Wu, M.; Wang, F.; Zhang, C.; Xie, Y.; Han, B.; Huang, J.; Shen, W. Heme oxygenase-1 is involved in nitric oxide-and cGMP-induced α-Amy2/54 gene expression in GA-treated wheat aleurone layers. Plant Mol. Biol. 2013, 81, 27–40. [Google Scholar] [CrossRef]
- Albertos, P.; Romero-Puertas, M.C.; Tatematsu, K.; Mateos, I.; Sánchez-Vicente, I.; Nambara, E.; Lorenzo, O. S-nitrosylation triggers ABI5 degradation to promote seed germination and seedling growth. Nat. Commun. 2015, 6, 8669. [Google Scholar] [CrossRef]
- Clark, D.; Durner, J.; Navarre, D.A.; Klessig, D.F. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol. Plant-Microbe Interact. 2000, 13, 1380–1384. [Google Scholar] [CrossRef]
- Peng, R.; Bian, Z.; Zhou, L.; Cheng, W.; Hai, N.; Yang, C.; Yang, T.; Wang, X.; Wang, C. Hydrogen sulfide enhances nitric oxide-induced tolerance of hypoxia in maize (Zea mays L.). Plant Cell Rep. 2016, 35, 2325–2340. [Google Scholar]
- Liu, F.; Guo, F.-Q. Nitric oxide deficiency accelerates chlorophyll breakdown and stability loss of thylakoid membranes during dark-induced leaf senescence in Arabidopsis. PLoS ONE 2013, 8, e56345. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liu, H.; Li, C.; Zhu, Y.; Tian, X.; Ma, G.; Zou, H. Effects of nitric oxide on some physiological characteristics of maize seedlings under waterlogging. Afr. J. Agric. Res. 2011, 6, 4501–4504. [Google Scholar]
- Pan, Q.-N.; Geng, C.-C.; Li, D.-D.; Xu, S.-W.; Mao, D.-D.; Umbreen, S.; Loake, G.J.; Cui, B.-M. Nitrate reductase-mediated nitric oxide regulates the leaf shape in Arabidopsis by mediating the homeostasis of reactive oxygen species. Int. J. Mol. Sci. 2019, 20, 2235. [Google Scholar] [CrossRef]
- Gupta, K.J.; Kumari, A.; Florez-Sarasa, I.; Fernie, A.R.; Igamberdiev, A.U. Interaction of nitric oxide with the components of the plant mitochondrial electron transport chain. J. Exp. Bot. 2018, 69, 3413–3424. [Google Scholar]
- Jayawardhane, J.; Cochrane, D.W.; Vyas, P.; Bykova, N.V.; Vanlerberghe, G.C.; Igamberdiev, A.U. Roles for plant mitochondrial alternative oxidase under normoxia, hypoxia, and reoxygenation conditions. Front. Plant Sci. 2020, 11, 566. [Google Scholar] [CrossRef]
- Rutten, P.J.; Poole, P.S. Oxygen regulatory mechanisms of nitrogen fixation in rhizobia. Adv. Microb. Physiol. 2019, 75, 325–389. [Google Scholar]
- He, L.; Li, B.; Lu, X.; Yuan, L.; Yang, Y.; Yuan, Y.; Du, J.; Guo, S. The effect of exogenous calcium on mitochondria, respiratory metabolism enzymes and ion transport in cucumber roots under hypoxia. Sci. Rep. 2015, 5, 11391. [Google Scholar] [CrossRef]
- He, L.; Yu, L.; Li, B.; Du, N.; Guo, S. The effect of exogenous calcium on cucumber fruit quality, photosynthesis, chlorophyll fluorescence, and fast chlorophyll fluorescence during the fruiting period under hypoxic stress. BMC Plant Biol. 2018, 18, 180. [Google Scholar]
- Besson-Bard, A.; Pugin, A.; Wendehenne, D. New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 2008, 59, 21–39. [Google Scholar] [CrossRef]
- Lamotte, O.; Courtois, C.; Dobrowolska, G.; Besson, A.; Pugin, A.; Wendehenne, D. Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free. Radic. Biol. Med. 2006, 40, 1369–1376. [Google Scholar]
- Loitto, V.M.; Nilsson, H.; Sundqvist, T.; Magnusson, K.E. Nitric oxide induces dose-dependent Ca2+ transients and causes temporal morphological hyperpolarization in human neutrophils. J. Cell. Physiol. 2000, 182, 402–413. [Google Scholar] [CrossRef]
- Rich, S.M.; Ludwig, M.; Pedersen, O.; Colmer, T.D. Aquatic adventitious roots of the wetland plant Meionectes brownii can photosynthesize: Implications for root function during flooding. New Phytol. 2011, 190, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Gibberd, M.R.; Gray, J.D.; Cocks, P.S.; Colmer, T.D. Waterlogging tolerance among a diverse range of Trifolium accessions is related to root porosity, lateral root formation and ‘aerotropic rooting’. Ann. Bot. 2001, 88, 579–589. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tanaka, A.; Tsutsumi, N.; Inukai, Y.; Nakazono, M. A role for auxin in ethylene-dependent inducible aerenchyma formation in rice roots. Plants 2020, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. Nitric oxide and cell death. Biochim. Biophys. Acta (BBA) Bioenerg. 1999, 1411, 401–414. [Google Scholar] [CrossRef]
- Deng, Y.; Jia, F.; Chen, S.; Shen, Z.; Jin, Q.; Fu, G.; Ji, J. Nitric oxide as an all-rounder for enhanced photodynamic therapy: Hypoxia relief, glutathione depletion and reactive nitrogen species generation. Biomaterials 2018, 187, 55–65. [Google Scholar] [CrossRef]
- Hebelstrup, K.H.; Møller, I.M. Mitochondrial signaling in plants under hypoxia: Use of reactive oxygen species (ROS) and reactive nitrogen species (RNS). In Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants; Springer: Cham, Switzerland, 2015; pp. 63–77. [Google Scholar]
- Nguyen, T.; Brunson, D.; Crespi, C.L.; Penman, B.W.; Wishnok, J.S.; Tannenbaum, S.R. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc. Natl. Acad. Sci. USA 1992, 89, 3030–3034. [Google Scholar] [CrossRef]
- Bai, S.; Li, M.; Yao, T.; Wang, H.; Zhang, Y.; Xiao, L.; He, Y. Nitric oxide restrain root growth by DNA damage induced cell cycle arrest in Arabidopsis thaliana. Nitric Oxide 2012, 26, 54–60. [Google Scholar] [CrossRef]
- Fernández-Marcos, M.; Sanz, L.; Lewis, D.R.; Muday, G.K.; Lorenzo, O. Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc. Natl. Acad. Sci. USA 2011, 108, 18506–18511. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Luo, S.; Zhang, G.-C.; Feng, L.-Y.; Zheng, C.; Zhou, Y.-H.; He, Y.-K. Nitric oxide induces monosaccharide accumulation through enzyme S-nitrosylation. Plant Cell Environ. 2017, 40, 1834–1848. [Google Scholar] [CrossRef]
- Novikova, G.V.; Mur, L.A.; Nosov, A.V.; Fomenkov, A.A.; Mironov, K.S.; Mamaeva, A.S.; Hall, M.A. Nitric oxide has a concentration-dependent effect on the cell cycle acting via EIN2 in Arabidopsis thaliana cultured cells. Front. Physiol. 2017, 8, 142. [Google Scholar] [CrossRef]
- Vandelle, E.; Delledonne, M. Peroxynitrite formation and function in plants. Plant Sci. 2011, 181, 534–539. [Google Scholar] [CrossRef]
- Castella, C.; Mirtziou, I.; Seassau, A.; Boscari, A.; Montrichard, F.; Papadopoulou, K.; Brouquisse, R. Post-translational modifications of Medicago truncatula glutathione peroxidase 1 induced by nitric oxide. Nitric Oxide 2017, 68, 125–136. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Graziano, M.; Lamattina, L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004, 218, 900–905. [Google Scholar] [CrossRef]
- Morot-Gaudry-Talarmain, Y.; Rockel, P.; Moureaux, T.; Quillere, I.; Leydecker, M.; Kaiser, W.; Morot-Gaudry, J. Nitrite accumulation and nitric oxide emission in relation to cellular signaling in nitrite reductase antisense tobacco. Planta 2002, 215, 708–715. [Google Scholar] [PubMed]
- Lazalt, A.M.; Beligni, M.V.; Lamattina, L. Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans. Eur. J. Plant Pathol. 1997, 103, 643–651. [Google Scholar] [CrossRef]
- He, Y.; Tang, R.-H.; Hao, Y.; Stevens, R.-D.; Cook, C.-W.; Ahn, S.-M.; Pei, Z.-M. Nitric oxide represses the Arabidopsis floral transition. Science 2004, 305, 1968–1971. [Google Scholar] [CrossRef] [PubMed]
- Phoa, N.; Epe, B. Influence of nitric oxide on the generation and repair of oxidative DNA damage in mammalian cells. Carcinogenesis 2002, 23, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, R.; Alexandre, D.; Benoit, V.; Mohamed, H.S.; Remi, N. Nitric oxide scavenging modulates mitochondrial dysfunction induced by hypoxia/reoxygenation. Pharmacol. Rep. 2011, 63, 1189–1194. [Google Scholar] [CrossRef]
- Timilsina, A.; Bizimana, F.; Pandey, B.; Yadav, R.K.P.; Dong, W.; Hu, C. Nitrous oxide emissions from paddies: Understanding the role of rice plants. Plants 2020, 9, 180. [Google Scholar] [CrossRef]
- Einarsdottir, O.; Caughey, W.S. Interactions of the anesthetic nitrous oxide with bovine heart cytochrome c oxidase. Effects on protein structure, oxidase activity, and other properties. J. Biol. Chem. 1988, 263, 9199–9205. [Google Scholar] [CrossRef]
- Sowa, S.; Towill, L.E. Effects of nitrous oxide on mitochondrial and cell respiration and growth in Distichlis spicata suspension cultures. Plant Cell Tissue Organ Cult. 1991, 27, 197–201. [Google Scholar] [CrossRef]
- Zhao, X.-J.; Sampath, V.; Caughey, W.S. Cytochrome c oxidase catalysis of the reduction of nitric oxide to nitrous oxide. Biochem. Biophys. Res. Commun. 1995, 212, 1054–1060. [Google Scholar] [CrossRef]
- Goshima, N.; Mukai, T.; Suemori, M.; Takahashi, M.; Caboche, M.; Morikawa, H. Emission of nitrous oxide (N2O) from transgenic tobacco expressing antisense NiR mRNA. Plant J. 1999, 19, 75–80. [Google Scholar] [CrossRef]
- Bellido-Pedraza, C.M.; Calatrava, V.; Llamas, A.; Fernandez, E.; Sanz-Luque, E.; Galvan, A. Nitrous Oxide Emissions from Nitrite Are Highly Dependent on Nitrate Reductase in the Microalga Chlamydomonas reinhardtii. Int. J. Mol. Sci. 2022, 23, 9412. [Google Scholar] [CrossRef]
- Li, G.; Zhu, S.; Wu, W.; Zhang, C.; Peng, Y.; Wang, Q.; Shi, J. Exogenous nitric oxide induces disease resistance against Monilinia fructicola through activating the phenylpropanoid pathway in peach fruit. J. Sci. Food Agric. 2017, 97, 3030–3038. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Li, X.; Wei, J.; Wu, B. Effect of nitrous oxide against Botrytis cinerea and phenylpropanoid pathway metabolism in table grapes. Sci. Hortic. 2019, 254, 99–105. [Google Scholar]
- Palomer, X.; Roig-Villanova, I.; Grima-Calvo, D.; Vendrell, M. Effects of nitrous oxide (N2O) treatment on the postharvest ripening of banana fruit. Postharvest Biol. Technol. 2005, 36, 167–175. [Google Scholar]
- Zhu, S.-H.; Zhou, J. Effect of nitric oxide on ethylene production in strawberry fruit during storage. Food Chem. 2007, 100, 1517–1522. [Google Scholar]
- Timilsina, A.; Oenema, O.; Luo, J.; Wang, Y.; Dong, W.; Pandey, B.; Hu, C. Plants are a natural source of nitrous oxide even in field conditions as explained by 15N site preference. Sci. Total Environ. 2022, 805, 150262. [Google Scholar]
- Yan, X.-Y.; Shi, S.-L.; Du, L.-J.; Xing, G. Pathways of N2O emission from rice paddy soil. Soil Biol. Biochem. 2000, 32, 437–440. [Google Scholar]
- Bruhn, D.; Albert, K.R.; Mikkelsen, T.N.; Ambus, P. UV-induced N2O emission from plants. Atmos. Environ. 2014, 99, 206–214. [Google Scholar] [CrossRef]
- Smart, D.R.; Bloom, A.J. Wheat leaves emit nitrous oxide during nitrate assimilation. Proc. Natl. Acad. Sci. USA 2001, 98, 7875–7878. [Google Scholar] [CrossRef]
- Machacova, K.; Vainio, E.; Urban, O.; Pihlatie, M. Seasonal dynamics of stem N2O exchange follow the physiological activity of boreal trees. Nat. Commun. 2019, 10, 4989. [Google Scholar]
- Gouble, B.; Fath, D.; Soudain, P. Nitrous oxide inhibition of ethylene production in ripening and senescing climacteric fruits. Postharvest Biol. Technol. 1995, 5, 311–321. [Google Scholar] [CrossRef]
- Lichanporn, I.; Techavuthiporn, C. The effects of nitric oxide and nitrous oxide on enzymatic browning in longkong (Aglaia dookkoo Griff.). Postharvest Biol. Technol. 2013, 86, 62–65. [Google Scholar] [CrossRef]
- Cunha, L.C., Jr.; Jacomino, Â.P.; Trevisan, M.J.; de Almeida Teixeira, G.H. Storage of ‘Oso Grande’ Strawberries in Controlled Atmosphere Containing Nitrous Oxide (N2O). HortScience 2013, 48, 1283–1287. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, J. Various electron donors for biological nitrate removal: A review. Sci. Total Environ. 2021, 794, 148699. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.N.; Zebarth, B.J.; Dandie, C.E.; Burton, D.L.; Goyer, C.; Trevors, J.T. Influence of liquid manure on soil denitrifier abundance, denitrification, and nitrous oxide emissions. Soil Sci. Soc. Am. J. 2009, 73, 760–768. [Google Scholar] [CrossRef]
- Gupta, K.J.; Kaiser, W.M. Production and scavenging of nitric oxide by barley root mitochondria. Plant Cell Physiol. 2010, 51, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Timilsina, A.; Dong, W.; Luo, J.; Lindsey, S.; Wang, Y.; Hu, C. Nitrogen isotopic signatures and fluxes of N2O in response to land-use change on naturally occurring saline-alkaline soil. Sci. Rep. 2020, 10, 21253. [Google Scholar] [CrossRef]
- Bizimana, F.; Timilsina, A.; Dong, W.; Uwamungu, J.Y.; Li, X.; Wang, Y.; Pandey, B.; Qin, S.; Hu, C. Effects of long-term nitrogen fertilization on N2O, N2 and their yield-scaled emissions in a temperate semi-arid agro-ecosystem. J. Soils Sediments 2021, 21, 1659–1671. [Google Scholar] [CrossRef]
- Bizimana, F.; Luo, J.; Timilsina, A.; Dong, W.; Gaudel, G.; Ding, K.; Qin, S.; Hu, C. Estimating field N2 emissions based on laboratory-quantified N2O/(N2O + N2) ratio and field quantified N2O emissions. J. Soils Sediments 2022, 22, 2196–2208. [Google Scholar]
- Machacova, K.; Papen, H.; Kreuzwieser, J.; Rennenberg, H. Inundation strongly stimulates nitrous oxide emissions from stems of the upland tree Fagus sylvatica and the riparian tree Alnus glutinosa. Plant Soil 2013, 364, 287–301. [Google Scholar] [CrossRef]
- Liu, B.; Rennenberg, H.; Kreuzwieser, J. Hypoxia induces stem and leaf nitric oxide (NO) emission from poplar seedlings. Planta 2015, 241, 579–589. [Google Scholar]
- Zafari, S.; Hebelstrup, K.H.; Igamberdiev, A.U. Transcriptional and metabolic changes associated with phytoglobin expression during germination of barley seeds. Int. J. Mol. Sci. 2020, 21, 2796. [Google Scholar] [CrossRef]
- Perazzolli, M.; Dominici, P.; Romero-Puertas, M.C.; Zago, E.; Zeier, J.; Sonoda, M.; Delledonne, M. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell 2004, 16, 2785–2794. [Google Scholar]
- Gupta, K.J.; Stoimenova, M.; Kaiser, W.M. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J. Exp. Bot. 2005, 56, 2601–2609. [Google Scholar]
- Rubio, M.C.; Calvo-Begueria, L.; Díaz-Mendoza, M.; Elhiti, M.; Moore, M.; Matamoros, M.A.; Becana, M. Phytoglobins in the nuclei, cytoplasm and chloroplasts modulate nitric oxide signaling and interact with abscisic acid. Plant J. 2019, 100, 38–54. [Google Scholar]
- Lenhart, K.; Behrendt, T.; Greiner, S.; Steinkamp, J.; Well, R.; Giesemann, A.; Keppler, F. Nitrous oxide effluxes from plants as a potentially important source to the atmosphere. New Phytol. 2019, 221, 1398–1408. [Google Scholar]
- Plouviez, M.; Wheeler, D.; Shilton, A.; Packer, M.A.; McLenachan, P.A.; Sanz-Luque, E.; Guieysse, B. The biosynthesis of nitrous oxide in the green alga Chlamydomonas reinhardtii. Plant J. 2017, 91, 45–56. [Google Scholar]
- Chaki, M.; Begara-Morales, J.C.; Valderrama, R.; Mata-Pérez, C.; Padilla-Serrano, M.N.; Barroso, J.B. Role of nitric oxide—Dependent posttranslational modifications of proteins under abiotic stress. In Plant Life under Changing Environment; Academic Press: Cambridge, MA, USA, 2020; pp. 793–809. [Google Scholar]
- Oliveira, H.C.; Salgado, I. Role of plant mitochondria in nitric oxide homeostasis during oxygen deficiency. In Nitric Oxide in Plants: Metabolism and Role in Stress Physiology; Springer: Cham, Switzerland, 2014; pp. 57–74. [Google Scholar]
- Sturms, R.; DiSpirito, A.A.; Hargrove, M.S. Plant and cyanobacterial hemoglobins reduce nitrite to nitric oxide under anoxic conditions. Biochemistry 2011, 50, 3873–3878. [Google Scholar]
- Astier, J.; Rasul, S.; Koen, E.; Manzoor, H.; Besson-Bard, A.; Lamotte, O.; Wendehenne, D. S-nitrosylation: An emerging post-translational protein modification in plants. Plant Sci. 2011, 181, 527–533. [Google Scholar] [CrossRef]
- Feng, J.; Chen, L.; Zuo, J. Protein S-nitrosylation in plants: Current progresses and challenges. J. Integr. Plant Biol. 2019, 61, 1206–1223. [Google Scholar] [CrossRef]
- Kolbert, Z.; Feigl, G.; Bordé, Á.; Molnár, Á.; Erdei, L. Protein tyrosine nitration in plants: Present knowledge, computational prediction and future perspectives. Plant Physiol. Biochem. 2017, 113, 56–63. [Google Scholar]
- Corpas, F.J.; Palma, J.M.; Del Río, L.A.; Barroso, J.B. Protein tyrosine nitration in higher plants grown under natural and stress conditions. Front. Plant Sci. 2013, 4, 29. [Google Scholar] [CrossRef]
- Blanquet, P.; Silva, L.; Catrice, O.; Bruand, C.; Carvalho, H.; Meilhoc, E. Sinorhizobium meliloti controls nitric oxide—Mediated post-translational modification of a Medicago truncatula nodule protein. Mol. Plant-Microbe Interact. 2015, 28, 1353–1363. [Google Scholar]
- Foster, M.W.; McMahon, T.J.; Stamler, J.S. S-nitrosylation in health and disease. Trends Mol. Med. 2003, 9, 160–168. [Google Scholar]
- Chen, S.C.; Huang, B.; Liu, Y.C.; Shyu, K.G.; Lin, P.Y.; Wang, D.L. Acute hypoxia enhances proteins’ S-nitrosylation in endothelial cells. Biochem. Biophys. Res. Commun. 2008, 377, 1274–1278. [Google Scholar]
- Gupta, A.K.; Kumari, A.; Mishra, S.; Wany, A.; Gupta, K.J. The functional role of nitric oxide in plant mitochondrial metabolism. Adv. Bot. Res. 2016, 77, 145–163. [Google Scholar]
- Fancy, N.N.; Bahlmann, A.K.; Loake, G.J. Nitric oxide function in plant abiotic stress. Plant Cell Environ. 2017, 40, 462–472. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Liu, L.; Liu, X.; Li, B.; Jin, C.; Lin, X. Molecular functions of nitric oxide and its potential applications in horticultural crops. Hortic. Res. 2021, 8, 71. [Google Scholar]
- Zhan, N.; Wang, C.; Chen, L.; Yang, H.; Feng, J.; Gong, X.; Zuo, J. S-nitrosylation targets GSNO reductase for selective autophagy during hypoxia responses in plants. Mol. Cell 2018, 71, 142–154. [Google Scholar]
- Sainz, M.; Pérez-Rontomé, C.; Ramos, J.; Mulet, J.M.; James, E.K.; Bhattacharjee, U.; Becana, M. Plant hemoglobins may be maintained in functional form by reduced flavins in the nuclei, and confer differential tolerance to nitro-oxidative stress. Plant J. 2013, 76, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Freschi, L. Nitric oxide and phytohormone interactions: Current status and perspectives. Front. Plant Sci. 2013, 4, 398. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Lindermayr, C. Nitric oxide-dependent posttranslational modification in plants: An update. Int. J. Mol. Sci. 2012, 13, 15193–15208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besson-Bard, A.; Courtois, C.; Gauthier, A.; Dahan, J.; Dobrowolska, G.; Jeandroz, S.; Alain, P.; David, W. Nitric oxide in plants: Production and cross-talk with Ca2+ signaling. Mol. Plant 2008, 1, 218–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Activities/Defense Mechanisms | Conditions | References | |
|---|---|---|---|
| NO3− | Maintains photosynthesis and transpiration | Waterlogging | [21] |
| NO3− | Protection of mitochondrial ultrastructure for a longer time | Anoxia | [97] |
| NO3− | Maintains membrane stability | Hypoxia | [102] |
| NO3− | Higher activities of antioxidant enzymes such as SOD, APX, CAT, glutathione reductase (GR, EC 1.8.1.7), and guaiacol peroxidase (GPOD, EC 1.11.1.7) | Hypoxia | [79] |
| NO3− | Increases the various nutrient contents | Waterlogging | [72] |
| NO3− | Increases seed germination rate by regulating the ABA level | Normoxia | [66] |
| NR inhibition | Growth is disturbed | Waterlogging | [94,95] |
| NO3− | Increases ATP synthesis while decreasing fermentation | Waterlogging | [75] |
| NO3− | Maintains the level of metabolites such as sucrose, alanine, γ-aminobutyrate, lactate, and succinate and decreases fermentation | Waterlogging | [73] |
| NO3− | Increases in CO2 assimilation, stomatal conductance, transpiration rate, and shoot biomass | Waterlogging | [78] |
| NO3− | UV-radiation tolerance by reducing H2O2 and malondialdehyde (MDA) and increasing plants’ height and biomass | UV stress | [116] |
| NO3− and NR | Delay wilting and anoxia symptoms | Anoxia | [99] |
| NR-deficient mutant plant | Produces less NO that is more susceptible to bacterial and fungal attack through decreasing hypersensitive response | Pathogen attack | [117] |
| NO2− | Decreases fermentation that helps to reduce the toxicity of fermentative metabolites | Hypoxia | [10] |
| NO3− and NO2− | Improves cytoplasmic acidification | Hypoxia and Anoxia | [73,74] |
| NO2− | ATP synthesis through mitochondria ETCs | Anoxia | [118] |
| NO2− | Protects mitochondrial structure and functions | Hypoxia | [111] |
| NR-dependent NO production | Defense against pathogen through rapid development of hypersensitive response and lessening the effects of clorotic lesions and bacterial infection | Pathogen attack | [40,119] |
| NR-dependent NO production | Involved in cold acclimation and freezing tolerance through reductions in electrolyte leakage | Cold stress | [39] |
| NO | Decreases the mitochondrial oxidative damages through decreased ROS content and maintained the structure and function of mitochondria through increasing mitochondrial antioxidants enzymes, improving mitochondrial Ca2+ homeostasis, promoting genes related to C-repeat binding factors (CBFs), while reducing the peroxidation of mitochondrial fatty acids | Cold stress | [120,121] |
| NO | Maintains quality, delays ripening, and enhances resistance to pathogens through increasing the activities of antioxidants, gene regulation, and suppressing ethylene production | Postharvest storage | [122] |
| NR-dependent NO production | Aluminum-induced ROS and lipid peroxidation are reduced, while it improves root growth during the stress through the regulation of ascorbate–glutathione cycle | Metal stress | [123,124] |
| NR-dependent NO production | Copper tolerance through enhanced antioxidant activities | Metal stress | [125] |
| NO | Improved seed germination through upregulation of α-amylase, protease, enzymes of N assimilation, and antioxidants | Metal stress | [126] |
| NR-dependent NO production | Salt tolerance by balancing redox and ions, reducing ROS, and increasing antioxidants | Salt stress | [127] |
| NO | Increases activities of antioxidants and proline content | Salt stress | [128] |
| NR-dependent NO production | The rapid accumulation of UV-absorbing substances such as flavonoids | UV stress | [41,129] |
| NR-dependent NO production | Higher photosynthetic rates and stomatal conductance, and less ROS accumulation due to higher activities of various antioxidants | Drought stress | [43] |
| NO | Improved photosynthesis activities and promotes growth | Drought stress | [130] |
| NR-dependent NO formation and induction of non-symbiotic hemoglobin | Root elongation through the activities of actin cytoskeleton and hormonal signaling | Normoxia | [131] |
| NR-dependent NO production | Releases tuber dormancy and sprouting via the expression of genes involved in ABA catabolism | Normoxia | [132] |
| NO3− dependent NO production | Regulation of lateral root and seminal root growth by regulating auxin transport, while lateral root formation increases N uptake capacity during partial N availability | Normoxia | [133,134] |
| NO2− dependent NO production | Regulates O2 concentration and postpone anoxia | Hypoxia | [53] |
| NO2− and NO | Accelerates germination through decreasing lipid peroxidation and DNA fragmentation in germinating seeds | Physiological hypoxia | [55] |
| NO | Decreases cell membrane injuries and increases stomatal conductance and transpiration rate as compared to the control | Waterlogging | [135] |
| NO3−, NO2− and NO | Breaks dormancy in seeds through NO signaling | Normoxia | [109] |
| NO | Increases biomass and lint yield of cotton plants through reduced lipid peroxidation, the expression of waterlogging tolerance-related genes, and increasing photosynthesis process | Waterlogging | [136] |
| NO | Enhances adventitious root formation | Waterlogging | [20] |
| NO | Regulates genes belonging to phytohormones, Cytochrome P450 encoding genes (CYP72A14 and CYP707A3) that regulate ROS and genes related to cell wall synthesis, modification, and degradation | Hypoxia | [137] |
| Effects of Higher Level of NO | References |
|---|---|
| Decreases the root growth through DNA damage, induces cell cycle arrest and inhibits primary root growth by affecting root apical meristem activity and cell elongation. | [165,166] |
| Delayed flowering, retarded root development, and reduced starch granule formation through S-nitrosylation modification. | [167] |
| Cell death through increased electrolyte leakage, cell wall degradation, cytoplasmic streaming, and DNA fragmentation. | [27] |
| Decreases the expression of cyclins (CYC) and Cyclin-Dependent Kinases (CDKs), resulting in the downregulation of cell cycle progression. | [168] |
| NO can generate peroxynitrite, which is a mediator of cytochrome c loss, protein oxidation and nitration, lipid peroxidation, mitochondrial dysfunction, damage DNA, and cell death. | [26,169] |
| NO can inhibit antioxidants such as catalase, glutathione peroxidase (GPX), and ascorbate peroxidase in a reversible way and peroxynitrite in an irreversible way. | [145,170] |
| NO can change the redox state and promote cell death. | [33] |
| Inhibits lateral and primary root growth through reduced cell division and the expression of the auxin reporter markers DR5pro:GUS/GFP. | [166,171] |
| Inhibits growth of tobacco plants through peroxynitrite formation and tyrosine nitration. | [172] |
| Inhibits seed germination, while the scavenging of NO alleviates the effect. | [139] |
| Inhibits the shoot growth and decreases the chlorophyll contents of the plants. | [173,174] |
| Beneficial Activities of N2O | Reference |
|---|---|
| Using post-harvest technology, the storage of fruits under N2O can lower ethylene production and slow the ripening of fruits. | [185,192] |
| N2O can increase resistance to pathogens by improving the accumulation of total phenolic, flavonoids, and lignin, as well as increase the activities of key enzymes in the metabolism of phenylpropanol. | [184] |
| Can inhibit the browning activities of enzymes such as polyphenol oxidase (PPO) and/or peroxidase (POD) and delay browning in fruits. | [193] |
| Can delay decay, lower the respiratory rate, and maintain the quality of fruit. | [194] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timilsina, A.; Dong, W.; Hasanuzzaman, M.; Liu, B.; Hu, C. Nitrate–Nitrite–Nitric Oxide Pathway: A Mechanism of Hypoxia and Anoxia Tolerance in Plants. Int. J. Mol. Sci. 2022, 23, 11522. https://doi.org/10.3390/ijms231911522
Timilsina A, Dong W, Hasanuzzaman M, Liu B, Hu C. Nitrate–Nitrite–Nitric Oxide Pathway: A Mechanism of Hypoxia and Anoxia Tolerance in Plants. International Journal of Molecular Sciences. 2022; 23(19):11522. https://doi.org/10.3390/ijms231911522
Chicago/Turabian StyleTimilsina, Arbindra, Wenxu Dong, Mirza Hasanuzzaman, Binbin Liu, and Chunsheng Hu. 2022. "Nitrate–Nitrite–Nitric Oxide Pathway: A Mechanism of Hypoxia and Anoxia Tolerance in Plants" International Journal of Molecular Sciences 23, no. 19: 11522. https://doi.org/10.3390/ijms231911522







