Crotalphine Modulates Microglia M1/M2 Phenotypes and Induces Spinal Analgesia Mediated by Opioid-Cannabinoid Systems
Abstract
1. Introduction
2. Results
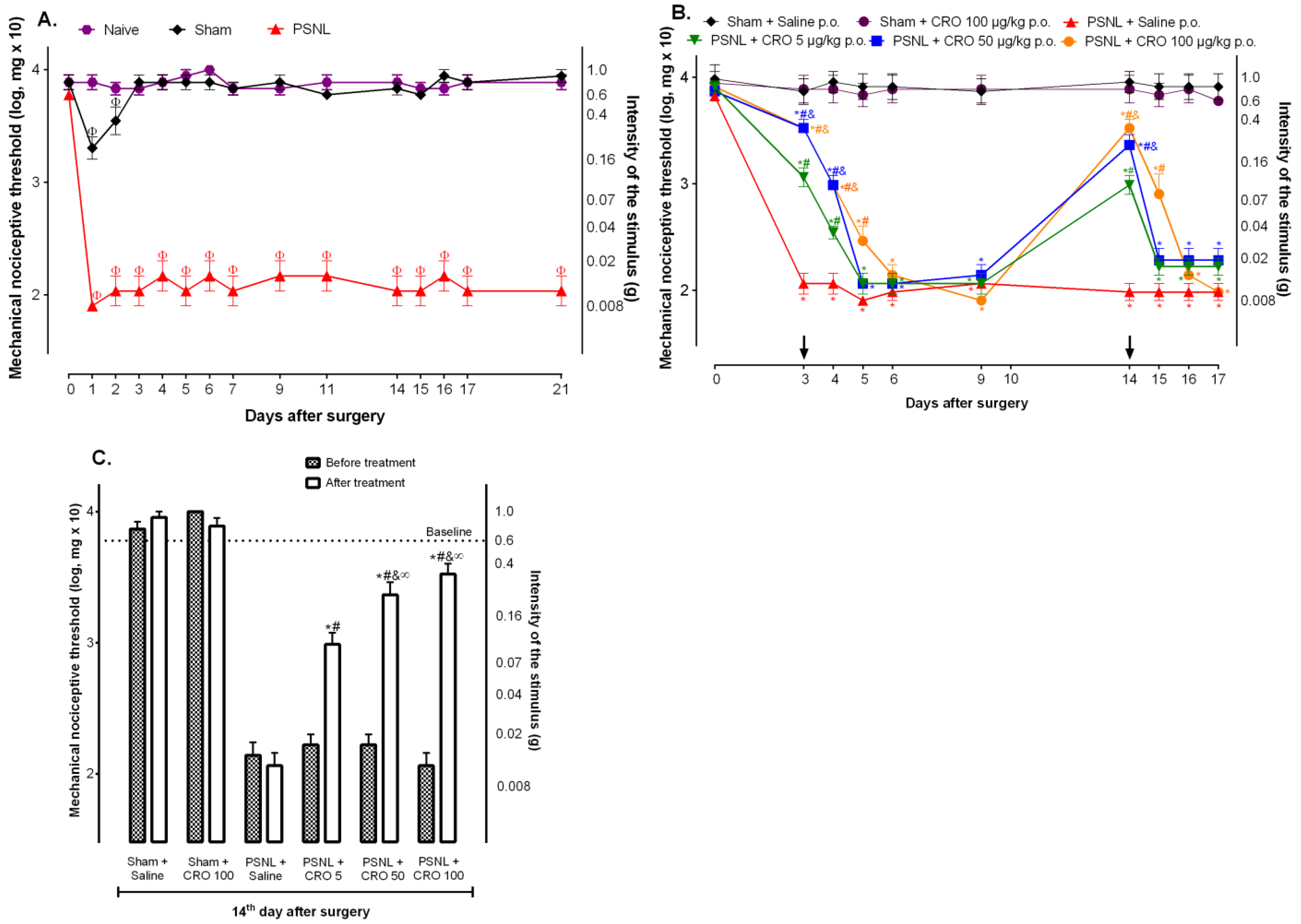
2.1. Crotalphine Induces Dose and Time-Dependent Analgesia in the PSNL Model of Neuropathic Pain
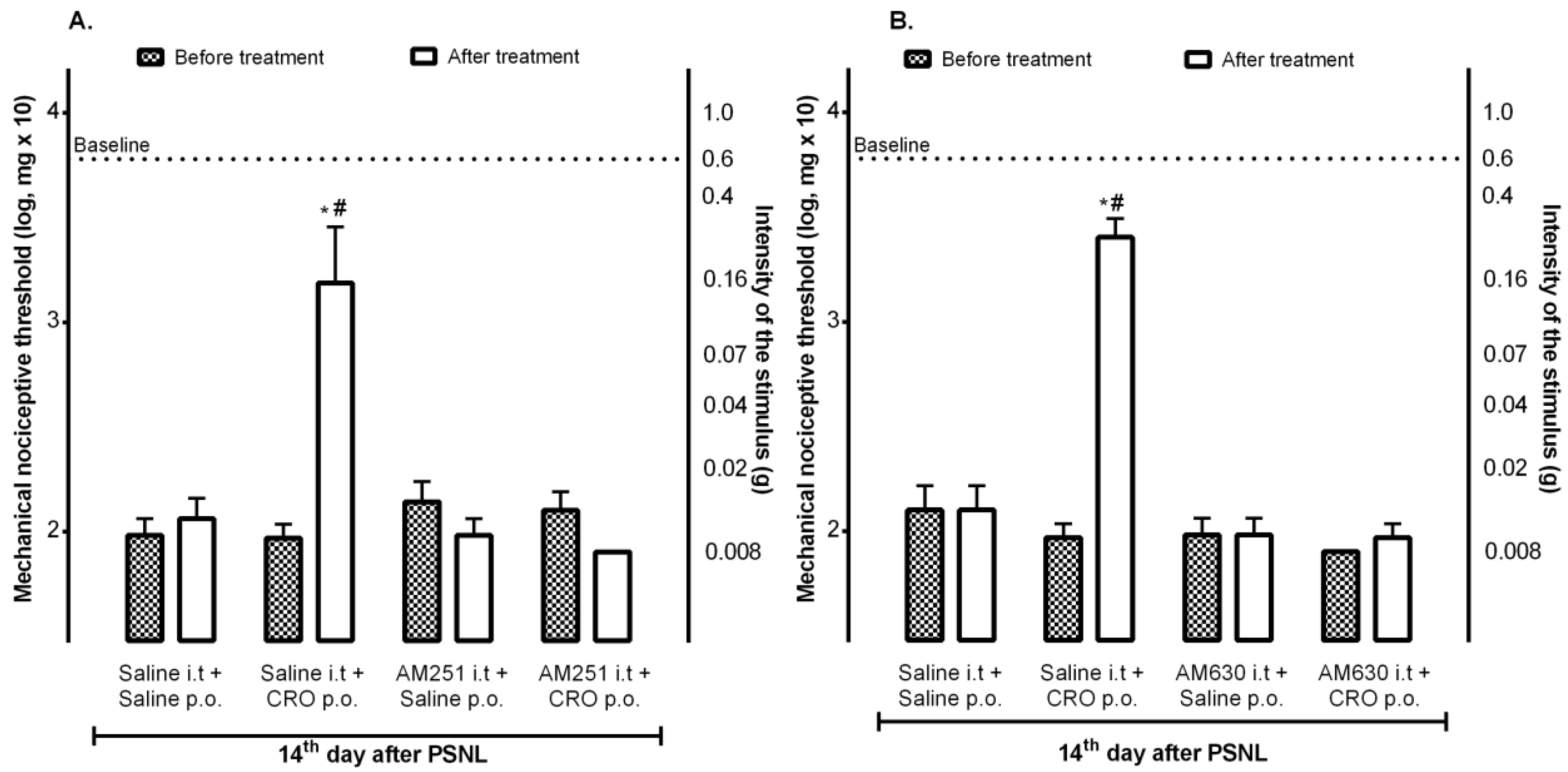
2.2. Spinal Cannabinoid Receptors Mediate the Analgesic Effect Induced by Crotalphine
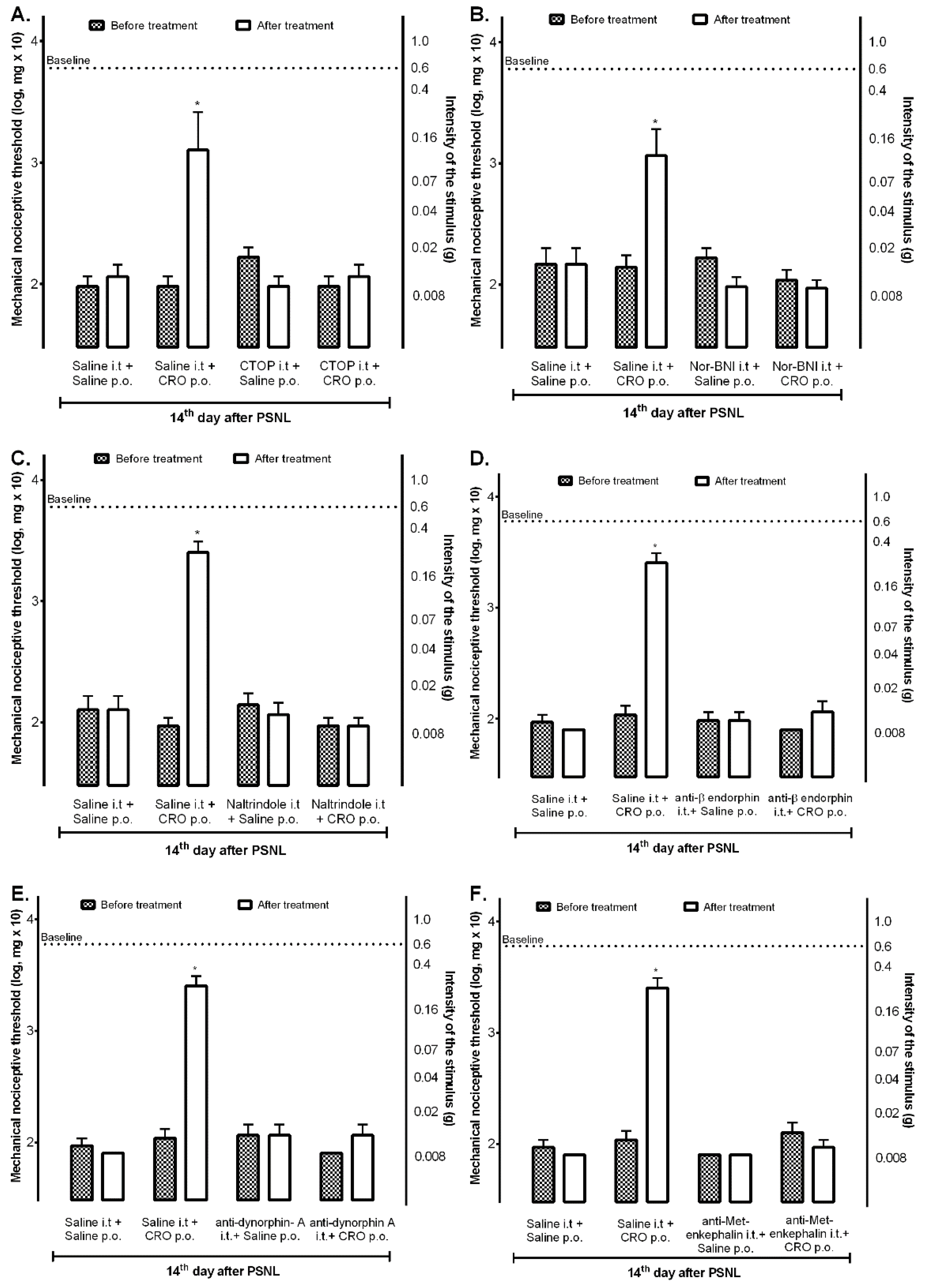
2.3. Spinal Opioid Pathway Is Involved in the Analgesic Effect Induced by Crotalphine
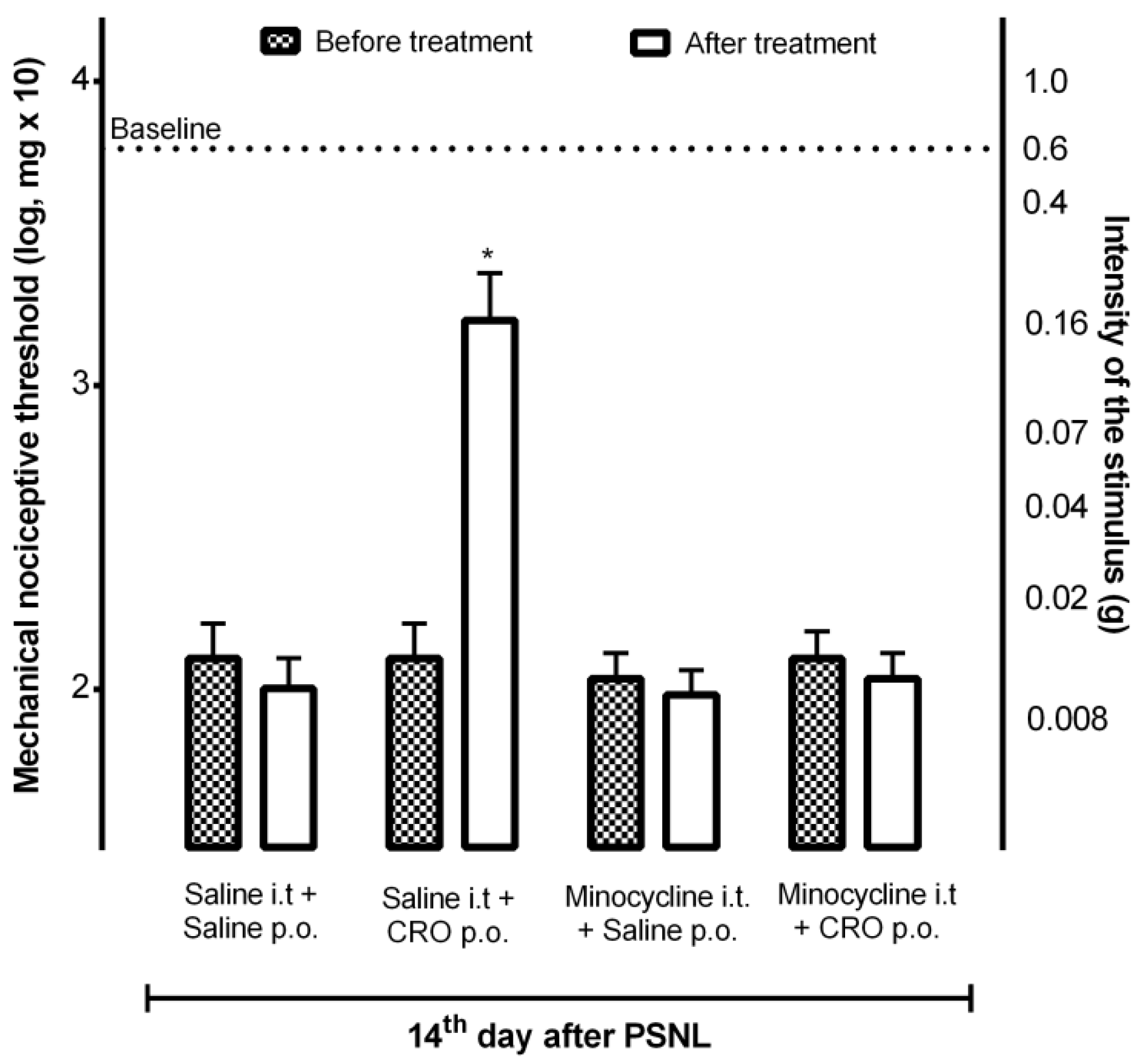
2.4. Participation of Microglia in the Analgesic Effect of Crotalphine
2.5. Quantification of Interleukin 6 Levels in the Spinal Cord of Mice with Neuropathy after Treatment with Crotalphine
2.6. Effect of Crotalphine on LPS-Induced BV2 Cells Polarization to M1 or M2 Phenotypes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Neuropathic Pain Induction—Partial Sciatic Nerve Ligation (PSNL) Model
4.3. Crotalphine Treatment
4.4. Pharmacological Treatments
4.5. Von Frey Filaments Test for Hypernociception Determination
4.6. Assessment of IL-6 Cytokine Levels
4.7. Cell Culture and Treatment
4.8. Flow Cytometry Assay
4.9. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Health 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.T.; Duong, V.; Ho, S.; Ngo, K.C.; Greer, C.L.; Weeks, D.L. side effects of commonly prescribed analgesic medications. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef]
- Gosselin, R.; Suter, M.; Ji, R.; Decosterd, I. Glial cells and chronic pain. Neuroscientist 2010, 16, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Gama, K.B.; Santos, D.S.; Evangelista, A.F.; Silva, D.N.; de Alcântara, A.C.; dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Conditioned medium of bone marrow-derived mesenchymal stromal cells as a therapeutic approach to neuropathic pain: A preclinical evaluation. Stem Cells Int. 2018, 2018, 8179013. [Google Scholar] [CrossRef]
- Miranda, H.F.; Noriega, V.; Prieto, J.C.; Zanetta, P.; Castillo, R.; Aranda, N.; Sierralta, F. Antinociceptive interaction of tramadol with gabapentin in experimental mononeuropathic pain. Basic Clin. Pharmacol. Toxicol. 2016, 119, 210–214. [Google Scholar] [CrossRef]
- Deng, M.Y.; Ahmad, K.A.; Han, Q.Q.; Wang, Z.Y.; Shoaib, R.M.; Li, X.Y.; Wang, Y.X. Thalidomide alleviates neuropathic pain through microglial IL-10/β-endorphin signaling pathway. Biochem. Pharmacol. 2021, 192, 114727. [Google Scholar] [CrossRef]
- Wang, Y.; Han, C.C.; Cui, D.; Li, Y.; Ma, Y.; Wei, W. Is macrophage polarization important in rheumatoid arthritis? Int. Immunopharmacol. 2017, 50, 345–352. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in pain: Detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Carrier, E.J.; Kearn, C.S.; Barkmeier, A.J.; Breese, N.M.; Yang, W.; Nithipatikom, K.; Pfister, S.L.; Campbell, W.B.; Hillard, C.J. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB 2 receptor-dependent mechanism. Mol. Pharmacol. 2004, 65, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Gong, N.; Li, T.F.; Ma, A.N.; Wu, X.Y.; Wang, M.W.; Wang, Y.X. The non-peptide GLP-1 receptor agonist WB4-24 Blocks inflammatory nociception by stimulating β-endorphin release from spinal microglia. Br. J. Pharmacol. 2015, 172, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Childers, S.R.; Pacheco, M.; Fleming, L.; Konkoy, C.; Ward, S.; Marckel, D.; Sexton, T. Opioid and cannabinoid receptor inhibition of adenylyl cyclase in brain. Ann. N. Y. Acad. Sci. 2006, 654, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Mackie, K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Viganò, D.; Rubino, T.; Parolaro, D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol. Biochem. Behav. 2005, 81, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, D.L. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004, 74, 1317–1324. [Google Scholar] [CrossRef]
- Devi, L.A. Heterodimerization of G-protein-coupled receptors: Pharmacology, signaling and trafficking. Trends Pharmacol. Sci. 2001, 22, 532–537. [Google Scholar] [CrossRef]
- Elikottil, J.; Gupta, P.; Gupta, K. The analgesic potential of cannabinoids. J. Opioid Manag. 2009, 5, 341–357. [Google Scholar] [CrossRef]
- Konno, K.; Picolo, G.; Gutierrez, V.P.; Brigatte, P.; Zambelli, V.O.; Camargo, A.C.M.; Cury, Y. Crotalphine, a novel potent analgesic peptide from the venom of the South American Rattlesnake Crotalus Durissus Terrificus. Peptides 2008, 29, 1293–1304. [Google Scholar] [CrossRef]
- Gutierrez, V.P.; Konno, K.; Chacur, M.; Sampaio, S.C.; Picolo, G.; Brigatte, P.; Zambelli, V.O.; Cury, Y. Crotalphine induces potent antinociception in neuropathic pain by acting at peripheral opioid receptors. Eur. J. Pharmacol 2008, 594, 84–92. [Google Scholar] [CrossRef]
- Brigatte, P.; Konno, K.; Pacciari, V.; Coccuzzo, S.; Olzon, V.; Picolo, G.; Curi, R.; Cury, Y. Pharmacology, biochemistry and behavior peripheral kappa and delta opioid receptors are involved in the antinociceptive effect of crotalphine in a rat model of cancer pain. Pharmacol. Biochem. Behav. 2013, 109, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.C.; Zambelli, V.O.; Fernandes, A.C.O.; Heimann, A.S.; Cury, Y.; Picolo, G. Peripheral interactions between cannabinoid and opioid systems contribute to the antinociceptive effect of crotalphine. Br. J. Pharmacol. 2014, 171, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Touska, F.; Vetter, I.; Kistner, K.; Kichko, T.I.; Teixeira, N.B.; Picolo, G.; Cury, Y.; Lewis, R.J.; Fischer, M.J.M.; et al. Crotalphine desensitizes TRPA1 ion channels to alleviate inflammatory hyperalgesia. Pain 2016, 157, 2504–2516. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, B.G.; Hösch, N.G.; Pereira, L.M.; Barbosa, T.C.; Picolo, G.; Zambelli, V.O.; Cury, Y. Pkcζ-mitogen-activated protein kinase signaling mediates crotalphine-induced antinociception. Toxins 2021, 13, 912. [Google Scholar] [CrossRef] [PubMed]
- Giardini, A.C.; Evangelista, B.G.; Sant’anna, M.B.; Martins, B.B.; Lancellotti, C.L.P.; Ciena, A.P.; Chacur, M.; Pagano, R.L.; Ribeiro, O.G.; Zambelli, V.O.; et al. Crotalphine attenuates pain and neuroinflammation induced by experimental autoimmune encephalomyelitis in mice. Toxins 2021, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, A.B.; Basbaum, A.I. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: Behavioral and neuroanatomical correlates. Pain 1998, 76, 215–222. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Publ. Group 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Kobayashi, K.; Imagama, S.; Ohgomori, T.; Hirano, K.; Uchimura, K.; Sakamoto, K.; Hirakawa, A.; Takeuchi, H.; Suzumura, A.; Ishiguro, N.; et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013, 4, e525–e529. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Machelska, H.; Celik, M. Opioid receptors in immune and glial cells—Implications for pain control. Front. Immunol. 2020, 11, 300. [Google Scholar] [CrossRef]
- Lang, G.P.; Li, C.; Han, Y.Y. Rutin pretreatment promotes microglial M1 to M2 phenotype polarization. Neural Regen. Res. 2021, 16, 2499–2504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-ΚB pathways in BV2 Cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, L.; Li, L.; Feng, W. Dihydromyricetin attenuates neuropathic pain via enhancing the transition from M1 to M2 phenotype polarization by potentially elevating ALDH2 activity in vitro and vivo. Ann. Transl. Med. 2020, 8, 1151. [Google Scholar] [CrossRef] [PubMed]
- Henschke, N.; Kamper, S.J.; Maher, C.G. The epidemiology and economic consequences of pain. Mayo. Clin. Proc. 2015, 90, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, V.P.; Zambelli, V.O.; Picolo, G.; Chacur, M.; Sampaio, S.C.; Brigatte, P.; Konno, K.; Cury, Y. The peripheral L-arginine–nitric oxide–cyclic GMP pathway and ATP-sensitive K+ channels are involved in the antinociceptive effect of crotalphine on neuropathic pain in rats. Behav. Pharmacol. 2012, 23, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Mannion, R.J. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Kim, C.F.; Moalem-Taylor, G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res. 2011, 1405, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Saijo, K.; Glass, C.K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011, 11, 775–787. [Google Scholar] [CrossRef]
- Ristoiu, V. Contribution of macrophages to peripheral neuropathic pain pathogenesis. Life Sci. 2013, 93, 870–881. [Google Scholar] [CrossRef]
- Bushlin, I.; Rozenfeld, R.; Devi, L.A. Cannabinoid-opioid interactions during neuropathic pain and analgesia. Curr. Opin. Pharmacol. 2010, 10, 80–86. [Google Scholar] [CrossRef]
- Green, J.M.; Sundman, M.H.; Chou, Y.H. Opioid-induced microglia reactivity modulates opioid reward, analgesia, and behavior. Neurosci. Biobehav. Rev. 2022, 135. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sandoval, E.A.; Horvath, R.; Landry, R.P.; DeLeo, J.A. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol. Pain 2009, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.Y.; Svensson, C.I.; Matsui, T.; Fitzsimmons, B.; Yaksh, T.L.; Webb, M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting P38 MAPK in Spinal Microglia. Eur. J. Neurosci. 2005, 22, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.M.; Park, K.S.; Oh, J.H.; Jung, S.Y.; Yang, K.H.; Song, Y.S.; Son, D.J.; Park, Y.H.; Yun, Y.P.; Lee, M.K.; et al. Activation of P38 mitogen-activated protein kinase and activator protein-1 during the promotion of neurite extension of PC-12 cells by 15-deoxy-12,14-prostaglandin J2. Mol. Pharmacol. 2003, 63, 607–616. [Google Scholar] [CrossRef]
- Carniglia, L.; Ramírez, D.; Durand, D.; Saba, J.; Turati, J.; Caruso, C.; Scimonelli, T.N.; Lasaga, M. Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases. Mediat. Inflamm. 2017, 2017, 5048616. [Google Scholar] [CrossRef]
- Ma, L.; Jia, J.; Liu, X.; Bai, F.; Wang, Q.; Xiong, L. Activation of murine microglial N9 Cells is attenuated through cannabinoid receptor CB2 signaling. Biochem. Biophys. Res. Commun. 2015, 458, 92–97. [Google Scholar] [CrossRef]
- Schmöle, A.C.; Lundt, R.; Ternes, S.; Albayram, Ö.; Ulas, T.; Schultze, J.L.; Bano, D.; Nicotera, P.; Alferink, J.; Zimmer, A. Cannabinoid receptor 2 deficiency results in reduced neuroinflammation in an Alzheimer’s Disease mouse model. Neurobiol. Aging 2015, 36, 710–719. [Google Scholar] [CrossRef]
- Braun, M.; Khan, Z.T.; Khan, M.B.; Kumar, M.; Ward, A.; Achyut, B.R.; Arbab, A.S.; Hess, D.C.; Hoda, M.N.; Baban, B.; et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav. Immun. 2018, 68, 224–237. [Google Scholar] [CrossRef]
- Simöes Da Gama, C.; Morin-Brureau, M. Study of BBB dysregulation in neuropathogenicity using integrative human model of blood-brain barrier. Front. Cell Neurosci. 2022, 16, 863836. [Google Scholar] [CrossRef]
- Smith, B.C.; Tinkey, R.A.; Shaw, B.C.; Williams, J.L. Targetability of the neurovascular unit in inflammatory diseases of the central nervous system. Immunol. Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Acevedo, J.F.; García-Pérez, V.; Cabezas-Pérez, R.; Losada-Barragán, M.; Vargas-Sánchez, K.; González-Reyes, R.E. Laminin as a biomarker of blood-brain barrier disruption under neuroinflammation: A systematic review. Int. J. Mol. Sci. 2022, 23, 6788. [Google Scholar] [CrossRef] [PubMed]
- Petty, M.A.; Lo, E.H. Junctional complexes of the blood-brain barrier: Permeability changes in neuroinflammation. Prog. Neurobiol. 2002, 68, 311–323. [Google Scholar] [CrossRef]
- Perry, V.H.; Anthony, D.C.; Bolton, S.J.; Brown, H.C. The blood-brain barrier and the inflammatory response. Mol. Med. Today 1997, 3, 335–341. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug and gene targeting to the brain with molecular trojan horses. Nat. Rev. Drug Discov. 2002, 1, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Romero, I.A. Transporting therapeutics across the blood-brain barrier. Mol. Med. Today 1996, 2, 106–113. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Dai, H.; Tan, S.; Mao, X.; Chen, Z. Dynorphin activation of kappa opioid receptor promotes microglial polarization toward M2 phenotype via TLR4/NF-ΚB pathway. Cell Biosci. 2020, 10, 42. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; Moore, A.; Raja, S.N.; Rice, A.S.C. Pharmacotherapy for neuropathic pain in adults: Systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet 2016, 14, 162–173. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Takagi, H.; Watanabe, C.; Yonezawa, A. Peptides involvement of multiple μ-opioid receptor subtypes on the presynaptic or postsynaptic inhibition of spinal pain transmission. Peptides 2014, 51, 15–25. [Google Scholar] [CrossRef]
- Wang, C.; Yang, D.; Yuan, B.; Wang, Y. Peptides C-terminal hydrazide modification changes the spinal antinociceptive profiles of endomorphins in mice. Peptides 2018, 99, 128–133. [Google Scholar] [CrossRef]
- Ochi, T.; Ohkubo, Y.; Mutoh, S. Blockade of the antinociceptive effect of spinally administered kyotorphin by naltrindole in mice. Neurosci. Lett. 2002, 322, 95–98. [Google Scholar] [CrossRef]
- Curto-reyes, V.; Boto, T.; Hidalgo, A.; Menéndez, L.; Baamonde, A. Antinociceptive effects induced through the stimulation of spinal cannabinoid type 2 receptors in chronically inflamed mice. Eur. J. Pharmacol. 2011, 668, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Emer, A.A.; Donatello, N.N.; Batisti, A.P.; Oliveira Belmonte, L.A.; Santos, A.R.S.; Martins, D.F. The role of the endocannabinoid system in the antihyperalgesic effect of cedrus atlantica essential oil inhalation in a mouse model of postoperative pain. J. Ethnopharmacol. 2018, 210, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Hylden, J.L.K.; Wilcox, G.L. Intrathecal morphine in mice: A new technique. Eur. J. Pharmacol. 1980, 67, 313–316. [Google Scholar] [CrossRef]
- Tal, M.; Bennett, G.J. Extra-territorial pain in rats with a peripheral mononeuropathy: Mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain 1994, 57, 375–382. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Li, B.; Lu, C.; Yang, S.; Long, J.; He, B.; Chen, H.; Huang, J. BBP predict: A web service for identifying blood-brain barrier penetrating peptides. Front. Genet. 2022, 13, 845747. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, F.S.R.; Giardini, A.C.; Sant’Anna, M.B.; Kimura, L.F.; Bufalo, M.C.; Vigerelli, H.; Zambelli, V.O.; Picolo, G. Crotalphine Modulates Microglia M1/M2 Phenotypes and Induces Spinal Analgesia Mediated by Opioid-Cannabinoid Systems. Int. J. Mol. Sci. 2022, 23, 11571. https://doi.org/10.3390/ijms231911571
Lopes FSR, Giardini AC, Sant’Anna MB, Kimura LF, Bufalo MC, Vigerelli H, Zambelli VO, Picolo G. Crotalphine Modulates Microglia M1/M2 Phenotypes and Induces Spinal Analgesia Mediated by Opioid-Cannabinoid Systems. International Journal of Molecular Sciences. 2022; 23(19):11571. https://doi.org/10.3390/ijms231911571
Chicago/Turabian StyleLopes, Flavia S. R., Aline C. Giardini, Morena B. Sant’Anna, Louise F. Kimura, Michelle C. Bufalo, Hugo Vigerelli, Vanessa O. Zambelli, and Gisele Picolo. 2022. "Crotalphine Modulates Microglia M1/M2 Phenotypes and Induces Spinal Analgesia Mediated by Opioid-Cannabinoid Systems" International Journal of Molecular Sciences 23, no. 19: 11571. https://doi.org/10.3390/ijms231911571
APA StyleLopes, F. S. R., Giardini, A. C., Sant’Anna, M. B., Kimura, L. F., Bufalo, M. C., Vigerelli, H., Zambelli, V. O., & Picolo, G. (2022). Crotalphine Modulates Microglia M1/M2 Phenotypes and Induces Spinal Analgesia Mediated by Opioid-Cannabinoid Systems. International Journal of Molecular Sciences, 23(19), 11571. https://doi.org/10.3390/ijms231911571





