The Isolation and Preparation of Samwinol from Dracocephalum heterophyllum and Prevention on Aβ25–35-Induced Neuroinflammation in PC-12 Cells
Abstract
:1. Introduction
2. Results
2.1. Sample Pretreatment with Medium-Pressure Liquid Chromatography
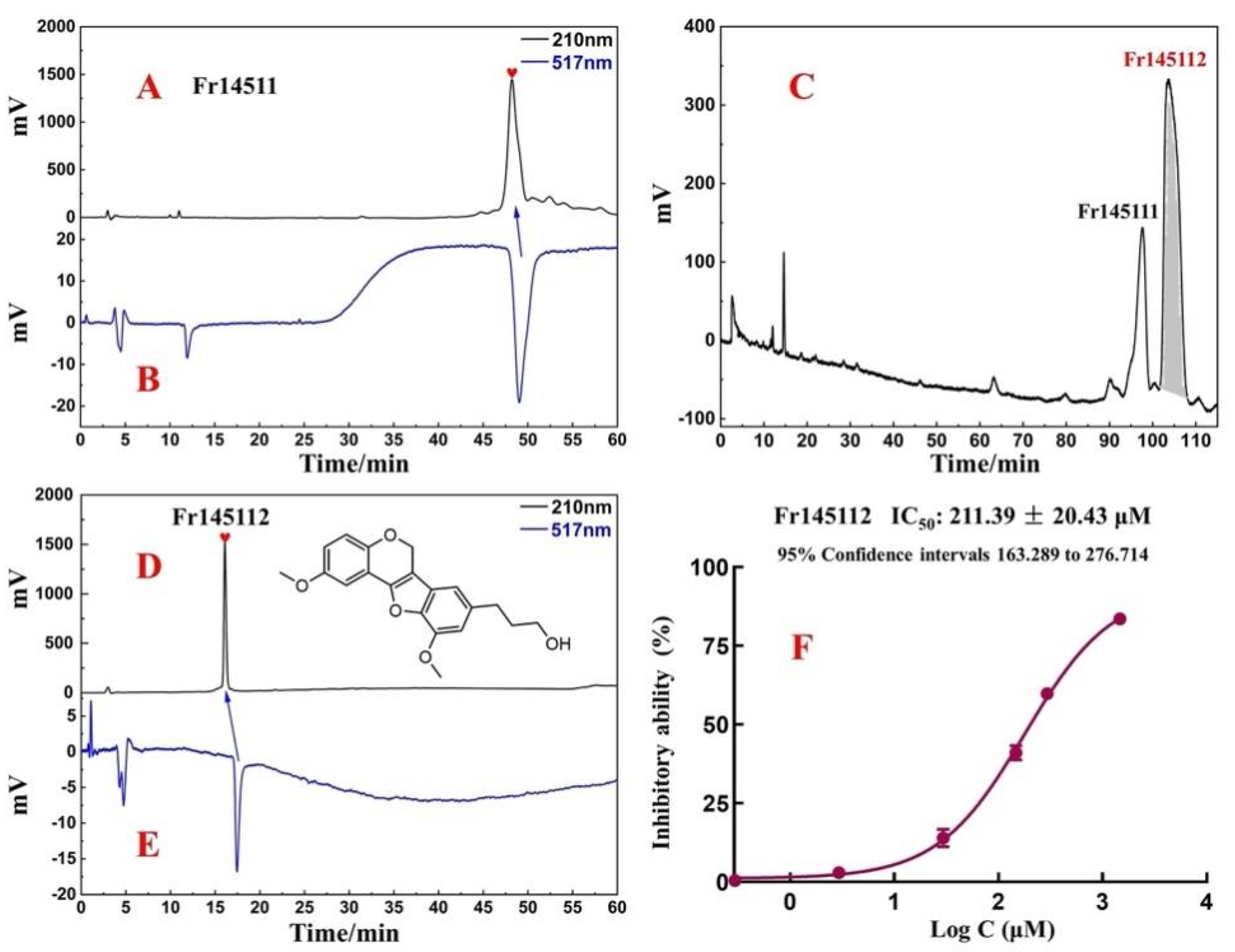
2.2. Target Preparation of Samwinol of Fr1451 with High-Pressure Liquid Chromatography
2.3. Purity, Activity, and Structure of Fr145112
2.4. Binding Ability of Samwinol to Antioxidant Proteins
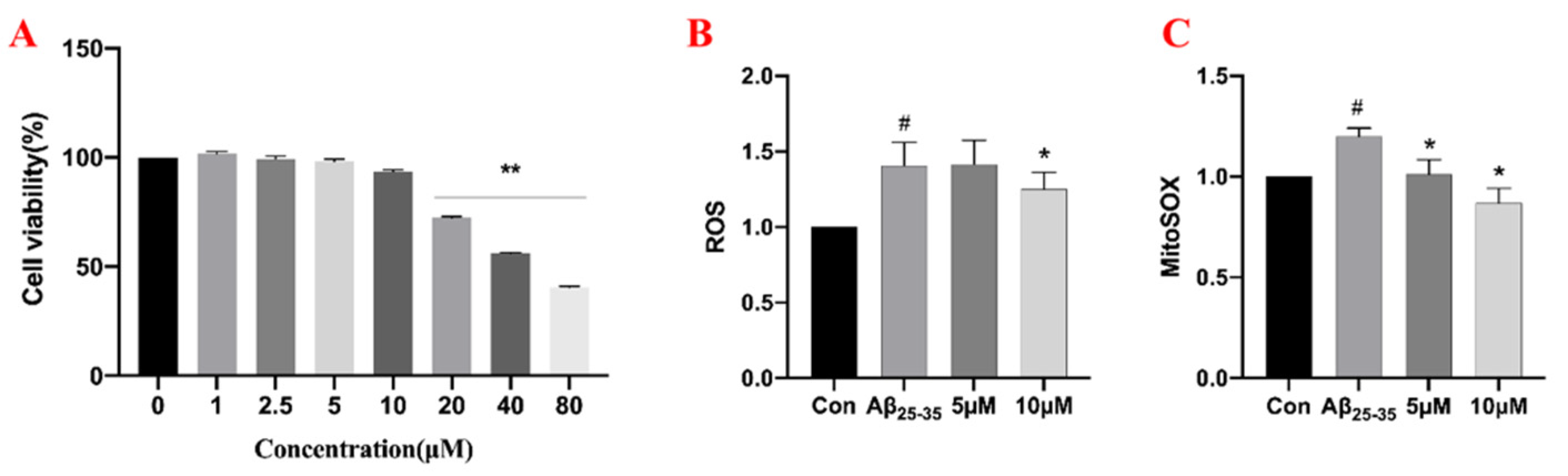
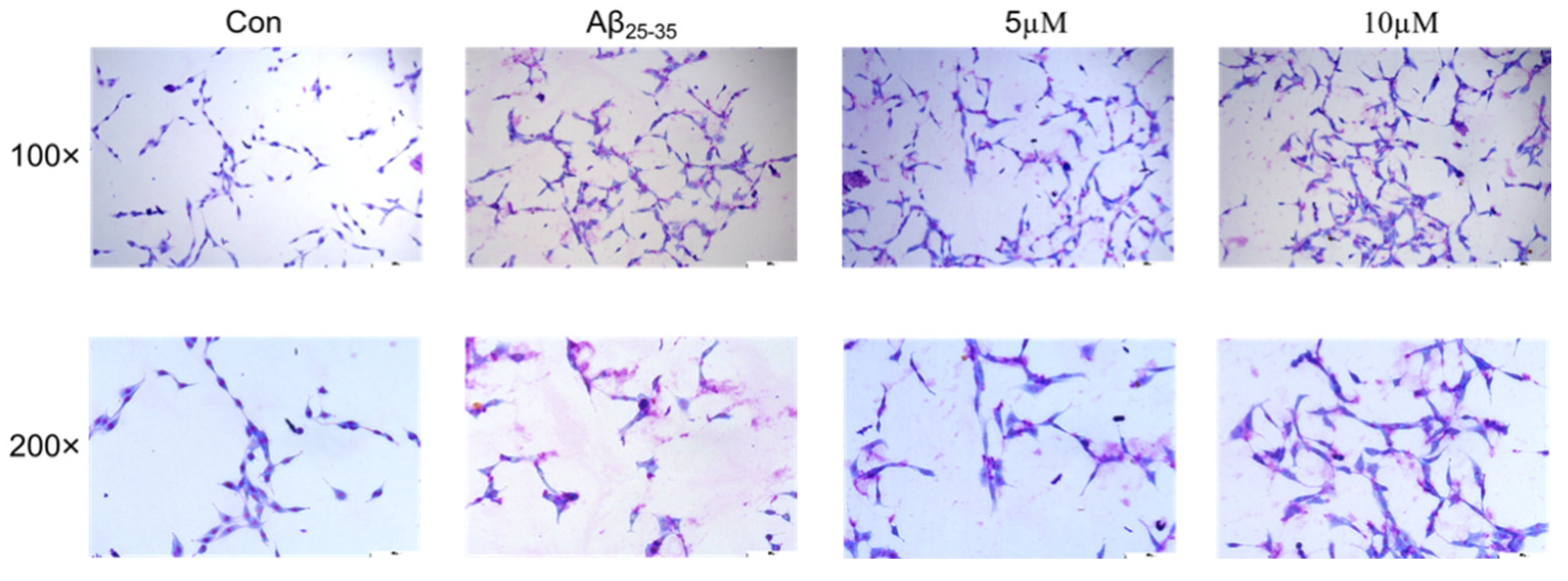
2.5. The Effects of Samwinol on PC12 Cells
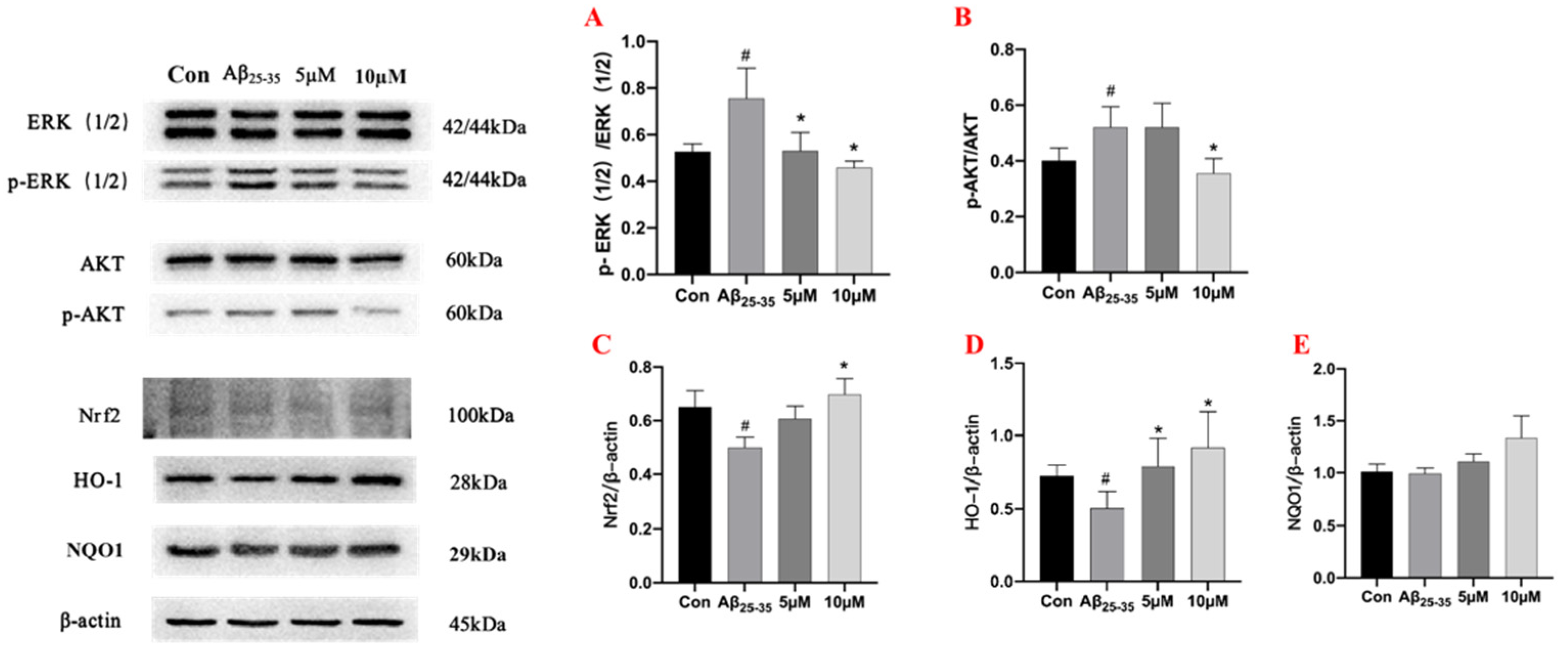
2.6. Western Blot Assay for Protein Expression in PC12 Cells
3. Discussion
4. Materials and Methods
4.1. Instrumentation and Reagents
4.2. Plant Sample Preparation and Medium Pressure Liquid Chromatography Pretreatment
4.3. High-Pressure Liquid Chromatography Separation and Purification of Samwinol from Fr1451
4.4. Assessment of Purity and Activity of Samwinol
4.5. Predicting the Binding Capacity of Samwinol to Antioxidant Proteins by Using Molecular Docking
4.6. Cellular Antioxidant Activity Assays
4.6.1. Culture of PC12 Cells and Aβ25–35 Induced AD Model
4.6.2. Effects of Samwinol on Cell Viability
4.6.3. Morphological Changes as Analyzed by GIEMSA Staining
4.6.4. Measurement of ROS and Mito-ROS Levels by Flow Cytometry
4.6.5. Detection of Protein Expression by Western Blot Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, W.; Wang, Q.; Lu, X.; Shi, Q.; Zou, J.; Tao, Y.; Wang, P. Protective Effects of Dracocephalum heterophyllum in ConA-Induced Acute Hepatitis. Mediat. Inflamm. 2016, 2016, 2684321. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.Q.; Dang, J.; Wen, H.X.; Yuan, X.; Tao, Y.D.; Wang, Q.L. Anti-hepatitis, antioxidant activities and bioactive compounds of Dracocephalum heterophyllum extracts. Bot. Stud. 2016, 57, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, H.; Yun, T.; Fu, Y.; Liu, C.; Gong, B.; Neng, B. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Tibetan herbal medicine Dracocephalum heterophyllum Benth. Nat. Prod. Res. 2008, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chander, R.; Maurya, A.K.; Kumar, K.; Kumari, S.; Kumar, R.; Agnihotri, V.K. In vitro antidiabetic and antimicrobial activity of Dracocephalum heterophyllum Benth. essential oil from different sites of North-western Himalayas India. Nat. Prod. Res. 2022, 36, 1–4. [Google Scholar] [CrossRef]
- Bian, J.; Wang, K.; Wang, Q.; Wang, P.; Wang, T.; Shi, W.; Ruan, Q. Dracocephalum heterophyllum (DH) Exhibits Potent Anti-Proliferative Effects on Autoreactive CD4(+) T Cells and Ameliorates the Development of Experimental Autoimmune Uveitis. Front. Immunol. 2020, 11, 575669. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, N.; Fang, Y.; Shaheen, N.; Wei, Y. An automatic on-line 2,2-diphenyl-1-picrylhydrazyl-high performance liquid chromatography method for high-throughput screening of antioxidants from natural products. J. Chromatogr. A 2017, 1521, 100–109. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, Z.; Wu, Q.; Fang, Y.; Wang, Q.; Li, G.; Dang, J. Preparation and Antioxidant Activities of Phenylethanoids from Dracocephalum heterophyllum. Separations 2022, 9, 111. [Google Scholar] [CrossRef]
- Dang, J.; Chen, C.; Ma, J.; Dawa, Y.; Wang, Q.; Tao, Y.; Wang, Q.; Ji, T. Preparative isolation of highly polar free radical inhibitor from Floccularia luteovirens using hydrophilic interaction chromatography directed by on-line HPLC-DPPH assay. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1142, 122043. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, Y.; Guo, Z.; Yu, D.; Jin, Y.; Liang, X. Purification of compounds from Lignum Dalbergia Odorifera using two-dimensional preparative chromatography with Click oligo (ethylene glycol) and C18 column. J. Sep. Sci. 2011, 34, 299–307. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [Green Version]
- Tarawneh, R.; Holtzman, D.M. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb. Perspect. Med. 2012, 2, a006148. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef]
- Katsumoto, A.; Takeuchi, H.; Takahashi, K.; Tanaka, F. Microglia in Alzheimer’s Disease: Risk Factors and Inflammation. Front. Neurol. 2018, 9, 978. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.I.; Ali, G.; Muhammad, T.; Ullah, R.; Umar, M.N.; Hashmi, A.N. Synthetic β-hydroxy ketone derivative inhibits cholinesterases, rescues oxidative stress and ameliorates cognitive deficits in 5XFAD mice model of AD. Mol. Biol. Rep. 2020, 47, 9553–9566. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. The amyloid beta peptide: A chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem. Rev. 2012, 112, 5147–5192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qian, C.; Zheng, Z.G.; Qian, F.; Wang, Y.; Thu, P.M.; Zhang, X.; Zhou, Y.; Tu, L.; Liu, Q.; et al. Jujuboside A promotes Aβ clearance and ameliorates cognitive deficiency in Alzheimer’s disease through activating Axl/HSP90/PPARγ pathway. Theranostics 2018, 8, 4262–4278. [Google Scholar] [CrossRef]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef]
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef]
- Mandrekar, S.; Jiang, Q.; Lee, C.Y.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Landreth, G.E. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J. Neurosci. 2009, 29, 4252–4262. [Google Scholar] [CrossRef] [Green Version]
- Griffin, W.S. Neuroinflammatory cytokine signaling and Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 770–771. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Li, L.J.; Dong, Q.X.; Zhu, J.; Huang, Y.R.; Hou, S.J.; Yu, X.L.; Liu, R.T. Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J. Neuroinflamm. 2021, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wei, Y.; Lautrup, S.; Yang, B.; Wang, Y.; Cordonnier, S.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc. Natl. Acad. Sci. USA 2021, 118, e2011226118. [Google Scholar] [CrossRef]
- Tang, L.; Xiang, Q.; Xiang, J.; Zhang, Y.; Li, J. Tripterygium glycoside ameliorates neuroinflammation in a mouse model of Aβ25-35-induced Alzheimer’s disease by inhibiting the phosphorylation of IκBα and p38. Bioengineered 2021, 12, 8540–8554. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.H.; Dai, Y.; Wong, M.S.; Yao, X.S. New lignans from the bioactive fraction of Sambucus williamsii Hance and proliferation activities on osteoblastic-like UMR106 cells. Fitoterapia 2014, 94, 29–35. [Google Scholar] [CrossRef]
- Bandoniene, D.; Murkovic, M. The detection of radical scavenging compounds in crude extract of borage (Borago officinalis L.) by using an on-line HPLC-DPPH method. J. Biochem. Biophys. Methods 2002, 53, 45–49. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Shi, S.Y.; Xiong, X.; Chen, X.Q.; Peng, M.J. Comparative evaluation of three methods based on high-performance liquid chromatography analysis combined with a 2,2’-diphenyl-1-picrylhydrazyl assay for the rapid screening of antioxidants from Pueraria lobata flowers. Anal. Bioanal. Chem. 2012, 402, 2965–2976. [Google Scholar] [CrossRef]
- Wang, W.; Jiao, L.; Tao, Y.; Shao, Y.; Wang, Q.; Yu, R.; Mei, L.; Dang, J. On-line HPLC-DPPH bioactivity-guided assay for isolated of antioxidative phenylpropanoids from Qinghai-Tibet Plateau medicinal plant Lancea tibetica. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1106–1107, 1–10. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 2014, 37, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.M.; Liu, F.; Chen, Y.M.; Liu, Y.J.; Wang, X.D.; Du, S.Y. CTGF-mediated ERK signaling pathway influences the inflammatory factors and intestinal flora in ulcerative colitis. Biomed. Pharmacother. 2019, 111, 1429–1437. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Hennig, P.; Garstkiewicz, M.; Grossi, S.; Di Filippo, M.; French, L.E.; Beer, H.D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018, 19, 562. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: Relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Yang, S.; Guo, X.; Lou, H.; Ren, D. Phenolic alkaloids from the aerial parts of Dracocephalum heterophyllum. Phytochemistry 2012, 82, 166–171. [Google Scholar] [CrossRef]
- Youn, K.; Jun, M. Geraniin Protects PC12 Cells Against Aβ(25-35)-Mediated Neuronal Damage: Involvement of NF-κB and MAPK Signaling Pathways. J. Med. Food 2020, 23, 928–937. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Ding, X.; Jian, T.; Wu, Y.; Zuo, Y.; Li, J.; Lv, H.; Ma, L.; Ren, B.; Zhao, L.; Li, W.; et al. Ellagic acid ameliorates oxidative stress and insulin resistance in high glucose-treated HepG2 cells via miR-223/keap1-Nrf2 pathway. Biomed. Pharmacother. 2019, 110, 85–94. [Google Scholar] [CrossRef]



| Proteins | Binding Energy (Kcal/mol) | Ligand Interactions |
|---|---|---|
| Nrf2 | −8.40 | VAL512 (Conventional Hydrogen Bond), ILE559 (Conventional Hydrogen Bond, Pi-Sigma), VAL608 (Conventional Hydrogen Bond), VAL606 (Carbon Hydrogen Bond, Alkyl), VAL463 (Carbon Hydrogen Bond), ALA510 (Carbon Hydrogen Bond), VAL418 (Carbon Hydrogen Bond), CYS513 (Alkyl, Pi-Alkyl) |
| HO-1 | −7.23 | ARG183 (Conventional Hydrogen Bond), LEU138(Pi-Sigma, Pi-Pi Stacked), GLY139 (Carbon Hydrogen Bond), HIS25 (Conventional Hydrogen Bond, Pi-Alkyl), PHE207 (Pi-Pi Stacked, Amide-Pi Stacked), ARG136 (Conventional Hydrogen Bond), ASN210 (Conventional Hydrogen Bond), TYR134 (Pi-Alkyl) |
| NQO1 | −5.33 | GLU185 (Conventional Hydrogen Bond), LYS270 (Conventional Hydrogen Bond), ILE269 (Carbon Hydrogen Bond), ARG210 (Alkyl, Pi-Alkyl), LYS209 (Alkyl, Pi-Alkyl) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Dang, J.; Lv, Y.; Fang, Y.; Ma, C.; Wang, Q.; Li, G. The Isolation and Preparation of Samwinol from Dracocephalum heterophyllum and Prevention on Aβ25–35-Induced Neuroinflammation in PC-12 Cells. Int. J. Mol. Sci. 2022, 23, 11572. https://doi.org/10.3390/ijms231911572
Li C, Dang J, Lv Y, Fang Y, Ma C, Wang Q, Li G. The Isolation and Preparation of Samwinol from Dracocephalum heterophyllum and Prevention on Aβ25–35-Induced Neuroinflammation in PC-12 Cells. International Journal of Molecular Sciences. 2022; 23(19):11572. https://doi.org/10.3390/ijms231911572
Chicago/Turabian StyleLi, Chengzhao, Jun Dang, Yue Lv, Yan Fang, Chengjun Ma, Qilan Wang, and Gang Li. 2022. "The Isolation and Preparation of Samwinol from Dracocephalum heterophyllum and Prevention on Aβ25–35-Induced Neuroinflammation in PC-12 Cells" International Journal of Molecular Sciences 23, no. 19: 11572. https://doi.org/10.3390/ijms231911572





