Methodological and Biological Factors Influencing Global DNA Methylation Results Measured by LINE-1 Pyrosequencing Assay in Colorectal Tissue and Liquid Biopsy Samples
Abstract
:1. Introduction
2. Results
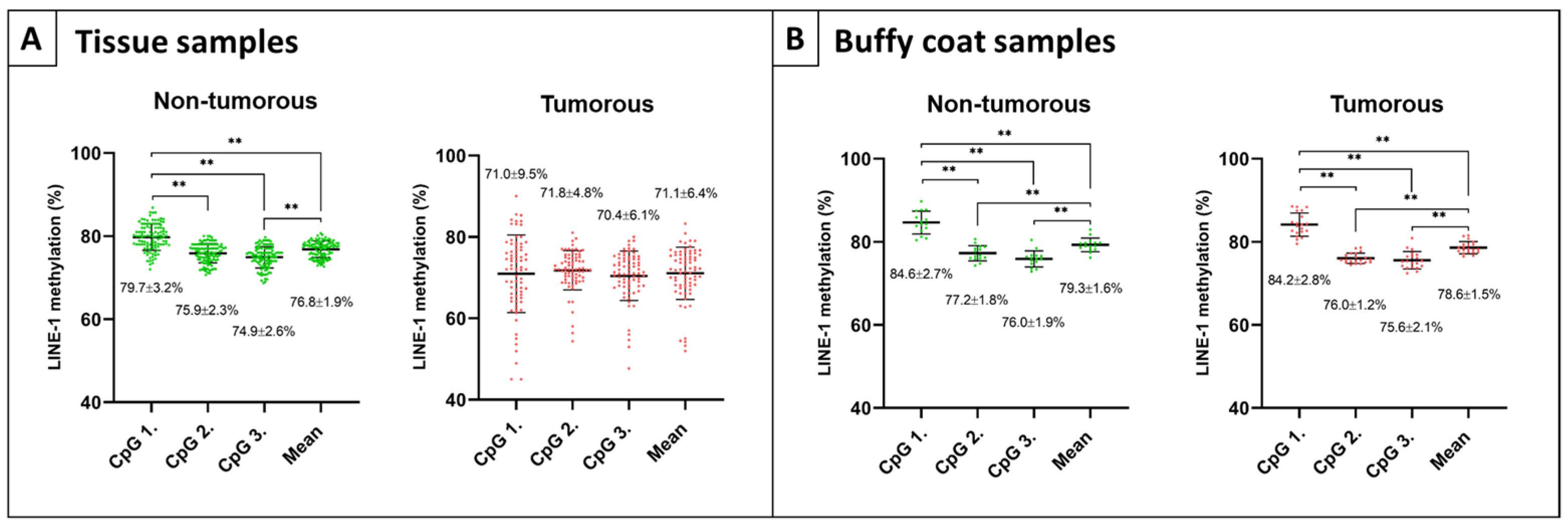
2.1. Methylation Values of Different LINE-1 CpG Positions
2.2. The Influence of Different Tissue Fixation and Sample Collection Methods
2.3. The Impact of Different Storage Conditions on LINE-1 Methylation
2.4. Methylation Values of Unmethylated and Methylated Standards
2.5. Tissue LINE-1 Methylation Alterations Related to Aging, Sex, and Physical Activity
2.6. LINE-1 Methylation Differences in MTHFR Mutations
3. Discussion
4. Materials and Methods
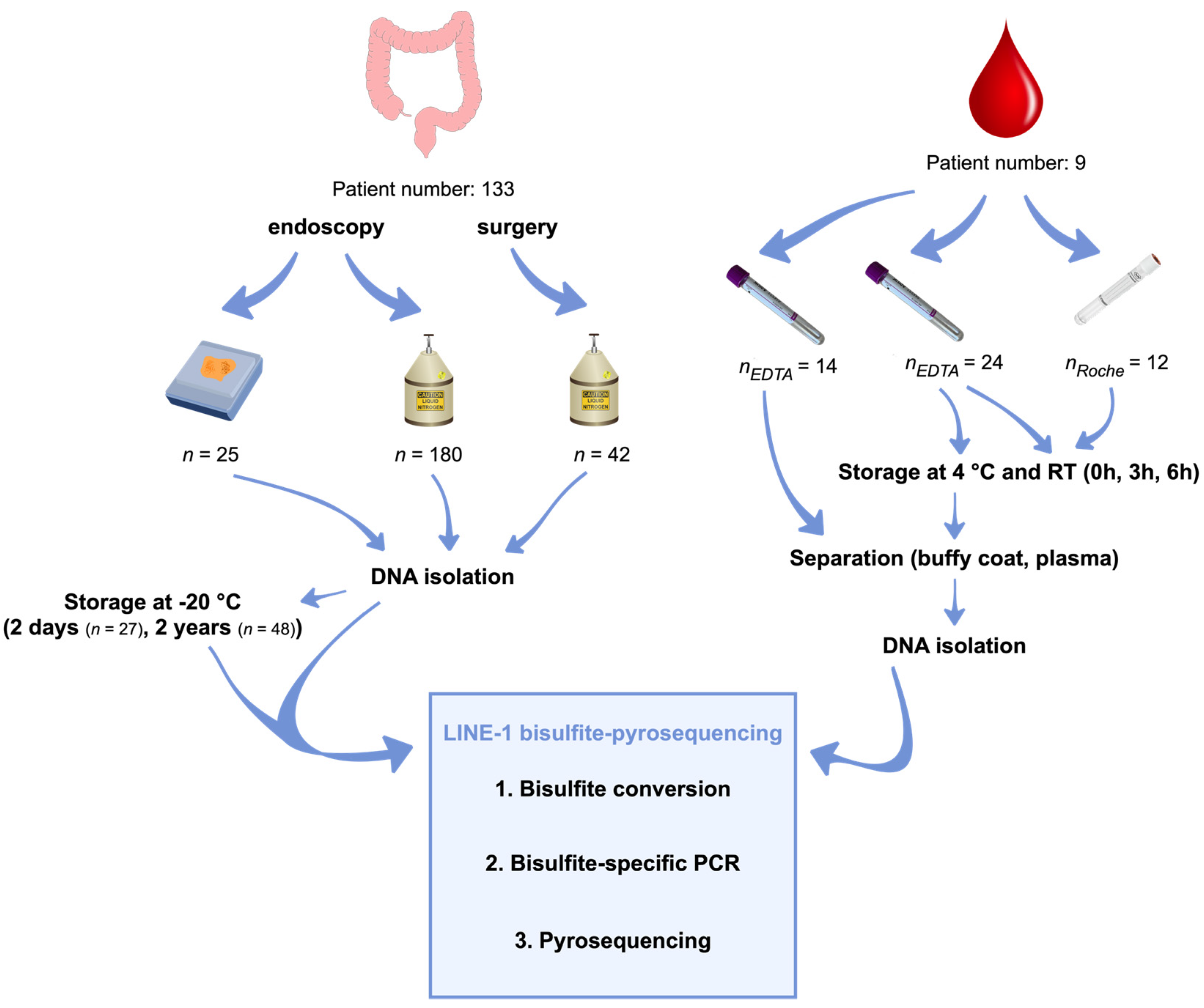
4.1. Sample Collection
4.2. Tissue Samples
4.3. Liquid Biopsy Samples
4.4. DNA Isolation from Fresh Frozen, Formalin-Fixed Paraffin-Embedded Tissue, Buffy Coat, and Plasma Samples
4.5. LINE-1 Bisulfite Pyrosequencing
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez, J.; Frigola, J.; Vendrell, E.; Risques, R.-A.; Fraga, M.F.; Morales, C.; Moreno, V.; Esteller, M.; Capellá, G.; Ribas, M.; et al. Chromosomal Instability Correlates with Genome-wide DNA Demethylation in Human Primary Colorectal Cancers. Cancer Res. 2006, 66, 8462–9468. [Google Scholar] [CrossRef] [Green Version]
- Suter, C.M.; Martin, D.I.; Ward, R.L. Hypomethylation of L1 retrotransposons in colorectal cancer and adjacent normal tissue. Int. J. Color. Dis. 2004, 19, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Yamashita, Y.-I.; Baba, Y.; Harada, K.; Yamao, T.; Umezaki, N.; Tsukamoto, M.; Kitano, Y.; Yamamura, K.; Arima, K.; et al. Prognostic value of LINE-1 methylation level in 321 patients with primary liver cancer including hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Oncotarget 2018, 9, 20795–20806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, D.; Jiang, D.; Li, Y.; Jin, M.; Chen, K. The role of LINE-1 methylation in predicting survival among colorectal cancer patients: A meta-analysis. Int. J. Clin. Oncol. 2017, 22, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Sigalotti, L.; Fratta, E.; Bidoli, E.; Covre, A.; Parisi, G.; Colizzi, F.; Coral, S.; Massarut, S.; Kirkwood, J.M.; Maio, M. Methylation levels of the “long interspersed nucleotide element-1” repetitive sequences predict survival of melanoma patients. J. Transl. Med. 2011, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Misawa, K.; Yamada, S.; Mima, M.; Nakagawa, T.; Kurokawa, T.; Imai, A.; Mochizuki, D.; Shinmura, D.; Yamada, T.; Kita, J.; et al. Long interspersed nuclear element 1 hypomethylation has novel prognostic value and potential utility in liquid biopsy for oral cavity cancer. Biomark. Res. 2020, 8, 53. [Google Scholar] [CrossRef]
- Pattamadilok, J.; Huapai, N.; Rattanatanyong, P.; Vasurattana, A.; Triratanachat, S.; Tresukosol, D.; Mutirangura, A. LINE-1 hypomethylation level as a potential prognostic factor for epithelial ovarian cancer. Int. J. Gynecol. Cancer. 2008, 18, 711–717. [Google Scholar] [CrossRef]
- Barták, B.K.; Fodor, T.; Kalmár, A.; Nagy, Z.B.; Zsigrai, S.; Szigeti, K.A.; Valcz, G.; Igaz, P.; Dank, M.; Takács, I.; et al. A Liquid Biopsy-Based Approach for Monitoring Treatment Response in Post-Operative Colorectal Cancer Patients. Int. J. Mol. Sci. 2022, 23, 3774. [Google Scholar] [CrossRef]
- Nagai, Y.; Sunami, E.; Yamamoto, Y.; Hata, K.; Okada, S.; Murono, K.; Yasuda, K.; Otani, K.; Nishikawa, T.; Tanaka, T.; et al. LINE-1 hypomethylation status of circulating cell-free DNA in plasma as a biomarker for colorectal cancer. Oncotarget 2017, 8, 11906–11916. [Google Scholar] [CrossRef] [Green Version]
- Szigeti, K.A.; Kalmár, A.; Galamb, O.; Valcz, G.; Barták, B.K.; Nagy, Z.B.; Zsigrai, S.; Felletár, I.; Patai, V.; Micsik, T.; et al. Global DNA hypomethylation of colorectal tumours detected in tissue and liquid biopsies may be related to decreased methyl-donor content. BMC Cancer 2022, 22, 605. [Google Scholar] [CrossRef]
- Wong, J.S.; Jadhav, T.; Young, E.; Wang, Y.; Xiao, M. Characterization of full-length LINE-1 insertions in 154 genomes. Genomics 2021, 113, 3804–3810. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P.; Tycko, B. The history of cancer epigenetics. Nat. Rev. Cancer 2004, 4, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M. DNA methylation in cancer: Too much, but also too little. Oncogene 2002, 21, 5400–5413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodić, N.; Burns, K.H. Long interspersed element-1 (LINE-1): Passenger or driver in human neoplasms? PLoS Genet. 2013, 9, e1003402. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tyler, J.K. Epigenetics and aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.F.; Cardarelli, R.; Carroll, J.; Fulda, K.G.; Kaur, M.; Gonzalez, K.; Vishwanatha, J.K.; Santella, R.M.; Morabia, A. Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics 2011, 6, 623–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, A.J.; Sandler, D.P.; Bolick, S.C.E.; Xu, Z.; Taylor, J.A.; DeRoo, L.A. Recreational and household physical activity at different time points and DNA global methylation. Eur. J. Cancer 2013, 49, 2199–2206. [Google Scholar] [CrossRef] [Green Version]
- Luttropp, K.; Nordfors, L.; Ekström, T.J.; Lind, L. Physical activity is associated with decreased global DNA methylation in Swedish older individuals. Scand. J. Clin. Lab. Investig. 2013, 73, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Vicenzi, M.; Tarantini, L.; Barretta, F.; Sironi, S.; Baccarelli, A.A.; Guazzi, M.; Bollati, V. Effects of Physical Exercise on Endothelial Function and DNA Methylation. Int. J. Environ. Res. Public Health 2019, 16, 2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.H.; den Heijer, M.; Kluijtmans, L.A.J.; van den Heuve, L.P.; et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef]
- van der Put, N.M.; Gabreëls, F.; Stevens, E.M.; Smeitink, J.A.; Trijbels, F.J.; Eskes, T.K.; Heuvel, L.P.V.D.; Blom, H.J. A second common mutation in the methylenetetrahydrofolate reductase gene: An additional risk factor for neural-tube defects? Am. J. Hum. Genet. 1998, 62, 1044–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friso, S.; Choi, S.-W. Gene-nutrient interactions and DNA methylation. J. Nutr. 2002, 132, 2382S–2387S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogino, S.; Wilson, R.B. Genotype and haplotype distributions of MTHFR 677C>T and 1298A>C single nucleotide polymorphisms: A meta-analysis. J. Hum. Genet. 2003, 48, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurdyukov, S.; Bullock, M. DNA Methylation Analysis: Choosing the Right Method. Biology 2016, 5, 3. [Google Scholar] [CrossRef]
- Phokaew, C.; Kowudtitham, S.; Subbalekha, K.; Shuangshoti, S.; Mutirangura, A. LINE-1 methylation patterns of different loci in normal and cancerous cells. Nucleic Acids Res. 2008, 36, 5704–5712. [Google Scholar] [CrossRef]
- Cordaux, R.; Batzer, M.A. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet. 2009, 10, 691–703. [Google Scholar] [CrossRef] [Green Version]
- Lisanti, S.; Omar, W.A.W.; Tomaszewski, B.; De Prins, S.; Jacobs, G.; Koppen, G.; Mathers, J.C.; Langie, S.A.S. Comparison of methods for quantification of global DNA methylation in human cells and tissues. PLoS ONE 2013, 8, e79044. [Google Scholar] [CrossRef] [Green Version]
- Irahara, N.; Nosho, K.; Baba, Y.; Shima, K.; Lindeman, N.I.; Hazra, A.; Schernhammer, E.S.; Hunter, D.J.; Fuchs, C.S.; Ogino, S. Precision of pyrosequencing assay to measure LINE-1 methylation in colon cancer, normal colonic mucosa, and peripheral blood cells. J. Mol. Diagn. 2010, 12, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, A.; North, B.; Barske, L.; Wang, X.; Bollati, V.; Weisenberger, D.; Yoo, C.; Tannir, N.; Horne, E.; Groshen, S.; et al. LINE-1 methylation in plasma DNA as a biomarker of activity of DNA methylation inhibitors in patients with solid tumors. Epigenetics 2009, 4, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Barone, G.; Mazzoleni, P.; Catalfo, A.; De Guidi, G.; Iemmolo, M.G.; Crimi, N.; Agodi, A. Mediterranean Diet and Particulate Matter Exposure Are Associated With LINE-1 Methylation: Results From a Cross-Sectional Study in Women. Front. Genet. 2018, 9, 514. [Google Scholar] [CrossRef] [Green Version]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Canto, C.; Marchese, A.E.; Vinciguerra, M. Low fruit consumption and folate deficiency are associated with LINE-1 hypomethylation in women of a cancer-free population. Genes Nutr. 2015, 10, 480. [Google Scholar] [CrossRef] [Green Version]
- Baba, Y.; Huttenhower, C.; Nosho, K.; Tanaka, N.; Shima, K.; Hazra, A.; Schernhammer, E.S.; Hunter, D.J.; Giovannucci, E.L.; Fuchs, C.S.; et al. Epigenomic diversity of colorectal cancer indicated by LINE-1 methylation in a database of 869 tumors. Mol. Cancer 2010, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Mima, K.; Nowak, J.A.; Qian, Z.R.; Cao, Y.; Song, M.; Masugi, Y.; Shi, Y.; da Silva, A.; Gu, M.; Li, W.; et al. Tumor LINE-1 methylation level and colorectal cancer location in relation to patient survival. Oncotarget 2016, 7, 55098–55109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajuddin, S.M.; Amaral, A.F.S.; Fernandez, A.F.; Rodríguez-Rodero, S.; Rodríguez, R.M.; Moore, L.E.; Tardon, A.; Carrato, A.; Garcia-Closas, M.; Silverman, D.T.; et al. Genetic and non-genetic predictors of LINE-1 methylation in leukocyte DNA. Environ. Health Perspect. 2013, 121, 650–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebrekiristos, M.; Melson, J.; Jiang, A.; Buckingham, L. DNA methylation and miRNA expression in colon adenomas compared with matched normal colon mucosa and carcinomas. Int. J. Exp. Pathol. 2022, 103, 74–82. [Google Scholar] [CrossRef]
- Akimoto, N.; Zhao, M.; Ugai, T.; Zhong, R.; Lau, M.; Fujiyoshi, K.; Kishikawa, J.; Haruki, K.; Arima, K.; Twombly, T.; et al. Tumor long interspersed nucleotide element-1 (LINE-1) hypomethylation in relation to age of colorectal cancer diagnosis and prognosis. Cancers 2021, 13, 2016. [Google Scholar] [CrossRef] [PubMed]
- QIAGEN. (EN)—PyroMark Q24 CpG LINE-1 Handbook. Available online: https://www.qiagen.com/au/resources/resourcedetail?id=d8398084-4746-4eb3-bca3-04ae6cb28429&lang=en (accessed on 15 September 2022).
- Chen, G.; Mosier, S.; Gocke, C.D.; Lin, M.-T.; Eshleman, J.R. Cytosine deamination is a major cause of baseline noise in next-generation sequencing. Mol. Diagn Ther. 2014, 18, 587–593. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, E.A.; Frey, B.L.; Smith, L.M.; Auble, D.T. Formaldehyde crosslinking: A tool for the study of chromatin complexes. J. Biol. Chem. 2015, 290, 26404–26411. [Google Scholar] [CrossRef] [Green Version]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Zeng, F.; Li, W.; He, Z.; Chen, W.; Zhu, W.; Zhang, B. The prognostic value of global DNA hypomethylation in cancer: A meta-analysis. PLoS ONE 2014, 9, e106290. [Google Scholar] [CrossRef] [PubMed]
- Nüsgen, N.; Goering, W.; Dauksa, A.; Biswas, A.; Jamil, M.A.; Dimitriou, I.; Sharma, A.; Singer, H.; Fimmers, R.; Fröhlich, H.; et al. Inter-locus as well as intra-locus heterogeneity in LINE-1 promoter methylation in common human cancers suggests selective demethylation pressure at specific CpGs. Clin. Epigenetics 2015, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Jamil, M.A.; Nuesgen, N.; Dauksa, A.; Gulbinas, A.; Schulz, W.; Oldenburg, J.; El-Maarri, O. Detailed methylation map of LINE-1 5’-promoter region reveals hypomethylated CpG hotspots associated with tumor tissue specificity. Mol. Genet. Genom. Med. 2019, 7, e601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, J.; Choi, B.; Han, T.-S.; Lee, H.-J.; Kong, S.-H.; Suh, Y.-S.; Kim, T.-H.; Choe, H.-N.; Kim, W.H.; Hur, K.; et al. Methylation Levels of LINE-1 As a Useful Marker for Venous Invasion in Both FFPE and Frozen Tumor Tissues of Gastric Cancer. Mol. Cells 2017, 40, 346–354. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Jeong, S.; Kim, Y.; Bae, J.M.; Cho, N.Y.; Kim, J.H.; Kang, G.H. Improved results of LINE-1 methylation analysis in formalin-fixed, paraffin-embedded tissues with the application of a heating step during the DNA extraction process. Clin. Epigenetics 2017, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Do, H.; Dobrovic, A. Sequence artifacts in DNA from formalin-fixed tissues: Causes and strategies for minimization. Clin. Chem. 2015, 61, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Chalitchagorn, K.; Shuangshoti, S.; Hourpai, N.; Kongruttanachok, N.; Tangkijvanich, P.; Thong-Ngam, D.; Voravud, N.; Sriuranpong, V.; Mutirangura, A. Distinctive pattern of LINE-1 methylation level in normal tissues and the association with carcinogenesis. Oncogene 2004, 23, 8841–8846. [Google Scholar] [CrossRef] [Green Version]
- Gosselt, H.R.; Griffioen, P.H.; van Zelst, B.D.; Oosterom, N.; de Jonge, R.; Heil, S.G. Global DNA (hydroxy)methylation is stable over time under several storage conditions and temperatures. Epigenetics 2021, 16, 45–53. [Google Scholar] [CrossRef]
- Barták, B.K.; Kalmár, A.; Galamb, O.; Wichmann, B.; Nagy, Z.B.; Tulassay, Z.; Dank, M.; Igaz, P.; Molnár, B. Blood Collection and Cell-Free DNA Isolation Methods Influence the Sensitivity of Liquid Biopsy Analysis for Colorectal Cancer Detection. Pathol. Oncol. Res. 2019, 25, 915–923. [Google Scholar] [CrossRef]
- Bulla, A.; De Witt, B.; Ammerlaan, W.; Betsou, F.; Lescuyer, P. Blood DNA Yield but Not Integrity or Methylation Is Impacted After Long-Term Storage. Biopreserv. Biobank. 2016, 14, 29–38. [Google Scholar] [CrossRef]
- Shiwa, Y.; Hachiya, T.; Furukawa, R.; Ohmomo, H.; Ono, K.; Kudo, H.; Hata, J.; Hozawa, A.; Iwasaki, M.; Matsuda, K.; et al. Adjustment of Cell-Type Composition Minimizes Systematic Bias in Blood DNA Methylation Profiles Derived by DNA Collection Protocols. PLoS ONE 2016, 11, e0147519. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-H.; Lin, P.-H.; Tsai, K.-W.; Wang, L.-J.; Huang, Y.-H.; Kuo, H.-C.; Li, S.-C. The effects of storage temperature and duration of blood samples on DNA and RNA qualities. PLoS ONE 2017, 12, e0184692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Paemel, R.; De Koker, A.; Caggiano, C.; Morlion, A.; Mestdagh, P.; De Wilde, B.; Vandesompele, J.; De Preter, K. Genome-wide study of the effect of blood collection tubes on the cell-free DNA methylome. Epigenetics 2021, 16, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, B.; Grieu, F.; Phillips, M.; Ruszkiewicz, A.; Moore, J.; Minamoto, T.; Kawakami, K. Methylation levels of LINE-1 repeats and CpG island loci are inversely related in normal colonic mucosa. Cancer Sci. 2007, 98, 1454–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaz, A.M.; Wong, C.-J.; Dzieciatkowski, S.; Luo, Y.; Schoen, R.E.; Grady, W.M. Patterns of DNA methylation in the normal colon vary by anatomical location, gender, and age. Epigenetics 2014, 9, 492–502. [Google Scholar] [CrossRef] [Green Version]
- El-Maarri, O.; Walier, M.; Behne, F.; Van Üüm, J.; Singer, H.; Diaz-Lacava, A.; Nüsgen, N.; Niemann, B.; Watzka, M.; Reinsberg, J.; et al. Methylation at global LINE-1 repeats in human blood are affected by gender but not by age or natural hormone cycles. PLoS ONE 2011, 6, e16252. [Google Scholar] [CrossRef]
- Sohn, K.-J.; Jang, H.; Campan, M.; Weisenberger, D.J.; Dickhout, J.; Wang, Y.-C.; Cho, R.C.; Yates, Z.; Lucock, M.; Chiang, E.; et al. The methylenetetrahydrofolate reductase C677T mutation induces cell-specific changes in genomic DNA methylation and uracil misincorporation: A possible molecular basis for the site-specific cancer risk modification. Int. J. Cancer 2009, 124, 1999–2005. [Google Scholar] [CrossRef] [Green Version]





| Tissue | Liquid Biopsy | |||||
|---|---|---|---|---|---|---|
| FF | FFPE | Buffy Coat | Plasma | |||
| Endoscopic Biopsy | Surgical Biopsy | Endoscopic Biopsy | ||||
| Healthy | Normal | 45 | - | 9 | 19 | 4 |
| Athletes | - | - | - | - | 5 | |
| Normal Adjacent Tissue to Colorectal Adenoma | 23 | - | - | - | - | |
| Normal Adjacent Tissue to Colorectal Carcinoma | 24 | 21 | - | - | - | |
| Colorectal Adenoma | 37 | - | 12 | 10 | - | |
| Colorectal Carcinoma | 36 | 21 | 4 | 10 | - | |
| Inflammatory Bowel Disease | 15 | - | - | - | - | |
| Total | 180 | 42 | 25 | 39 | 9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szigeti, K.A.; Barták, B.K.; Nagy, Z.B.; Zsigrai, S.; Papp, M.; Márkus, E.; Igaz, P.; Takács, I.; Molnár, B.; Kalmár, A. Methodological and Biological Factors Influencing Global DNA Methylation Results Measured by LINE-1 Pyrosequencing Assay in Colorectal Tissue and Liquid Biopsy Samples. Int. J. Mol. Sci. 2022, 23, 11608. https://doi.org/10.3390/ijms231911608
Szigeti KA, Barták BK, Nagy ZB, Zsigrai S, Papp M, Márkus E, Igaz P, Takács I, Molnár B, Kalmár A. Methodological and Biological Factors Influencing Global DNA Methylation Results Measured by LINE-1 Pyrosequencing Assay in Colorectal Tissue and Liquid Biopsy Samples. International Journal of Molecular Sciences. 2022; 23(19):11608. https://doi.org/10.3390/ijms231911608
Chicago/Turabian StyleSzigeti, Krisztina A, Barbara K Barták, Zsófia B Nagy, Sára Zsigrai, Márton Papp, Eszter Márkus, Peter Igaz, István Takács, Béla Molnár, and Alexandra Kalmár. 2022. "Methodological and Biological Factors Influencing Global DNA Methylation Results Measured by LINE-1 Pyrosequencing Assay in Colorectal Tissue and Liquid Biopsy Samples" International Journal of Molecular Sciences 23, no. 19: 11608. https://doi.org/10.3390/ijms231911608






