Physiological Cooperation between Aquaporin 5 and TRPV4
Abstract
:1. Introduction
1.1. Aquaporins
1.2. Transient Receptor Potential Vanilloid Channels
2. Cooperation of AQP5-TRPV4 Channels
2.1. Lung
2.2. Salivary Gland Cells
2.3. Uterus
2.4. Adipose Tissues
2.5. Lens
2.6. Skin
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z.; Zhu, C.; Bazer, F.W.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Aquaporins in the female reproductive system of mammals. Front. Biosci. 2015, 20, 838–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Li, C.; Wang, W. Molecular aspects of aquaporins. Vitam Horm. 2020, 113, 129–181. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of Water Channels in Xenopus Oocytes Expressing Red Cell CHIP28 Protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agre, P.; Preston, G.M.; Smith, B.L.; Jung, J.S.; Raina, S.; Moon, C.; Guggino, W.B.; Nielsen, S. Aquaporin CHIP: The archetypal molecular water channel. Am. J. Physiol. 1993, 265 Pt 2, F463–F476. [Google Scholar] [CrossRef] [Green Version]
- Kreida, S.; Törnroth-Horsefield, S. Structural insights into aquaporin selectivity and regulation. Curr. Opin. Struct. Biol. 2015, 33, 126–134. [Google Scholar] [CrossRef]
- Verkman, A.S. More than just water channels: Unexpected cellular roles of aquaporins. J. Cell Sci. 2005, 118 Pt 15, 3225–3232. [Google Scholar] [CrossRef] [Green Version]
- Moon, C.S.; Moon, D.; Kang, S.K. Aquaporins in Cancer Biology. Front. Oncol. 2022, 12, 782829. [Google Scholar] [CrossRef]
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta BBA—Gen. Subj. 2014, 1840, 1492–1506. [Google Scholar] [CrossRef] [Green Version]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Svokos, A.A.; Svokos, K.A. Aquaporins in health and disease. Adv. Clin. Chem. 2020, 98, 149–171. [Google Scholar] [CrossRef]
- Ishibashi, K.; Hara, S.; Kondo, S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 2009, 13, 107–117. [Google Scholar] [CrossRef]
- Song, Y.; Verkman, A.S. Aquaporin-5 Dependent Fluid Secretion in Airway Submucosal Glands. J. Biol. Chem. 2001, 276, 41288–41292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsubota, K.; Hirai, S.; King, L.S.; Agre, P.; Ishida, N. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjorgen’s syndrome. Lancet 2001, 357, 688–689. [Google Scholar] [CrossRef]
- Ducza, E.; Csányi, A.; Gáspár, R. Aquaporins during Pregnancy: Their Function and Significance. Int. J. Mol. Sci. 2017, 18, 2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducza, E.; Seres, A.B.; Hajagos-Tóth, J.; Falkay, G.; Gáspár, R. Oxytocin regulates the expression of aquaporin 5 in the late-pregnant rat uterus. Mol. Reprod. Dev. 2014, 81, 524–530. [Google Scholar] [CrossRef] [Green Version]
- González-Ramírez, R.; Chen, Y.; Liedtke, W.B.; Morales-Lázaro, S.L. TRP Channels and Pain. In Neurobiology of TRP Channels; Emir, T.L.R., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017; Chapter 8. [Google Scholar]
- Darby, W.; Grace, M.; Baratchi, S.; McIntyre, P. Modulation of TRPV4 by diverse mechanisms. Int. J. Biochem. Cell Biol. 2016, 78, 217–228. [Google Scholar] [CrossRef]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000, 2, 695–702. [Google Scholar] [CrossRef]
- Liedtke, W.; Choe, Y.; Marti-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Wissenbach, U.; Bödding, M.; Freichel, M.; Flockerzi, V. Trp12, a novel Trp related protein from kidney. FEBS Lett. 2000, 485, 127–134. [Google Scholar] [CrossRef]
- Garcia-Elias, A.; Mrkonjić, S.; Jung, C.; Pardo-Pastor, C.; Vicente, R.; Valverde, M.A. The TRPV4 channel. In Mammalian Transient Receptor Potential (TRP) Cation Channels. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 293–319. [Google Scholar]
- Michalick, L.; Kuebler, W.M. TRPV4—A Missing Link Between Mechanosensation and Immunity. Front. Immunol. 2020, 11, 413. [Google Scholar] [CrossRef] [Green Version]
- Arniges, M.; Vázquez, E.; Fernández-Fernández, J.M.; Valverde, M.A. Swelling-activated Ca2+ Entry via TRPV4 Channel Is Defective in Cystic Fibrosis Airway Epithelia. J. Biol. Chem. 2004, 279, 54062–54068. [Google Scholar] [CrossRef] [Green Version]
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in trpv4−/− mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar] [CrossRef] [Green Version]
- Voets, T.; Prenen, J.; Vriens, J.; Watanabe, H.; Janssens, A.; Wissenbach, U.; Bödding, M.; Droogmans, G.; Nilius, B. Molecular Determinants of Permeation through the Cation Channel TRPV4. J. Biol. Chem. 2002, 277, 33704–33710. [Google Scholar] [CrossRef] [Green Version]
- Everaerts, W.; Nilius, B.; Owsianik, G. The vanilloid transient receptor potential channel TRPV4: From structure to disease. Prog. Biophys. Mol. Biol. 2010, 103, 2–17. [Google Scholar] [CrossRef]
- Ducza, E.; Csányi, A.; Szőke, É.; Pohóczky, K.; Hajagos-Tóth, J.; Kothencz, A.; Tiszai, Z.; Gáspár, R. Significance of transient receptor potential vanilloid 4 and aquaporin 5 co-expression in the rat uterus at term. Heliyon 2019, 5, e02697. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Wu, J.; Chen, Y.; Zhao, J. Channels that Cooperate with TRPV4 in the Brain. J. Mol. Neurosci. 2020, 70, 1812–1820. [Google Scholar] [CrossRef]
- Pizzoni, A.; Bazzi, Z.; Di Giusto, G.; Alvarez, C.L.; Rivarola, V.; Capurro, C.; Schwarzbaum, P.J.; Ford, P. Release of ATP by TRPV4 activation is dependent upon the expression of AQP2 in renal cells. J. Cell. Physiol. 2021, 236, 2559–2571. [Google Scholar] [CrossRef]
- Wittekindt, O.H.; Dietl, P. Aquaporins in the lung. Pflug. Arch. 2019, 471, 519–532. [Google Scholar] [CrossRef]
- Cui, X.; Chen, W.; Zhou, H.; Gong, Y.; Zhu, B.; Lv, X.; Guo, H.; Duan, J.; Zhou, J.; Marcon, E.; et al. Pulmonary Edema in COVID-19 Patients: Mechanisms and Treatment Potential. Front. Pharmacol. 2021, 12, 664349. [Google Scholar] [CrossRef]
- Sidhaye, V.K.; Güler, A.D.; Schweitzer, K.S.; D’Alessio, F.; Caterina, M.J.; King, L.S. Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc. Natl. Acad. Sci. USA 2006, 103, 4747–4752. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.; Rajan, S.; Schremmer, C.; Chao, Y.-K.; Krasteva-Christ, G.; Kannler, M.; Yildirim, A.Ö.; Brosien, M.; Schredelseker, J.; Weissmann, N.; et al. TRPV4 channels are essential for alveolar epithelial barrier function as protection from lung edema. JCI Insight 2020, 5, e134464. [Google Scholar] [CrossRef]
- Rajan, S.; Schremmer, C.; Weber, J.; Alt, P.; Geiger, F.; Dietrich, A. Ca2+ Signaling by TRPV4 Channels in Respiratory Function and Disease. Cells 2021, 10, 822. [Google Scholar] [CrossRef]
- Watanabe, H.; Davis, J.B.; Smart, D.; Jerman, J.C.; Smith, G.D.; Hayes, P.; Vriens, J.; Cairns, W.; Wissenbach, U.; Prenen, J.; et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 2002, 277, 13569–13577. [Google Scholar] [CrossRef] [Green Version]
- Sidhaye, V.K.; Schweitzer, K.S.; Caterina, M.J.; Shimoda, L.; King, L.S. Shear stress regulates aquaporin-5 and airway epithelial barrier function. Proc. Natl. Acad. Sci. USA 2008, 105, 3345–3350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towne, J.E.; Krane, C.M.; Bachurski, C.J.; Menon, A.G. Tumor Necrosis Factor-α Inhibits Aquaporin 5 Expression in Mouse Lung Epithelial Cells. J. Biol. Chem. 2001, 276, 18657–18664. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, D.F.; King, J.A.; Weber, D.; Addison, E.; Liedtke, W.; Townsley, M.I. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: A novel mechanism of acute lung injury. Circ. Res. 2006, 99, 988–995. [Google Scholar] [CrossRef]
- Hui, D.S.C.; Zumla, A. Severe Acute Respiratory Syndrome: Historical, Epidemiologic, and Clinical Features. Infect. Dis. Clin. N. Am. 2019, 33, 869–889. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bandyopadhyay, B.C.; Nakamoto, T.; Singh, B.B.; Liedtke, W.; Melvin, J.E.; Ambudkar, I.S. A role for AQP5 in activation of TRPV4 by hypotonicity: Concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J. Biol. Chem. 2006, 283, 3688. [Google Scholar] [CrossRef]
- He, X.; Tse, C.-M.; Donowitz, M.; Alper, S.L.; Gabriel, S.E.; Baum, B.J. Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflug. Arch. 1997, 433, 260–268. [Google Scholar] [CrossRef]
- Hosoi, K. Physiological role of aquaporin 5 in salivary glands. Pflug. Arch. 2016, 468, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Csányi, A.; Bóta, J.; Falkay, G.; Gáspár, R.; Ducza, E. The Effects of Female Sexual Hormones on the Expression of Aquaporin 5 in the Late-Pregnant Rat Uterus. Int. J. Mol. Sci. 2016, 17, 1300. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Ram, M.; Kandasamy, K.; Thangamalai, R.; Choudhary, S.; Dash, J.R.; Kumar, D.; Parida, S.; Singh, T.U.; Mishra, S.K. Molecular and functional characterization of TRPV4 channels in pregnant and nonpregnant mouse uterus. Life Sci. 2015, 122, 51–58. [Google Scholar] [CrossRef]
- Ying, L.; Becard, M.; Lyell, D.; Han, X.; Shortliffe, L.; Husted, C.I.; Alvira, C.M.; Cornfield, D.N. The transient receptor potential vanilloid 4 channel modulates uterine tone during pregnancy. Sci. Transl. Med. 2015, 7, 319ra204. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wu, Y.; Zhu, Q.; Zhang, H.-Y.; Huang, Z.; Zhang, D.; Qi, H.; Liang, G.-L.; He, X.-Q.; Wang, X.-F.; et al. TRPV4 is involved in levonorgestrel-induced reduction in oviduct ciliary beating. J. Pathol. 2019, 248, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Boroditsky, R.S.; Reyes, F.I.; Winter, J.S.; Faiman, C. Maternal serum estrogen and progesterone concentrations preceding normal labor. Obstet. Gynecol. 1978, 51, 686–691. [Google Scholar] [PubMed]
- Seres-Bokor, A.; Kemény, K.K.; Taherigorji, H.; Schaffer, A.; Kothencz, A.; Gáspár, R.; Ducza, E. The Effect of Citral on Aquaporin 5 and Trpv4 Expressions and Uterine Contraction in Rat—An Alternative Mechanism. Life 2021, 11, 897. [Google Scholar] [CrossRef]
- Dobrin, R.; Zhu, J.; Molony, C.; Argman, C.; Parrish, M.L.; Carlson, S.; Allan, M.F.; Pomp, D.; Schadt, E.E. Multi-tissue coexpression networks reveal unexpected subnetworks associated with disease. Genome Biol. 2009, 10, R55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkman, A.S.; Yang, B.; Song, Y.; Manley, G.T.; Ma, T. Role of water channels in fluid transport studied by phenotype analysis of aquaporin knockout mice. Exp. Physiol. 2000, 85, 233s–241s. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Mósca, A.F.; Moura, T.F.; Soveral, G. Aquaporin-5 is expressed in adipocytes with implications in adipose differentiation. IUBMB Life 2015, 67, 54–60. [Google Scholar] [CrossRef]
- Ye, L.; Kleiner, S.; Wu, J.; Sah, R.; Gupta, R.K.; Banks, A.S.; Cohen, P.; Khandekar, M.J.; Boström, P.; Mepani, R.J.; et al. TRPV4 Is a Regulator of Adipose Oxidative Metabolism, Inflammation, and Energy Homeostasis. Cell 2012, 151, 96–110. [Google Scholar] [CrossRef] [Green Version]
- Petrova, R.S.; Webb, K.F.; Vaghefi, E.; Walker, K.; Schey, K.L.; Donaldson, P.J. Dynamic functional contribution of the water channel AQP5 to the water permeability of peripheral lens fiber cells. Am. J. Physiol. Physiol. 2018, 314, C191–C201. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Di, G.; Hu, S.; Liu, Y.; Dai, Y.; Chen, P. AQP5 regulates vimentin expression via miR-124-3p.1 to protect lens transparency. Exp. Eye Res. 2021, 205, 108485. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Petrova, R.S.; Sugiyama, Y.; Nagai, N.; Tamura, H.; Donaldson, P.J. Regulation of the Membrane Trafficking of the Mechanosensitive Ion Channels TRPV1 and TRPV4 by Zonular Tension, Osmotic Stress and Activators in the Mouse Lens. Int. J. Mol. Sci. 2021, 22, 12658. [Google Scholar] [CrossRef]
- Petrova, R.S.; Bavana, N.; Zhao, R.; Schey, K.L.; Donaldson, P.J. Changes to Zonular Tension Alters the Subcellular Distribution of AQP5 in Regions of Influx and Efflux of Water in the Rat Lens. Investig. Opthalmology Vis. Sci. 2020, 61, 36. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, P.M.; Mentino, D.; De Marco, A.; Del Vecchio, C.; Garra, S.; Cazzato, G.; Foti, C.; Crovella, S.; Calamita, G. Aquaporins Are One of the Critical Factors in the Disruption of the Skin Barrier in Inflammatory Skin Diseases. Int. J. Mol. Sci. 2022, 23, 4020. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, Y.; Liu, J.; Ren, J.; Wu, J.; Zhu, N. AQP5 regulates the proliferation and differentiation of epidermal stem cells in skin aging. Braz. J. Med Biol. Res. 2020, 53, e10009. [Google Scholar] [CrossRef]
- Sokabe, T.; Tominaga, M. The TRPV4 cation channel: A molecule linking skin temperature and barrier function. Commun. Integr. Biol. 2010, 3, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Diana CBlaydon David, P. Kelsell Defective channels lead to an impaired skin barrier. J. Cell Sci. 2014, 127, 4343–4350. [Google Scholar]
- Cao, X.; Yin, J.; Wang, H.; Zhao, J.; Zhang, J.; Dai, L.; Zhang, J.; Jiang, H.; Lin, Z.; Yang, Y. Mutation in AQP5, Encoding Aquaporin 5, Causes Palmoplantar Keratoderma Bothnia Type. J. Investig. Dermatol. 2014, 134, 284–287. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, J.; Li, Y.; Pan, C.; Zhou, W.; Cao, Q.; Yao, Z.; Han, J.; Li, M. AQP5 pathogenic variants induce palmoplantar keratoderma Bothnia type in two Chinese families. J. Dermatol. 2022, 49, 463–468. [Google Scholar] [CrossRef] [PubMed]
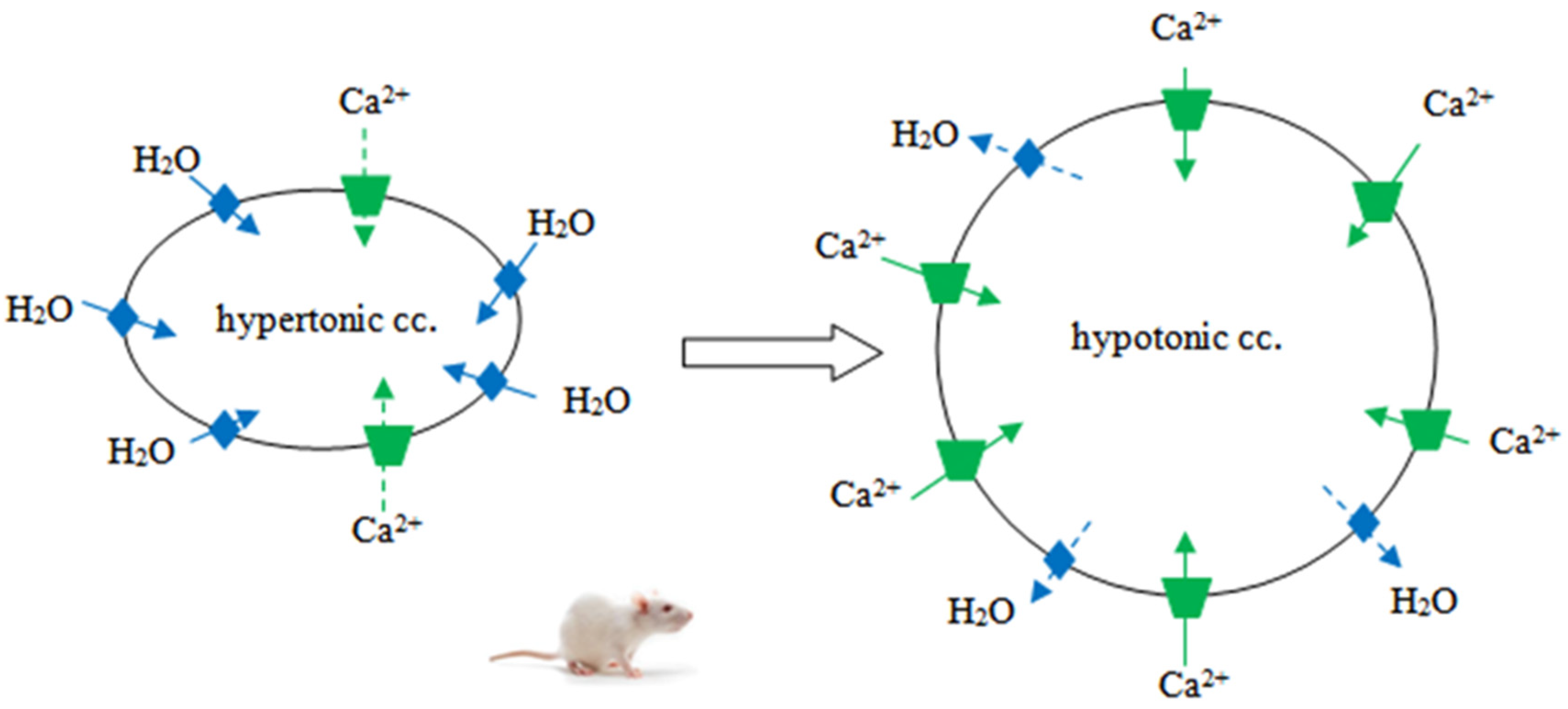
 , TRPV4:
, TRPV4:  .
.
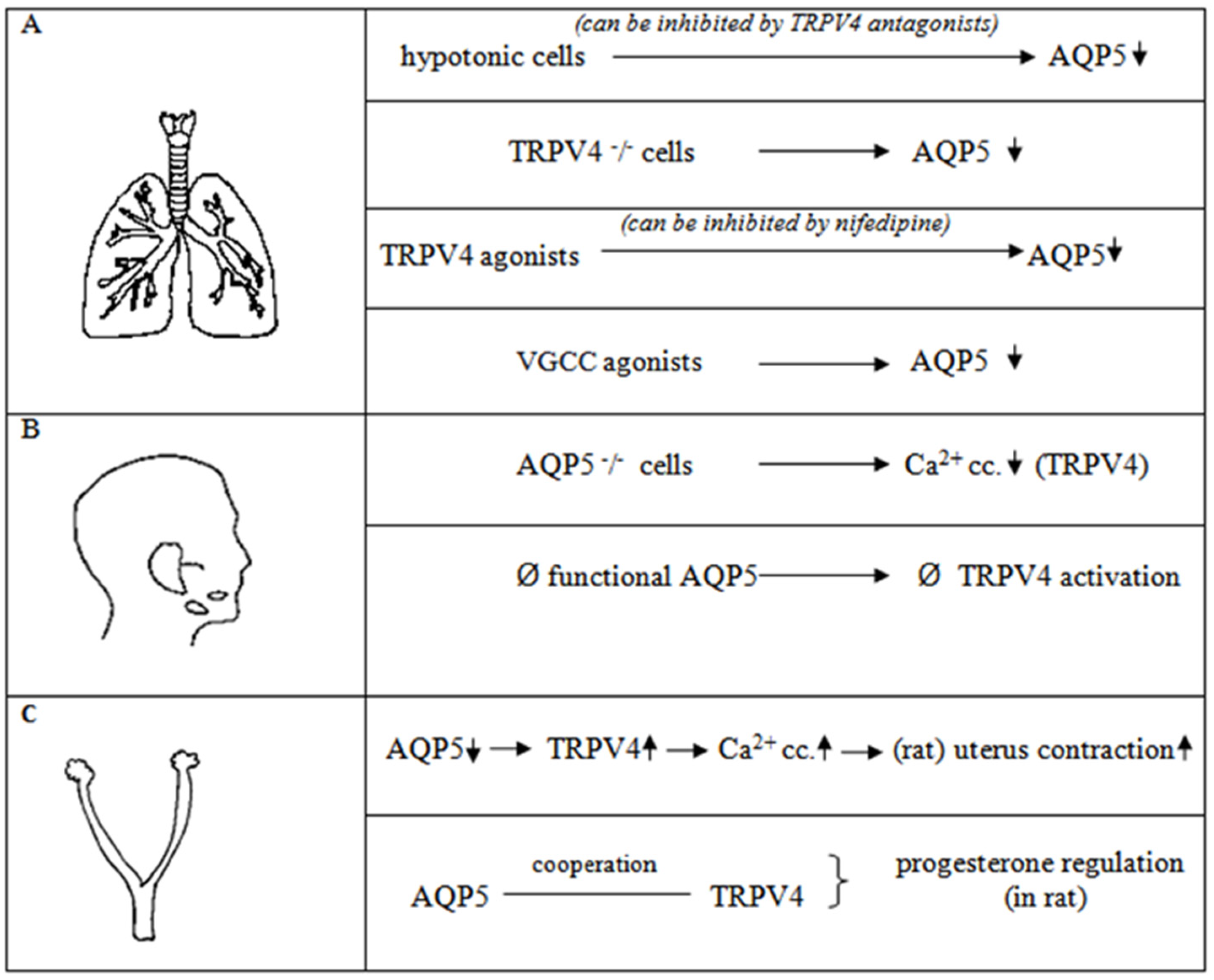
 , TRPV4:
, TRPV4:  .
.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemény, K.K.; Ducza, E. Physiological Cooperation between Aquaporin 5 and TRPV4. Int. J. Mol. Sci. 2022, 23, 11634. https://doi.org/10.3390/ijms231911634
Kemény KK, Ducza E. Physiological Cooperation between Aquaporin 5 and TRPV4. International Journal of Molecular Sciences. 2022; 23(19):11634. https://doi.org/10.3390/ijms231911634
Chicago/Turabian StyleKemény, Kata Kira, and Eszter Ducza. 2022. "Physiological Cooperation between Aquaporin 5 and TRPV4" International Journal of Molecular Sciences 23, no. 19: 11634. https://doi.org/10.3390/ijms231911634
APA StyleKemény, K. K., & Ducza, E. (2022). Physiological Cooperation between Aquaporin 5 and TRPV4. International Journal of Molecular Sciences, 23(19), 11634. https://doi.org/10.3390/ijms231911634






