An Insight into Molecular Targets of Breast Cancer Brain Metastasis
Abstract
:1. Introduction
2. Brain Metastases (BMs)
3. Breast Cancer Brain Metastases (BCBM)
4. Challenges of BM
5. The Blood-Brain Barrier (BBB) and Blood-Tumor Barrier (BTB): A Physiological Barrier in Brain Cancer Treatment
6. Treatment Modalities and Challenges
7. Complete Systemic Targeted Therapy
8. Local Therapy Modalities
9. Surgical Approaches
10. Radiation Targeted Therapy
11. Stereotactic Radiotherapy
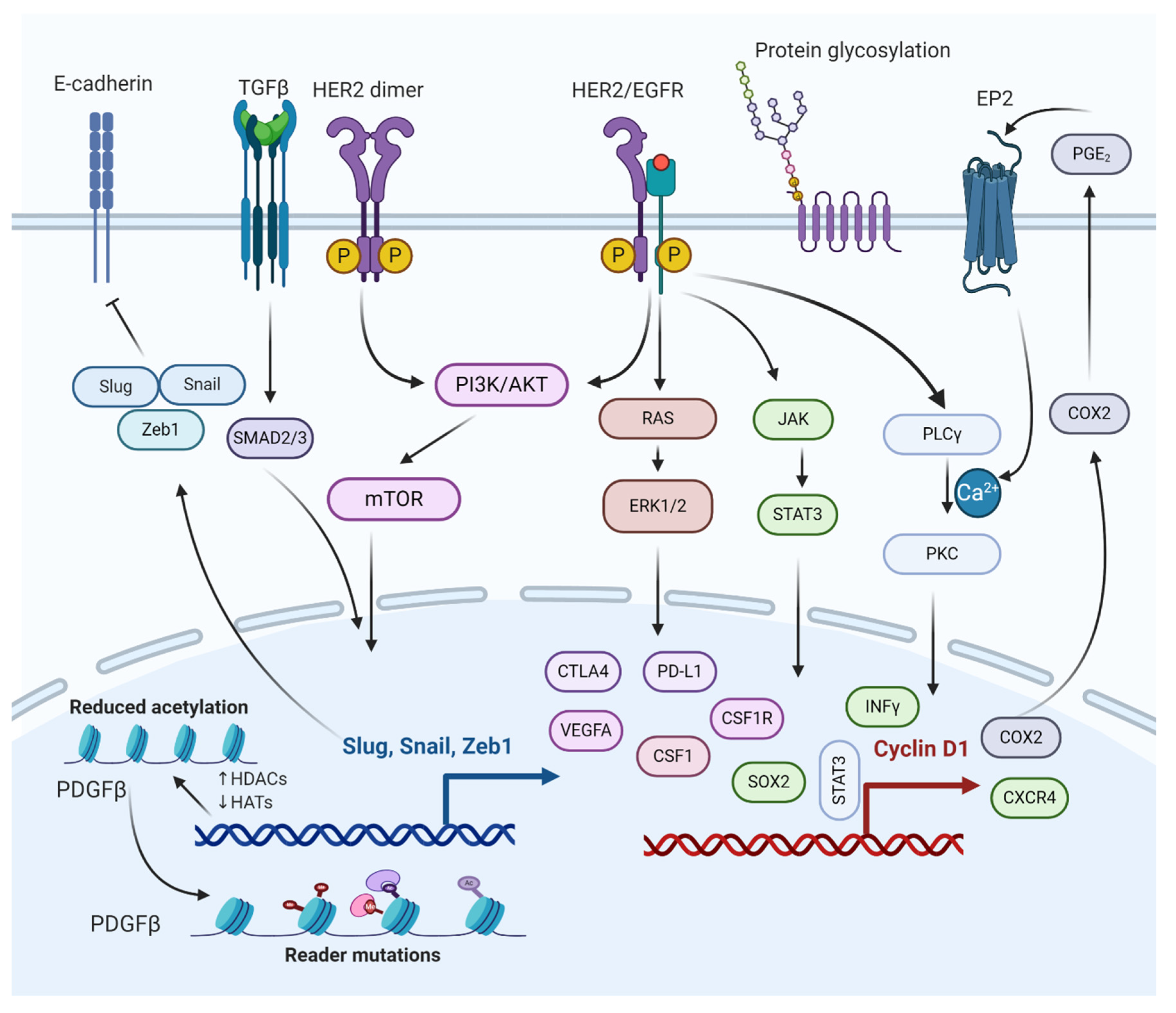
12. Molecular Pathways Involved in the Regulation of BCBM
12.1. TGFβ/SMAD Signaling Pathway
12.2. PI3K/mTOR Signaling Pathway
12.3. HER2/Epidermal Growth Factor Receptor (EGFR) Signaling Pathway
12.4. JAK/Signal Transducer and Activator of Transcription 3 (STAT3) Signaling Pathway
13. Role of Oncogenes Regulating Brain Metastasis through Breast Cancer Cells Growth Inhibition
13.1. Role of CXCR4 Gene
13.2. Role of SOX2 Gene
13.3. Role of BRCA1/2 Gene
14. Emerging Trends of Therapeutics to Treat BMs
15. Forecast for BCBM Cure
16. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| BC | Breast cancer |
| BCBM | Breast cancer brain metastasis |
| bHER2-ATC | Biparatopic anti-HER2 antibody-tubulysin conjugate |
| BMs | Brain metastases |
| BMsBC | Brain metastases breast cancer |
| BRCA1 | Breast cancer type 1 |
| cANGPTL4 | Angiopoietin-like 4 fibrinogen-like domain |
| CNS | Central nervous system |
| COX-2/MMP1 | Cyclooxygenase 2/Matrix metalloproteinase 1 |
| CXCR4 | CXC chemokine receptor 4 |
| DTI | Diffusion tensor imaging |
| ER | Estrogen |
| GPA | Graded prognostic assessment |
| HDACs | Histone deacetylases |
| HER2 | Human epidermal growth factor receptor 2 |
| IGF1R | Type 1 insulin-like growth factor receptor |
| LncRNA | Long noncoding RNA |
| MBC | Metastatic breast cancer |
| MEF2 | Myocyte enhancer factor 2 |
| miRNAs | MicroRNAs |
| MMP-2 | Matrix metalloproteinase-2 |
| mTOR | Mammalian target of rapamycin |
| NPNT | Nephronectin |
| PCI | Prophylactic cranial irradiation |
| PDGF | Platelet-derived growth factor |
| PDGFRβ | Platelet-derived growth factor receptor-beta |
| PD-1 | Programmed death receptor 1 |
| PDXs | Patient-derived xenografts |
| PI3K/AKT | Phosphatidylinositol-3-kinase/Protein kinase B |
| PR | Progesterone |
| RRM2 | Ribonucleotide reductase M2 |
| SRS | Stereotactic radiosurgery |
| T-DM1 | Trastuzumab emtansine |
| TGLI1 | Truncated glioma-associated oncogene homolog 1 |
| TNBC | Triple-negative breast cancer |
| VEGF | Vascular endothelial growth factor |
| WBRT | Whole-brain radiation targeting |
References
- Alitheen, N.B.; Yeap, S.K.; Faujan, N.H.; Ho, W.Y.; Beh, B.K.; Mashitoh, A.R. Leukemia and therapy. Am. J. Immunol. 2011, 7, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Kaleem, M.; Perwaiz, M.; Nur, S.M.; Abdulrahman, A.O.; Ahmad, W.; Al-Abbasi, F.A.; Kumar, V.; Kamal, M.A.; Anwar, F. Epigenetics of Triple-Negative Breast Cancer via Natural Compounds. Curr. Med. Chem. 2021, 28, 1436–1458. [Google Scholar] [CrossRef] [PubMed]
- Alhosin, M.; Sharif, T.; Mousli, M.; Etienne-Selloum, N.; Fuhrmann, G.; Schini-Kerth, V.B.; Bronner, C. Down-regulation of UHRF1, associated with re-expression of tumor suppressor genes, is a common feature of natural compounds exhibiting anti-cancer properties. J. Exp. Clin. Cancer Res. 2011, 30, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Sharma, J.D.; Sarma, A.; Ahmed, S.; Kataki, A.C.; Saxena, R.; Sharma, D. Triple negative breast cancer in people of North East India: Critical insights gained at a regional cancer centre. Asian Pac. J. Cancer Prev. 2014, 15, 4507–4511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbarzadeh Kaboli, P.; Salimian, F.; Aghapour, S.; Xiang, S.; Zhao, Q.; Li, M.; Wu, X.; Du, F.; Zhao, Y.; Shen, J.; et al. Akt-targeted therapy as a promising strategy to overcome drug resistance in breast cancer—A comprehensive review from chemotherapy to immunotherapy. Pharmacol. Res. 2020, 156, 104806. [Google Scholar] [CrossRef]
- Michniak-Kohn, B.B.; Deol, P.K.; Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Kaleem, M.; Dalhat, M.H. Thermosensitive Hydrogels Loaded with Resveratrol Nanoemulsion: Formulation Optimization by Central Composite Design and Evaluation in MCF-7 Human Breast Cancer Cell Lines. Gels 2022, 8, 450. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Ensenyat-Mendez, M.; Llinàs-Arias, P.; Orozco, J.I.J.; Íñiguez-Muñoz, S.; Salomon, M.P.; Sesé, B.; DiNome, M.L.; Marzese, D.M. Current Triple-Negative Breast Cancer Subtypes: Dissecting the Most Aggressive Form of Breast Cancer. Front. Oncol. 2021, 11, 681476. [Google Scholar] [CrossRef]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belykh, E.; Shaffer, K.V.; Lin, C.; Byvaltsev, V.A.; Preul, M.C.; Chen, L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 2020, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Krizbai, I.A.; Nyúl-Tóth, Á.; Bauer, H.H.; Farkas, A.E.; Traweger, A.; Haskó, J.; Wilhelm, I.; Bauer, H.C. Pharmaceutical Targeting of the Brain. Curr. Pharm. Des. 2016, 22, 5442–5462. [Google Scholar] [CrossRef] [PubMed]
- Quattrocchi, C.C.; Errante, Y.; Gaudino, C.; Mallio, C.A.; Giona, A.; Santini, D.; Tonini, G.; Zobel, B.B. Spatial brain distribution of intra-axial metastatic lesions in breast and lung cancer patients. J. Neurooncol. 2012, 110, 79–87. [Google Scholar] [CrossRef]
- Takano, K.; Kinoshita, M.; Takagaki, M.; Sakai, M.; Tateishi, S.; Achiha, T.; Hirayama, R.; Nishino, K.; Uchida, J.; Kumagai, T.; et al. Different spatial distributions of brain metastases from lung cancer by histological subtype and mutation status of epidermal growth factor receptor. Neur. Oncol. 2016, 18, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Izutsu, N.; Kinoshita, M.; Ozaki, T.; Sakai, M.; Nakanishi, K.; Nakayama, T.; Tamaki, Y.; Kishima, H. Cerebellar preference of luminal a and b type and basal ganglial preference of her2-positive type breast cancer-derived brain metastases. Mol. Clin. Oncol. 2021, 15, 175. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Prim. 2019, 5, 5. [Google Scholar] [CrossRef]
- Matzenauer, M.; Vrana, D.; Melichar, B. Treatment of brain metastases. Biomed. Pap. 2016, 160, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Bollig-Fischer, A.; Michelhaugh, S.; Ali-Fehmi, R.; Mittal, S. The molecular genomics of metastatic brain tumours. OA Mol. Oncol. 2013, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Hosonaga, M.; Saya, H.; Arima, Y. Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev. 2020, 39, 711–720. [Google Scholar] [CrossRef]
- Franchino, F.; Rudà, R.; Soffietti, R. Mechanisms and therapy for cancer metastasis to the brain. Front. Oncol. 2018, 8, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knier, N.N.; Hamilton, A.M.; Foster, P.J. Comparing the fate of brain metastatic breast cancer cells in different immune compromised mice with cellular magnetic resonance imaging. Clin. Exp. Metastasis 2020, 37, 465–475. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Breast Cancer Facts & Figures 2019–2020. Am. Cancer Soc. 2019, 1–44. [Google Scholar]
- Zhang, L.; Ridgway, L.D.; Wetzel, M.D.; Ngo, J.; Yin, W.; Kumar, D.; Goodman, J.C.; Groves, M.D.; Marchetti, D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 2013, 5, 180ra48. [Google Scholar] [CrossRef] [Green Version]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, W.; Plock, J.A. Breast Cancer Metastasis. Introd. Cancer Metastasis 2017, 2017, 13–31. [Google Scholar] [CrossRef]
- Chia, S.K.; Ellard, S.L.; Mates, M.; Welch, S.; Mihalcioiu, C.; Miller, W.H.; Gelmon, K.; Lohrisch, C.; Kumar, V.; Taylor, S.; et al. A phase-I study of lapatinib in combination with foretinib, a c-MET, AXL and vascular endothelial growth factor receptor inhibitor, in human epidermal growth factor receptor 2 (HER-2)-positive metastatic breast cancer. Breast Cancer Res. 2017, 19, 54. [Google Scholar] [CrossRef] [Green Version]
- Venur, V.A.; Leone, J.P. Targeted therapies for brain metastases from breast cancer. Int. J. Mol. Sci. 2016, 17, 1543. [Google Scholar] [CrossRef]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, M.; Swords, D.; Terjung Thill, M.; Baum, S.; Bischoff, J. Breast cancer and metastases of the central nervous system. Eur. J. Gynaecol. Oncol. 2017, 38, 653–656. [Google Scholar] [CrossRef]
- Watase, C.; Shiino, S.; Shimoi, T.; Noguchi, E.; Kaneda, T.; Yamamoto, Y.; Yonemori, K.; Takayama, S.; Suto, A. Breast cancer brain metastasis—Overview of disease state, treatment options and future perspectives. Cancers 2021, 13, 1078. [Google Scholar] [CrossRef] [PubMed]
- Bailleux, C.; Eberst, L.; Bachelot, T. Treatment strategies for breast cancer brain metastases. Br. J. Cancer 2021, 124, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: A view of metastasis. J. Intern. Med. 2013, 274, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Soffietti, R.; Abacioglu, U.; Baumert, B.; Combs, S.E.; Kinhult, S.; Kros, J.M.; Marosi, C.; Metellus, P.; Radbruch, A.; Freixa, S.S.V.; et al. Diagnosis and treatment of brain metastases from solid tumors: Guidelines from the European Association of neuro-oncology (EANO). Neur. Oncol. 2017, 19, 162–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurtsever, I.; Sari, L.; Gultekin, M.A.; Toprak, H.; Turk, H.M.; Aliyev, A.; Peker, A.A.; Yabaci, A.; Alkan, A. Diffusion Tensor Imaging of Brain Metastases in Patients with Breast Cancer According to Molecular Subtypes. Curr. Med. Imaging Former. Curr. Med. Imaging Rev. 2020, 17, 120–128. [Google Scholar] [CrossRef]
- Harris, J.R.; Lippman, M.E.; Veronesi, U.; Willett, W. Breast cancer. N. Engl. J. Med. 1992, 327, 319–328. [Google Scholar] [CrossRef]
- Riecke, K.; Müller, V.; Weide, R.; Schmidt, M.; Park-simon, T.W.; Möbus, V.; Mundhenke, C.; Polasik, A.; Lübbe, K.; Hesse, T.; et al. Predicting prognosis of breast cancer patients with brain metastases in the BMBC registry—Comparison of three different GPA prognostic scores. Cancers 2021, 13, 844. [Google Scholar] [CrossRef]
- Goss, G.; Tsai, C.M.; Shepherd, F.A.; Ahn, M.J.; Bazhenova, L.; Crinò, L.; de Marinis, F.; Felip, E.; Morabito, A.; Hodge, R.; et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: Pooled data from two phase II trials. Ann. Oncol. 2018, 29, 687–693. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood—brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef]
- O’Brown, N.M.; Pfau, S.J.; Gu, C. Bridging barriers: A comparative look at the blood—brain barrier across organisms. Genes Dev. 2018, 32, 466–478. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood—brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neur. Oncol. 2018, 20, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood--brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef] [Green Version]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Giaume, C.; Koulakoff, A.; Roux, L.; Holcman, D.; Rouach, N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010, 11, 87–99. [Google Scholar] [CrossRef]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte—Vascular coupling and the blood—Brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 4196. [Google Scholar] [CrossRef] [Green Version]
- Lannes, N.; Eppler, E.; Etemad, S.; Yotovski, P.; Filgueira, L. Microglia at center stage: A comprehensive review about the versatile and unique residential macrophages of the central nervous system. Oncotarget 2017, 8, 114393. [Google Scholar] [CrossRef] [Green Version]
- Shemer, A.; Erny, D.; Jung, S.; Prinz, M. Microglia plasticity during health and disease: An immunological perspective. Trends Immunol. 2015, 36, 614–624. [Google Scholar] [CrossRef]
- Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.E.; Kooij, G.; Frenkel, D.; Georgopoulos, S.; Monsonego, A.; Janigro, D. Inflammatory events at blood—Brain barrier in neuroinflammatory and neurodegenerative disorders: Implications for clinical disease. Epilepsia 2012, 53, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groothuis, D.R.; Molnar, P.; Blasberg, R.G. Regional blood flow and blood-to-tissue transport in five brain tumor models. Brain Tumor Biol. 1984, 27, 132–153. [Google Scholar]
- Gerstner, E.R.; Duda, D.G.; di Tomaso, E.; Ryg, P.A.; Loeffler, J.S.; Sorensen, A.G.; Ivy, P.; Batchelor, T.T. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat. Rev. Clin. Oncol. 2009, 6, 229–236. [Google Scholar] [CrossRef]
- Machein, M.R.; Kullmer, J.; Fiebich, B.L.; Plate, K.H.; Warnke, P.C. Vascular endothelial growth factor expression, vascular volume, and capillary permeability in human brain tumors. Neurosurgery 1999, 44, 732–741. [Google Scholar] [CrossRef]
- Blasberg, R.G.; Nakagawa, H.; Bourdon, M.A.; Groothuis, D.R.; Patlak, C.S.; Bigner, D.D. Regional localization of a glioma-associated antigen defined by monoclonal antibody 81C6 in vivo: Kinetics and implications for diagnosis and therapy. Cancer Res. 1987, 47, 4432–4443. [Google Scholar]
- Takamiya, Y.; Abe, Y.; Tanaka, Y.; Tsugu, A.; Kazuno, M.; Oshika, Y.; Maruo, K.; Ohnishi, Y.; Sato, O.; Yamazaki, H.; et al. Murine P-glycoprotein on stromal vessels mediates multidrug resistance in intracerebral human glioma xenografts. Br. J. Cancer 1997, 76, 445–450. [Google Scholar] [CrossRef]
- la Fougere, C.; Suchorska, B.; Bartenstein, P.; Kreth, F.-W.; Tonn, J.-C. Molecular imaging of gliomas with PET: Opportunities and limitations. Neur. Oncol. 2011, 13, 806–819. [Google Scholar] [CrossRef] [Green Version]
- Hardee, M.E.; Zagzag, D. Mechanisms of glioma-associated neovascularization. Am. J. Pathol. 2012, 181, 1126–1141. [Google Scholar] [CrossRef] [Green Version]
- Plate, K.H.; Scholz, A.; Dumont, D.J. Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol. 2012, 124, 763–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juillerat-Jeanneret, L. The targeted delivery of cancer drugs across the blood—Brain barrier: Chemical modifications of drugs or drug-nanoparticles? Drug Discov. Today 2008, 13, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Ningaraj, N.S.; Rao, M.; Hashizume, K.; Asotra, K.; Black, K.L. Regulation of blood-brain tumor barrier permeability by calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 2002, 301, 838–851. [Google Scholar] [CrossRef]
- Nussbaum, E.S.; Djalilian, H.R.; Cho, K.H.; Hall, W.A. Brain metastases: Histology, multiplicity, surgery, and survival. Cancer 1996, 78, 1781–1788. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Baik, C.S.; Gadi, V.K.; Bhatia, S.; Chow, L.Q.M. Systemic therapy of brain metastases: Non-small cell lung cancer, breast cancer, and melanoma. Neur. Oncol. 2017, 19, i1–i24. [Google Scholar] [CrossRef] [Green Version]
- Halasz, L.M.; Uno, H.; Hughes, M.; D’Amico, T.; Dexter, E.U.; Edge, S.B.; Hayman, J.A.; Niland, J.C.; Otterson, G.A.; Pisters, K.M.W.; et al. Comparative effectiveness of stereotactic radiosurgery versus whole-brain radiation therapy for patients with brain metastases from breast or non–small cell lung cancer. Cancer 2016, 122, 2091–2100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Chen Jian Yu, X.; Cai, G.; Yang, Z.; Cao, L.; Hu, C.; Guo, X.; Sun, J.; Chen, J. Survival benefit of anti-HER2 therapy after whole-brain radiotherapy in HER2-positive breast cancer patients with brain metastasis. Breast Cancer 2016, 23, 732–739. [Google Scholar] [CrossRef]
- Lee, J.S.; Toktas, O.; Soran, A. Role of Locoregional Treatment in De Novo Stage IV Breast Cancer. Clin. Med. Insights Oncol. 2020, 14, 1–5. [Google Scholar] [CrossRef]
- Yoshimura, M. Radiation therapy for primary tumor of de novo stage IV breast cancer. Transl. Cancer Res. 2020, 9, 5108. [Google Scholar] [CrossRef]
- Santori, F.; Vanni, G.; Buonomo, O.C.; De Majo, A.; Rho, M.; Granai, A.V.; Pellicciaro, M.; Cotesta, M.; Assogna, M.; D’Angelillo, R.M.; et al. Ulcerated breast cancer with single brain metastasis: A combined surgical approach. Clinical presentation at one year follow up—A case report. Int. J. Surg. Case Rep. 2020, 73, 75–78. [Google Scholar] [CrossRef]
- Pérez, M.; Schootman, M.; Hall, L.E.; Jeffe, D.B. Accelerated partial breast irradiation compared with whole breast radiation therapy: A breast cancer cohort study measuring change in radiation side-effects severity and quality of life. Breast Cancer Res. Treat. 2017, 162, 329–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Possanzini, M.; Greco, C. Stereotactic radiotherapy in metastatic breast cancer. Breast 2018, 41, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sanchez-Duffhues, G.; Goumans, M.J.; ten Dijke, P. TGF-β-Induced Endothelial to Mesenchymal Transition in Disease and Tissue Engineering. Front. Cell Dev. Biol. 2020, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Arteaga, C.L.; Wang, S.E. When tumor suppressor TGFβ meets the HER2 (ERBB2) oncogene. J. Mammary Gland. Biol. Neoplasia 2011, 16, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grusch, M.; Petz, M.; Metzner, T.; Ozturk, D.; Schneller, D.; Mikulits, W. The crosstalk of RAS with the TGF-β family during carcinoma progression and its implications for targeted cancer therapy. Curr. Cancer Drug Targets 2010, 10, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, B. The Roles of Epithelial-to-Mesenchymal Transition (EMT) and Mesenchymal-to-Epithelial Transition (MET) in Breast Cancer Bone Metastasis: Potential Targets for Prevention and Treatment. J. Clin. Med. 2013, 2, 264–282. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Gu, L.N.; Shan, B.E.; Geng, C.Z.; Sang, M.X. Biomarkers for EMT and MET in breast cancer: An update (review). Oncol. Lett. 2016, 12, 4869–4876. [Google Scholar] [CrossRef] [Green Version]
- Witzel, I.; Oliveira-Ferrer, L.; Pantel, K.; Müller, V.; Wikman, H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. 2016, 18, 8. [Google Scholar] [CrossRef] [Green Version]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Krizbai, I.A.; Gasparics, Á.; Nagyőszi, P.; Fazakas, C.; Molnár, J.; Wilhelm, I.; Bencs, R.; Rosivall, L.; Sebe, A. Endothelial-mesenchymal transition of brain endothelial cells: Possible role during metastatic extravasation. PLoS ONE 2015, 10, e0119655. [Google Scholar] [CrossRef] [Green Version]
- Drappatz, J.; Brenner, A.; Wong, E.T.; Eichler, A.; Schiff, D.; Groves, M.D.; Mikkelsen, T.; Rosenfeld, S.; Sarantopoulos, J.; Meyers, C.A.; et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin. Cancer Res. 2013, 19, 1567–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Tang, S.C. Targeting metastatic breast cancer with ANG1005, a novel peptide-paclitaxel conjugate that crosses the blood-brain-barrier (BBB). Genes Dis. 2017, 4, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.U.; Khan, M.S.; Husain, F.M.; Rehman, M.T.; Zughaibi, T.A.; Abuzenadah, A.M.; Urooj, M.; Kamal, M.A.; Tabrez, S. mTOR Targeted Cancer Chemoprevention by Flavonoids. Curr. Med. Chem. 2020, 8, 8068–8082. [Google Scholar] [CrossRef]
- ul Islam, B.; Suhail, M.; Khan, M.S.; Ahmad, A.; Zughaibi, T.A.; Husain, F.M.; Rehman, M.T.; Tabrez, S. Flavonoids and PI3K/Akt/mTOR Signaling Cascade: A Potential Crosstalk in Anticancer Treatment. Curr. Med. Chem. 2021, 28, 8083–8097. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Suhail, M.; Tarique, M.; Tabrez, S. Targeting pi3k/akt/mtor pathway by different flavonoids: A cancer chemopreventive approach. Int. J. Mol. Sci. 2021, 22, 12455. [Google Scholar] [CrossRef]
- Blazquez, R.; Wlochowitz, D.; Wolff, A.; Seitz, S.; Wachter, A.; Perera-Bel, J.; Bleckmann, A.; Beißbarth, T.; Salinas, G.; Riemenschneider, M.J.; et al. PI3K: A master regulator of brain metastasis-promoting macrophages/microglia. Glia 2018, 66, 2438–2455. [Google Scholar] [CrossRef]
- Hohensee, I.; Chuang, H.-N.N.; Grottke, A.; Werner, S.; Schulte, A.; Horn, S.; Lamszus, K.; Bartkowiak, K.; Witzel, I.; Westphal, M.; et al. PTEN mediates the cross talk between breast and glial cells in brain metastases leading to rapid disease progression. Oncotarget 2017, 8, 6155–6168. [Google Scholar] [CrossRef] [Green Version]
- Corti, C.; Antonarelli, G.; Criscitiello, C.; Lin, N.U.; Carey, L.A.; Cortés, J.; Poortmans, P.; Curigliano, G. Targeting brain metastases in breast cancer. Cancer Treat. Rev. 2022, 103, 102324. [Google Scholar] [CrossRef]
- Schmit, F.; Utermark, T.; Zhang, S.; Wang, Q.; Von, T.; Roberts, T.M.; Zhao, J.J. PI3K isoform dependence of PTEN-deficient tumors can be altered by the genetic context. Proc. Natl. Acad. Sci. USA 2014, 111, 6395–6400. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Ramkissoon, S.H.; Xie, S.; Goel, S.; Stover, D.G.; Guo, H.; Luu, V.; Marco, E.; Ramkissoon, L.A.; Kang, Y.J.; et al. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat. Med. 2016, 22, 723–726. [Google Scholar] [CrossRef]
- Elmenier, F.M.; Lasheen, D.S.; Abouzid, K.A.M. Phosphatidylinositol 3 kinase (PI3K) inhibitors as new weapon to combat cancer. Eur. J. Med. Chem. 2019, 183, 111718. [Google Scholar] [CrossRef] [PubMed]
- Kodack, D.P.; Askoxylakis, V.; Ferraro, G.B.; Sheng, Q.; Badeaux, M.; Goel, S.; Qi, X.; Shankaraiah, R.; Cao, Z.A.; Ramjiawan, R.R.; et al. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci. Transl. Med. 2017, 9, eaal4682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Lin, M.; Zhang, J.; Wang, B.; Tao, Z.; Du, Y.; Zhang, S.; Cao, J.; Wang, L.; Hu, X. Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis. Cancer Res Treat. 2020, 52, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.; Simpson, P.T.; Smart, C.E.; Cocciardi, S.; Waddell, N.; Lane, A.; Morrison, B.J.; Vargas, A.C.; Healey, S.; Beesley, J.; et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 2010, 12, R46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giri, D.K.; Ali-Seyed, M.; Li, L.-Y.; Lee, D.-F.; Ling, P.; Bartholomeusz, G.; Wang, S.-C.; Hung, M.-C. Endosomal Transport of ErbB-2: Mechanism for Nuclear Entry of the Cell Surface Receptor. Mol. Cell. Biol. 2005, 25, 11005–11018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-C.C.; Lien, H.-C.C.; Xia, W.; Chen, I.-F.F.; Lo, H.-W.W.; Wang, Z.; Ali-Seyed, M.; Lee, D.-F.F.; Bartholomeusz, G.; Ou-Yang, F.; et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer cell 2004, 6, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Bryan, S.; Witzel, I.; Borgmann, K.; Oliveira-Ferrer, L. Molecular mechanisms associated with brain metastases in her2-positive and triple negative breast cancers. Cancers 2021, 13, 4137. [Google Scholar] [CrossRef]
- Sirkisoon, S.R.; Carpenter, R.L.; Rimkus, T.; Miller, L.; Metheny-Barlow, L.; Lo, H.W. EGFR and HER2 signaling in breast cancer brain metastasis. Front. Biosci. (Elite Ed.) 2016, 8, 245–263. [Google Scholar] [CrossRef] [Green Version]
- Balch, S.M.; Vaz-Luis, I.; Li, T.; Tayob, N.; Jain, E.; Helvie, K.; Buendia-Buendia, J.E.; Shannon, E.; Isakoff, S.J.; Tung, N.M.; et al. A phase II study of efficacy, toxicity, and the potential impact of genomic alterations on response to eribulin mesylate in combination with trastuzumab and pertuzumab in women with human epidermal growth factor receptor 2 (HER2)+ metastatic breast cancer. Breast Cancer Res. Treat. 2021, 189, 411–423. [Google Scholar] [CrossRef]
- Batra, A.; Kong, S.; Cheung, W.Y. Eligibility of real-world patients with metastatic breast cancer for clinical trials. Breast 2020, 54, 171–178. [Google Scholar] [CrossRef]
- Lo, H.-W.W.; Hsu, S.-C.C.; Xia, W.; Cao, X.; Shih, J.-Y.Y.; Wei, Y.; Abbruzzese, J.L.; Hortobagyi, G.N.; Hung, M.-C.C. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007, 67, 9066–9076. [Google Scholar] [CrossRef] [Green Version]
- Heiland, D.H.; Ravi, V.M.; Behringer, S.P.; Frenking, J.H.; Wurm, J.; Joseph, K.; Garrelfs, N.W.C.; Strähle, J.; Heynckes, S.; Grauvogel, J.; et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat. Commun. 2019, 10, 2541. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation AIDS central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Priego, N.; Zhu, L.; Monteiro, C.; Mulders, M.; Wasilewski, D.; Bindeman, W.; Doglio, L.; Martinez-Saez, E.; y Cajal, S.R.; Megías, D. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med. 2018, 24, 1024–1035. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, J.-S.; Park, E.S.; Lee, J.-S.; Lin, Q.; Langley, R.R.; Maya, M.; He, J.; Kim, S.-W.; Weihua, Z.; et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia 2011, 13, 286–298. [Google Scholar] [CrossRef] [Green Version]
- Crinò, L.; Bronte, G.; Bidoli, P.; Cravero, P.; Minenza, E.; Cortesi, E.; Garassino, M.C.; Proto, C.; Cappuzzo, F.; Grossi, F.; et al. Nivolumab and brain metastases in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer 2019, 129, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Lorger, M.; Andreou, T.; Fife, C.; James, F. Immune Checkpoint Blockade—How Does It Work in Brain Metastases? Front. Mol. Neurosci. 2019, 12, 282. [Google Scholar] [CrossRef]
- Guo, X.; Deng, G.; Liu, J.; Zou, P.; Du, F.; Liu, F.; Chen, A.T.; Hu, R.; Li, M.; Zhang, S.; et al. Thrombin-responsive, brain-targeting nanoparticles for improved stroke therapy. ACS Nano 2018, 12, 8723–8732. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, S.; Chen, Z.; Bao, Y.; Chen, A.T.; Sheu, W.C.; Liu, F.; Jiang, Z.; Zhou, J. Targeted Delivery of Secretory Promelittin via Novel Poly(lactone-co-β-amino ester) Nanoparticles for Treatment of Breast Cancer Brain Metastases. Adv. Sci. 2020, 7, 1901866. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Zheng, S.; Xie Xinhua Li, X.; Zhang, L.; Yang, A.; Wang, J.; Tang, H.; Xie, X. SOX2 Promotes Brain Metastasis of Breast Cancer by Upregulating the Expression of FSCN1 and HBEGF. Mol. Ther. Oncolytics 2020, 17, 118–129. [Google Scholar] [CrossRef]
- Claus, E.B.; Schildkraut, J.M.; Thompson, W.D.; Risch, N.J. The genetic attributable risk of breast and ovarian cancer. Cancer 1996, 77, 2318–2324. [Google Scholar] [CrossRef]
- Song, Y.; Barry, W.T.; Seah, D.S.; Tung, N.M.; Garber, J.E.; Lin, N.U. Patterns of recurrence and metastasis in BRCA1/BRCA2-associated breast cancers. Cancer 2020, 126, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albiges, L.; André, F.; Balleyguier, C.; Gomez-Abuin, G.; Chompret, A.; Delaloge, S. Spectrum of breast cancer metastasis in BRCA1 mutation carriers: Highly increased incidence of brain metastases. Ann. Oncol. 2005, 16, 1846–1847. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Alexander, B.; Schnitt, S.J.; Comander, A.; Gallagher, B.; Garber, J.E.; Tung, N. Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and non-carriers. Cancer 2011, 117, 3093–3100. [Google Scholar] [CrossRef] [Green Version]
- Tsou, H.-R.; Overbeek-Klumpers, E.G.; Hallett, W.A.; Reich, M.F.; Floyd, M.B.; Johnson, B.D.; Michalak, R.S.; Nilakantan, R.; Discafani, C.; Golas, J.; et al. Optimization of 6, 7-disubstituted-4-(arylamino) quinoline-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor receptor-2 kinase activity. J. Med. Chem. 2005, 48, 1107–1131. [Google Scholar] [CrossRef]
- Jiang, Z.; Yan, M.; Hu, X.; Zhang, Q.; Ouyang, Q.; Feng, J.; Yin, Y.; Sun, T.; Tong, Z.; Wang, X.; et al. Pyrotinib combined with capecitabine in women with HER2+ metastatic breast cancer previously treated with trastuzumab and taxanes: A randomized phase III study. J. Clin. Oncol. 2019, 37, 1001. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Wan, H.; Zhang, G.; Feng, J.; Zhang Lei Chen, X.; Zhong, D.; Lou, L.; Tao, W.; Zhang, L. Discovery and development of pyrotinib: A novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Elsevier 2017, 110, 51–61. [Google Scholar] [CrossRef]
- Ma, F.; Li, Q.; Chen, S.; Zhu, W.; Fan, Y.; Wang, J.; Luo, Y.; Xing, P.; Lan, B.; Li, M.; et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible Pan-ERBB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2—positive metastatic breast cancer. J. Clin. Oncol. 2017, 35, 3105–3112. [Google Scholar] [CrossRef]
- Ma, F.; Ouyang, Q.; Li, W.; Jiang, Z.; Tong, Z.; Liu, Y.; Li, H.; Yu, S.; Feng, J.; Wang, S.; et al. Pyrotinib or lapatinib combined with capecitabine in HER2–positive metastatic breast cancer with prior taxanes, anthracyclines, and/ or trastuzumab: A randomized, phase II study. J. Clin. Oncol. 2019, 37, 2610–2619. [Google Scholar] [CrossRef]
- Lindholm, E.M.; Krohn, M.; Iadevaia, S.; Kristian, A.; Mills, G.B.; Mælandsmo, G.M.; Engebraaten, O. Proteomic characterization of breast cancer xenografts identifies early and late bevacizumab-induced responses and predicts effective drug combinations. Clin. Cancer Res. 2014, 20, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Karaman, S.; Leppänen, V.M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, dev151019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigtlaender, M.; Schneider-Merck, T.; Trepel, M. Lapatinib. Recent Results Cancer Res. 2018, 211, 19–44. [Google Scholar] [CrossRef]
- Fares, J.; Kanojia, D.; Rashidi, A.; Ulasov, I.; Lesniak, M.S. Landscape of combination therapy trials in breast cancer brain metastasis. Int J Cancer 2020, 147, 1939–1952. [Google Scholar] [CrossRef]
- Amiri-Kordestani, L.; Blumenthal, G.M.; Xu, Q.C.; Zhang, L.; Tang, S.W.; Ha, L.; Weinberg, W.C.; Chi, B.; Candau-Chacon, R.; Hughes, P.; et al. FDA approval: Ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin. Cancer Res. 2014, 20, 4436–4441. [Google Scholar] [CrossRef] [Green Version]
- Hecht, J.R.; Bang, Y.J.; Qin, S.K.; Chung, H.C.; Xu, J.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—A randomized phase III trial. J. Clin. Oncol. 2016, 34, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Swain, S.M.; Baselga, J.; Miles, D.; Im, Y.H.; Quah, C.; Lee, L.F.; Cortés, J. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: Results from the randomized phase III study CLEOPATRA. Ann. Oncol. 2014, 25, 1116–1121. [Google Scholar] [CrossRef]
- Bartsch, R.; Berghoff, A.S.; Vogl, U.; Rudas, M.; Bergen, E.; Dubsky, P.; Dieckmann, K.; Pinker, K.; Bago-Horvath, Z.; Galid, A.; et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin. Exp. Metastasis. 2015, 32, 729–737. [Google Scholar] [CrossRef]
- Jacot, W.; Pons, E.; Frenel, J.S.; Guiu, S.; Levy, C.; Heudel, P.E.; Bachelot, T.; D’Hondt, V.; Darlix, A.; Firmin, N.; et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res. Treat. 2016, 157, 307–318. [Google Scholar] [CrossRef]
- Jerusalem, G.; Park, Y.H.; Yamashita, T.; Hurvitz, S.A.; Chen, S.; Cathcart, J.; Lee, C.; Perrin, C. 138O CNS metastases in HER2-positive metastatic breast cancer treated with trastuzumab deruxtecan: DESTINY-Breast01 subgroup analyses. Ann. Oncol. 2020, 31, S63–S64. [Google Scholar] [CrossRef]
- Modi, S. Trastuzumab deruxtecan in previously treated HER2-positive metastatic breast cancer: Plain language summary of the DESTINY-Breast01 study. Futur. Oncol. 2021, 17, 3415–3423. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Di Leo, A.; Johnston, S.; Lee, K.S.; Ciruelos, E.; Lønning, P.E.; Janni, W.; O’Regan, R.; Mouret-Reynier, M.A.; Kalev, D.; Egle, D.; et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 87–100. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib keynote-012 study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Syriac, A.K.; Nandu, N.S.; Leone, J.P. Central Nervous System Metastases from Triple-Negative Breast Cancer: Current Treatments and Future Prospective. Breast Cancer (Dove Med. Press) 2022, 14, 1–13. [Google Scholar] [CrossRef]
- Hussain, M.H.A.; Powles, T.; Albers, P.; Castellano, D.; Daneshmand, S.; Gschwend, J.; Nishiyama, H.; Oudard, S.; Tayama, D.; Davarpanah, N.N.; et al. IMvigor010: Primary analysis from a phase III randomized study of adjuvant atezolizumab (atezo) versus observation (obs) in high-risk muscle-invasive urothelial carcinoma (MIUC). J. Clin. Oncol. 2020, 38, 5000. [Google Scholar] [CrossRef]
- Régina, A.; Demeule, M.; Ché, C.; Lavallée, I.; Poirier, J.; Gabathuler, R.; Béliveau, R.; Castaigne, J.P. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br. J. Pharmacol. 2008, 155, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Goetz, M.P.; Toi, M.; Campone, M.; Trédan, O.; Bourayou, N.; Sohn, J.; Park, I.H.; Paluch-Shimon, S.; Huober, J.; Chen, S.C.; et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Kourie, H.R.; Chaix, M.; Gombos, A.; Aftimos, P.; Awada, A. Pharmacodynamics, pharmacokinetics and clinical efficacy of neratinib in HER2-positive breast cancer and breast cancer with HER2 mutations. Expert Opin. Drug Metab. Toxicol. 2016, 12, 947–957. [Google Scholar] [CrossRef]
- Yardley, D.A.; Hart, L.L.; Ward, P.J.; Wright, G.L.; Shastry, M.; Finney, L.; DeBusk, L.M.; Hainsworth, J.D. Cabazitaxel Plus Lapatinib as Therapy for HER2 + Metastatic Breast Cancer with Intracranial Metastases: Results of a Dose-finding Study. Clin. Breast Cancer 2018, 18, E781–E787. [Google Scholar] [CrossRef]
- White, J.; Moughan, J.; Kim, I.; Peereboom, D.; De Los Santos, J.; Sperduto, P.; Mehta, M. Abstract OT1-04-02: NRG oncology/RTOG 1119: Phase II randomized study of whole brain radiotherapy with concurrent lapatinib in patients with brain met from HER2-positive breast cancer—A collaborative study of RTOG & KROG (NCT01622868). Cancer Res. 2017, 77, OT1-04-02. [Google Scholar] [CrossRef]
- Pistilli, B.; Pluard, T.; Urruticoechea, A.; Farci, D.; Kong, A.; Bachelot, T.; Chan, S.; Han, H.S.; Jerusalem, G.; Urban, P.; et al. Phase II study of buparlisib (BKM120) and trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer resistant to trastuzumab-based therapy. Breast Cancer Res. Treat. 2018, 168, 357–364. [Google Scholar] [CrossRef] [PubMed]
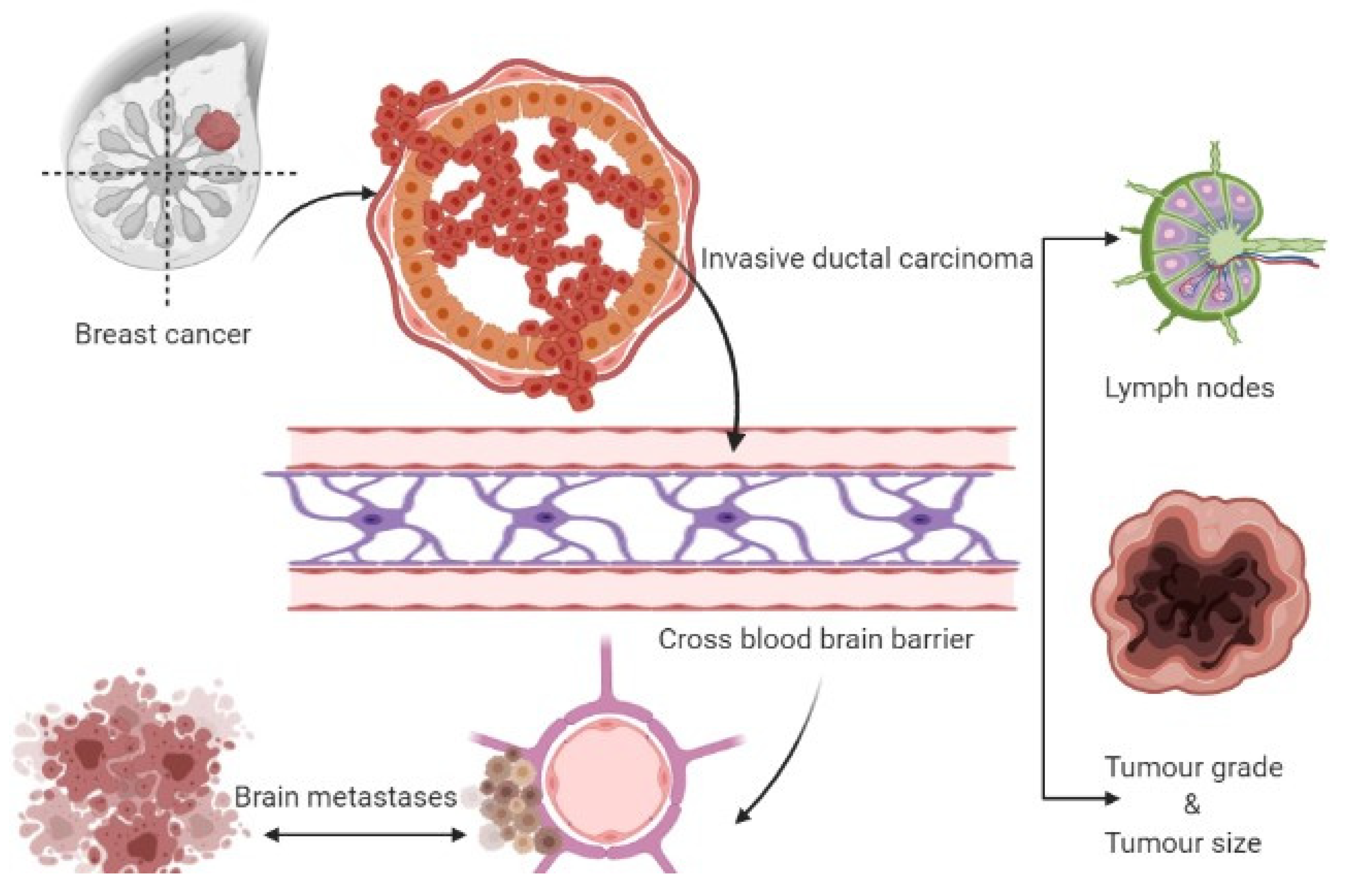
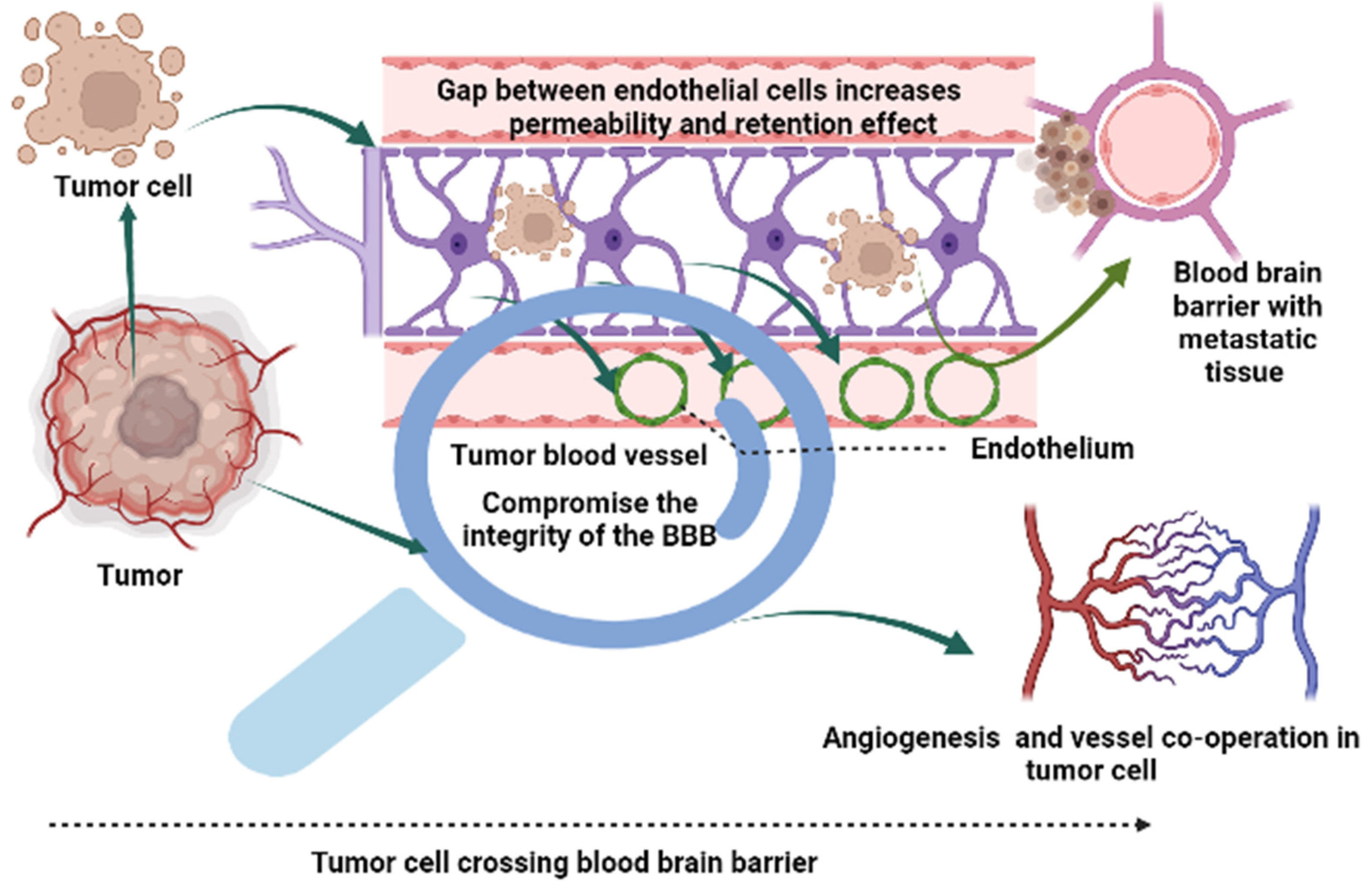

| Risk Factors | Identification | Brain Metastasis Risk | References |
|---|---|---|---|
| Lymph nodes | Histopathology | Positive lymphnodes-4, HR = 2.5, p = 0.029 | [23] |
| Tumor grade | Histopathology | The grade of the tumor is 3; Rate is 7.9% after 10-years completion of follow-up | [24] |
| Tumor size | Histopathology | The size of the tumor is 2 cm, after 10 years with a 7% rate. Size of tumor greater than 2 cm have a high risk of BM | [25] |
| Luminal A and B subtypes | Molecular biology | Over-expresses of epidermal growth factor of a human determined as HER-2 | [26] |
| AlphaB-crystallin (CRYAB) | Genetic biomarker | Occurrence of BM | [27] |
| Phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK/ERK) | Molecular biomarker | Overexpression of HER 3 receptor MAPK signaling pathway preferentially activated in the BM of cancer patients | [23] |
| VEGF and CXCR-4 | Molecular biomarker | Disrupt the BBB with migration in the parenchyma region | [28] |
| A2,6-sialyltransferase | Genetic driver | Cancer cell extravasations in the course of the BBB | [29] |
| Adjuvant trastuzumab trials | Targeted therapy for HER2-positive | BM risk was increased with a range of 1.32 to 1.9 | [30] |
| Target Drug | Treatment Type | Tumor Type | Mode of Action | References |
|---|---|---|---|---|
| EP versus IC | Chemotherapy | BCBM subgroup | Topoisomerase-I inhibitor-polymer conjugate | [136] |
| Ang-1005 | Chemotherapy | BCBM with leptomeningeal carcinomatosis | Peptide-paclitaxel conjugate | [137] |
| Abemaciclib | Targeted therapy | HER2-BC | CDK 4 and 6 inhibitor | [138] |
| Neratinib monotherapy | Targeted therapy | HER2+ progression within CNS | Pan-HER inhibitor | [139] |
| Cabazitaxel + lapatinib | Chemotherapy | HER2+ progression | HER2 positive | [140] |
| Lapatinib + WBRT | Chemotherapy | Effective therapeutic EGFR family target | HER2 | [141] |
| BKM120 + capecitabine | Triple-negative | Capability toward penetration to the BBB | PI3K | [142] |
| Pertuzumab | Systemic therapy | HER2+ BCBM | Monoclonal antibody | [93,126] |
| Everolimus and Buparlisib | BCBM | mTOR and PI3K inhibitors | [133,38] | |
| Trastuzumab emtansine | HER2+ BCBM | Monoclonal antibody | [127,128] | |
| Trastuzumab deruxtecan | Systemic therapy | BCBM HER2+ metastatic breast cancer | Topoisomerase I inhibitor | [129,130,131] |
| Trastuzumab, capecitabine, with tucatinib | Targeted therapy | HER2+ BCBM | Tyrosine kinase inhibitor | [99,132] |
| Pembrolizumab | High affinity and great selectivity | Advance TNBC | Monoclonal IgG4- antibody | [32,134] |
| Stereotactic radiosurgery (SRS) and atezolizumab | TNB with BCBM | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaleem, M.; Dalhat, M.H.; Azmi, L.; Asar, T.O.; Ahmad, W.; Alghanmi, M.; Almostadi, A.; Zughaibi, T.A.; Tabrez, S. An Insight into Molecular Targets of Breast Cancer Brain Metastasis. Int. J. Mol. Sci. 2022, 23, 11687. https://doi.org/10.3390/ijms231911687
Kaleem M, Dalhat MH, Azmi L, Asar TO, Ahmad W, Alghanmi M, Almostadi A, Zughaibi TA, Tabrez S. An Insight into Molecular Targets of Breast Cancer Brain Metastasis. International Journal of Molecular Sciences. 2022; 23(19):11687. https://doi.org/10.3390/ijms231911687
Chicago/Turabian StyleKaleem, Mohammed, Mahmood Hassan Dalhat, Lubna Azmi, Turky Omar Asar, Wasim Ahmad, Maimonah Alghanmi, Amal Almostadi, Torki A. Zughaibi, and Shams Tabrez. 2022. "An Insight into Molecular Targets of Breast Cancer Brain Metastasis" International Journal of Molecular Sciences 23, no. 19: 11687. https://doi.org/10.3390/ijms231911687







