Research Progress on the Therapeutic Effect of Polysaccharides on Non-Alcoholic Fatty Liver Disease through the Regulation of the Gut–Liver Axis
Abstract
:1. Introduction
2. The Pathogenesis of NAFLD Based on the Gut–Liver Axis
2.1. Intestinal Dysbiosis and NAFLD
2.2. Gut Barrier Permeability and NAFLD
3. Ameliorative Effects of Polysaccharides on NAFLD via the Gut–Liver Axis
3.1. Ameliorative Effects of Polysaccharides on NAFLD
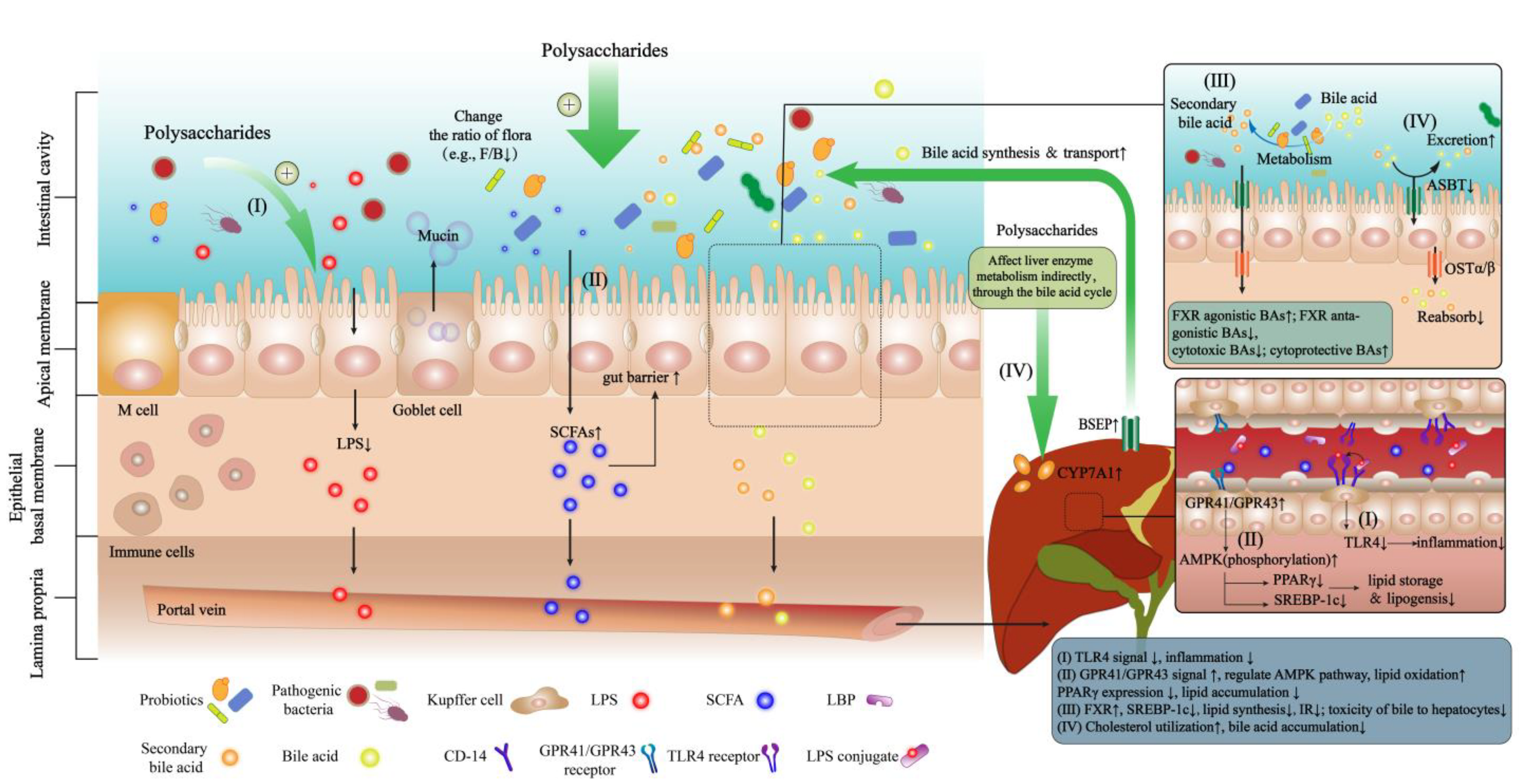
3.2. The Mechanism of Action of Polysaccharides in the Prevention and Treatment of NAFLD through the Gut–Liver Axis
3.2.1. Maintaining the Ecological Balance of GM
3.2.2. Regulating the Metabolites of GM
LPS
SCFAs
Bile acids (Primary and Secondary BAs)
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| ALP | Alkaline phosphatase |
| ALT | Alanine transaminase |
| AMPK | Adenosine monophosphate-activated protein kinase |
| Ara | arabinose |
| ASBT | Apical sodium-dependent bile acid transporter |
| ASC | Apoptosis-associated speck-like protein |
| AST | Serum aspartate transaminase |
| BWs | Body weights |
| CA | Cholic acid |
| CAT | Catalase |
| CCL5 | Chemokine ligand 5 |
| CD-68 | Cluster of differentiation 68 |
| CDCA | Chenodeoxycholic acid |
| COX-2 | Cyclooxygenase-2 |
| CYP7A1 | Cytochrome P450 enzyme7A1 |
| DCA | Deoxycholic acid |
| D-GalN | D-galactosamine |
| eWAT | epididymal white adipose tissue |
| FAS | Fatty acid synthase |
| FGFR4 | Fibroblast growth factors receptor 4 |
| Fru | Fructose |
| Fuc | Fucose |
| Gal | Galactose |
| GalA | Galacturonic acid |
| GalN | N-acetyl-galactose |
| GDCA | Glycodeoxycholic acid |
| GlcA | Gluconic acid |
| Glc | Glucose |
| GPR43 | G protein-coupled receptor43 |
| GPR45 | G protein-coupled receptor45 |
| GSDMD | Gasdermin D |
| GSH-Px | Glutathione peroxidas |
| GUDCA | Glycoursodesoxycholic acid |
| HDL-C | Glutathione peroxidas |
| HOMA-IR | Homeostatic model assessment of insulin resistance |
| IL -1β | Interleukin -1β |
| IL-10 | Interleukin -10 |
| IL-17A | Interleukin -17A |
| IL-4 | Interleukin -4 |
| IL-6 | Interleukin -6 |
| i-NOS | Inducible nitric oxide synthase |
| JNK | C-Jun N-terminal kinase |
| LDL-C | Low-density lipoprotein cholesterol |
| LDL-L | High density lipoprotein cholesterol |
| LPS | Lipopolysaccharide |
| LxRα | Liver X receptor α |
| MDA | Malondialdehyde |
| Man | Mannose |
| MPO | Myeloperoxidase |
| Mψs | Macrophages |
| NAFLD | Nonalcoholic fatty liver disease |
| NEFA | Non-esterified fatty acid |
| NLRP3 | Nod-like receptor protein 3 |
| OSTα/β | Organic solute transporter-α/β |
| PARP-1 | Poly ADP-ribose polymerase-1 |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| REK | Extracellular signal-regulated kinase |
| Rha | Rhamnose |
| Rib | Ribose |
| SCFAs | Short chain fatty acids |
| SOCS2 | Suppressor of cytokine signaling 2 |
| SOD | Glutathione peroxidas |
| SREBP-1c | Sterol regulatory element-binding protein 1c |
| TBA | Total bile acids |
| TC | Total cholesterol |
| TCDCA | Taurochenodeoxycholic acid |
| TEAC | Trolox equivalent antioxidant capacity |
| TG | Lipid triglycerides |
| TLCA | Taurolithocholic acid |
| TLR4 | Toll-like receptor 4 |
| T-αMCA | Taurine-α-mouse cholic acid |
| TNF-α | Tumor necrosis factor alpha |
| TUDCA | Tauroursodesoxycholic acid |
| UDCA | Ursodesoxycholic acid |
| Xyl | Xylose |
References
- Ajmera, V.; Loomba, R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol. Metab. 2021, 50, 101167. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makri, E.; Goulas, A.; Polyzos, S.A. Epidemiology, pathogenesis, diagnosis and emerging treatment of nonalcoholic fatty liver disease. Arch. Med. Res. 2021, 52, 25–37. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, J.; Wang, W.; Zhang, X.J.; Ji, Y.X.; Zhang, P.; She, Z.G.; Zhu, L.; Cai, J.; Li, H. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: A systematic review and meta-analysis. Hepatology 2019, 70, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Fianchi, F.; Liguori, A.; Gasbarrini, A.; Grieco, A.; Miele, L. Nonalcoholic fatty liver disease (NAFLD) as model of gut-liver axis interaction: From pathophysiology to potential target of treatment for personalized therapy. Int. J. Mol. Sci. 2021, 22, 6485. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [Green Version]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut microbiota, and its modulation in the management of liver diseases: A review of the literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef] [Green Version]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef]
- Lyu, F.; Xu, X.; Zhang, L. Natural polysaccharides with different conformations: Extraction, structure and anti-tumor activity. J. Mater. Chem. B 2020, 8, 9652–9667. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, P.; Li, C.; Xu, F.; Chen, J. A polysaccharide from Rosa roxburghii Tratt fruit attenuates high-fat diet-induced intestinal barrier dysfunction and inflammation in mice by modulating the gut microbiota. Food Funct. 2022, 13, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Wu, F.; Wang, X.; Feng, Y.; Wang, Y. MDG, an Ophiopogon japonicus polysaccharide, inhibits non-alcoholic fatty liver disease by regulating the abundance of Akkermansia muciniphila. Int. J. Biol. Macromol. 2022, 196, 23–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Zhao, N.; Hong, Z.; Cai, B.; Le, Q.; Yang, T.; Shi, L.; He, J. Soluble polysaccharide derived from Laminaria japonica attenuates obesity-related nonalcoholic fatty liver disease associated with gut microbiota regulation. Mar. Drugs 2021, 19, 699. [Google Scholar] [CrossRef]
- Ren, F.; Chen, Q.P.; Meng, C.; Chen, H.M.; Zhou, Y.J.; Zhang, H.; Chen, W.J. Serum metabonomics revealed the mechanism of Ganoderma amboinense polysaccharides in preventing non-alcoholic fatty liver disease (NAFLD) induced by high-fat diet. J. Funct. Foods 2021, 82, 104496. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Gu, Y.; Wang, M.; Guo, S.; Liu, J.; Zhang, X.; Zhao, Z.; Qian, B.; Yan, Y.; et al. Hepatoprotection of Lycii Fructus polysaccharide against oxidative stress in hepatocytes and larval zebrafish. Oxidative Med. Cell. Longev. 2021, 2021, 3923625. [Google Scholar] [CrossRef]
- Wang, W.; Xu, A.L.; Li, Z.C.; Li, Y.; Xu, S.F.; Sang, H.C.; Zhi, F. Combination of probiotics and Salvia miltiorrhiza polysaccharide alleviates hepatic steatosis via gut microbiota modulation and insulin resistance improvement in high fat-induced NAFLD mice. Diabetes Metab. J. 2020, 44, 336–348. [Google Scholar] [CrossRef] [Green Version]
- Kai, L.; Zong, X.; Jiang, Q.; Lu, Z.; Wang, F.; Wang, Y.; Wang, T.; Jin, M. Protective effects of polysaccharides from Atractylodes macrocephalae Koidz. against dextran sulfate sodium induced intestinal mucosal injury on mice. Int. J. Biol. Macromol. 2022, 195, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, A.; Hundertmark, J.; Guillot, A.; Tacke, F. Molecular and cellular mediators of the gut-liver axis in the progression of liver diseases. Front. Med. 2021, 8, 725390. [Google Scholar] [CrossRef] [PubMed]
- Leamy, A.K.; Egnatchik, R.A.; Young, J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res. 2013, 52, 165–174. [Google Scholar] [CrossRef]
- Arab, J.P.; Arrese, M.; Trauner, M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu. Rev. Pathol. 2018, 13, 321–350. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, X. The role of gut-liver axis in gut microbiome dysbiosis associated NAFLD and NAFLD-HCC. Biomedicines 2022, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yin, Y.; Li, Z.; Zhang, W. Gut microbiota-derived components and metabolites in the progression of non-alcoholic fatty liver disease (NAFLD). Nutrients 2019, 11, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clement, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Bibbo, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Bezek, K.; Petelin, A.; Praznikar, J.; Nova, E.; Redondo, N.; Marcos, A.; Jenko Praznikar, Z. Obesity measures and dietary parameters as predictors of gut microbiota phyla in healthy individuals. Nutrients 2020, 12, 2695. [Google Scholar] [CrossRef]
- Da Silva, H.E.; Teterina, A.; Comelli, E.M.; Taibi, A.; Arendt, B.M.; Fischer, S.E.; Lou, W.; Allard, J.P. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci. Rep. 2018, 8, 1466. [Google Scholar] [CrossRef] [Green Version]
- Park, G.; Jung, S.; Wellen, K.E.; Jang, C. The interaction between the gut microbiota and dietary carbohydrates in nonalcoholic fatty liver disease. Exp. Mol. Med. 2021, 53, 809–822. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. Gut Microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int. J. Mol. Sci. 2020, 21, 5214. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wang, K.; Zhi, Y.; Shen, W.; Huang, L. Gypenosides improves nonalcoholic fatty liver disease induced by high-fat diet induced through regulating LPS/TLR4 signaling pathway. Cell Cycle 2020, 19, 3042–3053. [Google Scholar] [CrossRef]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Niess, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The role of gut-derived lipopolysaccharides and the intestinal barrier in fatty liver diseases. J. Gastrointest. Surg. 2022, 26, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, A.; Canbay, A. Why bile acids are so important in non-alcoholic fatty liver disease (NAFLD) progression. Cells 2019, 8, 1358. [Google Scholar] [CrossRef]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.U.; Backhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Lu, M.; Xu, Y.; Wang, Q.; Gu, X.; Li, Y.; Zhuang, T.; Xia, C.; Zhang, T.; Gou, X.J.; et al. The role of gut microbiota-bile acids axis in the progression of non-alcoholic fatty liver disease. Front. Microbiol. 2022, 13, 908011. [Google Scholar] [CrossRef]
- Wang, D.; Doestzada, M.; Chen, L.; Andreu-Sanchez, S.; van den Munckhof, I.C.L.; Augustijn, H.E.; Koehorst, M.; Ruiz-Moreno, A.J.; Bloks, V.W.; Riksen, N.P.; et al. Characterization of gut microbial structural variations as determinants of human bile acid metabolism. Cell Host Microbe 2021, 29, 1802–1814.e1805. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macnaughtan, J.; Jalan, R. Clinical and pathophysiological consequences of alterations in the microbiome in cirrhosis. Am. J. Gastroenterol. 2015, 110, 1399–1410, quiz 1411. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, C.; Singanayagam, A.; Patel, V.C. Modulating the gut-liver axis and the pivotal role of the faecal microbiome in cirrhosis. Clin. Med. 2020, 20, 493–500. [Google Scholar] [CrossRef]
- Gruner, N.; Mattner, J. Bile acids and microbiota: Multifaceted and versatile regulators of the liver-gut axis. Int. J. Mol. Sci. 2021, 22, 1397. [Google Scholar] [CrossRef]
- Jia, B.; Park, D.; Chun, B.H.; Hahn, Y.; Jeon, C.O. Diet-related alterations of gut bile salt hydrolases determined using a metagenomic analysis of the human microbiome. Int. J. Mol. Sci. 2021, 22, 3652. [Google Scholar] [CrossRef] [PubMed]
- Duszka, K. Versatile Triad Alliance: Bile acid, taurine and microbiota. Cells 2022, 11, 2337. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. -Gastrointest. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 2014, 66, 948–983. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E. Bile acids in glucose metabolism in health and disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlstrom, A.; Ashby, J.W.; et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021, 33, 1671–1684.e1674. [Google Scholar] [CrossRef]
- Klindt, C.; Reich, M.; Hellwig, B.; Stindt, J.; Rahnenfuhrer, J.; Hengstler, J.G.; Kohrer, K.; Schoonjans, K.; Haussinger, D.; Keitel, V. The G protein-coupled bile acid receptor TGR5 (Gpbar1) modulates endothelin-1 signaling in liver. Cells. 2019, 8, 1467. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.D.; Chen, W.D.; Yu, D.; Forman, B.M.; Huang, W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology 2011, 54, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Donepudi, A.C.; Boehme, S.; Li, F.; Chiang, J.Y. G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. Hepatology 2017, 65, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the role of the gut microbiome and microbial metabolites in non-alcoholic fatty liver disease: Current evidence and perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- Coppola, S.; Avagliano, C.; Calignano, A.; Berni Canani, R. The protective role of butyrate against obesity and obesity-related diseases. Molecules. 2021, 26, 682. [Google Scholar] [CrossRef]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Gunther, C.; Bischoff, S.C. Prebiotic inulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front. Immunol. 2021, 12, 678360. [Google Scholar] [CrossRef]
- Michail, S.; Lin, M.; Frey, M.R.; Fanter, R.; Paliy, O.; Hilbush, B.; Reo, N.V. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol. Ecol. 2015, 91, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Goh, Y.Q.; Cheam, G.; Wang, Y. Understanding choline bioavailability and utilization: First step toward personalizing choline nutrition. J. Agric. Food Chem. 2021, 69, 10774–10789. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019, 11. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Long, J.; Chen, S.; Liao, G.; Wu, S.; Li, C.; Wang, L.; Ling, W.; Zhu, H. Trimethylamine N-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease. Mol. Nutr. Food Res. 2019, 63, e1900257. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q.; Chang, R.; Zhou, X.; Xu, C. Intestinal barrier function-non-alcoholic fatty liver disease interactions and possible role of gut microbiota. J. Agric. Food Chem. 2019, 67, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cantos, M.V.; Garcia-Morena, D.; Iannone, V.; El-Nezami, H.; Kolehmainen, M.; Kuipers, O.P. Role of microbiota and related metabolites in gastrointestinal tract barrier function in NAFLD. Tissue Barriers 2021, 9, 1879719. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Cui, S.X.; Zheng, J.M.; Li, Y.Q.; Li, P.P.; Hou, H.T. Berberine ameliorates intestinal mucosal barrier dysfunction in nonalcoholic fatty liver disease (NAFLD) rats. J. King Saud Univ. -Sci. 2020, 32, 2534–2539. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xie, Q.S.; Chen, G.C.; Huang, C.L.; Yu, T.; Chen, Q.K.; Li, J.Y. Impaired intestinal barrier function in type 2 diabetic patients measured by serum LPS, Zonulin, and IFABP. J. Diabetes Complicat. 2021, 35, 107766. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Kawabe, T.; Suzuki, N.; Yamaki, S.; Sun, S.L.; Asao, A.; Okuyama, Y.; So, T.; Iwakura, Y.; Ishii, N. Mesenteric lymph nodes contribute to proinflammatory Th17-cell generation during inflammation of the small intestine in mice. Eur. J. Immunol. 2016, 46, 1119–1131. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Wu, Z.; Chi, Y.; Song, Y.; Xu, J.; Tan, J.; Cong, X.; Liu, Y. Mesenteric lymph node CD4(+) T lymphocytes migrate to liver and contribute to non-alcoholic fatty liver disease. Cell Immunol. 2019, 337, 33–41. [Google Scholar] [CrossRef]
- Inamine, T.; Schnabl, B. Immunoglobulin A and liver diseases. J. Gastroenterol. 2018, 53, 691–700. [Google Scholar] [CrossRef]
- Yang, J.M.; Sun, Y.; Wang, M.; Zhang, X.L.; Zhang, S.J.; Gao, Y.S.; Chen, L.; Wu, M.Y.; Zhou, L.; Zhou, Y.M.; et al. Regulatory effect of a chinese herbal medicine formula on non-alcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 5105–5119. [Google Scholar] [CrossRef]
- Leon, E.D.; Francino, M.P. Roles of secretory immunoglobulin A in host-microbiota interactions in the gut ecosystem. Front. Microbiol. 2022, 13, 880484. [Google Scholar] [CrossRef]
- Turula, H.; Wobus, C.E. The Role of the Polymeric immunoglobulin receptor and secretory immunoglobulins during mucosal infection and immunity. Viruses 2018, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, B. Role of gut barrier function in the pathogenesis of nonalcoholic fatty liver disease. Gastroenterol. Res. Pract. 2015, 2015, 287348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanniyasi, E.; Venkatasubramanian, G.; Anbalagan, M.M.; Raj, P.P.; Gopal, R.K. In vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana J.V.Lamouroux and Turbinaria decurrens Bory). Sci. Rep. 2019, 9, 12185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Jiang, X.; Xie, H.; Li, X.; Shi, L. Structural characterization and antitumor activity of a polysaccharide from ramulus mori. Carbohydr. Polym. 2018, 190, 232–239. [Google Scholar] [CrossRef]
- Cui, M.; Wu, J.; Wang, S.; Shu, H.; Zhang, M.; Liu, K.; Liu, K. Characterization and anti-inflammatory effects of sulfated polysaccharide from the red seaweed Gelidium pacificum Okamura. Int. J. Biol. Macromol. 2019, 129, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Seedevi, P.; Ramu Ganesan, A.; Moovendhan, M.; Mohan, K.; Sivasankar, P.; Loganathan, S.; Vairamani, S.; Shanmugam, A. Anti-diabetic activity of crude polysaccharide and rhamnose-enriched polysaccharide from G. lithophila on Streptozotocin (STZ)-induced in Wistar rats. Sci. Rep. 2020, 10, 556. [Google Scholar] [CrossRef] [Green Version]
- Che, J.; Yang, S.; Qiao, Z.; Li, H.; Sun, J.; Zhuang, W.; Chen, J.; Wang, C. Schisandra chinensis acidic polysaccharide partialy reverses acetaminophen-induced liver injury in mice. J. Pharmacol. Sci. 2019, 140, 248–254. [Google Scholar] [CrossRef]
- Xu, H.; Liu, L.; Chen, Y.; Ma, H.; Li, M.; Qu, W.; Yin, J.; Zhang, X.; Gao, Y.; Shan, J.; et al. The chemical character of polysaccharides from processed Morindae officinalis and their effects on anti-liver damage. Int. J. Biol. Macromol. 2019, 141, 410–421. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Yang, L.; Pan, Q.; Li, J.; Wu, Y.; Chen, M.; Cui, S.; Yu, J. Protective effect of Anoectochilus roxburghii polysaccharide against CCl4-induced oxidative liver damage in mice. Int. J. Biol. Macromol. 2017, 96, 442–450. [Google Scholar] [CrossRef]
- Qu, H.; Gao, X.; Cheng, C.; Zhao, H.; Wang, Z.; Yi, J. Hepatoprotection mechanism against alcohol-induced liver injury in vivo and structural characterization of Pinus koraiensis pine nut polysaccharide. Int. J. Biol. Macromol. 2020, 154, 1007–1021. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, H.; Xu, W.; Liu, C.; Hu, B.; Guo, Y.; Cheng, Y.; Qian, H. Echinacea purpurea polysaccharide prepared by fractional precipitation prevents alcoholic liver injury in mice by protecting the intestinal barrier and regulating liver-related pathways. Int. J. Biol. Macromol. 2021, 187, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Huang, P.; Zhang, L.; Qiu, Y.; Qi, H.; Leng, A.; Shang, D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 161, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Wang, L.; Chen, W.D.; Duan, Y.T.; Sun, M.J.; Huang, J.J.; Peng, D.Y.; Yu, N.J.; Wang, Y.Y.; Zhang, Y. Poria cocos polysaccharide prevents alcohol-induced hepatic injury and inflammation by repressing oxidative stress and gut leakiness. Front. Nutr. 2022, 9, 963598. [Google Scholar] [CrossRef]
- Qu, H.; Liu, S.; Cheng, C.; Zhao, H.; Gao, X.; Wang, Z.; Yi, J. Hepatoprotection of pine nut polysaccharide via NRF2/ARE/MKP1/JNK signaling pathways against carbon tetrachloride-induced liver injury in mice. Food Chem. Toxicol. 2020, 142, 111490. [Google Scholar] [CrossRef]
- Tang, J.; Wen, J.; Cheng, M. Application of Ligularia. hodgsonii. Hook polysaccharide in preparation of medicine for treating and/or preventing liver disease using pharmaceutically acceptable excipients. C.N. Patent 112089725A, 18 December 2020. [Google Scholar]
- Fan, R.; Ma, G.H.; Yu, S.S. Protective effect of polysaccharide from Gastrodia elata Blume on non-alcoholic fatty liver induced by high fat diet. Sci. Technol. Food Ind. 2022, 43, 381–391. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, F.; Jiang, H.; Wang, Z.; Hua, C.; Zhang, Y. Chicory (Cichorium intybus L.) polysaccharides attenuate high-fat diet induced non-alcoholic fatty liver disease via AMPK activation. Int. J. Biol. Macromol. 2018, 118, 886–895. [Google Scholar] [CrossRef]
- Du, T.; Fang, Q.; Zhang, Z.; Zhu, C.; Xu, R.; Chen, G.; Wang, Y. Lentinan protects against nonalcoholic fatty liver disease by reducing oxidative stress and apoptosis via the PPARalpha pathway. Metabolites 2022, 12, 55. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Guo, Y.Q.; Fan, Y.N.; Tao, X.J.; Gao, Q.H.; Yang, J.J. Lycium barbarum polysaccharides promotes mitochondrial biogenesis and energy balance in a NAFLD cell model. Chin. J. Integr. Med. 2021. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Sarker, M.M.R.; Yan, X.; Yang, C.; Zhao, L.; Lv, X.; Liu, B.; Zhao, C. Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohydr. Polym. 2018, 198, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, F.; Huang, Y.; Liu, B. The Positive Effects of Grifola frondosa heteropolysaccharide on NAFLD and regulation of the gut microbiota. Int. J. Mol. Sci. 2019, 20, 5302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.Y.; Wang, Y.X.; Yang, X.B.; Yin, H.; Nie, S.P.; Wu, X.Y. Structure characterization of a polysaccharide extracted from noni (Morinda citrifolia L.) and its protective effect against DSS-induced bowel disease in mice. Food Hydrocoll. 2019, 90, 189–197. [Google Scholar] [CrossRef]
- Yang, X.; Mo, W.; Zheng, C.; Li, W.; Tang, J.; Wu, X. Alleviating effects of noni fruit polysaccharide on hepatic oxidative stress and inflammation in rats under a high-fat diet and its possible mechanisms. Food. Funct. 2020, 11, 2953–2968. [Google Scholar] [CrossRef]
- Wu, J.; Shao, H.; Zhang, J.; Ying, Y.; Cheng, Y.; Zhao, D.; Dou, X.; Lv, H.; Li, S.; Liu, F.; et al. Mussel polysaccharide alpha-D-glucan (MP-A) protects against non-alcoholic fatty liver disease via maintaining the homeostasis of gut microbiota and regulating related gut-liver axis signaling pathways. Int. J. Biol. Macromol. 2019, 130, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, L.; Wang, X.; Feng, Y.; Wang, Y. MDG-1, an Ophiopogon polysaccharide, restrains process of non-alcoholic fatty liver disease via modulating the gut-liver axis. Int. J. Biol. Macromol. 2019, 141, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Tawfick, M.M.; Xie, H.; Zhao, C.; Shao, P.; Farag, M.A. Inulin fructans in diet: Role in gut homeostasis, immunity, health outcomes and potential therapeutics. Int. J. Biol. Macromol. 2022, 208, 948–961. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Bao, T.; He, F.; Zhang, X.; Zhu, L.; Wang, Z.; Lu, H.; Wang, T.; Li, Y.; Yang, S.; Wang, H. Inulin exerts beneficial effects on non-alcoholic fatty liver disease via modulating gut microbiome and suppressing the lipopolysaccharide-toll-like receptor 4-Mpsi-nuclear factor-kappaB-nod-like receptor protein 3 pathway via gut-liver axis in mice. Front. Pharmacol. 2020, 11, 558525. [Google Scholar] [CrossRef]
- Puluhulawa, L.E.; Joni, I.M.; Mohammed, A.F.A.; Arima, H.; Wathoni, N. The use of megamolecular polysaccharide Sacran in food and biomedical applications. Molecules 2021, 26, 3362. [Google Scholar] [CrossRef]
- Goto, M.; Azuma, K.; Arima, H.; Kaneko, S.; Higashi, T.; Motoyama, K.; Michihara, A.; Shimizu, T.; Kadowaki, D.; Maruyama, T.; et al. Sacran, a sulfated polysaccharide, suppresses the absorption of lipids and modulates the intestinal flora in non-alcoholic steatohepatitis model rats. Life Sci. 2021, 268, 118991. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Sheng, L.; Zhong, J.; Tao, X.; Zhu, W.; Ma, J.; Yan, J.; Zhao, A.; Zheng, X.; Wu, G.; et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, J.; Yan, C.; Zhang, C.; Pan, W.; Zhang, W.; Lu, Y.; Chen, L.; Chen, Y. Sarcodon aspratus polysaccharides ameliorated obesity-induced metabolic disorders and modulated gut microbiota dysbiosis in mice fed a high-fat diet. Food. Funct. 2020, 11, 2588–2602. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.S.; Wang, K.; Ma, K.; Bao, L.; Liu, H.W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 2019, 17, 3–14. [Google Scholar] [CrossRef]
- Finley, J.W.; Soto-Vaca, A.; Heimbach, J.; Rao, T.P.; Juneja, L.R.; Slavin, J.; Fahey, G.C. Safety assessment and caloric value of partially hydrolyzed guar gum. J. Agric. Food Chem. 2013, 61, 1756–1771. [Google Scholar] [CrossRef]
- Takayama, S.; Katada, K.; Takagi, T.; Iida, T.; Ueda, T.; Mizushima, K.; Higashimura, Y.; Morita, M.; Okayama, T.; Kamada, K.; et al. Partially hydrolyzed guar gum attenuates non-alcoholic fatty liver disease in mice through the gut-liver axis. World J. Gastroenterol. 2021, 27, 2160–2176. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and applications of pectin in food, biomedical, and pharmaceutical industry: A review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An emerging new bioactive food polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Houron, C.; Ciocan, D.; Trainel, N.; Mercier-Nome, F.; Hugot, C.; Spatz, M.; Perlemuter, G.; Cassard, A.M. Gut microbiota reshaped by Pectin treatment improves liver steatosis in obese mice. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Zhong, M.; Yan, Y.; Yuan, H.; Rong, A.; Xu, G.; Cai, F.; Yang, Y.; Wang, Y.; Zhang, W. Astragalus mongholicus polysaccharides ameliorate hepatic lipid accumulation and inflammation as well as modulate gut microbiota in NAFLD rats. Food Funct. 2022, 13, 7287–7301. [Google Scholar] [CrossRef]
- Huang, S.; Pang, D.; Li, X.; You, L.; Zhao, Z.; Cheung, P.C.; Zhang, M.; Liu, D. A sulfated polysaccharide from Gracilaria Lemaneiformis regulates cholesterol and bile acid metabolism in high-fat diet mice. Food Funct. 2019, 10, 3224–3236. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Bonfrate, L.; Portincasa, P. The role of microbiota in nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2022, 52, e13768. [Google Scholar] [CrossRef]
- Saltzman, E.T.; Palacios, T.; Thomsen, M.; Vitetta, L. Intestinal microbiome shifts, dysbiosis, inflammation, and non-alcoholic fatty liver disease. Front. Microbiol. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Ma, S.; Qiu, Z.; Huang, S.; Deng, G.; Li, Y.; Xu, S.; Yang, M.; Shi, H.; Wu, C.; et al. Poria cocos polysaccharides rescue pyroptosis-driven gut vascular barrier disruption in order to alleviates non-alcoholic steatohepatitis. J. Ethnopharmacol. 2022, 296, 115457. [Google Scholar] [CrossRef] [PubMed]
- Ho Do, M.; Seo, Y.S.; Park, H.Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 1212–1224. [Google Scholar] [CrossRef]
- Rastall, R.A.; Diez-Municio, M.; Forssten, S.D.; Hamaker, B.; Meynier, A.; Moreno, F.J.; Respondek, F.; Stah, B.; Venema, K.; Wiese, M. Structure and function of non-digestible carbohydrates in the gut microbiome. Benef. Microbes 2022, 13, 95–168. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martin, R.; Bermudez-Humaran, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, D.; Tsujimoto, G.; Kimura, I. Regulation of energy homeostasis by GPR41. Front. Endocrinol. 2014, 5, 81. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Marques, F.Z. How Dietary fibre, acting via the gut microbiome, lowers blood pressure. Curr. Hypertens. Rep. 2022. Advance online publication. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Xie, F.; He, H.; Johnston, L.J.; Dai, X.; Wu, C.; Ma, X. Dietary fiber-derived short-chain fatty acids: A potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes. Rev. 2021, 22, e13316. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, B. The gut-liver axis in health and disease: The role of gut microbiota-derived signals in liver injury and regeneration. Front. Immunol. 2021, 12, 775526. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.T.; Martens, E.C. The Critical roles of polysaccharides in gut microbial ecology and physiology. Annu. Rev. Microbiol. 2017, 71, 349–369. [Google Scholar] [CrossRef]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Lombard, V.; Henrissat, B. Complex carbohydrate utilization by the healthy human microbiome. PLoS ONE 2012, 7, e28742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.L.; Nie, S.P.; Li, C.; Xie, M.Y. In vitro fermentation of polysaccharide from the seeds of Plantago asiatica L. by human fecal microbiota. Food Hydrocoll. 2013, 33, 384–392. [Google Scholar] [CrossRef]
- Yao, J.; Chen, Y.; Xu, M. The critical role of short-chain fatty acids in health and disease: A subtle focus on cardiovascular disease-NLRP3 inflammasome-angiogenesis axis. Clin. Immunol. 2022, 238, 109013. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARgamma-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, T.Y.; Ma, Y.D.; Guo, X.Z.; Ye, Y.F.; Xie, C. Bile acid and receptors: Biology and drug discovery for nonalcoholic fatty liver disease. Acta Pharmacol. Sin. 2022, 43, 1103–1119. [Google Scholar] [CrossRef]
- Wang, R.; Ren, Y.; Bao, T.; Wang, T.; Li, Y.; Liu, Y.; Zhang, X.; Yang, S.; Wang, H. Inulin activates FXR-FGF15 signaling and further increases bile acids excretion in non-alcoholic fatty liver disease mice. Biochem. Biophys. Res. Commun. 2022, 600, 156–162. [Google Scholar] [CrossRef]
- Zhong, D.; Xie, Z.; Huang, B.; Zhu, S.; Wang, G.; Zhou, H.; Lin, S.; Lin, Z.; Yang, B. Ganoderma Lucidum polysaccharide peptide alleviates hepatoteatosis via modulating bile acid metabolism dependent on FXR-SHP/FGF. Cell Physiol. Biochem. 2018, 49, 1163–1179. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, A.; Kosters, A.; Mells, J.E.; Zhang, W.; Setchell, K.D.; Amanso, A.M.; Wynn, G.M.; Xu, T.; Keller, B.T.; Yin, H.; et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci. Transl. Med. 2016, 8, 357ra122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma. Hepatology 2021, 73 (Suppl. 1), 4–13. [Google Scholar] [CrossRef]
- Zhuang, Y.P.; Zhang, Y.T.; Zhang, R.X.; Zhong, H.J.; He, X.X. The Gut-liver axis in nonalcoholic fatty liver disease: Association of intestinal permeability with disease severity and treatment outcomes. Int. J. Clin. Pract. 2022, 2022, 4797453. [Google Scholar] [CrossRef]
- Dai, X.; Feng, J.; Chen, Y.; Huang, S.; Shi, X.; Liu, X.; Sun, Y. Traditional chinese medicine in nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Chin. Med. 2021, 16, 68. [Google Scholar] [CrossRef]
- Peng, S.; Liu, L.; Xie, Z.; Zhang, X.; Xie, C.; Ye, S.; Zhang, X.; Liang, X.; Wang, H.; Liu, Y. Chinese herbal medicine for type 2 diabetes mellitus with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 863839. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Cao, Q.; Ye, L.; Wang, J.; Guo, M. The function of natural polysaccharides in the treatment of ulcerative colitis. Front. Pharmacol. 2022, 13, 927855. [Google Scholar] [CrossRef]
- Sun, C.Y.; Zheng, Z.L.; Chen, C.W.; Lu, B.W.; Liu, D. Targeting gut microbiota with natural polysaccharides: Effective interventions against high-fat diet-induced metabolic diseases. Front. Microbiol. 2022, 13, 859206. [Google Scholar] [CrossRef]
- Zhao, W.X.; Wang, T.; Zhang, Y.N.; Chen, Q.; Wang, Y.; Xing, Y.Q.; Zheng, J.; Duan, C.C.; Chen, L.J.; Zhao, H.J.; et al. Molecular mechanism of polysaccharides extracted from Chinese medicine targeting gut microbiota for promoting health. Chin. J. Integr. Med. 2022, 1–10. [Google Scholar] [CrossRef]


| Polysaccharides | Structural Information * | Dose | Disease Modelling Method | Related Biochemical Information, Signal Factor and Histological Change (Compared with the Model Group) # | Variation of Gut Microbiota (Compared with the Model Group) | Reference |
|---|---|---|---|---|---|---|
| Grifola frondosa heteropolysaccharide (GFP) | (1) Mw:66.1 kDa (2) Monosaccharide composition:Fuc:Xyl:Man:Glc:Gal = 1.2:1.4:1.4:1.1:1.0 (3) Glycosidic linkage type:β-D-GlcpA→, 1,2,6-α-Gal, →2)-α-Manp→, →3)-α-L-Fucp-(1→ [92] | 150 mg/kg·bw | HFD for 8 weeks | (1) BW gain rates↓ (2) Attenuate liver damage on a histological level (3) AST, TG, TC, LDL-c↓; HDL-c↑ (4) ACC, TNF-α, SOCS2, CYP4A1↓; GSH-Px, SOD, CYP7A1↑ | Firmicutes↓ Bacteroidetes↑ Allobaculum, Bacteroides, Bifidobacterium, Blautia, Coprococcus, Phascolarctobacterium, Prevotella, Roseburia ↑ Acetatifactor, Alistipes, Flavonifractor, Paraprevotella, Oscillibacter ↓ | [93] |
| Noni fruit polysaccharide (NFP) | (1) Mw:456 kDa (2) Monosaccharide composition:GalA:Gal:Glc:Rha:Ara = 27.1:2.1:9.8:2.2:1.0 (3) Glycosidic linkage type:O-acetylated-(1→4)-α-GalAp (backbone), (1→2)-Rhap, (1→4)-β-Galp, (1→5)/(1→ 3)-Ara [94] | 100 mg/kg·bw | HFD for 4 weeks | (1) BW, LW, abdominal fat weight, spleen weight, BW gain rates↓ (2) Attenuate liver damage on a histological level (3) AST, ALT, TG, TC, LDL-c↓; HDL-c↑ (4) CCL5, TNF-α, IL-1β, liver IL-10, GPR43↓; MDA, hepatic TEAC, SOD, GSH-Px, CAT, ZO-1, occludin, total SCFA↑ | Firmicutes↑ Lactobacillus, [Eubacterium] coprostanoligenes group, Ruminococcus_1, Alloprevotella, Ruminococcaceae_UCG_014, Parasutterella, Muribaculaceae↑ Actinobacteria, Prevotella_9, Turicibacter↓ | [95] |
| Mussel polysaccharide α-D-glucan (MP-A) | (1) Mw:1.2 × 103 kDa (2) Monosaccharide composition:Glc (3) Glycosidic linkage type:(1→4)-α-D-Glc, (1, 2)-α-D-Glc | 600 mg/kg·bw | HFD for 3 weeks | (1) BW, BW gain rates↓ (2) Attenuate liver damage on a histological level (3) AST, ALT, TG, TC, LDL-L, ALP, plasma ethanol↓ (4) LPS, TNF-α, IL-1, IL-6, TLR4, NF-κB, SREBP-1c, PPARγ↓ (5) SCFAs↑ | Firmicutes↓ Bacteroides↑ Mailhella, Alloprevotella, Butyricimonas, Parabacteroides, Akkermansia, Bifidobacterium↑ Allobaculum, Pseudomonas, Hydrogenophaga, Romboutsia, Turicibacter, Ruthenibacterium, Faecalibaculum↓ | [96] |
| Ophiopogon polysaccharide (MDG-1) | (1) Mw:3.4 kDa (2) Monosaccharide composition:Fru, trace of GLc (3) Glycosidic linkage type:Fruf (2→1), Fruf (2→6) Fruf (2→) | HFD with MDG-1 (2‰, 4‰, 8‰) | HFD for 16 weeks | (1) BW↓ (2) Attenuate liver damage on a histological level (3) TG, TC, AST, ALT↓ (4) IL-1β, IL-4, TNF-α, CD68, SREBP-1c, FAS, ACC-1, PPARc↓; IL-10, AMPK, GPR41, GPR43↑ (5) SCFAs↑ | Firmicutes/Bacteroidetes (F/B) ratio↓ Lactococcus, Enterorhabdus, Turicibacter, Clostridium-sensu-stricto-1, Tyzzerella, Oscillibacter↓ Alistipes, Ruminiclostridium, Ricenella↑ | [97] |
| Inulin (INU) | (1) Mw:different degrees of polymerization (2-60 Fru units) (2) Monosaccharide composition:Glc, Fru (3) Glycosidic linkage type:(2→1)-β-D-Fru, α-Glu-(1→2)- β-D-Fru [98,99] | 5000 mg/kg·bw | HFD for 14 weeks | (1) BW, LW, liver index↓ (2) Attenuate liver damage on a histological level (3) ALT, AST, TG, TC, plasma insulin, HOMA-IR↓ (4) F4/80+ Mψs, TLR4, NLRP3, ASC, caspase-1, NF-κB, IL-1β, IL-18, IL-6, LPS↓; IL-10↑ (5) SCFAs↑ | F/B ratio↓ Bififidobacterium, Akkermansia↑ | [100] |
| Sacran polysaccharide | (1) Mw:1–2.2 × 104 kDa (2) Monosaccharide composition:Glc:Gal:Man:Xyl:Rha:Fuc:GalA:GlcA = 25.9:11.0:10.0:16.2:10.2:6.9:4.0:4.2 (traces of Ara, GalN, Muramic acid) (3) Glycosidic linkage type:→4)-6-deoxy acid/pentose-(1,4)-uronic acid-(1,4)-uronic acid--(1,4)-hexopyranose-(1,4)- hexopyranose-(1,4)-sulfated muramic acid-(1→ [101] | 80 mg/kg·bw | HFC for 4 and 8 weeks | (1) BW, LW, liver index↓ (2) Attenuate liver damage on a histological level (3) TC, TG, AST, ALT, ALP↓ (4) TGF-β1, TNF-α↓ | F/B ratio ↓ Blautia↑ Prevotella↓ | [102] |
| Astragalus polysaccharides (APS) | Monosaccharide composition:Ara:Xyl:Glc:Gal:Rha = 1.0:14.6:0.5:44.1:2.2 | HFD with APS (4% in finial concentration) | HFD for 14 weeks | (1) BW, LW, liver index↓ (2) Attenuate liver damage on a histological level (3) ALT, AST, TG, TC, serum insulin↓ (4) IL-1β, FASN, CPT-1α↓ (5) SCFAs, acetic acid level↑ | The bacterial richness and evenness↑ F/B ratio↓ Desulfovibrio vulgaris (Traditional sulfate-reducing bacteria, producing acetic acid) ↑ | [103] |
| Sarcodon aspratus, polysaccharides (SATP) | Monosaccharide composition:Gal:Ara:Man:Glc = 24.2:8.4:3.3:1.0 | 100, 200, 400 mg/kg·bw | HFD for 14 weeks | (1) Attenuate liver/ileum damage on a histological level (2) TC, TG, NEFA, ALT, AST↓; HDL-c↑ (3) LPO, MDA↓; SOD, GSH-Px↑ (4) IL-1β, TNF-α, LPS↓ (5) SCFAs ↑ | F/B ratio↓ Lactobacillus, Bacteroides, Akkermansia↑ | [104] |
| An insoluble polysaccharide from the sclerotium of Poria cocos (WIP) | (1) Mw:4.486 × 103 kDa (2) Monosaccharide composition:Glc (3) Glycosidic linkage type:(1, 3)-β-D-Glc (main chain) | 500, 1000 mg/kg·bw | ob/ob mice (8 weeks old) | (1) Attenuate liver damage on a histological level (2) AST, ALT, TC, TG, LDL-c↓; glucose tolerance insulin resistance↑ (3) LPS↓; SOD, Muc-5, ZO-1, Occludin, SCFAs ↑ | Lachnospiracea, Clostridium IV, Alloprevotella, Parabacteroides, Ruminococcus, Bacteroides↑ | [105] |
| Partially hydrolyzed guar gum (PHGG) | (1) Mw:1-100 kDa (2) Monosaccharide composition:Man:Gal = 2:1 (repeating unit of PHGG) (3) Glycosidic linkage type:(1,4)-β-D-Man, (1,6)-α-D-Gal [106] | Given an atherogenic diet with 5% PHGG | Atherogenic diet for 8 weeks; Administration of 0.5% DSS | (1) Attenuate liver damage on a histological level (2) AST, ALT, MPO activity↓ (3) TNF-α, Collagen 1a1, MCP-1, TLR4, TLR9, endotoxin levels in the portal vein, FD4 flux↓ (4) formic acid↑ | Bacteroides, Clostridium subcluster XIVa↑ Prevotella, Bifidobacterium, Clostridium cluster XVIII↓ (Compare to control group) | [107] |
| Pectin | (1) Sub-domains composition:Homogalacturonan (HG, 65%), Rhammogalacturonan I (RG I, 20-35%), Rhamnogalacturonan II (<10%), Xilogalacturonan (<10%) (2) Glycosidic linkage type:partially methyl-esterified (1, 4)-α-D-GalA (HG), →4)-α-D-GalA-(1, 2)-α-L-Rha-(1→(RG I) [108,109] | 2000 mg/kg·bw | HFD for 16 weeks | (1) TG, liver/body weight ratio↓ (2) Attenuate liver damage on a histological level | Firmicutes↓ Bacteroidetes↑ Prevotellaceae, Turicibacteraceae↑Desulfovibrionaceae, Ruminococcus↓ | [110] |
| Astragalus mongholicus polysaccharides (mAPS) | Monosaccharide composition:Glc:Ara:Gal:Rib = 26.0:1.4:1.2:1.0 | 200 mg/kg·bw | HFD for 6 weeks | (1) BW, liver index, eWAT weight↓ (2) Attenuate liver damage on a histological level (3) AST, ALT, TG, TC, LDL-c, HOMA-IR↓ (4) ZO-1, occludin↑ (5) LPS, TLR4, NLRP3, TNF-α, IL-1β, NF-κB↓ (6) AMPK, SREBP-1, PPAR-α, GPR41, GPR43↓ | F/B ratio↓ Proteobacteria, Episilonbacteria ↑ | [111] |
| Sulfated Gracilaria lemaneiformis polysaccharide (GLP) | (1) Molecular weight:31.5 kDa (2) Monosaccharide composition:Gal:Glc:Fuc:Man = 9.2:6.6:1.0:0.6 (3) Glycosidic linkage type:→3,4)-Fucp-(1→, →3,4,6)-Galp-(1→, →4)-Glcp-(1→, →4,6)-Manp-(1→, →6)-Glcp-(1→, →6)-Galp-(1→, Galp-(1→ | 60, 225 mg/kg·bw | High-fat and high-cholesterol diet for 40 days | (1) BW, LW, epididymal fat weight↓ (2) Attenuate liver damage on a histological level (3) TG, TC, FFA↓ (4) TLCA, GDCA, GUDCA, TUDCA↑, AMPKα, CYP7A1↑; CA, CDCA, TCDCA, DCA↓, SBREP-2↓ | F/B ratio↑ Lachnospiraceae_NK4A136_group, Bacteroides, Ruminococcus_1, Lactobacillus↑ Prevotellaceae_UCG-001, Corprococcus_1, Alistipes, Roseburia↑ | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, M.; Tang, J.; Wang, N.; Feng, Y.; Ma, H. Research Progress on the Therapeutic Effect of Polysaccharides on Non-Alcoholic Fatty Liver Disease through the Regulation of the Gut–Liver Axis. Int. J. Mol. Sci. 2022, 23, 11710. https://doi.org/10.3390/ijms231911710
Chen X, Liu M, Tang J, Wang N, Feng Y, Ma H. Research Progress on the Therapeutic Effect of Polysaccharides on Non-Alcoholic Fatty Liver Disease through the Regulation of the Gut–Liver Axis. International Journal of Molecular Sciences. 2022; 23(19):11710. https://doi.org/10.3390/ijms231911710
Chicago/Turabian StyleChen, Xiang, Menghan Liu, Jun Tang, Ning Wang, Yibin Feng, and Haotian Ma. 2022. "Research Progress on the Therapeutic Effect of Polysaccharides on Non-Alcoholic Fatty Liver Disease through the Regulation of the Gut–Liver Axis" International Journal of Molecular Sciences 23, no. 19: 11710. https://doi.org/10.3390/ijms231911710
APA StyleChen, X., Liu, M., Tang, J., Wang, N., Feng, Y., & Ma, H. (2022). Research Progress on the Therapeutic Effect of Polysaccharides on Non-Alcoholic Fatty Liver Disease through the Regulation of the Gut–Liver Axis. International Journal of Molecular Sciences, 23(19), 11710. https://doi.org/10.3390/ijms231911710






