Two Synthetic Peptides Corresponding to the Human Follicle-Stimulating Hormone β-Subunit Promoted Reproductive Functions in Mice
Abstract
:1. Introduction
2. Result
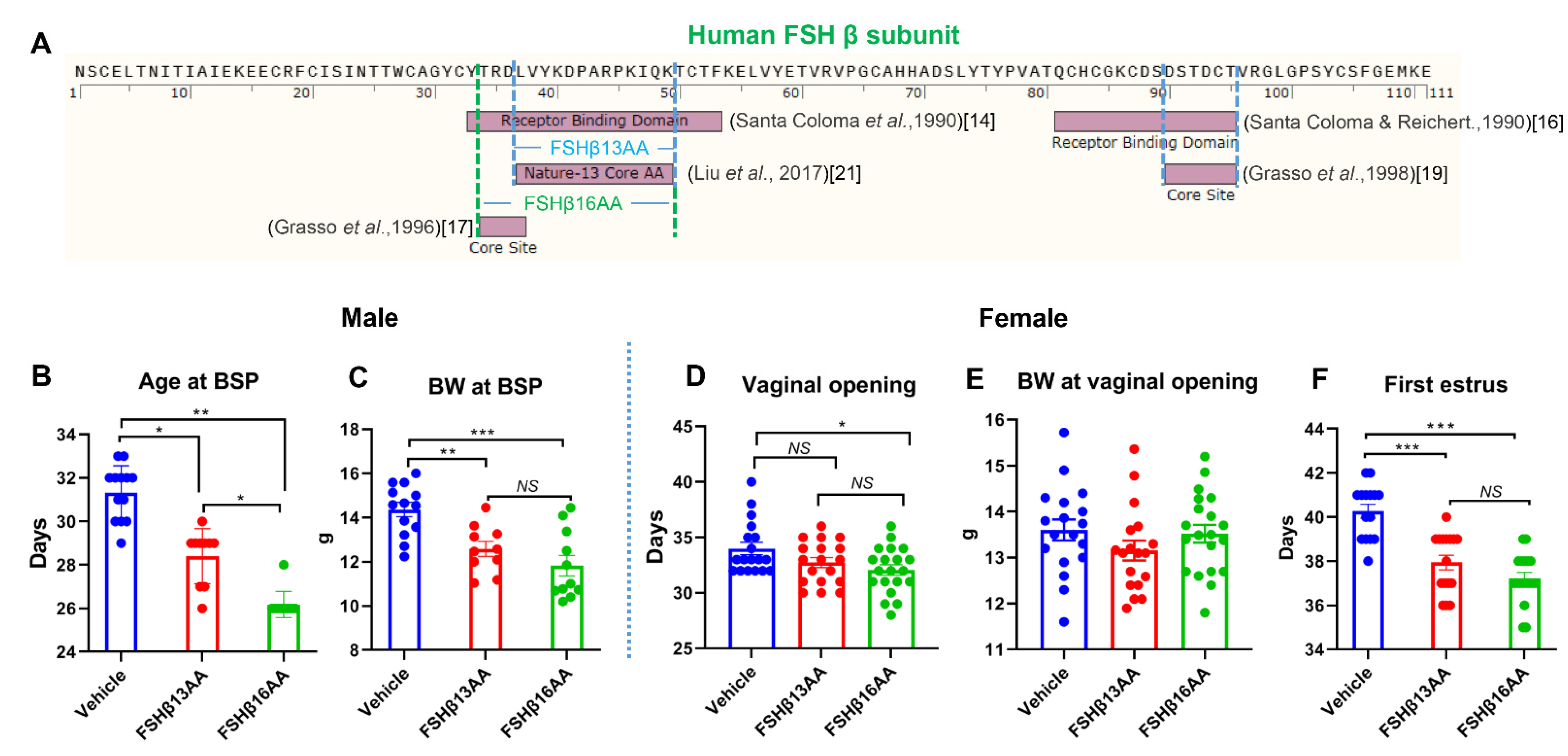
2.1. FSHβ13AA/FSHβ16AA Accelerated the Onset of Puberty in Both Sexes
2.2. Daily FSHβ13AA/FSHβ16AA Promoted Gonadal Development in Both Sexes
2.3. FSHβ13AA/FSHβ16AA Promoted the GC Proliferation and the 17β-Estradiol Production In Vitro
2.4. FSHβ13AA/FSHβ16AA Antagonized Endogenous FSH Actions in the Adult Female Mice
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis, Purification and Characterization
4.2. Mice and Experimental Design
4.2.1. Experiment 1: Effects of FSHβ13AA/FSHβ16AA on the Pubertal Onset
4.2.2. Experiment 2: Effects of FSHβ13AA/FSHβ16AA on the Gonadal Functions in the Prepubertal Mice
4.2.3. Experiment 3: Effects of FSHβ13AA/FSHβ16AA on the Granulosa Cell Function and Proliferation In Vitro
4.2.4. Experiment 4: Effects of FSHβ13AA/FSHβ16AA on the Endogenous FSH Action in Adult Females
4.2.5. Serum and Cell Culture Media Hormones Assays
4.2.6. Gonadal Histology
4.2.7. Quantitative Analysis of the mRNA Expression
4.2.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Casarini, L.; Crépieux, P. Molecular Mechanisms of Action of FSH. Front. Endocrinol. 2019, 10, 305–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsueh, A.J.; Kawamura, K.; Cheng, Y.; Fauser, B.C. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, T.R.; Wang, Y.; Lu, N.; Matzuk, M.M. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 1997, 15, 201–204. [Google Scholar] [CrossRef]
- Themmen, A.P.N.; Huhtaniemi, I.T. Mutations of gonadotropins and gonadotropin receptors: Elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr. Rev. 2000, 21, 551–583. [Google Scholar] [CrossRef]
- Dierich, A.; Sairam, M.R.; Monaco, L.; Fimia, G.M.; Gansmuller, A.; LeMeur, M.; Sassone-Corsi, P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. USA 1998, 95, 13612–13617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of Follicle-Stimulating Hormone in Spermatogenesis. Front. Endocrinol. 2018, 9, 763. [Google Scholar] [CrossRef] [Green Version]
- Santi, D.; Crépieux, P.; Reiter, E.; Spaggiari, G.; Brigante, G.; Casarini, L.; Rochira, V.; Simoni, M. Follicle-stimulating Hormone (FSH) Action on Spermatogenesis: A Focus on Physiological and Therapeutic Roles. J. Clin. Med. 2020, 9, 1014. [Google Scholar] [CrossRef] [Green Version]
- Ben-Menahem, D. Preparation, characterization and application of long-acting FSH analogs for assisted reproduction. Theriogenology 2018, 112, 11–17. [Google Scholar] [CrossRef]
- Lunenfeld, B. Historical perspectives in gonadotrophin therapy. Hum. Reprod. Update 2004, 10, 453–467. [Google Scholar] [CrossRef] [Green Version]
- Lunenfeld, B.; Bilger, W.; Longobardi, S.; Alam, V.; Sunkara, S.K. The Development of Gonadotropins for Clinical Use in the Treatment of Infertility. Front. Endocrinol. 2019, 10, 429–443. [Google Scholar] [CrossRef]
- Bergandi, L.; Canosa, S.; Carosso, A.R.; Paschero, C.; Gennarelli, G.; Silvagno, F.; Benedetto, C.; Revelli, A. Human Recombinant FSH and Its Biosimilars: Clinical Efficacy, Safety, and Cost-Effectiveness in Controlled Ovarian Stimulation for In Vitro Fertilization. Pharmaceuticals 2020, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Bousfield, G.R.; May, J.V.; Davis, J.S.; Dias, J.A.; Kumar, T.R. In Vivo and In Vitro Impact of Carbohydrate Variation on Human Follicle-Stimulating Hormone Function. Front. Endocrinol. 2018, 9, 216–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vloeberghs, V.; Peeraer, K.; Pexsters, A.; D’Hooghe, T. Ovarian hyperstimulation syndrome and complications of ART. Best Pract. Res. Clin. Obstet. Gynaecol. 2009, 23, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Santa Coloma, T.A.; Dattatreyamurty, B.; Reichert, L.E., Jr. A synthetic peptide corresponding to human FSH beta-subunit 33–53 binds to FSH receptor, stimulates basal estradiol biosynthesis, and is a partial antagonist of FSH. Biochemistry 1990, 29, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Grasso, P.; Crabb, J.W.; Reichert, L.E., Jr. An explanation for the disparate effects of synthetic peptides corresponding to human follicle-stimulating hormone beta-subunit receptor binding regions (33–53) and (81–95) and their serine analogs on steroidogenesis in cultured rat Sertoli cells. Biochem. Biophys. Res. Commun. 1993, 190, 56–62. [Google Scholar] [CrossRef]
- Santa Coloma, T.A.; Reichert, L.E., Jr. Identification of a follicle-stimulating hormone receptor-binding region in hFSH-beta-(81–95) using synthetic peptides. J. Biol. Chem. 1990, 265, 5037–5042. [Google Scholar] [CrossRef]
- Grasso, P.; Reichert, L.E., Jr. In vivo effects of follicle-stimulating hormone-related synthetic peptides on the mouse estrous cycle. Endocrinology 1996, 137, 5370–5375. [Google Scholar] [CrossRef]
- Grasso, P.; Rozhavskaya, M.; Reichert, L.E., Jr. A synthetic peptide corresponding to amino acid residues 34 to 37 of human follicle-stimulating hormone beta-subunit accelerates the onset of puberty in male and female mice. Endocrinology 1997, 138, 4215–4219. [Google Scholar] [CrossRef]
- Grasso, P.; Rozhavskaya, M.; Reichert, L.E., Jr. In vivo effects of human follicle-stimulating hormone-related synthetic peptide hFSH-beta-(81–95) and its subdomain hFSH-beta-(90–95) on the mouse estrous cycle. Biol. Reprod. 1998, 58, 821–825. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Liu, P.; Yuen, T.; Haider, S.; He, J.; Romero, R.; Chen, H.; Bloch, M.; Kim, S.M.; Lizneva, D.; et al. Epitope-specific monoclonal antibodies to FSHβ increase bone mass. Proc. Natl. Acad. Sci. USA 2018, 115, 2192–2197. [Google Scholar] [CrossRef]
- Liu, P.; Ji, Y.; Yuen, T.; Rendina-Ruedy, E.; DeMambro, V.E.; Dhawan, S.; Abu-Amer, W.; Izadmehr, S.; Zhou, B.; Shin, A.C.; et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 2017, 546, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guan, Z.; Xu, M.; Zhang, Y.; Yao, H.; Meng, F.; Zhuo, Y.; Yu, G.; Cao, X.; Du, X.; et al. A novel follicle-stimulating hormone vaccine for controlling fat accumulation. Theriogenology 2020, 148, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yao, H.; Li, J.; Zhuo, Y.; Yu, G.; Bu, G.; Cao, X.; Du, X.; Liang, Q.; Zeng, X.; et al. Effects of active immunization against a 13-amino acid receptor-binding epitope of FSHβ on fertility regulation in female mice. Reprod. Biol. 2022, 22, 100669. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Zhuo, Y.; Jiang, D.; Li, J.; Huang, X.; Zhu, Y.; Li, Z.; Yan, L.; Jin, C.; Jiang, X.; et al. Identification of hepatic fibroblast growth factor 21 as a mediator in 17β-estradiol-induced white adipose tissue browning. FASEB J. 2018, 32, 5602–5611. [Google Scholar] [CrossRef] [Green Version]
- Walker, W.H.; Cheng, J. FSH and testosterone signaling in Sertoli cells. Reproduction 2005, 130, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Godmann, M.; Katz, J.P.; Guillou, F.; Simoni, M.; Kaestner, K.H.; Behr, R. Krüppel-like factor 4 is involved in functional differentiation of testicular Sertoli cells. Dev. Biol. 2008, 315, 552–566. [Google Scholar] [CrossRef] [Green Version]
- Sridharan, S.; Simon, L.; Meling, D.D.; Cyr, D.G.; Gutstein, D.E.; Fishman, G.I.; Guillou, F.; Cooke, P.S. Proliferation of adult sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol. Reprod. 2007, 76, 804–812. [Google Scholar] [CrossRef] [Green Version]
- Yeste, M.; Morató, R.; Rodríguez-Gil, J.E.; Bonet, S.; Prieto-Martínez, N. Aquaporins in the male reproductive tract and sperm: Functional implications and cryobiology. Reprod. Domest. Anim. 2017, 52 (Suppl. 4), 12–27. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Rajareddy, S.; Reddy, P.; Du, C.; Jagarlamudi, K.; Shen, Y.; Gunnarsson, D.; Selstam, G.; Boman, K.; Liu, K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development 2007, 134, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Rajkovic, A.; Pangas, S.A.; Ballow, D.; Suzumori, N.; Matzuk, M.M. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004, 305, 1157–1159. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Visser, J.A.; Themmen, A.P. Regulation of ovarian function: The role of anti-Müllerian hormone. Reproduction 2002, 124, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Gifford, J.A. The role of WNT signaling in adult ovarian folliculogenesis. Reproduction 2015, 150, R137–R148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicinski, P.; Donaher, J.L.; Geng, Y.; Parker, S.B.; Gardner, H.; Park, M.Y.; Robker, R.L.; Richards, J.S.; McGinnis, L.K.; Biggers, J.D.; et al. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 1996, 384, 470–474. [Google Scholar] [CrossRef]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, J.S.; Pangas, S.A. The ovary: Basic biology and clinical implications. J. Clin. Investig. 2010, 120, 963–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, Q.; Liu, R.; Yang, C.; Wang, X.; Ran, Z.; Zhou, S.; Li, X.; He, C. A Prepubertal Mice Model to Study the Growth Pattern of Early Ovarian Follicles. Int. J. Mol. Sci. 2021, 22, 5130. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5–19. [Google Scholar] [CrossRef] [Green Version]
- Taneja, C.; Gera, S.; Kim, S.M.; Iqbal, J.; Yuen, T.; Zaidi, M. FSH-metabolic circuitry and menopause. J. Mol. Endocrinol. 2019, 63, R73–R80. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, W.; Li, P.; Wei, J.; Cheng, Y.; Liu, P.; Yan, Q.; Xu, X.; Cui, Y.; Gu, Z.; et al. Follicular Stimulating Hormone Accelerates Atherogenesis by Increasing Endothelial VCAM-1 Expression. Theranostics 2017, 7, 4671–4688. [Google Scholar] [CrossRef]
- Kumar, A.; Alhassan, M.; Lopez, J.; Albericio, F.; de la Torre, B.G. N-Butylpyrrolidinone for Solid-Phase Peptide Synthesis is Environmentally Friendlier and Synthetically Better than DMF. ChemSusChem 2020, 13, 5288–5294. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Aguirre, A.; Zariñán, T.; Jardón-Valadez, E.; Gutiérrez-Sagal, R.; Dias, J.A. Structure-Function Relationships of the Follicle-Stimulating Hormone Receptor. Front. Endocrinol. 2018, 9, 707–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franca, M.M.; Han, X.; Funari, M.F.A.; Lerario, A.M.; Nishi, M.Y.; Fontenele, E.G.P.; Domenice, S.; Jorge, A.A.L.; Garcia-Galiano, D.; Elias, C.F.; et al. Exome Sequencing Reveals the POLR3H Gene as a Novel Cause of Primary Ovarian Insufficiency. J. Clin. Endocrinol. Metab. 2019, 104, 2827–2841. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Li, L.; Liu, H.; Sun, S.C. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy 2017, 13, 1364–1385. [Google Scholar] [CrossRef]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef]









| Gene | Genbank Accession No. | Primer Sequence (5′–3′) | Amplification Length (bp) |
|---|---|---|---|
| Fshr | NM_013523.3 | F: GTGCATTCAACGGAACCCAG R: TCTAAGCCATGGTTGGGCAG | 154 |
| Lhcgr | M81310.1 | F: CTGAAAACTCTGCCCTCCAG R: AATCGTAATCCCAGCCACTG | 281 |
| Star | NM_011485.5 | F: TCCCTCGCAGGACCTTGATCT R: TGGATGGGTCAAGTTCGACG | 337 |
| Cyp11a1 | AF195119.1 | F: AGGTCCTTCAATGAGATCCCTT R: TCCCTGTAAATGGGGCCATAC | 137 |
| Hsd3β1 | AK147114.1 | F: GCGGCTGCTGCACAGGAATA R: GACGCATGCCTGCTTCGTGA | 99 |
| Cyp17a1 | NM_007809.3 | F: GATCGGTTTATGCCTGAGCG R: TCCGAAGGGCAAATAACTGG | 81 |
| Cyp19a1 | BC103670.1 | F: CGGGCTACGTGGATGTGTT R: GAGCTTGCCAGGCGTTAAAG | 135 |
| Creb | X67728.1 | F: ACTGGCTTGGCACAACCAGA R: GGCAGAAGTCTCTTCATGATT | 202 |
| Wnt2 | NM_023653.5 | F: GCTGAAGTCCTGCTCCTGTG R: CGGTTGTTGTGGAGGTTCAT | 183 |
| Klf4 | JF277566.1 | F: TGGTGCAGCTTGCAGCAGT R: TGGGTTAGCGAGTTGGAAAGG | 108 |
| Gja1 | XM_036155617.1 | F: TTACAACAAGCAAGCCAGCG R: CGTCAGGGAAATCAAACGGC | 119 |
| Aqp8 | AF018952.1 | F: TTGCTACCTTGGGGAACATC R: CCAAATAGCTGGGAGATCCA | 121 |
| Erα | AB560752.1 | F: GCTCCTAACTTGCTCCTGGAC R: CAGCAACATGTCAAAGATCTCC | 75 |
| Inha | NM_010564.5 | F:TGAACCAGAGGAGGAAGATGTCTC R:GTCACTGGTCAACTCCAGCAC | 82 |
| Amh | NM_007445.3 | F:ATCTGGCTGAAGTGATATGG R:CAGGGTATAGCACTAACAGG | 105 |
| Foxo3a | NM_001376967.1 | F:ACTGAGGAAAGGGGAAATGG R:CAAAGGTGTCAAGCTGTAAACG | 123 |
| Bmp15 | NM_009757.5 | F:GCACGATTGGAGCGAAAATG R:CGTACGCTACCTGGTTTGATGC | 123 |
| Cdh1 | NM_007988.3 | F: TTGGTGTGGGTCAGGAAATC R: GTGTCCCTCCAAATCCGATAC | 91 |
| Ar | NM_013476.4 | F: GGCAGTCATTCAGTATTCC R: AGTAGAGCATCCTAGAGTTG | 89 |
| Ccnd2 | XM_036165787.1 | F: CAGAAGGACATCCAGCCGTAC R: TCGGGACTCCAGCCAAGAA | 136 |
| Nobox | XM_030255220.1 | F:CTATCCTGACAGTGACAAACGCC R:CACCCTCTCAGCACCCTCATTAT | 331 |
| 18S | NR_003278.3 | F: TGACTCAACACGGGAAACCT R: AACCAGACAAATCGCTCCAC | 125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Bai, X.; Yao, H.; Chen, W.; Meng, F.; Cao, X.; Zhuo, Y.; Hua, L.; Bu, G.; Du, X.; et al. Two Synthetic Peptides Corresponding to the Human Follicle-Stimulating Hormone β-Subunit Promoted Reproductive Functions in Mice. Int. J. Mol. Sci. 2022, 23, 11735. https://doi.org/10.3390/ijms231911735
Han X, Bai X, Yao H, Chen W, Meng F, Cao X, Zhuo Y, Hua L, Bu G, Du X, et al. Two Synthetic Peptides Corresponding to the Human Follicle-Stimulating Hormone β-Subunit Promoted Reproductive Functions in Mice. International Journal of Molecular Sciences. 2022; 23(19):11735. https://doi.org/10.3390/ijms231911735
Chicago/Turabian StyleHan, Xingfa, Xinyu Bai, Huan Yao, Weihao Chen, Fengyan Meng, Xiaohan Cao, Yong Zhuo, Lun Hua, Guixian Bu, Xiaogang Du, and et al. 2022. "Two Synthetic Peptides Corresponding to the Human Follicle-Stimulating Hormone β-Subunit Promoted Reproductive Functions in Mice" International Journal of Molecular Sciences 23, no. 19: 11735. https://doi.org/10.3390/ijms231911735
APA StyleHan, X., Bai, X., Yao, H., Chen, W., Meng, F., Cao, X., Zhuo, Y., Hua, L., Bu, G., Du, X., Liang, Q., & Zeng, X. (2022). Two Synthetic Peptides Corresponding to the Human Follicle-Stimulating Hormone β-Subunit Promoted Reproductive Functions in Mice. International Journal of Molecular Sciences, 23(19), 11735. https://doi.org/10.3390/ijms231911735








