Cytokine Storm—Definition, Causes, and Implications
Abstract
1. Introduction
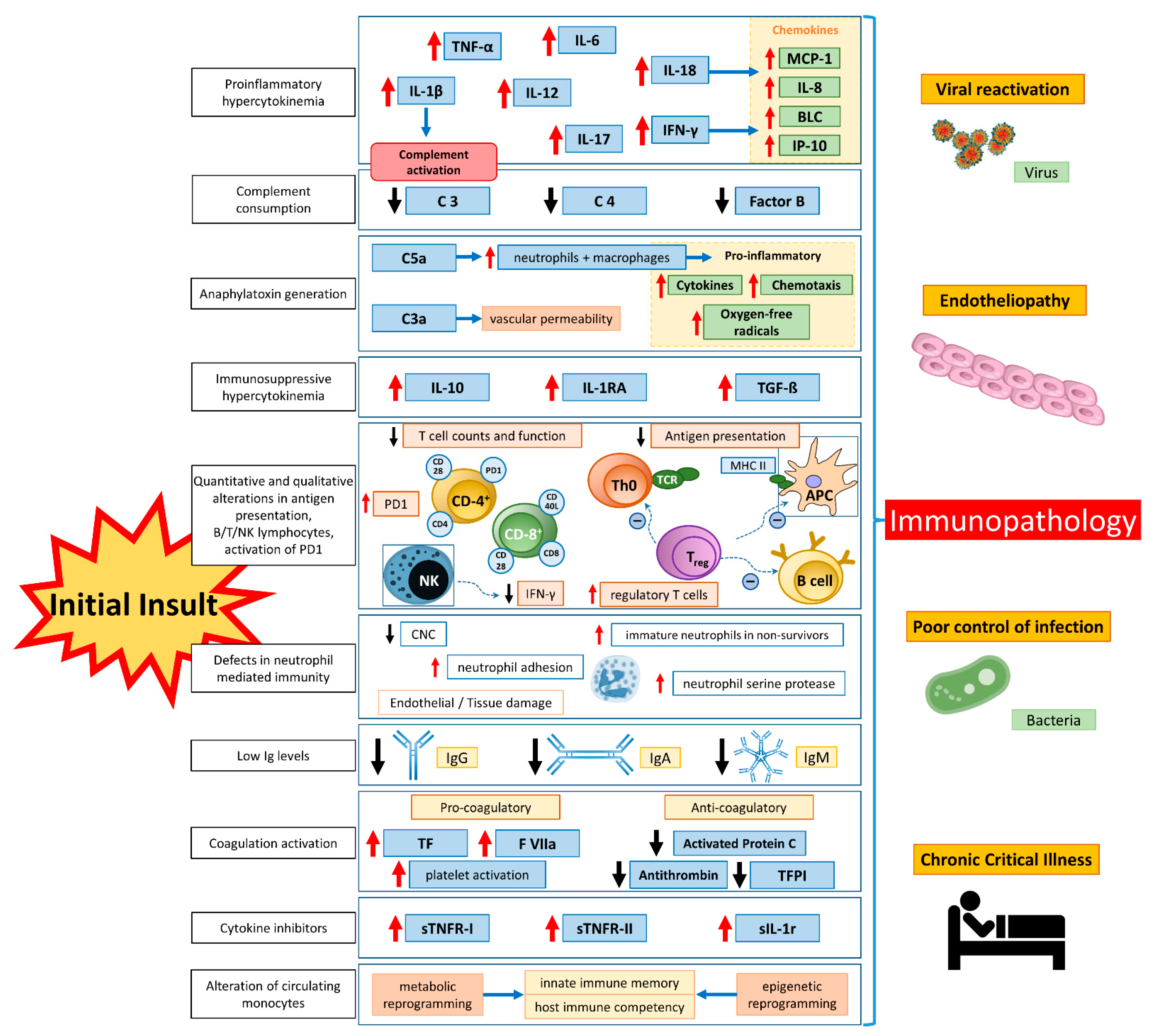
2. Pathophysiology of CS
3. Inflammation Due to Sepsis
4. Unleashing the Cytokine Cascade
4.1. Sepsis
4.2. Post-Cardiac Arrest Syndrome
4.3. Endotoxin
4.4. Post-Splenectomy Syndrome
4.5. Invasive Meningococcal Disease (IMD)
4.6. Viral and Parasitic Infections
4.7. Sterile Inflammation and Iatrogenic CS
5. After the Storm
6. Viral- vs. Non-Viral-Induced CS
7. Age-Related Changes of the Immune System
8. Endotypes, GWAS, and Transcriptome Analysis
9. Future Perspectives
- (a)
- Preclinical models for both basic and translational research need to be improved; current animal models do not adequately represent inflammation. New models are needed to comprehensively represent the complex processes and numerous feedback loops and redundant pathways should be considered. The individual reactions of the components of the immune system within different compartments need to be further elucidated. Local microenvironments with different molecular and cellular characteristics can result in different polarizations of the immune response in different tissues and organs as mentioned above. It has been shown that the ex vivo phenotype of leukocytes is strongly dependent on their compartment of origin. Blood leukocytes may show limited proliferation and secretion capacity, with reduced expression of HLA-DR mRNA in monocytes, while recruited monocytes in the lungs express greater than threefold more HLA-DR on their membrane [208,209]. Further, lung, spleen, and peritoneal macrophages have disparate transcriptomes and cell surface marker expression [210];
- (b)
- Development of a personalized approach combining clinical phenotypes, endotypes, and biomarkers, deciphered through the combination of “-omics” technologies and artificial intelligence (AI), is needed.
- (c)
- Further, there is a significant need for diagnostic testing procedures that can quickly and reliably help differentiate, for example, between bacterial and viral bases and, thus, possibly guide the use of anti-infectives and ultimately counteract the development of resistance. The current routine involves culture, isolation, and identification of pathogens from patient material—a time-consuming and not necessarily successful process. Contamination, mixed cultures, and unsuccessful cultivation are common risks. In addition, depending on the (presumed) focus, obtaining appropriate samples is not always without risk (intrapulmonary, cerebrospinal fluid). Although detection and cultivation conditions are constantly being developed, the method of direct detection, e.g., by PCR or immunoassays, also has limitations—previously unknown variants may evade detection and the method cannot reliably distinguish between already dysfunctional/dead pathogens and inflammatory-active material [216,217]. Further research and an increased understanding of the interrelationships between what has been considered viral and bacterial defense cascades may lead to further blurring of the boundaries and to therapeutic options in which the exact origin of inflammation may turn out to be less relevant [218,219].
10. Summary
- We support the comprehensive and clinical definition of CS that was recently suggested by Fajgenbaum and June [5]:
- (i)
- Elevated circulating cytokine levels;
- (ii)
- Acute systemic inflammatory symptoms;
- (iii)
- Severe secondary organ dysfunction.
- In CS, both excessive hyperinflammation and uncontrolled anti-inflammation occur simultaneously. Survivors of the initial phase often develop acquired immunosuppression, which is an additional risk factor for unfavorable outcomes and long-term morbidity;
- A wide array of infectious and non-infectious disease may cause CS; the most common cause is sepsis due to invasive microbial infection;
- The biomarker signature profiles of different types of CS are rather distinct. However, to use these signatures to diagnose the origin of CS is currently not yet feasible;
- CS characteristics differ between different compartments (blood, CSF, pleural effusion, lung tissue, etc.) In clinical routine, the blood compartment is almost exclusively used for analysis; this may lead to wrong conclusions and misconceptions of the underlying pathophysiology;
- The earliest possible detection of CS is of outstanding importance, as this may be related to therapeutic decisions and, ultimately, prognosis and outcome;
- Transcriptome analysis and GWAS represent promising opportunities for future development. These techniques enable improved knowledge of different phenotypes and may help to implement “precision medicine”, very similar to what is already done in modern oncology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| ALL | acute lymphocytic leukemia |
| AP-1 | activator protein-1 |
| APC | antigen-presenting cell |
| ARDS | acute respiratory distress syndrome |
| BBB | blood–brain barrier |
| CAM | cell adhesion molecule |
| CARS | compensatory anti-inflammatory response syndrome |
| CAR-T cell | chimeric antigen receptor-T cell |
| CMV | cytomegalovirus |
| COVID-19 | coronavirus disease 2019 |
| CRP | C-reactive protein |
| CRS | cytokine release syndrome |
| CS | cytokine storm |
| DAMP | damage-associated molecular pattern |
| DIC | disseminated intravascular coagulation |
| DNA | deoxyribonucleic acid |
| EBV | Epstein–Barr virus |
| fHbp | factor H-binding protein |
| GWAS | genome-wide association studies |
| GvHD | graft-versus-host disease |
| HLA-DR | human leukocyte antigen-DR |
| HLH | hemophagocytic lymphohistiocytosis |
| ICU | intensive care unit |
| IHCA | in-hospital cardiac arrest |
| IFN | interferon |
| IL | interleukin |
| IMD | invasive meningococcal disease |
| ISG | interferon-stimulated gene |
| ISTH | International Society on Thrombosis and Haemostasis |
| JAK | Janus kinase |
| LOS | lipooligosaccharide |
| LPS | lipopolysaccharide |
| MAS | macrophage activation syndrome |
| MDSC | myeloid-derived suppressor cell |
| MHC II | major histocompatibility complex II |
| MIF | migration inhibitory factor |
| NET | neutrophil extracellular traps |
| NHL | non-Hodgkin lymphoma |
| NF-κ | nuclear factor kappa-light-chain-enhancer |
| NK cell | natural killer cell |
| OHCA | out-of-hospital cardiac arrest |
| OPSI | overwhelming post-splenectomy infection |
| PAMP | pathogen-associated molecular pattern |
| PCAS | post-cardiac arrest syndrome |
| PCI | persistent critical illness |
| PCT | procalcitonin |
| PICS | persistent inflammation, immunosuppression, and catabolism |
| PRR | pattern recognition receptors |
| PT-INR | prothrombin time-International Normalized Ratio |
| ROSC | return of spontaneous circulation |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus type 2 |
| SIC | sepsis-induced coagulopathy |
| SIRS | systemic inflammatory response syndrome |
| STAT | signal transducer and activator of transcription |
| T4P | type IV pili |
| TF | tissue factor |
| TFPI | tissue factor pathway inhibitor |
| TGF-β | transforming growth factor β |
| TIR | Toll–interleukin-1 receptor domain |
| TLR | Toll-like receptor |
| TNF | tumor necrosis factor |
References
- Ferrara, J.L.; Abhyankar, S.; Gilliland, D.G. Cytokine storm of graft-versus-host disease: A critical effector role for interleukin-1. Transplant. Proc. 1993, 25, 1216–1217. [Google Scholar] [PubMed]
- Chatenoud, L.; Ferran, C.; Bach, J.F. The anti-CD3-induced syndrome: A consequence of massive in vivo cell activation. Curr. Top. Microbiol. Immunol. 1991, 174, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Marshall, J.C.; Reinhart, K.; International Sepsis, F. Biomarkers of sepsis. Crit. Care Med. 2009, 37, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Biron, B.M.; Ayala, A.; Lomas-Neira, J.L. Biomarkers for Sepsis: What Is and What Might Be? Biomark Insights 2015, 10, 7–17. [Google Scholar] [CrossRef]
- Cavaillon, J.M.; Annane, D. Compartmentalization of the inflammatory response in sepsis and SIRS. J. Endotoxin. Res. 2006, 12, 151–170. [Google Scholar] [CrossRef]
- Cavaillon, J.M.; Munoz, C.; Fitting, C.; Misset, B.; Carlet, J. Circulating cytokines: The tip of the iceberg? Circ. Shock. 1992, 38, 145–152. [Google Scholar]
- Kweon, O.J.; Choi, J.H.; Park, S.K.; Park, A.J. Usefulness of presepsin (sCD14 subtype) measurements as a new marker for the diagnosis and prediction of disease severity of sepsis in the Korean population. J. Crit. Care 2014, 29, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, Y.; Li, C.; Liu, C.; Yao, Y.; Su, M.; Shou, S. The utility of presepsin in diagnosis and risk stratification for the emergency patients with sepsis. Am. J. Emerg. Med. 2018, 36, 1341–1345. [Google Scholar] [CrossRef]
- Aksaray, S.; Alagoz, P.; Inan, A.; Cevan, S.; Ozgultekin, A. Diagnostic value of sTREM-1 and procalcitonin levels in the early diagnosis of sepsis. N. Clin. Istanb. 2016, 3, 175–182. [Google Scholar] [CrossRef][Green Version]
- Brenner, T.; Uhle, F.; Fleming, T.; Wieland, M.; Schmoch, T.; Schmitt, F.; Schmidt, K.; Zivkovic, A.R.; Bruckner, T.; Weigand, M.A.; et al. Soluble TREM-1 as a diagnostic and prognostic biomarker in patients with septic shock: An observational clinical study. Biomarkers 2017, 22, 63–69. [Google Scholar] [CrossRef]
- Khater, W.S.; Salah-Eldeen, N.N.; Khater, M.S.; Saleh, A.N. Role of suPAR and Lactic Acid in Diagnosing Sepsis and Predicting Mortality in Elderly Patients. Eur. J. Microbiol. Immunol. (Bp.) 2016, 6, 178–185. [Google Scholar] [CrossRef]
- Yin, W.P.; Li, J.B.; Zheng, X.F.; An, L.; Shao, H.; Li, C.S. Effect of neutrophil CD64 for diagnosing sepsis in emergency department. World J. Emerg. Med. 2020, 11, 79–86. [Google Scholar] [CrossRef]
- Larsson, A.; Tyden, J.; Johansson, J.; Lipcsey, M.; Bergquist, M.; Kultima, K.; Mandic-Havelka, A. Calprotectin is superior to procalcitonin as a sepsis marker and predictor of 30-day mortality in intensive care patients. Scand. J. Clin. Lab. Investig. 2020, 80, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Spoto, S.; Cella, E.; de Cesaris, M.; Locorriere, L.; Mazzaroppi, S.; Nobile, E.; Lanotte, A.M.; Pedicino, L.; Fogolari, M.; Costantino, S.; et al. Procalcitonin and MR-Proadrenomedullin Combination with SOFA and qSOFA Scores for Sepsis Diagnosis and Prognosis: A Diagnostic Algorithm. Shock 2018, 50, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.; Behnes, M.; Pauly, D.; Lepiorz, D.; Barre, M.; Becher, T.; Lang, S.; Akin, I.; Borggrefe, M.; Bertsch, T.; et al. Diagnostic value of Pentraxin-3 in patients with sepsis and septic shock in accordance with latest sepsis-3 definitions. BMC Infect. Dis. 2017, 17, 554. [Google Scholar] [CrossRef]
- Casagranda, I.; Vendramin, C.; Callegari, T.; Vidali, M.; Calabresi, A.; Ferrandu, G.; Cervellin, G.; Cavazza, M.; Lippi, G.; Zanotti, I.; et al. Usefulness of suPAR in the risk stratification of patients with sepsis admitted to the emergency department. Intern. Emerg. Med. 2015, 10, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, X.; Kong, G.; Liu, X.; Tian, H.; Lyu, B.; Ning, F.; Wang, T.; Hao, D. Significance of high mobility group box 1, von Willebrand factor and other cytokines in the evaluation of severity and prognosis of sepsis patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020, 32, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Andaluz-Ojeda, D.; Nguyen, H.B.; Meunier-Beillard, N.; Cicuendez, R.; Quenot, J.P.; Calvo, D.; Dargent, A.; Zarca, E.; Andres, C.; Nogales, L.; et al. Superior accuracy of mid-regional proadrenomedullin for mortality prediction in sepsis with varying levels of illness severity. Ann. Intensive Care 2017, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hur, M.; Struck, J.; Bergmann, A.; Di Somma, S. Circulating Biologically Active Adrenomedullin Predicts Organ Failure and Mortality in Sepsis. Ann. Lab. Med. 2019, 39, 454–463. [Google Scholar] [CrossRef]
- Seol, C.H.; Yong, S.H.; Shin, J.H.; Lee, S.H.; Leem, A.Y.; Park, M.S.; Kim, Y.S.; Chung, K.S. The ratio of plasma angiopoietin-2 to angiopoietin-1 as a prognostic biomarker in patients with sepsis. Cytokine 2020, 129, 155029. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, C.; Shao, R.; Yu, H.; Zhang, Q.; Zhao, L. Prognostic significance of the angiopoietin-2/angiopoietin-1 and angiopoietin-1/Tie-2 ratios for early sepsis in an emergency department. Crit. Care 2015, 19, 367. [Google Scholar] [CrossRef]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transpl. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Rouce, R.H. The earlier the better: Timely mitigation of CRS. Blood 2019, 134, 2119–2120. [Google Scholar] [CrossRef] [PubMed]
- Iskander, K.N.; Osuchowski, M.F.; Stearns-Kurosawa, D.J.; Kurosawa, S.; Stepien, D.; Valentine, C.; Remick, D.G. Sepsis: Multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol. Rev. 2013, 93, 1247–1288. [Google Scholar] [CrossRef] [PubMed]
- Brands, X.; Haak, B.W.; Klarenbeek, A.M.; Otto, N.A.; Faber, D.R.; Lutter, R.; Scicluna, B.P.; Wiersinga, W.J.; van der Poll, T. Concurrent Immune Suppression and Hyperinflammation in Patients With Community-Acquired Pneumonia. Front. Immunol. 2020, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M.; Adib-Conquy, M.; Fitting, C.; Adrie, C.; Payen, D. Cytokine cascade in sepsis. Scand. J. Infect. Dis. 2003, 35, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; van Engelen, T.S.R.; Alp, E.; Calandra, T.; Cattoir, V.; Kern, W.V.; Netea, M.G.; Nseir, S.; Opal, S.M.; van de Veerdonk, F.L.; et al. Towards precision medicine in sepsis: A position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2018, 24, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Barochia, A.; Solomon, S.; Cui, X.; Natanson, C.; Eichacker, P.Q. Eritoran tetrasodium (E5564) treatment for sepsis: Review of preclinical and clinical studies. Expert Opin. Drug Metab. Toxicol. 2011, 7, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Kong, L.; Fink, M.P.; Weissfeld, L.A.; Yealy, D.M.; Pinsky, M.R.; Fine, J.; Krichevsky, A.; Delude, R.L.; Angus, D.C.; et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch. Intern. Med. 2007, 167, 1655–1663. [Google Scholar] [CrossRef]
- van der Poll, T.; Shankar-Hari, M.; Wiersinga, W.J. The immunology of sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar] [CrossRef]
- Kuypers, F.A. Hyperinflammation, apoptosis, and organ damage. Exp. Biol. Med. 2022, 247, 15353702221090454. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Fajgenbaum, D.C. Overview of Castleman disease. Blood 2020, 135, 1353–1364. [Google Scholar] [CrossRef]
- Stearns-Kurosawa, D.J.; Osuchowski, M.F.; Valentine, C.; Kurosawa, S.; Remick, D.G. The pathogenesis of sepsis. Annu. Rev. Pathol. 2011, 6, 19–48. [Google Scholar] [CrossRef]
- Lykens, J.E.; Terrell, C.E.; Zoller, E.E.; Risma, K.; Jordan, M.B. Perforin is a critical physiologic regulator of T-cell activation. Blood 2011, 118, 618–626. [Google Scholar] [CrossRef]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Tamayo, E.; Fernandez, A.; Almansa, R.; Carrasco, E.; Heredia, M.; Lajo, C.; Goncalves, L.; Gomez-Herreras, J.I.; de Lejarazu, R.O.; Bermejo-Martin, J.F. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur. Cytokine Netw. 2011, 22, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed]
- Walton, A.H.; Muenzer, J.T.; Rasche, D.; Boomer, J.S.; Sato, B.; Brownstein, B.H.; Pachot, A.; Brooks, T.L.; Deych, E.; Shannon, W.D.; et al. Reactivation of multiple viruses in patients with sepsis. PLoS ONE 2014, 9, e98819. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Zucoloto, A.Z.; Jenne, C.N. Platelet-Neutrophil Interplay: Insights Into Neutrophil Extracellular Trap (NET)-Driven Coagulation in Infection. Front. Cardiovasc. Med. 2019, 6, 85. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef]
- Lu, T.; Kobayashi, S.D.; Quinn, M.T.; Deleo, F.R. A NET Outcome. Front. Immunol. 2012, 3, 365. [Google Scholar] [CrossRef]
- Czaikoski, P.G.; Mota, J.M.; Nascimento, D.C.; Sonego, F.; Castanheira, F.V.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Barroso-Sousa, R.; Souto, F.O.; et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE 2016, 11, e0148142. [Google Scholar] [CrossRef]
- Daix, T.; Guerin, E.; Tavernier, E.; Mercier, E.; Gissot, V.; Herault, O.; Mira, J.P.; Dumas, F.; Chapuis, N.; Guitton, C.; et al. Multicentric Standardized Flow Cytometry Routine Assessment of Patients With Sepsis to Predict Clinical Worsening. Chest 2018, 154, 617–627. [Google Scholar] [CrossRef]
- Cox, L.E.; Walstein, K.; Vollger, L.; Reuner, F.; Bick, A.; Dotsch, A.; Engler, A.; Peters, J.; von Kockritz-Blickwede, M.; Schafer, S.T. Neutrophil extracellular trap formation and nuclease activity in septic patients. BMC Anesthesiol. 2020, 20, 15. [Google Scholar] [CrossRef]
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PLoS ONE 2012, 7, e48111. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.A.; Gao, H. Sepsis, complement and the dysregulated inflammatory response. J. Cell. Mol. Med. 2009, 13, 4154–4160. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Denk, S.; Taylor, R.P.; Wiegner, R.; Cook, E.M.; Lindorfer, M.A.; Pfeiffer, K.; Paschke, S.; Eiseler, T.; Weiss, M.; Barth, E.; et al. Complement C5a-Induced Changes in Neutrophil Morphology During Inflammation. Scand. J. Immunol. 2017, 86, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Oehmcke-Hecht, S.; Kohler, J. Interaction of the Human Contact System with Pathogens-An Update. Front. Immunol. 2018, 9, 312. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- Delvaeye, M.; Conway, E.M. Coagulation and innate immune responses: Can we view them separately? Blood 2009, 114, 2367–2374. [Google Scholar] [CrossRef]
- Taylor, F.B., Jr.; Toh, C.H.; Hoots, W.K.; Wada, H.; Levi, M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001, 86, 1327–1330. [Google Scholar] [CrossRef]
- Iba, T.; Levi, M.; Levy, J.H. Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. Semin. Thromb. Hemost. 2020, 46, 89–95. [Google Scholar] [CrossRef]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascon, G.A.; Hernandez, G.; Murray, P.; De Backer, D.; Workgroup, A.X. The Endothelium in Sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Alouf, J.E.; Muller-Alouf, H. Staphylococcal and streptococcal superantigens: Molecular, biological and clinical aspects. Int. J. Med. Microbiol. 2003, 292, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Proft, T.; Fraser, J.D. Streptococcal Superantigens: Biological properties and potential role in disease. In Streptococcus Pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma City Science Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Sriskandan, S.; Moyes, D.; Cohen, J. Detection of circulating bacterial superantigen and lymphotoxin-alpha in patients with streptococcal toxic-shock syndrome. Lancet 1996, 348, 1315–1316. [Google Scholar] [CrossRef]
- Zhang, M.; Bracaglia, C.; Prencipe, G.; Bemrich-Stolz, C.J.; Beukelman, T.; Dimmitt, R.A.; Chatham, W.W.; Zhang, K.; Li, H.; Walter, M.R.; et al. A Heterozygous RAB27A Mutation Associated with Delayed Cytolytic Granule Polarization and Hemophagocytic Lymphohistiocytosis. J. Immunol. 2016, 196, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Terrell, C.E.; Jordan, M.B. Perforin deficiency impairs a critical immunoregulatory loop involving murine CD8(+) T cells and dendritic cells. Blood 2013, 121, 5184–5191. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Nichol, G.; Thomas, E.; Callaway, C.W.; Hedges, J.; Powell, J.L.; Aufderheide, T.P.; Rea, T.; Lowe, R.; Brown, T.; Dreyer, J.; et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 2008, 300, 1423–1431. [Google Scholar] [CrossRef]
- Nolan, J.P.; Neumar, R.W.; Adrie, C.; Aibiki, M.; Berg, R.A.; Bottiger, B.W.; Callaway, C.; Clark, R.S.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008, 79, 350–379. [Google Scholar] [CrossRef] [PubMed]
- Adrie, C.; Adib-Conquy, M.; Laurent, I.; Monchi, M.; Vinsonneau, C.; Fitting, C.; Fraisse, F.; Dinh-Xuan, A.T.; Carli, P.; Spaulding, C.; et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation 2002, 106, 562–568. [Google Scholar] [CrossRef]
- Peberdy, M.A.; Andersen, L.W.; Abbate, A.; Thacker, L.R.; Gaieski, D.; Abella, B.S.; Grossestreuer, A.V.; Rittenberger, J.C.; Clore, J.; Ornato, J.; et al. Inflammatory markers following resuscitation from out-of-hospital cardiac arrest-A prospective multicenter observational study. Resuscitation 2016, 103, 117–124. [Google Scholar] [CrossRef]
- Araujo, D.M.; Cotman, C.W. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: Potential involvement of glial-derived factors. Brain Res. 1993, 600, 49–55. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Johansson, P.I.; Hassager, C.; Wanscher, M.; Ostrowski, S.R.; Bjerre, M.; Kjaergaard, J. Endothelial activation/injury and associations with severity of post-cardiac arrest syndrome and mortality after out-of-hospital cardiac arrest. Resuscitation 2016, 107, 71–79. [Google Scholar] [CrossRef]
- Niemann, J.T.; Rosborough, J.P.; Youngquist, S.; Shah, A.P.; Lewis, R.J.; Phan, Q.T.; Filler, S.G. Cardiac function and the proinflammatory cytokine response after recovery from cardiac arrest in swine. J. Interferon Cytokine Res. 2009, 29, 749–758. [Google Scholar] [CrossRef]
- Annborn, M.; Dankiewicz, J.; Erlinge, D.; Hertel, S.; Rundgren, M.; Smith, J.G.; Struck, J.; Friberg, H. Procalcitonin after cardiac arrest—An indicator of severity of illness, ischemia-reperfusion injury and outcome. Resuscitation 2013, 84, 782–787. [Google Scholar] [CrossRef]
- Chelazzi, C.; Villa, G.; Mancinelli, P.; De Gaudio, A.R.; Adembri, C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care 2015, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C. Sepsis, the sepsis syndrome, multi-organ failure: A plea for comparable definitions. Ann. Intern. Med. 1991, 114, 332–333. [Google Scholar] [CrossRef]
- Morrison, D.C.; Ryan, J.L. Endotoxins and disease mechanisms. Annu. Rev. Med. 1987, 38, 417–432. [Google Scholar] [CrossRef]
- van Deuren, M.; Brandtzaeg, P.; van der Meer, J.W. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 2000, 13, 144–166. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 and interleukin-1 antagonism. Blood 1991, 77, 1627–1652. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.I.; Ernst, R.K.; Bader, M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005, 3, 36–46. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Kadokami, T.; McTiernan, C.F.; Kubota, T.; Frye, C.S.; Bounoutas, G.S.; Robbins, P.D.; Watkins, S.C.; Feldman, A.M. Effects of soluble TNF receptor treatment on lipopolysaccharide-induced myocardial cytokine expression. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2281–H2291. [Google Scholar] [CrossRef]
- Hacham, M.; Cristal, N.; White, R.M.; Segal, S.; Apte, R.N. Complementary organ expression of IL-1 vs. IL-6 and CSF-1 activities in normal and LPS-injected mice. Cytokine 1996, 8, 21–31. [Google Scholar] [CrossRef]
- Taveira da Silva, A.M.; Kaulbach, H.C.; Chuidian, F.S.; Lambert, D.R.; Suffredini, A.F.; Danner, R.L. Brief report: Shock and multiple-organ dysfunction after self-administration of Salmonella endotoxin. N. Engl. J. Med. 1993, 328, 1457–1460. [Google Scholar] [CrossRef]
- Cowan, J.R.; Salyer, L.; Wright, N.T.; Kinnamon, D.D.; Amaya, P.; Jordan, E.; Bamshad, M.J.; Nickerson, D.A.; Hershberger, R.E. SOS1 Gain-of-Function Variants in Dilated Cardiomyopathy. Circ. Genom. Precis. Med. 2020, 13, e002892. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Stamatos, N.M.; Dragan, A.I.; Medvedev, A.; Whitford, M.; Zhang, L.; Song, C.; Rallabhandi, P.; Cole, L.; Nhu, Q.M.; et al. Sialyl residues modulate LPS-mediated signaling through the Toll-like receptor 4 complex. PLoS ONE 2012, 7, e32359. [Google Scholar] [CrossRef][Green Version]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- van Deuren, M.; van der Ven-Jongekrijg, J.; Bartelink, A.K.; van Dalen, R.; Sauerwein, R.W.; van der Meer, J.W. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J. Infect. Dis. 1995, 172, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Bjerre, A.; Ovstebo, R.; Brusletto, B.; Joo, G.B.; Kierulf, P. Neisseria meningitidis lipopolysaccharides in human pathology. J. Endotoxin. Res. 2001, 7, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Osnes, L.; Ovstebo, R.; Joo, G.B.; Westvik, A.B.; Kierulf, P. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: Identification of interleukin-10 as a major functional deactivator of human monocytes. J. Exp. Med. 1996, 184, 51–60. [Google Scholar] [CrossRef]
- Moller, A.S.; Bjerre, A.; Brusletto, B.; Joo, G.B.; Brandtzaeg, P.; Kierulf, P. Chemokine patterns in meningococcal disease. J. Infect. Dis. 2005, 191, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Zandvoort, A.; Timens, W. The dual function of the splenic marginal zone: Essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin. Exp. Immunol. 2002, 130, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M.; Lewis, M.P.; Wimperis, J.; Rafi, I.; Ladhani, S.; Bolton-Maggs, P.H. British Committee for Standards in Health Care, Review of guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen: Prepared on behalf of the British Committee for Standards in Haematology by a working party of the Haemato-Oncology task force. Br. J. Haematol. 2011, 155, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Luu, S.; Spelman, D.; Woolley, I.J. Post-splenectomy sepsis: Preventative strategies, challenges, and solutions. Infect. Drug Resist. 2019, 12, 2839–2851. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Ochani, M.; Parrish, W.R.; Ochani, K.; Harris, Y.T.; Huston, J.M.; Chavan, S.; Tracey, K.J. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. USA 2008, 105, 11008–11013. [Google Scholar] [CrossRef]
- Huston, J.M.; Ochani, M.; Rosas-Ballina, M.; Liao, H.; Ochani, K.; Pavlov, V.A.; Gallowitsch-Puerta, M.; Ashok, M.; Czura, C.J.; Foxwell, B.; et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 2006, 203, 1623–1628. [Google Scholar] [CrossRef]
- Cameron, P.U.; Jones, P.; Gorniak, M.; Dunster, K.; Paul, E.; Lewin, S.; Woolley, I.; Spelman, D. Splenectomy associated changes in IgM memory B cells in an adult spleen registry cohort. PLoS ONE 2011, 6, e23164. [Google Scholar] [CrossRef] [PubMed]
- Moeniralam, H.S.; Bemelman, W.A.; Endert, E.; Koopmans, R.; Sauerwein, H.P.; Romijn, J.A. The decrease in nonsplenic interleukin-6 (IL-6) production after splenectomy indicates the existence of a positive feedback loop of IL-6 production during endotoxemia in dogs. Infect. Immun. 1997, 65, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Kohno, H.; Yamamoto, M.; Iimuro, Y.; Fujii, H.; Matsumoto, Y. The role of splenic macrophages in plasma tumor necrosis factor levels in endotoxemia. Eur. Surg. Res. 1997, 29, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Francke, E.L.; Neu, H.C. Postsplenectomy infection. Surg. Clin. N. Am. 1981, 61, 135–155. [Google Scholar] [CrossRef]
- Lyczko, K.; Borger, J. Meningococcal Prophylaxis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Coureuil, M.; Bourdoulous, S.; Marullo, S.; Nassif, X. Invasive meningococcal disease: A disease of the endothelial cells. Trends Mol. Med. 2014, 20, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Deghmane, A.E.; Taha, S.; Taha, M.K. Global epidemiology and changing clinical presentations of invasive meningococcal disease: A narrative review. Infect. Dis. 2021, 54, 1971289. [Google Scholar] [CrossRef] [PubMed]
- Gabutti, G.; Stefanati, A.; Kuhdari, P. Epidemiology of Neisseria meningitidis infections: Case distribution by age and relevance of carriage. J. Prev. Med. Hyg. 2015, 56, E116–E120. [Google Scholar] [PubMed]
- Sridhar, S.; Greenwood, B.; Head, C.; Plotkin, S.A.; Safadi, M.A.; Saha, S.; Taha, M.K.; Tomori, O.; Gessner, B.D. Global incidence of serogroup B invasive meningococcal disease: A systematic review. Lancet Infect. Dis. 2015, 15, 1334–1346. [Google Scholar] [CrossRef]
- Reher, D.; Fuhrmann, V.; Kluge, S.; Nierhaus, A. A rare case of septic shock due to Neisseria meningitidis serogroup B infection despite prior vaccination in a young adult with paroxysmal nocturnal haemoglobinuria receiving eculizumab. Vaccine 2018, 36, 2507–2509. [Google Scholar] [CrossRef]
- Waage, A.; Brandtzaeg, P.; Halstensen, A.; Kierulf, P.; Espevik, T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J. Exp. Med. 1989, 169, 333–338. [Google Scholar] [CrossRef]
- Waage, A.; Espevik, T.; Lamvik, J. Detection of tumour necrosis factor-like cytotoxicity in serum from patients with septicaemia but not from untreated cancer patients. Scand. J. Immunol. 1986, 24, 739–743. [Google Scholar] [CrossRef]
- Girardin, E.; Grau, G.E.; Dayer, J.M.; Roux-Lombard, P.; Lambert, P.H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N. Engl. J. Med. 1988, 319, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Girardin, E.; Roux-Lombard, P.; Grau, G.E.; Suter, P.; Gallati, H.; Dayer, J.M. Imbalance between tumour necrosis factor-alpha and soluble TNF receptor concentrations in severe meningococcaemia. The J5 Study Group. Immunology 1992, 76, 20–23. [Google Scholar] [PubMed]
- van Deuren, M.; van der Ven-Jongekrijg, J.; Vannier, E.; van Dalen, R.; Pesman, G.; Bartelink, A.K.; Dinarello, C.A.; van der Meer, J.W. The pattern of interleukin-1beta (IL-1beta) and its modulating agents IL-1 receptor antagonist and IL-1 soluble receptor type II in acute meningococcal infections. Blood 1997, 90, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Riordan, F.A.; Marzouk, O.; Thomson, A.P.; Sills, J.A.; Hart, C.A. Proinflammatory and anti-inflammatory cytokines in meningococcal disease. Arch. Dis. Child 1996, 75, 453–454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coureuil, M.; Join-Lambert, O.; Lecuyer, H.; Bourdoulous, S.; Marullo, S.; Nassif, X. Pathogenesis of meningococcemia. Cold Spring Harb. Perspect. Med. 2013, 3, a012393. [Google Scholar] [CrossRef]
- Bille, E.; Ure, R.; Gray, S.J.; Kaczmarski, E.B.; McCarthy, N.D.; Nassif, X.; Maiden, M.C.; Tinsley, C.R. Association of a bacteriophage with meningococcal disease in young adults. PLoS ONE 2008, 3, e3885. [Google Scholar] [CrossRef]
- Capel, E.; Barnier, J.P.; Zomer, A.L.; Bole-Feysot, C.; Nussbaumer, T.; Jamet, A.; Lecuyer, H.; Euphrasie, D.; Virion, Z.; Frapy, E.; et al. Peripheral blood vessels are a niche for blood-borne meningococci. Virulence 2017, 8, 1808–1819. [Google Scholar] [CrossRef]
- Berry, J.L.; Pelicic, V. Exceptionally widespread nanomachines composed of type IV pilins: The prokaryotic Swiss Army knives. FEMS Microbiol. Rev. 2015, 39, 134–154. [Google Scholar] [CrossRef]
- Dos Santos Souza, I.; Ziveri, J.; Bouzinba-Segard, H.; Morand, P.; Bourdoulous, S. Meningococcus, this famous unknown. C. R. Biol. 2021, 344, 127–143. [Google Scholar] [CrossRef]
- Bernard, S.C.; Simpson, N.; Join-Lambert, O.; Federici, C.; Laran-Chich, M.P.; Maissa, N.; Bouzinba-Segard, H.; Morand, P.C.; Chretien, F.; Taouji, S.; et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat. Med. 2014, 20, 725–731. [Google Scholar] [CrossRef]
- Xiong, L.; Edwards, C.K., 3rd; Zhou, L. The biological function and clinical utilization of CD147 in human diseases: A review of the current scientific literature. Int. J. Mol. Sci. 2014, 15, 17411. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein. Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef]
- Huang, K.J.; Su, I.J.; Theron, M.; Wu, Y.C.; Lai, S.K.; Liu, C.C.; Lei, H.Y. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005, 75, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.W.; Lu, J.; Wu, C.L.; Yi, L.N.; Xie, X.; Shi, X.D.; Fang, S.S.; Zan, H.; Kung, H.F.; He, M.L. Three fatal cases of pandemic 2009 influenza A virus infection in Shenzhen are associated with cytokine storm. Respir. Physiol. Neurobiol. 2011, 175, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Guihot, A.; Luyt, C.E.; Parrot, A.; Rousset, D.; Cavaillon, J.M.; Boutolleau, D.; Fitting, C.; Pajanirassa, P.; Mallet, A.; Fartoukh, M.; et al. Low titers of serum antibodies inhibiting hemagglutination predict fatal fulminant influenza A(H1N1) 2009 infection. Am. J. Respir. Crit. Care Med. 2014, 189, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, C.; Zhan, F.; Wang, X.; Liang, M.; Zhang, Q.; Ding, S.; Guan, X.; Huo, X.; Li, C.; et al. Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J. Infect. Dis. 2012, 206, 1085–1094. [Google Scholar] [CrossRef]
- Rana, R.; Kant, R.; Kaul, D.; Sachdev, A.; Ganguly, N.K. Integrated view of molecular diagnosis and prognosis of dengue viral infection: Future prospect of exosomes biomarkers. Mol. Cell. Biochem. 2022, 477, 815–832. [Google Scholar] [CrossRef]
- Mandala, W.L.; Msefula, C.L.; Gondwe, E.N.; Drayson, M.T.; Molyneux, M.E.; MacLennan, C.A. Cytokine Profiles in Malawian Children Presenting with Uncomplicated Malaria, Severe Malarial Anemia, and Cerebral Malaria. Clin. Vaccine Immunol. CVI 2017, 24, e00533-16. [Google Scholar] [CrossRef]
- Santos-Oliveira, J.R.; Regis, E.G.; Leal, C.R.; Cunha, R.V.; Bozza, P.T.; Da-Cruz, A.M. Evidence that lipopolisaccharide may contribute to the cytokine storm and cellular activation in patients with visceral leishmaniasis. PLoS Negl. Trop. Dis. 2011, 5, e1198. [Google Scholar] [CrossRef]
- Puelles, V.G.; Lutgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Lopes-Pacheco, M.; Silva, P.L.; Cruz, F.F.; Battaglini, D.; Robba, C.; Pelosi, P.; Morales, M.M.; Caruso Neves, C.; Rocco, P.R.M. Pathogenesis of Multiple Organ Injury in COVID-19 and Potential Therapeutic Strategies. Front. Physiol. 2021, 12, 593223. [Google Scholar] [CrossRef] [PubMed]
- Stolarski, A.E.; Kim, J.; Zhang, Q.; Remick, D.G. Cytokine Drizzle-The Rationale for Abandoning “Cytokine Storm”. Shock 2021, 56, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Remy, K.E.; Mazer, M.; Striker, D.A.; Ellebedy, A.H.; Walton, A.H.; Unsinger, J.; Blood, T.M.; Mudd, P.A.; Yi, D.J.; Mannion, D.A.; et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight 2020, 5, e140329. [Google Scholar] [CrossRef] [PubMed]
- Kox, M.; Waalders, N.J.B.; Kooistra, E.J.; Gerretsen, J.; Pickkers, P. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. JAMA 2020, 324, 1565–1567. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. Cytokine “storm”, cytokine “breeze”, or both in COVID-19? Clin. Chem. Lab Med. 2020, 59, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883–890.e2. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, Y.; Cao, L.; Wang, D.; Guo, M.; Jiang, A.; Guo, D.; Hu, W.; Yang, J.; Tang, Z.; et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020, 9, 761–770. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet. Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Webb, B.J.; Peltan, I.D.; Jensen, P.; Hoda, D.; Hunter, B.; Silver, A.; Starr, N.; Buckel, W.; Grisel, N.; Hummel, E.; et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: A cohort study. Lancet Rheumatol. 2020, 2, e754–e763. [Google Scholar] [CrossRef]
- Suntharalingam, G.; Perry, M.R.; Ward, S.; Brett, S.J.; Castello-Cortes, A.; Brunner, M.D.; Panoskaltsis, N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006, 355, 1018–1028. [Google Scholar] [CrossRef]
- Alegre, M.L.; Gastaldello, K.; Abramowicz, D.; Kinnaert, P.; Vereerstraeten, P.; De Pauw, L.; Vandenabeele, P.; Moser, M.; Leo, O.; Goldman, M. Evidence that pentoxifylline reduces anti-CD3 monoclonal antibody-induced cytokine release syndrome. Transplantation 1991, 52, 674–679. [Google Scholar] [CrossRef]
- Winkler, U.; Jensen, M.; Manzke, O.; Schulz, H.; Diehl, V.; Engert, A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 1999, 94, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, J.; Yakoub-Agha, I. Chimeric antigen-receptor T-cell therapy for hematological malignancies and solid tumors: Clinical data to date, current limitations and perspectives. Curr. Res. Transl. Med. 2017, 65, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Maus, M.V.; Grupp, S.A.; Porter, D.L.; June, C.H. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014, 123, 2625–2635. [Google Scholar] [CrossRef]
- Porter, D.L.; Hwang, W.T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef]
- Makhija, R.; Kingsnorth, A.N. Cytokine storm in acute pancreatitis. J. Hepatobiliary Pancreat. Surg 2002, 9, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shao, M.; Zeng, X.; Qian, P.; Huang, H. Signaling pathways in the regulation of cytokine release syndrome in human diseases and intervention therapy. Signal. Transduct. Target Ther. 2021, 6, 367. [Google Scholar] [CrossRef]
- Bermejo-Martin, J.F.; Andaluz-Ojeda, D.; Almansa, R.; Gandia, F.; Gomez-Herreras, J.I.; Gomez-Sanchez, E.; Heredia-Rodriguez, M.; Eiros, J.M.; Kelvin, D.J.; Tamayo, E. Defining immunological dysfunction in sepsis: A requisite tool for precision medicine. J. Infect. 2016, 72, 525–536. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Schmieg, R.E., Jr.; Hui, J.J.; Chang, K.C.; Osborne, D.F.; Freeman, B.D.; Cobb, J.P.; Buchman, T.G.; et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001, 166, 6952–6963. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, B.G.; Park, C.J.; Kim, S.; Kim, D.H.; Jang, S.; Hong, S.K.; Chi, H.S. An extended leukocyte differential count (16 types of circulating leukocytes) using the cytodiff flow cytometric system can provide informations for the discrimination of sepsis severity and prediction of outcome in sepsis patients. Cytom. B Clin. Cytom. 2013, 86, 244–256. [Google Scholar] [CrossRef]
- Monserrat, J.; de Pablo, R.; Diaz-Martin, D.; Rodriguez-Zapata, M.; de la Hera, A.; Prieto, A.; Alvarez-Mon, M. Early alterations of B cells in patients with septic shock. Crit. Care 2013, 17, R105. [Google Scholar] [CrossRef]
- Darden, D.B.; Bacher, R.; Brusko, M.A.; Knight, P.; Hawkins, R.B.; Cox, M.C.; Dirain, M.L.; Ungaro, R.; Nacionales, D.C.; Rincon, J.C.; et al. Single Cell RNA-SEQ of Human Myeloid Derived Suppressor Cells in Late Sepsis Reveals Multiple Subsets with Unique Transcriptional Responses: A Pilot Study. Shock 2020, 55, 587–595. [Google Scholar] [CrossRef]
- Davis, D.M.; Dustin, M.L. What is the importance of the immunological synapse? Trends Immunol. 2004, 25, 323–327. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Datta, D.; Wilson, J.; Assi, V.; Stephen, J.; Weir, C.J.; Rennie, J.; Antonelli, J.; Bateman, A.; Felton, J.M.; et al. Early PREdiction of sepsis using leukocyte surface biomarkers: The ExPRES-sepsis cohort study. Intens. Care Med. 2018, 44, 1836–1848. [Google Scholar] [CrossRef] [PubMed]
- Pena, O.M.; Hancock, D.G.; Lyle, N.H.; Linder, A.; Russell, J.A.; Xia, J.; Fjell, C.D.; Boyd, J.H.; Hancock, R.E. An Endotoxin Tolerance Signature Predicts Sepsis and Organ Dysfunction at Initial Clinical Presentation. EBioMedicine 2014, 1, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Mathur, P.; Kumar, S.; Gupta, A.; Gupta, D.; John, N.V.; Varghese, P.; Misra, M.C. Depressed Monocytic Activity may be a Predictor for Sepsis. J. Lab. Phys. 2015, 7, 26–31. [Google Scholar] [CrossRef]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef]
- Deutschman, C.S.; Tracey, K.J. Sepsis: Current dogma and new perspectives. Immunity 2014, 40, 463–475. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef]
- Rossman, J.S.; Lamb, R.A. Influenza virus assembly and budding. Virology 2011, 411, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Pillai, P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014, 14, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Durbin, R.K.; Kotenko, S.V.; Durbin, J.E. Interferon induction and function at the mucosal surface. Immunol. Rev. 2013, 255, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Pothlichet, J.; Meunier, I.; Davis, B.K.; Ting, J.P.; Skamene, E.; von Messling, V.; Vidal, S.M. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog. 2013, 9, e1003256. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zuo, X.; Zhang, S.; Ouyang, Z.; Jiang, S.; Wang, F.; Wang, G. The Mechanism behind Influenza Virus Cytokine Storm. Viruses 2021, 13, 1362. [Google Scholar] [CrossRef]
- Crosse, K.M.; Monson, E.A.; Beard, M.R.; Helbig, K.J. Interferon-Stimulated Genes as Enhancers of Antiviral Innate Immune Signaling. J. Innate. Immun. 2018, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Boxx, G.M.; Cheng, G. The Roles of Type I Interferon in Bacterial Infection. Cell Host Microbe 2016, 19, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, P.; Castiglia, V.; Ivin, M.; Ebner, F. Type I Interferons in Bacterial Infections: A Balancing Act. Front. Immunol. 2016, 7, 652. [Google Scholar] [CrossRef] [PubMed]
- Green, E.R.; Mecsas, J. Bacterial Secretion Systems: An Overview. Microbiol. Spectr. 2016, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Reber, A.J.; Chirkova, T.; Kim, J.H.; Cao, W.; Biber, R.; Shay, D.K.; Sambhara, S. Immunosenescence and Challenges of Vaccination against Influenza in the Aging Population. Aging Dis. 2012, 3, 68–90. [Google Scholar] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef]
- Mannick, J.B.; Morris, M.; Hockey, H.P.; Roma, G.; Beibel, M.; Kulmatycki, K.; Watkins, M.; Shavlakadze, T.; Zhou, W.; Quinn, D.; et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. 2018, 10, aaq1564. [Google Scholar] [CrossRef]
- Bektas, A.; Schurman, S.H.; Gonzalez-Freire, M.; Dunn, C.A.; Singh, A.K.; Macian, F.; Cuervo, A.M.; Sen, R.; Ferrucci, L. Age-associated changes in human CD4(+) T cells point to mitochondrial dysfunction consequent to impaired autophagy. Aging 2019, 11, 9234–9263. [Google Scholar] [CrossRef]
- Salminen, A. Activation of immunosuppressive network in the aging process. Ageing Res. Rev. 2020, 57, 100998. [Google Scholar] [CrossRef]
- He, W.; Xiao, K.; Fang, M.; Xie, L. Immune Cell Number, Phenotype, and Function in the Elderly with Sepsis. Aging Dis. 2021, 12, 277–296. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef]
- Gavazzi, G.; Krause, K.H. Ageing and infection. Lancet. Infect. Dis. 2002, 2, 659–666. [Google Scholar] [CrossRef]
- Werner, H.; Kuntsche, J. Infection in the elderly--what is different? Z. Gerontol. Geriatr. 2000, 33, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Girard, T.D.; Opal, S.M.; Ely, E.W. Insights into severe sepsis in older patients: From epidemiology to evidence-based management. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 40, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Girard, T.D.; Ely, E.W. Bacteremia and sepsis in older adults. Clin. Geriatr. Med. 2007, 23, 633–647. [Google Scholar] [CrossRef]
- Norman, D.C. Fever in the elderly. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000, 31, 148–151. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Nicholson, D.W. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 2006, 6, 813–822. [Google Scholar] [CrossRef]
- Delano, M.J.; Ward, P.A. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol. Rev. 2016, 274, 330–353. [Google Scholar] [CrossRef]
- Hansen, S.; Baptiste, K.E.; Fjeldborg, J.; Horohov, D.W. A review of the equine age-related changes in the immune system: Comparisons between human and equine aging, with focus on lung-specific immune-aging. Ageing Res. Rev. 2015, 20, 11–23. [Google Scholar] [CrossRef]
- Drew, W.; Wilson, D.V.; Sapey, E. Inflammation and neutrophil immunosenescence in health and disease: Targeted treatments to improve clinical outcomes in the elderly. Exp. Gerontol. 2018, 105, 70–77. [Google Scholar] [CrossRef]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef]
- Hinojosa, C.A.; Akula Suresh Babu, R.; Rahman, M.M.; Fernandes, G.; Boyd, A.R.; Orihuela, C.J. Elevated A20 contributes to age-dependent macrophage dysfunction in the lungs. Exp. Gerontol. 2014, 54, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Oliveira, A.; Smith-Carvalho, M.; Porto, L.C.; Cardoso-Oliveira, J.; Ribeiro Ados, S.; Falcao, R.R.; Abdelhay, E.; Bouzas, L.F.; Thuler, L.C.; Ornellas, M.H.; et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum. Immunol. 2011, 72, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Pera, A.; Sanchez-Correa, B.; Alonso, C.; Lopez-Fernandez, I.; Morgado, S.; Tarazona, R.; Solana, R. Effect of age and CMV on NK cell subpopulations. Exp. Gerontol. 2014, 54, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Chidrawar, S.M.; Khan, N.; Chan, Y.L.; Nayak, L.; Moss, P.A. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun. Ageing 2006, 3, 10. [Google Scholar] [CrossRef]
- Le Garff-Tavernier, M.; Beziat, V.; Decocq, J.; Siguret, V.; Gandjbakhch, F.; Pautas, E.; Debre, P.; Merle-Beral, H.; Vieillard, V. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell 2010, 9, 527–535. [Google Scholar] [CrossRef]
- Hazeldine, J.; Hampson, P.; Lord, J.M. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell 2012, 11, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Verschoor, C.P.; Johnstone, J.; Millar, J.; Dorrington, M.G.; Habibagahi, M.; Lelic, A.; Loeb, M.; Bramson, J.L.; Bowdish, D.M. Blood CD33(+)HLA-DR(-) myeloid-derived suppressor cells are increased with age and a history of cancer. J. Leukoc. Biol. 2013, 93, 633–637. [Google Scholar] [CrossRef]
- Lu, X.; Yang, Y.M.; Lu, Y.Q. Immunosenescence: A Critical Factor Associated With Organ Injury After Sepsis. Front. Immunol. 2022, 13, 917293. [Google Scholar] [CrossRef]
- Davenport, E.E.; Burnham, K.L.; Radhakrishnan, J.; Humburg, P.; Hutton, P.; Mills, T.C.; Rautanen, A.; Gordon, A.C.; Garrard, C.; Hill, A.V.; et al. Genomic landscape of the individual host response and outcomes in sepsis: A prospective cohort study. Lancet. Respir. Med. 2016, 4, 259–271. [Google Scholar] [CrossRef]
- Nierhaus, A.; Montag, B.; Timmler, N.; Frings, D.P.; Gutensohn, K.; Jung, R.; Schneider, C.G.; Pothmann, W.; Brassel, A.K.; Schulte Am Esch, J. Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive Care Med. 2003, 29, 646–651. [Google Scholar] [CrossRef]
- Monneret, G.; Lepape, A.; Voirin, N.; Bohe, J.; Venet, F.; Debard, A.L.; Thizy, H.; Bienvenu, J.; Gueyffier, F.; Vanhems, P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006, 32, 1175–1183. [Google Scholar] [CrossRef]
- Meisel, C.; Schefold, J.C.; Pschowski, R.; Baumann, T.; Hetzger, K.; Gregor, J.; Weber-Carstens, S.; Hasper, D.; Keh, D.; Zuckermann, H.; et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: A double-blind, randomized, placebo-controlled multicenter trial. Am. J. Respir. Crit. Care Med. 2009, 180, 640–648. [Google Scholar] [CrossRef]
- Scicluna, B.P.; van Vught, L.A.; Zwinderman, A.H.; Wiewel, M.A.; Davenport, E.E.; Burnham, K.L.; Nurnberg, P.; Schultz, M.J.; Horn, J.; Cremer, O.L.; et al. Classification of patients with sepsis according to blood genomic endotype: A prospective cohort study. Lancet. Respir. Med. 2017, 5, 816–826. [Google Scholar] [CrossRef]
- Kreitmann, L.; Bodinier, M.; Fleurie, A.; Imhoff, K.; Cazalis, M.A.; Peronnet, E.; Cerrato, E.; Tardiveau, C.; Conti, F.; Llitjos, J.F.; et al. Mortality Prediction in Sepsis With an Immune-Related Transcriptomics Signature: A Multi-Cohort Analysis. Front. Med. 2022, 9, 930043. [Google Scholar] [CrossRef]
- Wong, H.R.; Cvijanovich, N.Z.; Anas, N.; Allen, G.L.; Thomas, N.J.; Bigham, M.T.; Weiss, S.L.; Fitzgerald, J.; Checchia, P.A.; Meyer, K.; et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am. J. Respir. Crit. Care Med. 2015, 191, 309–315. [Google Scholar] [CrossRef]
- Skirecki, T.; Mikaszewska-Sokolewicz, M.; Hoser, G.; Zielinska-Borkowska, U. The Early Expression of HLA-DR and CD64 Myeloid Markers Is Specifically Compartmentalized in the Blood and Lungs of Patients with Septic Shock. Mediat. Inflamm. 2016, 2016, 3074902. [Google Scholar] [CrossRef]
- Rasid, O.; Cavaillon, J.M. Compartment diversity in innate immune reprogramming. Microbes Infect. 2018, 20, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Azad, T.D.; Donato, M.; Haynes, W.A.; Perumal, T.M.; Henao, R.; Bermejo-Martin, J.F.; Almansa, R.; Tamayo, E.; Howrylak, J.A.; et al. Unsupervised Analysis of Transcriptomics in Bacterial Sepsis Across Multiple Datasets Reveals Three Robust Clusters. Crit. Care Med. 2018, 46, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Konig, R.; Kolte, A.; Ahlers, O.; Oswald, M.; Krauss, V.; Roell, D.; Sommerfeld, O.; Dimopoulos, G.; Tsangaris, I.; Antoniadou, E.; et al. Use of IFNgamma/IL10 Ratio for Stratification of Hydrocortisone Therapy in Patients With Septic Shock. Front. Immunol. 2021, 12, 607217. [Google Scholar] [CrossRef]
- Shakoory, B.; Carcillo, J.A.; Chatham, W.W.; Amdur, R.L.; Zhao, H.; Dinarello, C.A.; Cron, R.Q.; Opal, S.M. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit. Care Med. 2016, 44, 275–281. [Google Scholar] [CrossRef]
- Panacek, E.A.; Marshall, J.C.; Albertson, T.E.; Johnson, D.H.; Johnson, S.; MacArthur, R.D.; Miller, M.; Barchuk, W.T.; Fischkoff, S.; Kaul, M.; et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit. Care Med. 2004, 32, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Antcliffe, D.B.; Burnham, K.L.; Al-Beidh, F.; Santhakumaran, S.; Brett, S.J.; Hinds, C.J.; Ashby, D.; Knight, J.C.; Gordon, A.C. Transcriptomic Signatures in Sepsis and a Differential Response to Steroids. From the VANISH Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 980–986. [Google Scholar] [CrossRef]
- Storch, G.A. Diagnostic virology. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000, 31, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Cobo, F. Application of molecular diagnostic techniques for viral testing. Open Virol. J. 2012, 6, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewski, R.A.; Jones, H.E.; Gersuk, V.H.; Russell, P.; Simpson, A.; Brealey, D.; Walker, J.; Thomas, M.; Whitehouse, T.; Ostermann, M.; et al. Presymptomatic diagnosis of postoperative infection and sepsis using gene expression signatures. Intensive Care Med. 2022, 48, 1133–1143. [Google Scholar] [CrossRef]
- Leticia Fernandez-Carballo, B.; Escadafal, C.; MacLean, E.; Kapasi, A.J.; Dittrich, S. Distinguishing bacterial versus non-bacterial causes of febrile illness—A systematic review of host biomarkers. J. Infect. 2021, 82, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Wong, H.R.; Khatri, P. Robust classification of bacterial and viral infections via integrated host gene expression diagnostics. Sci. Transl. Med. 2016, 8, 346ra391. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Crosby, S.D.; Storch, G.A. Gene expression profiles in febrile children with defined viral and bacterial infection. Proc. Natl. Acad. Sci. USA 2013, 110, 12792–12797. [Google Scholar] [CrossRef]
- Parnell, G.P.; McLean, A.S.; Booth, D.R.; Armstrong, N.J.; Nalos, M.; Huang, S.J.; Manak, J.; Tang, W.; Tam, O.Y.; Chan, S.; et al. A distinct influenza infection signature in the blood transcriptome of patients with severe community-acquired pneumonia. Crit. Care 2012, 16, R157. [Google Scholar] [CrossRef]
- Sampson, D.L.; Fox, B.A.; Yager, T.D.; Bhide, S.; Cermelli, S.; McHugh, L.C.; Seldon, T.A.; Brandon, R.A.; Sullivan, E.; Zimmerman, J.J.; et al. A Four-Biomarker Blood Signature Discriminates Systemic Inflammation Due to Viral Infection Versus Other Etiologies. Sci. Rep. 2017, 7, 2914. [Google Scholar] [CrossRef]
- Xu, N.; Hao, F.; Dong, X.; Yao, Y.; Guan, Y.; Yang, L.; Chen, F.; Zheng, F.; Li, Q.; Liu, W.; et al. A two-transcript biomarker of host classifier genes for discrimination of bacterial from viral infection in acute febrile illness: A multicentre discovery and validation study. Lancet Digit Health 2021, 3, e507–e516. [Google Scholar] [CrossRef]
| Mediator (Abbreviation) | Main Source | Major Function | |
|---|---|---|---|
| Cytokines | |||
| IL-1 | macrophages, pyroptotic cells, epithelial cells | Proinflammatory; pyrogenic function; activation of macrophage and TH17 cells | |
| IL-2 | T cells | Immune response; Teff and Treg cell growth factor; T-cell differentiation | |
| IL-4 | TH2 cells, basophils, eosinophils, mast cells, NK cells | Anti-inflammatory; TH2 differentiation; adhesion; chemotaxis | |
| IL-6 | T cells, macrophages, endothelial cells | Proinflammatory; pleiotropic; pyrogenic function; acute phase response; lymphoid differentiation; increased antibody production, | |
| IL-9 | TH9 cells | Pleiotropic; stimulation of B, T, and NK cells; protection from helminth infections; activation of mast cells; association with type I interferon in COVID-19 | |
| IL-10 | regulatory T cells, TH9 cells | Anti-inflammatory; inhibition of macrophage activation; inhibition of TH1 cells and cytokine release | |
| IL-12 | dendritic cells, macrophages | Stimulation of T and NK cells; activation of TH1 pathway; induction of interferon-γ from TH1 cells; cytotoxic T cells and NK cells; acting in synergy with interleukin-18 | |
| IL-13 | TH2 cells | Anti-inflammatory; differentiation of B cells; mediator of humoral immunity | |
| IL-17 | TH17 cells, NK cells, group 3 innate lymphoid cells | Protection from bacterial and fungal infections; promotion of neutrophilic inflammation | |
| IL-18 | monocytes, macrophages, dendritic cells | Proinflammatory; activation of TH1 pathway; synergistic with interleukin-12 | |
| IL-31 | TH2 cells, macrophages, mast cells, dendritic cells | Proinflammatory; cell-mediated immunity | |
| IL-33 | macrophages, dendritic cells, mast cells, epithelial cells | Proinflammatory; amplification of TH1 and TH2 cells; activation of cytotoxic T cells, NK cells, and mast cells | |
| Type I Interferon | virtually all body cells | Dendritic cell activation/maturation/migration/survival; enhancement of the activity of NK and T/B cells; induction of antiviral effector molecules; antagonism to the action of interferon-γ | |
| Interferon-γ(Type II IFN) | TH1 cells, cytotoxic T cells, group 1 innate lymphoid cells, NK cells | Proinflammatory; activation of monocytes and macrophages | |
| Lymphotoxin α | activated lymphocytes | Pleiotropic; activation of NF-κB pathway | |
| TGF-β | Treg cells, monocytes, macrophages, fibroblasts, epithelial cells, cancer cells | Immunosuppressive; regulation of proliferation, differentiation, apoptosis, and adhesion; inhibition of hematopoiesis | |
| Tumor necrosis factor | T cells, NK cells, mast cells, macrophages | Pyrogenic; increasing vascular permeability | |
| Chemokines | |||
| MCP-1 | CCL2 | macrophages, dendritic cells, cardiac myocytes | Pyrogenic; recruitment of TH1 cells, NK cells, macrophages, eosinophils, and dendritic cells |
| MIP-1α | CCL3 | monocytes, neutrophils, dendritic cells, NK cells, mast cells | Recruitment of TH1 cells, NK cells, macrophages, and dendritic cells |
| MIP-1β | CCL4 | macrophages, neutrophils, endothelium | Recruitment of B cells, CD4+ T cells, and dendritic cells |
| IL-8 | CXCL8 | macrophages, epithelial cells | Recruitment of neutrophils |
| MIG | CXCL9 | monocytes, endothelial cells, keratinocytes | Interferon-inducible chemokine; recruitment of TH1 cells, NK cells, and plasmacytoid dendritic cells |
| IP-10 | CXCL10 | monocytes, endothelial cells, keratinocytes | Interferon-inducible chemokine; recruitment of TH1 cells, NK cells, and macrophages |
| BLC | CXCL13 | B cells, follicular dendritic cells | Recruitment of TH1 cells, monocytes, dendritic cells, and basophils |
| Plasma proteins | |||
| CRP | hepatocytes | Interleukin-6 increases CRP expression, interleukin-8 and MCP-1 secretion; | |
| Complement | hepatocytes, other cells | In cytokine storm, activation of complement contributes to tissue damage, inhibition may reduce immunopathologic effects | |
| Study | Number of Patients | Diagnosis/Prediction | Commonly Used Markers/Comparators | New Biomarkers | Variables | AUC |
|---|---|---|---|---|---|---|
| Kweon et al. [11] | n = 118 (73 sepsis; 45 SIRS or healthy controls) | Sepsis | PCT IL-6 | sCD14-ST (Presepsin) | sCD14-ST PCT IL-6 hs-CRP | 0.937 0.915 0.869 0.853 |
| Lu et al. [12] | 115 patients (72 sepsis; 43 SIRS or healthy controls) | Sepsis | PCT CRP | sCD14-ST (Presepsin) | sCD14-ST PCT CRP | 0.954 0.847 0.859 |
| Aksaray et al. [13] | n = 90 (52 sepsis; 38 SIRS) | Differentiation Sepsis–SIRS | PCT APACHE II | sTREM-1 | sTREM-1 PCT APACHE II | 0.78 0.65 0.71 |
| Brenner et al. [14] | n = 90 (60 septic shock; 30 healthy controls) | Septic shock | PCT IL-6 CRP | sTREM-1 | sTREM-1 IL-6 PCT CRP | 0.955 0.898 0.844 0.791 |
| Khater et al. [15] | n = 80 (40 sepsis; 40 healthy controls) | Sepsis | Lactate | suPAR | suPAR Lactate | 0.99 0.84 |
| Yin et al. [16] | n = 171 (151 sepsis; 20 healthy controls) | Sepsis | PCT CRP SOFA | CD64 | CD64 PCT SOFA CRP | 0.879 0.868 0.701 0.609 |
| Larsson et al. [17] | n = 271 (77 sepsis; 194 non-sepsis) | Sepsis | PCT | Calprotectin | CaPT PCT | 0.67 0.55 |
| Spoto et al. [18] | n = 159 (109 sepsis; 50 healthy controls) | Sepsis | PCT SOFA | MR-proADM | MR-proADM PCT SOFA | 0.817 0.884 0.774 |
| Hamed et al. [19] | n = 290 (213 sepsis; 77 healthy controls) | Sepsis | PCT IL-6 CRP | PTX-3 | PTX-3 PCT IL-6 CRP | 0.92 0.92 0.91 0.82 |
| Casagranda et al. [20] | n = 130 (Sepsis) | 28-day mortality | Lactate | suPAR | suPAR Lactate | 0.77 0.70 |
| Chen et al. [21] | n = 66 (25 septic shock; 11 sepsis; 30 healthy controls) | 28-day mortality | APACHE II | HMGB-1 | HMGB-1 IL-10 APACHE II | 0.946 0.877 0.846 |
| Andaluz-Ojeda et al. [22] | n = 326 (Sepsis) | 28-day mortality | PCT CRP Lactate SOFA | MR-proADM | MR-proADM SOFA Lactate PCT CRP | 0.79 0.75 0.71 0.61 0.54 |
| Kim H et al. [23] | n = 215 (Sepsis) | 30-day mortality | SOFA | Bio-ADM | Bio-ADM SOFA | 0.827 0.830 |
| Seol et al. [24] | n = 145 (Sepsis) | 28-day mortality | SOFA | Angiopoietin | SOFA Angpt2/1 ratio | 0.745 0.736 |
| Fang et al. [25] | n = 388 (333 sepsis; 55 healthy controls) | 28-day mortality | PCT | Angiopoietin | Angpt2/1 ratio PCT | 0.845 0.732 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarczak, D.; Nierhaus, A. Cytokine Storm—Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. https://doi.org/10.3390/ijms231911740
Jarczak D, Nierhaus A. Cytokine Storm—Definition, Causes, and Implications. International Journal of Molecular Sciences. 2022; 23(19):11740. https://doi.org/10.3390/ijms231911740
Chicago/Turabian StyleJarczak, Dominik, and Axel Nierhaus. 2022. "Cytokine Storm—Definition, Causes, and Implications" International Journal of Molecular Sciences 23, no. 19: 11740. https://doi.org/10.3390/ijms231911740
APA StyleJarczak, D., & Nierhaus, A. (2022). Cytokine Storm—Definition, Causes, and Implications. International Journal of Molecular Sciences, 23(19), 11740. https://doi.org/10.3390/ijms231911740







